Mechanical Characteristics of Individualized Biodegradable Augmentation Scaffold—In Vitro Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
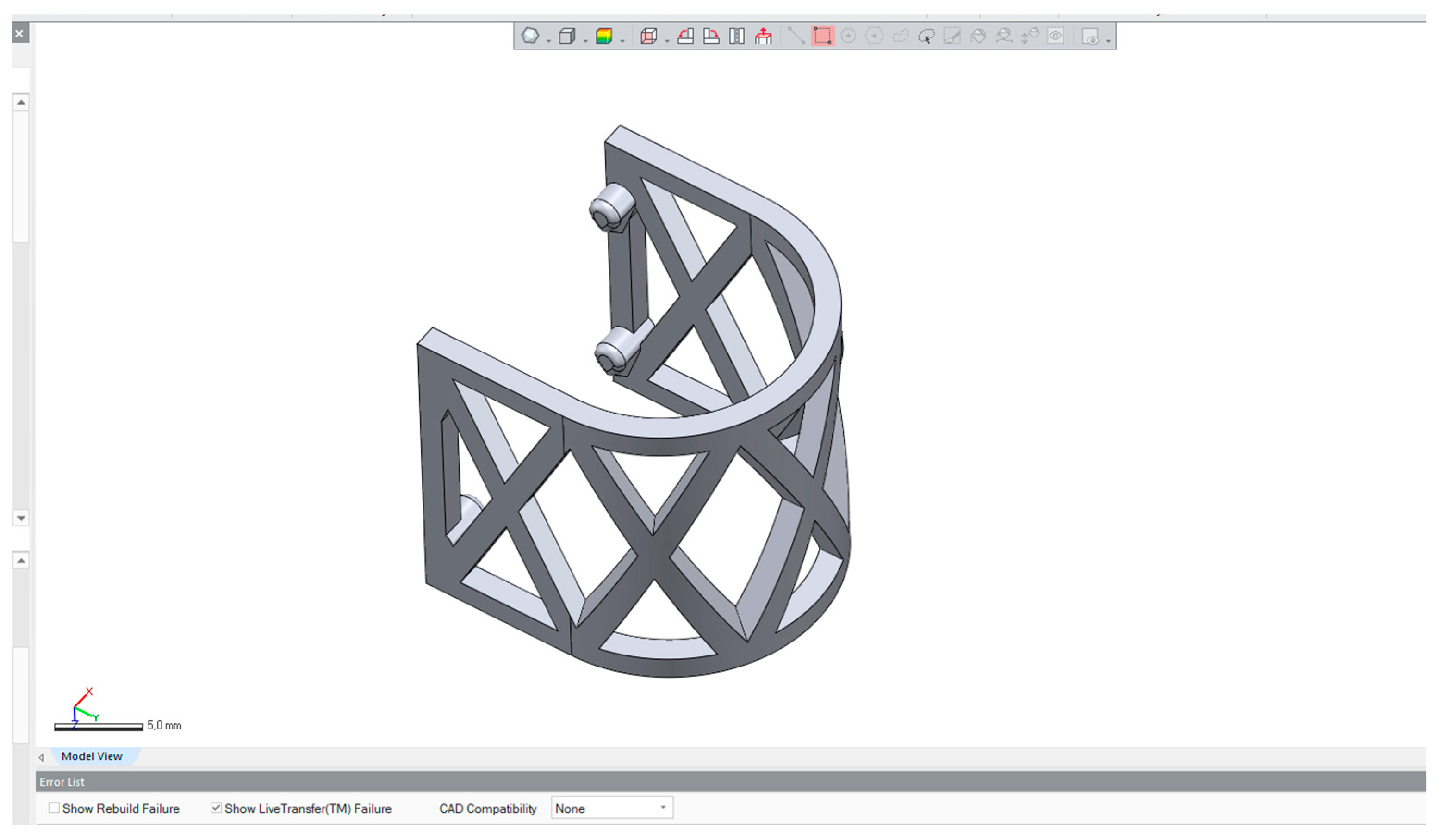
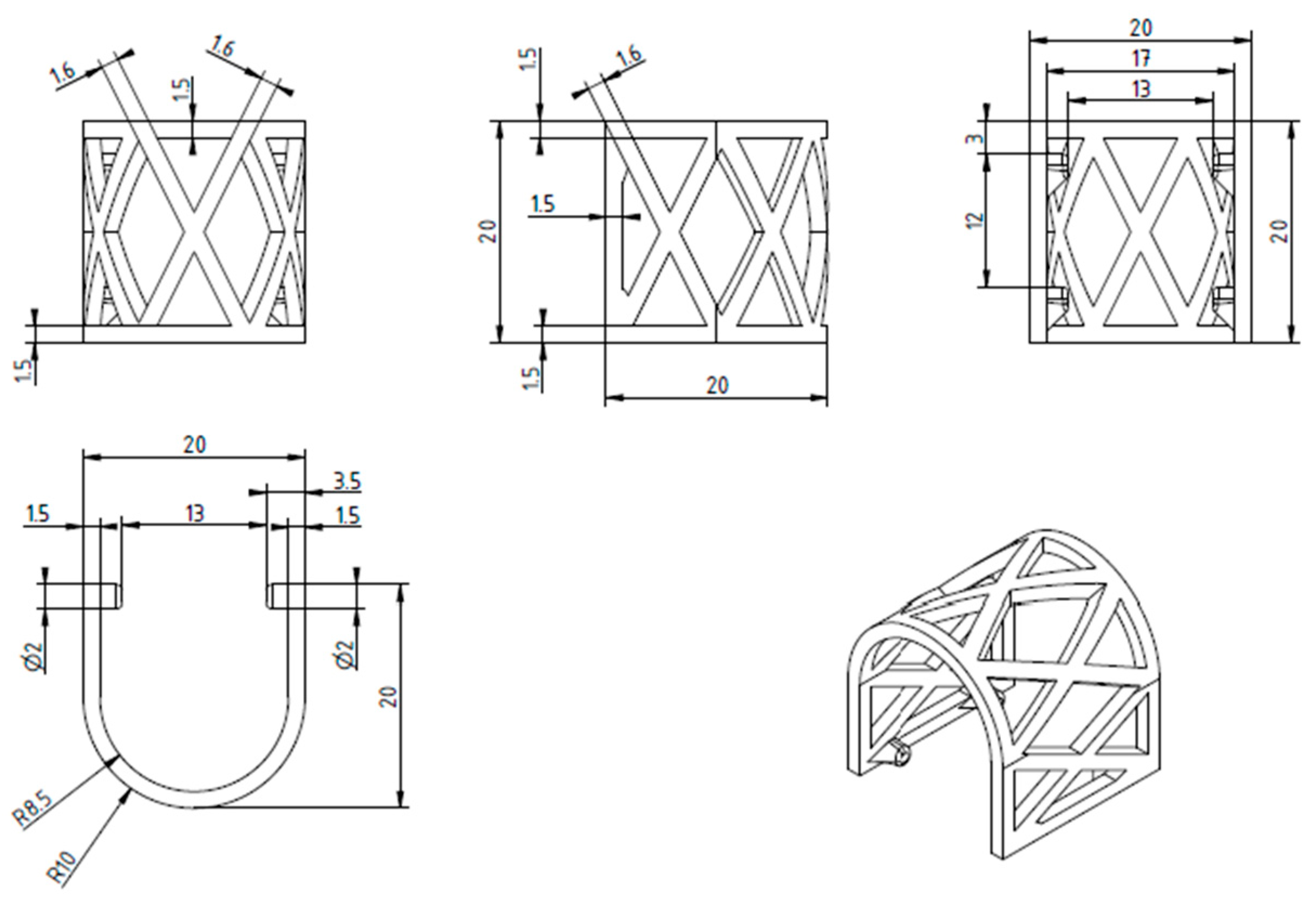
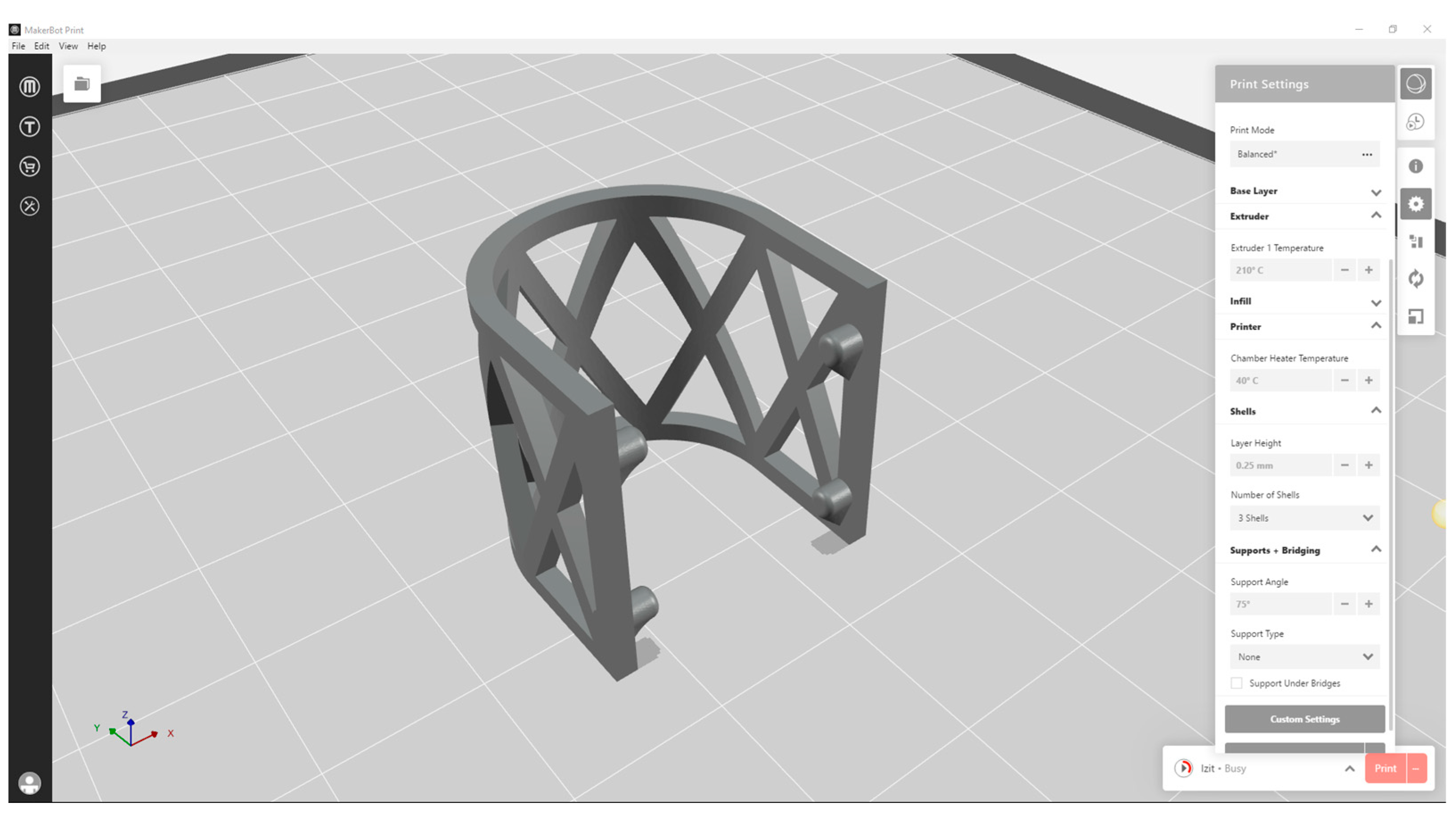
2.1. The Design and 3D Printing of PLA Scaffolds
2.2. Finite Element Method (FEM) Analysis of PLA Scaffold
2.2.1. Scaffold Geometry and Meshing
2.2.2. Physical and Mechanical Properties of Lactoprene® 7415
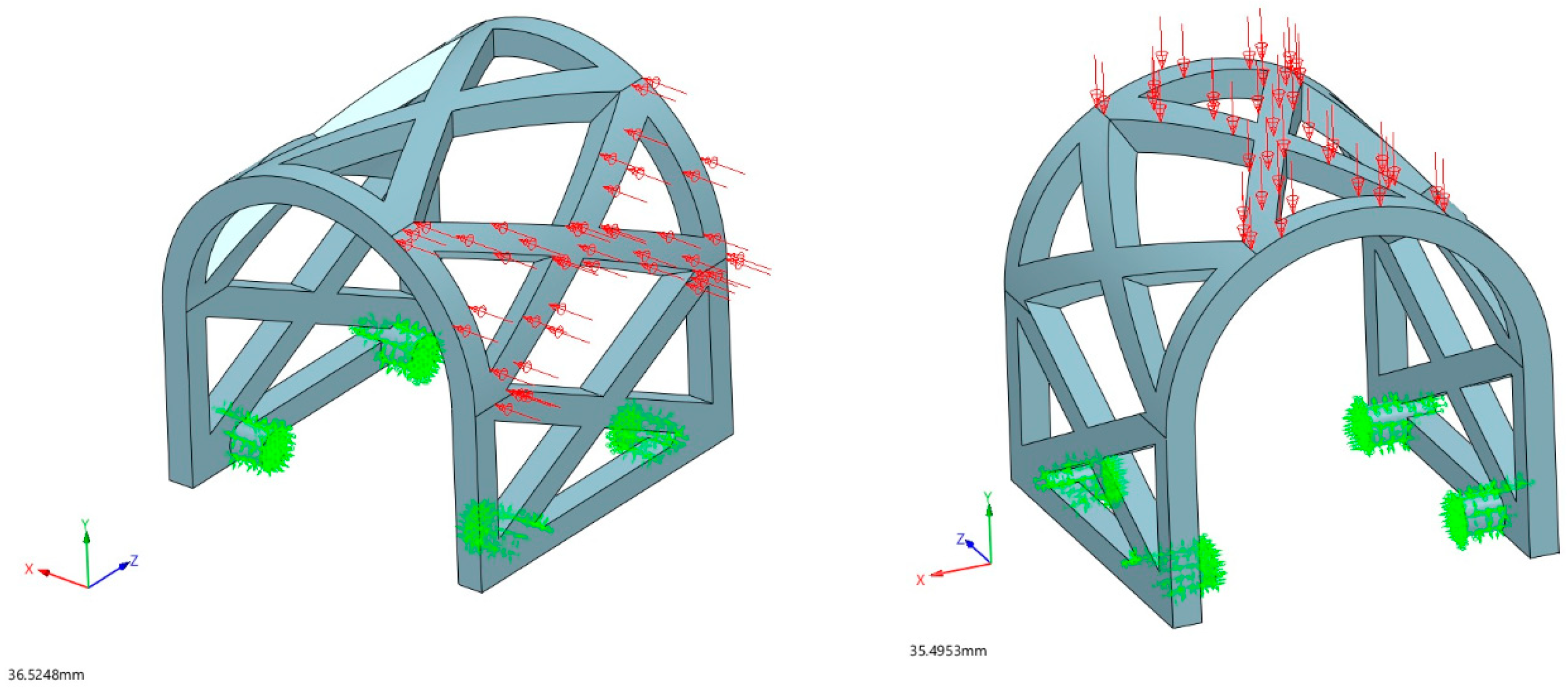
2.2.3. Loading Conditions
2.3. Compression Testing Procedure
- Group 1 comprised 27 Lactoprene® 7415 scaffolds for occlusal compression testing.
- Group 2 comprised 27 Lactoprene® 7415 scaffolds for lateral compression testing.
2.4. Biodegradation of the Scaffolds
2.5. Compressive Modulus of the Scaffold
2.6. Statistical Analysis
3. Results and Discussion
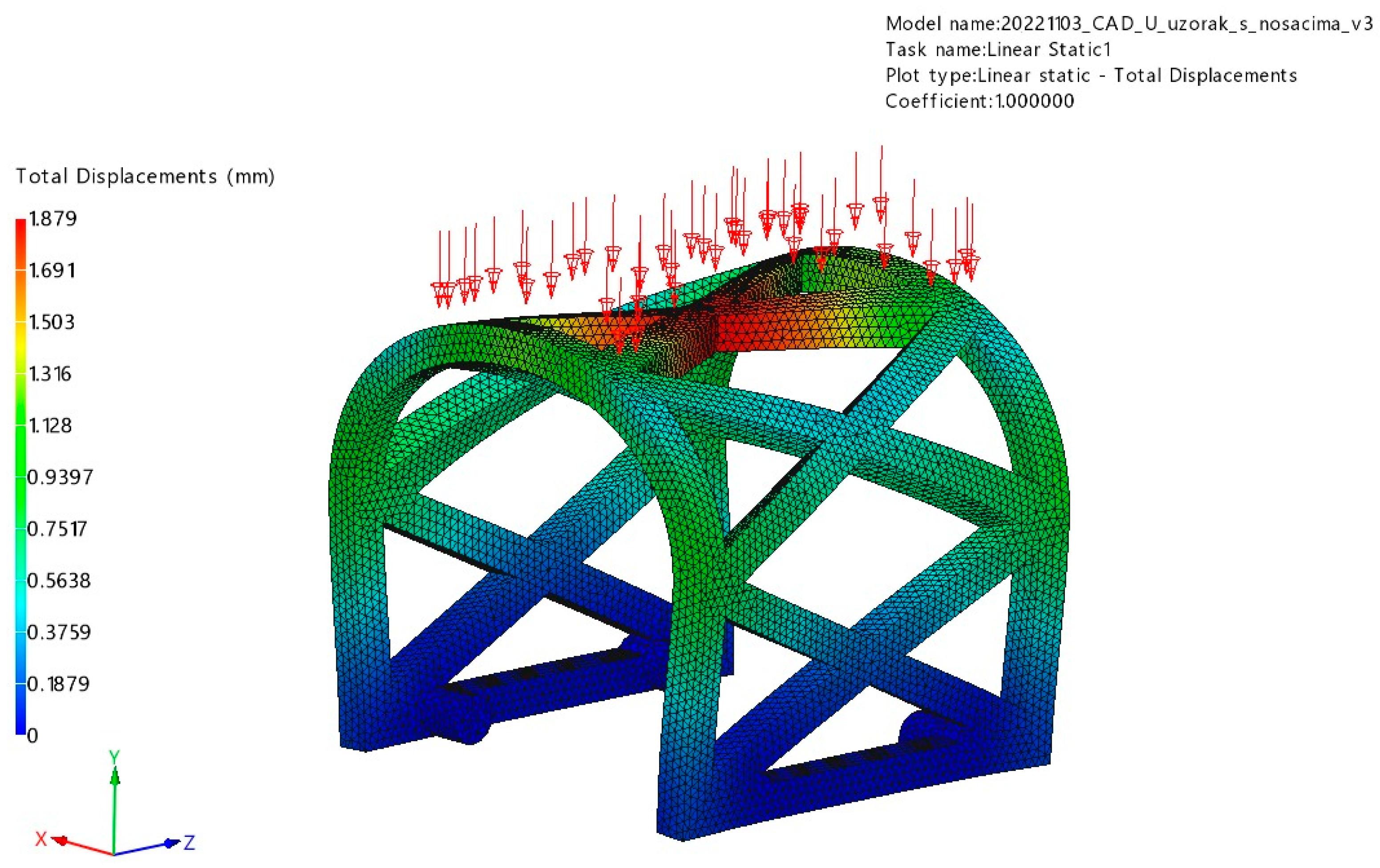
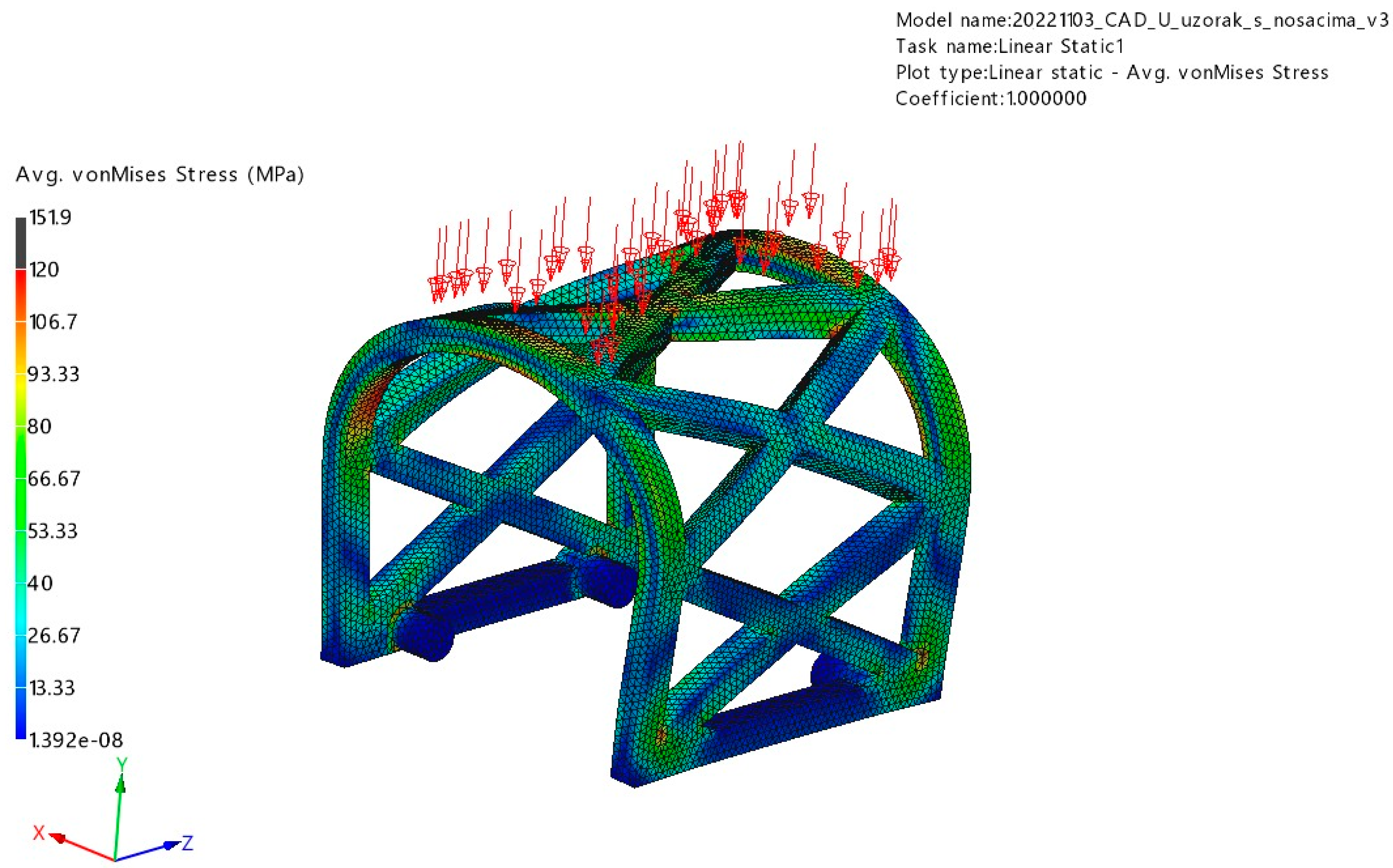
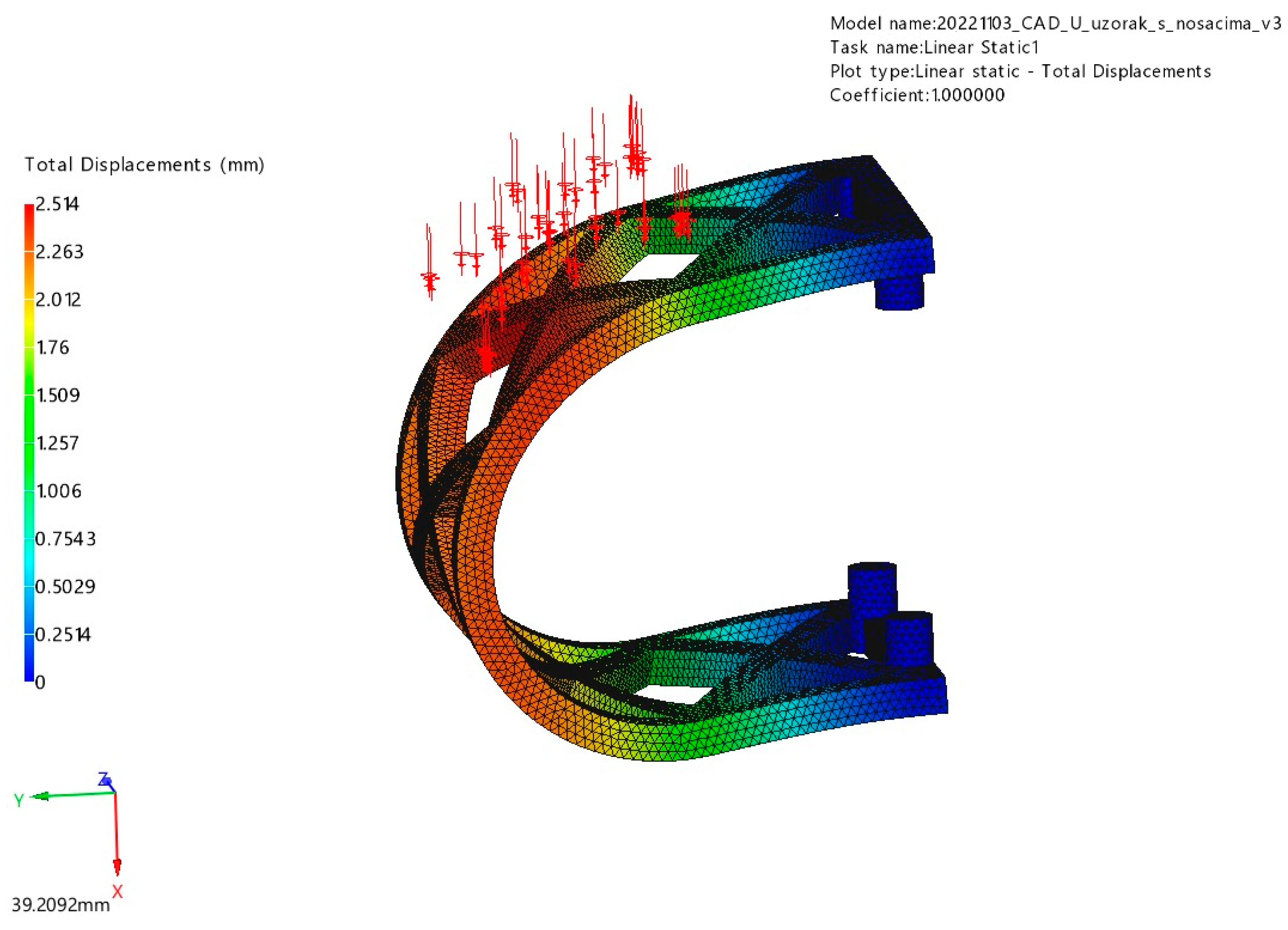
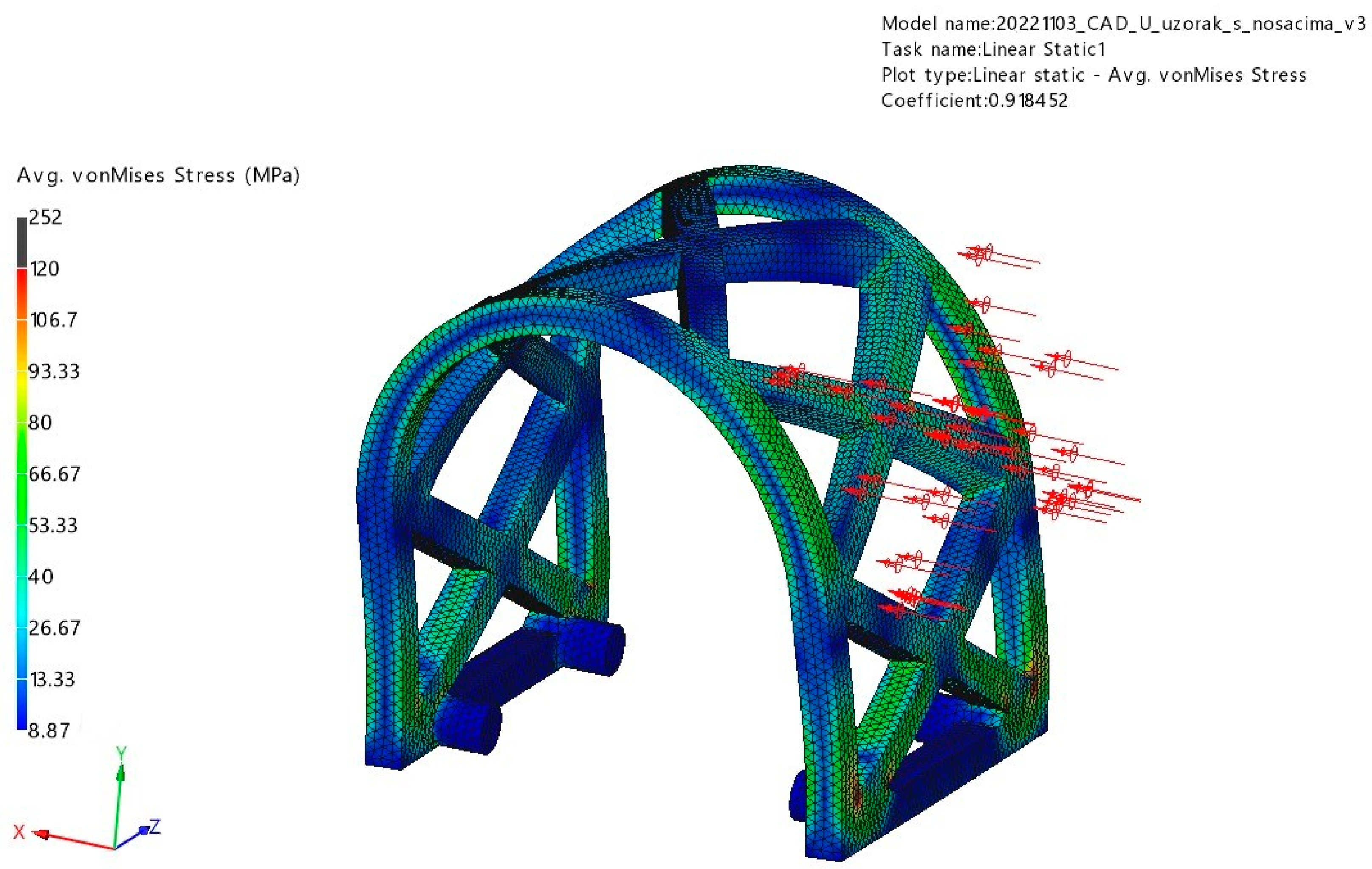
3.1. FEM Analysis
3.1.1. Occlusal Loading
3.1.2. Lateral Loading
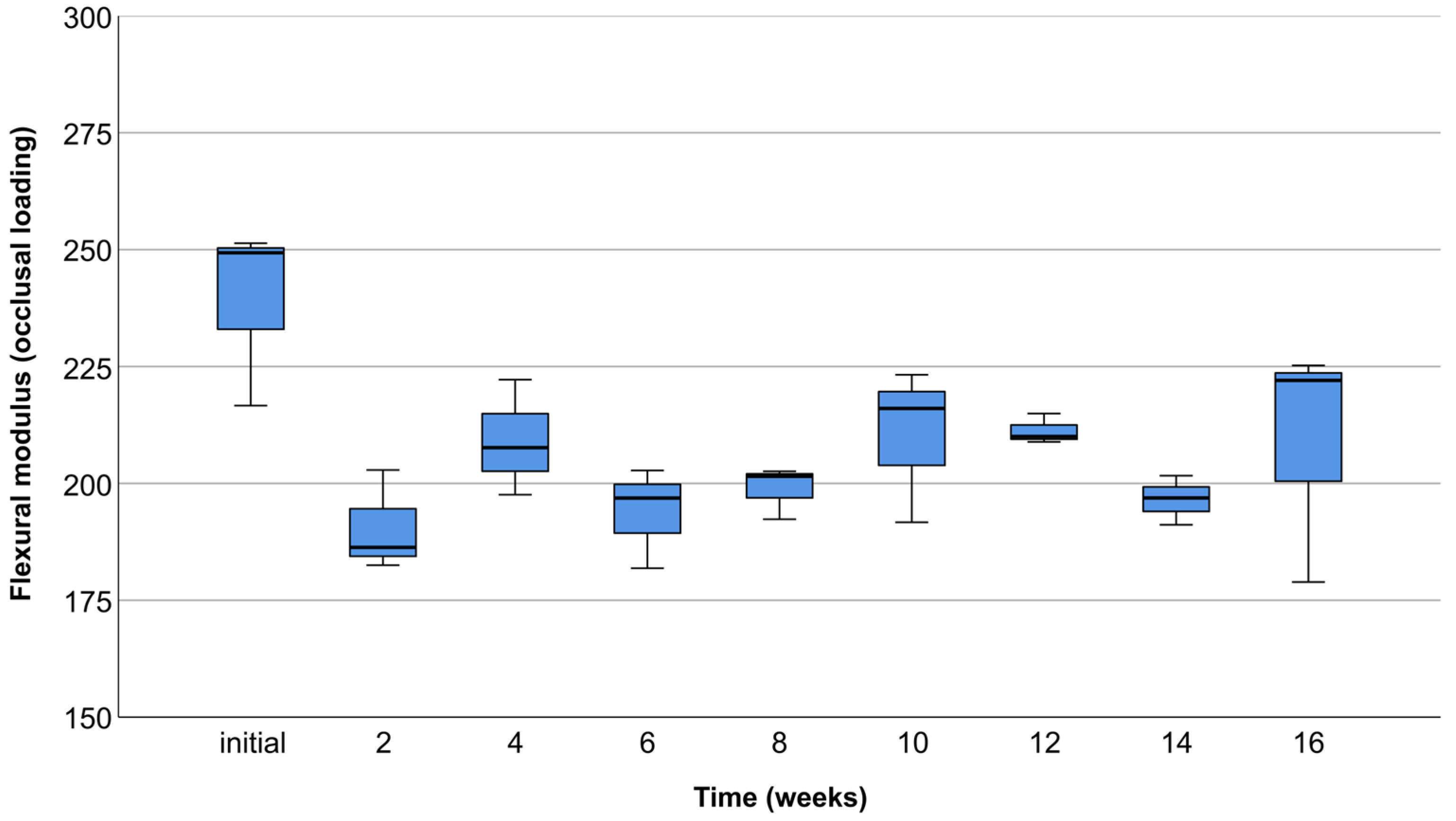
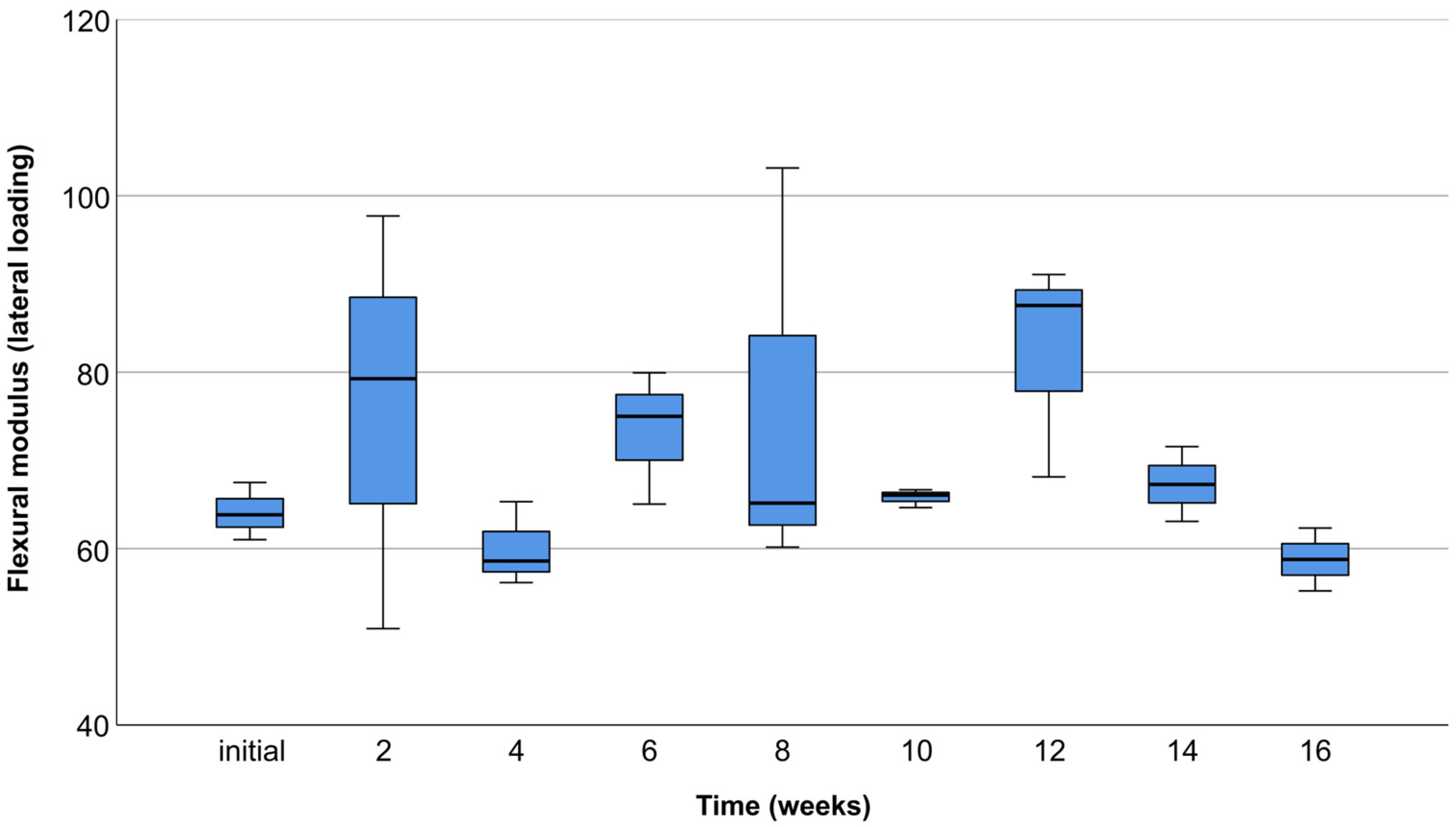
3.2. Occlusal Compression Test
3.3. Lateral Compression Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Araú Jo, M.; Lindhe, J. Dynamics of Bone Tissue Formation in Tooth Extraction Sites An Experimental Study in Dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef]
- Araújo, M.G.; Silva, C.O.; Misawa, M.; Sukekava, F. Alveolar Socket Healing: What Can We Learn? Periodontol. 2000 2015, 68, 122–134. [Google Scholar] [CrossRef]
- Allen, E.P.; Gainza, C.S.; Farthing, G.G.; Newbold, D.A. Improved Technique for Localized Ridge Augmentation: A Report of 21 Cases. J. Periodontol. 1985, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.F.; Zitzmann, N.U. Long-Term Outcome of Implants Placed with Guided Bone Regeneration (GBR) Using Resorbable and Non-Resorbable Membranes after 12–14 Years. Clin. Oral Implants Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Urban, I.A.; Montero, E.; Monje, A.; Sanz-Sánchez, I. Effectiveness of Vertical Ridge Augmentation Interventions: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2019, 46, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Andre, A.; Ogle, O.E. Vertical and Horizontal Augmentation of Deficient Maxilla and Mandible for Implant Placement. Dent. Clin. N. Am. 2021, 65, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, J.; Pickert, F.N.; Sánchez-Labrador, L.; Tresguerres, F.G.F.; Martínez-González, J.M.; Meniz-García, C. Horizontal Ridge Augmentation: A Comparison between Khoury and Urban Technique. Biology 2021, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Kang, T.; Fien, M.J. Titanium Mesh as an Alternative to a Membrane for Ridge Augmentation. J. Oral Maxillofac. Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef]
- Khoury, F.; Hanser, T. Mandibular Bone Block Harvesting from the Retromolar Region: A 10-Year Prospective Clinical Study. Int. J. Oral Maxillofac. Implant. 2015, 30, 688–697. [Google Scholar] [CrossRef]
- Ersanli, S.; Arisan, V.; Bedeloğlu, E. Evaluation of the Autogenous Bone Block Transfer for Dental Implant Placement: Symphysal or Ramus Harvesting? BMC Oral Health 2016, 16, 4. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Toledano, M.; Manzano-Moreno, F.J.; Vallecillo, C.; Vallecillo-Rivas, M.; Rodriguez-Archilla, A.; Osorio, R. Alveolar Bone Ridge Augmentation Using Polymeric Membranes: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 1172. [Google Scholar] [CrossRef]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials Design for Bone-Tissue Engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Xu, D.; Shi, J.; Qiu, R.; Lei, W.; Yu, W. Comparative Investigations on Properties of Three Kinds of FDM 3D-Printed Natural Plant Powder/Poly(Lactic Acid) Biocomposites. Polymers 2023, 15, 557. [Google Scholar] [CrossRef]
- Athanasiou, K.; Agrawal, C.; Barber, F.; Burkhart, S. Orthopaedic Applications for PLA-PGA Biodegradable Polymers. Arthroscopy 1998, 14, 726–737. [Google Scholar] [CrossRef]
- Madan, R.; Mohan, R.; Bains, V.K.; Gupta, V.; Singh, G.P.; Madan, M. Analysis of Socket Preservation Using Polylactide and Polyglycolide (PLA-PGA) Sponge: A Clinical, Radiographic, and Histologic Study. Int. J. Period. Res. Dent. 2014, 34, 36–42. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, B.; Liu, F.; Duan, J.; Wang, S.; Qiu, J.; Li, D.; Sang, Y.; Liu, C.; Liu, D.; et al. Polylactic Acid Nanopillar Array-Driven Osteogenic Differentiation of Human Adipose-Derived Stem Cells Determined by Pillar Diameter. Nano Lett. 2018, 18, 2243–2253. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Holmes, B.; Faucett, S.; Zhang, L.G. Three-Dimensional Printing of Nanomaterial Scaffolds for Complex Tissue Regeneration. Tissue Eng. Part. B Rev. 2015, 21, 103–114. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kariz, M.; Kwon, J.H.; Kitek Kuzman, M. Effect of Printing Layer Thickness on Water Absorption and Mechanical Properties of 3D-Printed Wood/PLA Composite Materials. Int. J. Adv. Manuf. Technol. 2019, 102, 2195–2200. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Ibad, H.; Suchal, Z.A.; Gosain, A.K. Implementation of 3D Printing and Computer-Aided Design and Manufacturing (CAD/CAM) in Craniofacial Reconstruction. J. Craniofac. Surg. 2022, 33, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Chiba, H.; Takahashi, H.; Toyoda, J.; Abukawa, H. Clinical Application of a Custom-Made Bioresorbable Raw Particulate Hydroxyapatite/Poly-L-Lactide Mesh Tray for Mandibular Reconstruction. Odontology 2010, 98, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Holmes, J.; Fernandes, R. Resorbable Mesh as a Containment System in Reconstruction of the Atrophic Mandible Fracture. J. Oral Maxillofac. Surg. 2004, 62, 719–723. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Du, M.; Zhang, S.; Liu, Y.; Yang, H.; Shi, R.; Guo, Y.; Song, F.; Zhao, Y.; et al. Customized Barrier Membrane (Titanium Alloy, Poly Ether-Ether Ketone and Unsintered Hydroxyapatite/Poly-l-Lactide) for Guided Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 916967. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Moya, M.P.; Rebolledo, C.; Haidar, Z.S.; Alister, J.P.; Olate, S. PLA/PGA and Its Co-Polymers in Alveolar Bone Regeneration. A Systematic Review. Int. J. Odontostomatol. 2019, 13, 258–265. [Google Scholar] [CrossRef]
- Żur, P. Finite Elements Analysis of PLA 3D-Printed Elements and Shape Optimization. Eur. J. Eng. Sci. Technol. 2019, 2, 59–64. [Google Scholar] [CrossRef]
- ISO 13485; Medical Devices-Quality Management Systems-Requirements for Regulatory Purposes. International Organization for Standardization (ISO): Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/59752.html (accessed on 12 July 2023).
- Zhang, Y.R.; Du, W.; Zhou, X.D.; Yu, H.Y. Review of Research on the Mechanical Properties of the Human Tooth. Int. J. Oral Sci. 2014, 6, 61–69. [Google Scholar] [CrossRef]
- Ulrich Sommer, J.; Birk, R.; Hörmann, K.; Stuck, B.A. Evaluation of the Maximum Isometric Tongue Force of Healthy Volunteers. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 3077–3084. [Google Scholar] [CrossRef]
- Noroozi, R.; Shamekhi, M.A.; Mahmoudi, R.; Zolfagharian, A.; Asgari, F.; Mousavizadeh, A.; Bodaghi, M.; Hadi, A.; Haghighipour, N. In Vitro Static and Dynamic Cell Culture Study of Novel Bone Scaffolds Based on 3D-Printed PLA and Cell-Laden Alginate Hydrogel. Biomed. Mater. 2022, 17, 045024. [Google Scholar] [CrossRef]
- ISO 604; Plastics—Determination of Compressive Properties. International Organization for Standardization (ISO): Geneva, Switzerland, 2002. Available online: https://www.iso.org/standard/31261.html (accessed on 12 July 2023).
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA Scaffolds for Bone Tissue Replacement Fabricated by Ordinary Commercial 3D Printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Lactoprene® 7415. 2022. Available online: https://poly-med.com/wp-content/uploads/2022/08/Lactoprene®-Polymer-Series.pdf (accessed on 12 July 2023).
- Lundgren, D.; Sennerby, L.; Falk, H.; Friberg, B.; Nyman, S. The Use of a New Bioresorbable Barrier for Guided Bone Regeneration in Connection with Implant Installation. Case Reports. Clin. Oral Implant. Res. 1994, 5, 177–184. [Google Scholar] [CrossRef]
- Serino, G.; Biancu, S.; Iezzi, G.; Piattelli, A. Ridge Preservation Following Tooth Extraction Using a Polylactide and Polyglycolide Sponge as Space Filler: A Clinical and Histological Study in Humans. Clin. Oral Implant. Res. 2003, 14, 651–658. [Google Scholar] [CrossRef]
- Suzuki, S.; Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Njuguna, M.M.; Nozawa, R.; Kakiuchi, Y.; Nakano, M.; Handa, K.; et al. Correction of Large Jawbone Defect in the Mouse Using Immature Osteoblast-like Cells and a 3D Polylactic Acid Scaffold. PNAS Nexus 2022, 1, 151. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Jin, X.; Xu, J.; Lu, J.; Zhang, C.; Tian, T.; Teng, L. Complications of Absorbable Fixation in Maxillofacial Surgery: A Meta-Analysis. PLoS ONE 2013, 8, 67449. [Google Scholar] [CrossRef]
- Rodríguez-Chessa, J.; Olate, S.; Netto, H.D.; Noia, C.; de Moraes, M.; Mazzonetto, R. In Vitro Resistance of Titanium and Resorbable (Poly l-Co-Dl Lactic Acid) Osteosynthesis in Mandibular Body Fracture. Int. J. Oral Maxillofac. Surg. 2014, 43, 362–366. [Google Scholar] [CrossRef]
- Gleeson, J.; Plunkett, N.; O’Brien, F. Addition of Hydroxyapatite Improves Stiffness, Interconnectivity and Osteogenic Potential of a Highly Porous Collagen-Based Scaffold for Bone Tissue Regeneration. Eur. Cell Mater. 2010, 20, 218–230. [Google Scholar] [CrossRef]
- Abe, G.L.; Sasaki, J.-I.; Katata, C.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Fabrication of Novel Poly(Lactic Acid/Caprolactone) Bilayer Membrane for GBR Application. Dent. Mater. 2020, 36, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.G.; Dalvi, Y.B.; Fijol, N.; Shilpa, J.; Unni, R.; Binsi, P.K.; Varghese, M.G.; Reshmy, R.; Mathew, A.; Sukumaran, A. Fish Scale Derived Hydroxyapatite Incorporated 3D Printed PLA Scaffold for Bone Tissue Engineering. New J. Chem. 2023. accepted. [Google Scholar] [CrossRef]
- Jung, U.; Cha, J.; Vignoletti, F.; Nuñez, J.; Sanz, J.; Sanz, M. Simultaneous Lateral Bone Augmentation and Implant Placement Using a Particulated Synthetic Bone Substitute around Chronic Peri-implant Dehiscence Defects in Dogs. J. Clin. Periodontol. 2017, 44, 1172–1180. [Google Scholar] [CrossRef] [PubMed]



| Material Property | Value | Unit |
|---|---|---|
| Elastic modulus | 3149 | MPa |
| Poisson’s ratio | 0.36 | |
| Shear modulus | 1157 | MPa |
| Mass density | 1240 | Kgm−3 |
| Tensile strength | 40 | MPa |
| Compressive strength | 65 | MPa |
| Yield strength | 77 | MPa |
| Thermal expansion coefficient | 0.000041 | K−1 |
| Thermal conductivity | 0.183 | Wm−1K−1 |
| Specific heat | 1300 | Jkg−1K−1 |
| Material damping ratio | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjelica, R.; Prpić, V.; Drvar, N.; Ćatić, A.; Gabrić, D. Mechanical Characteristics of Individualized Biodegradable Augmentation Scaffold—In Vitro Pilot Study. Materials 2024, 17, 1419. https://doi.org/10.3390/ma17061419
Bjelica R, Prpić V, Drvar N, Ćatić A, Gabrić D. Mechanical Characteristics of Individualized Biodegradable Augmentation Scaffold—In Vitro Pilot Study. Materials. 2024; 17(6):1419. https://doi.org/10.3390/ma17061419
Chicago/Turabian StyleBjelica, Roko, Vladimir Prpić, Nenad Drvar, Amir Ćatić, and Dragana Gabrić. 2024. "Mechanical Characteristics of Individualized Biodegradable Augmentation Scaffold—In Vitro Pilot Study" Materials 17, no. 6: 1419. https://doi.org/10.3390/ma17061419





