Advanced Solid Geopolymer Formulations for Refractory Applications
Abstract
:1. Introduction
2. Experimental Details
2.1. Raw Material Properties
2.2. Design Mix Proportions
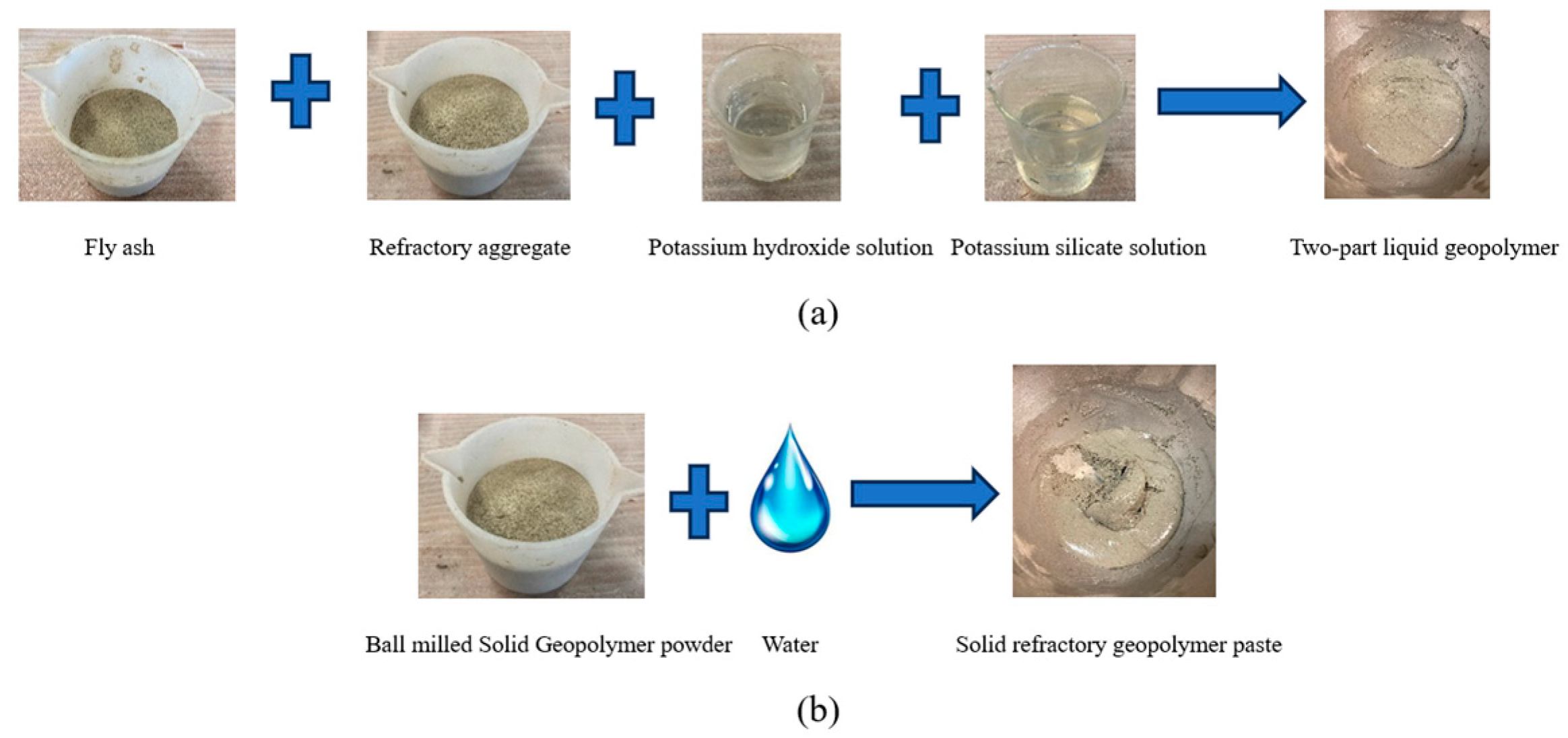
2.3. Experimental Methodology
3. Results and Discussion
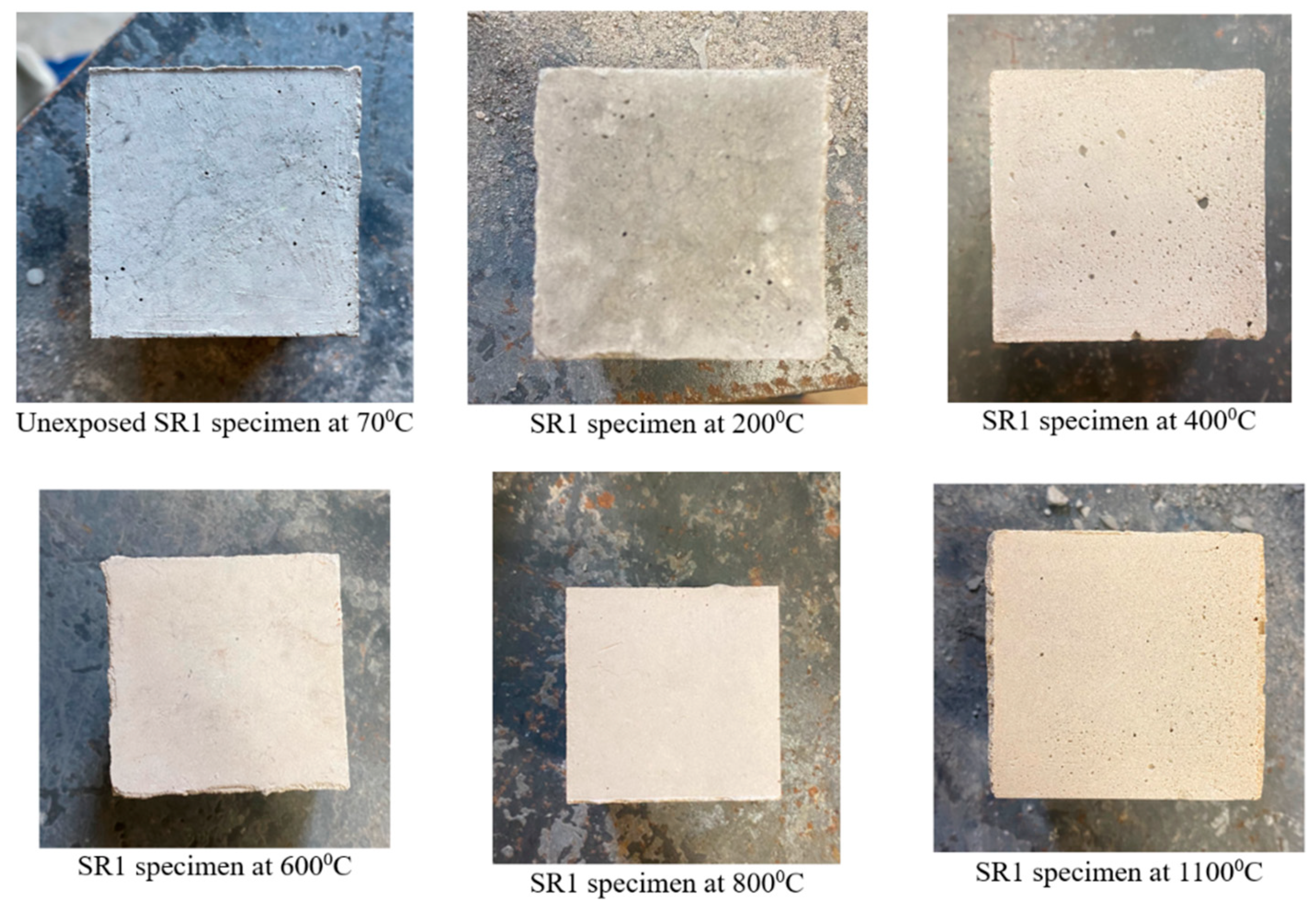
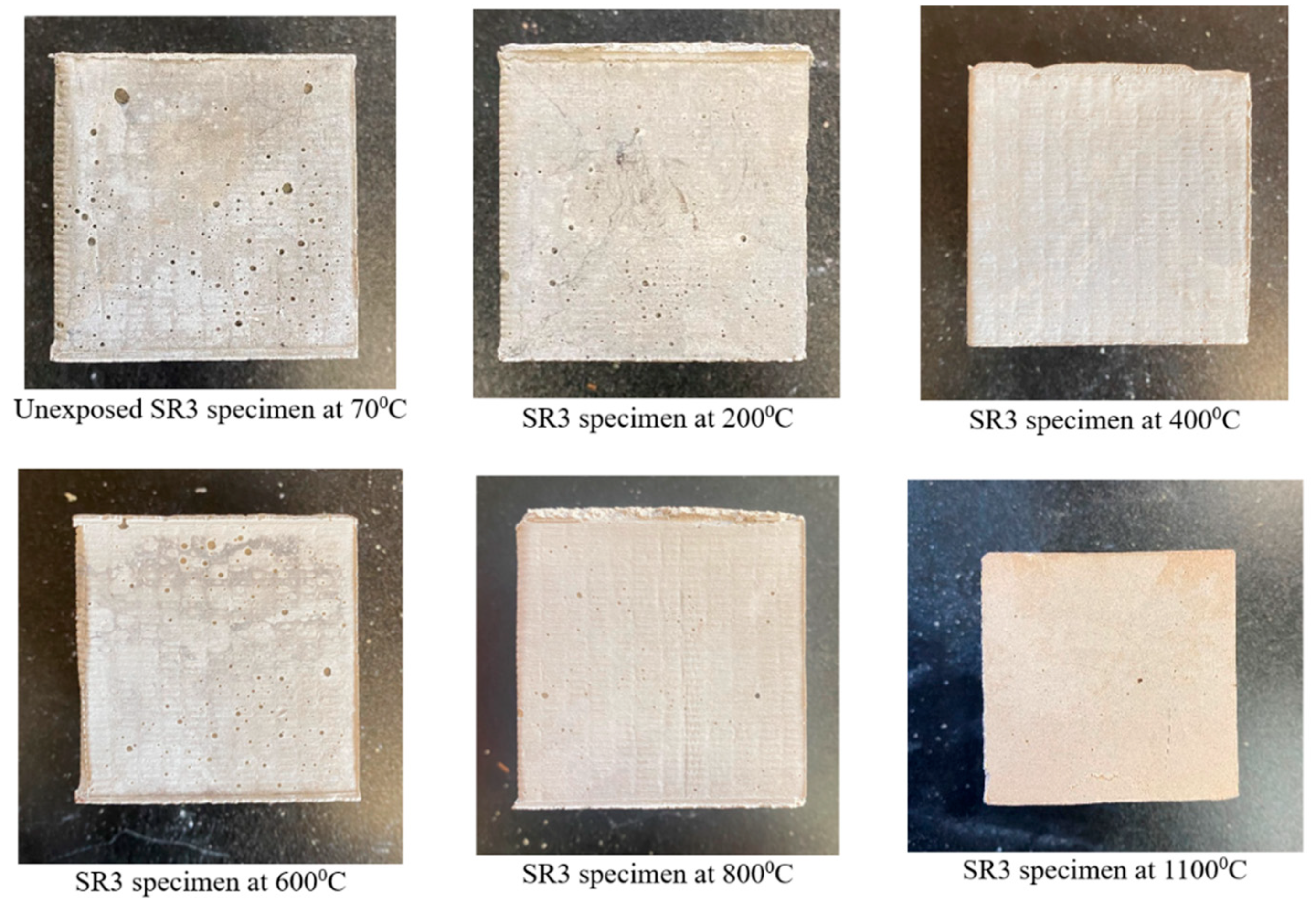
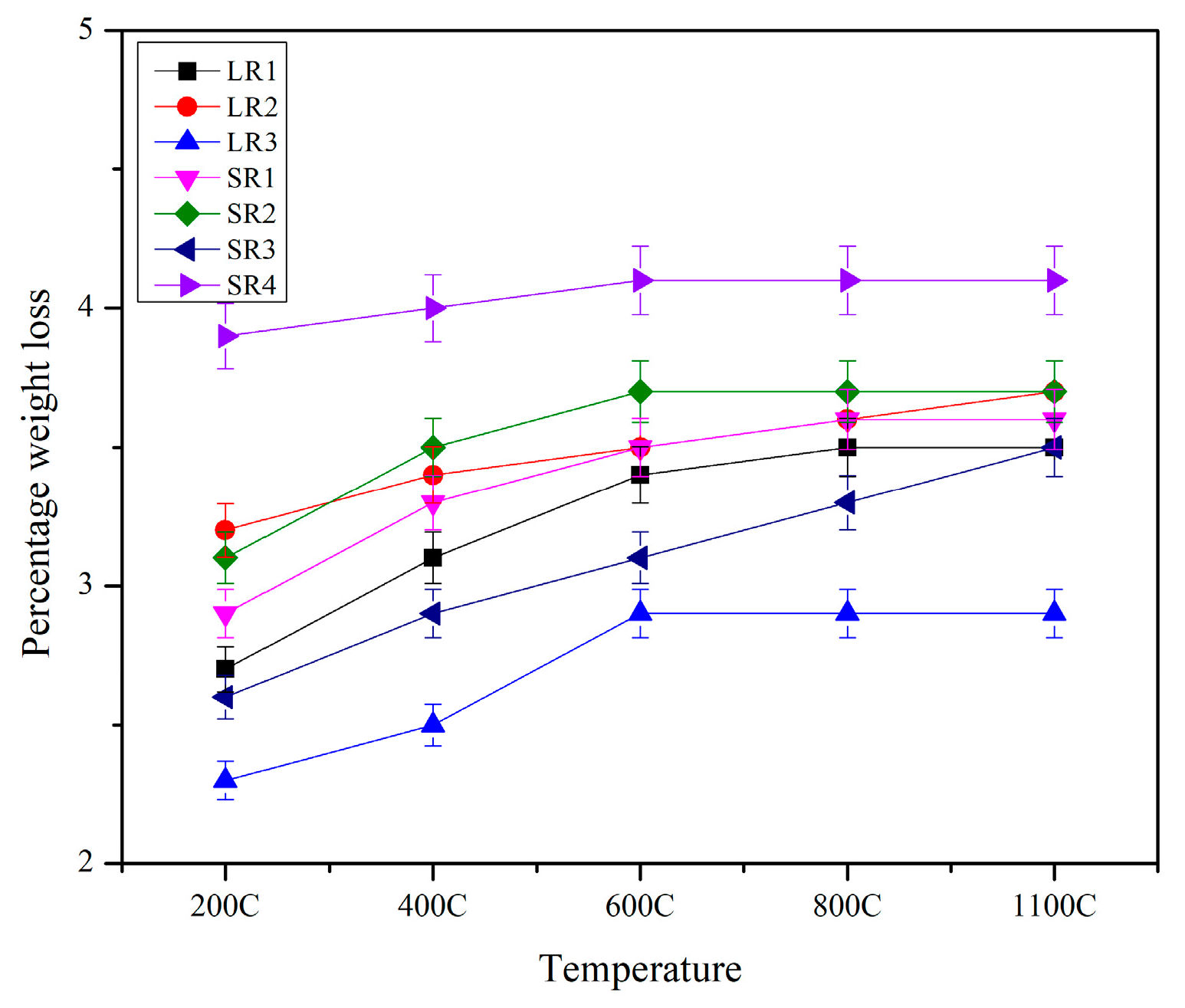
3.1. Physical Appearance and Weight Loss
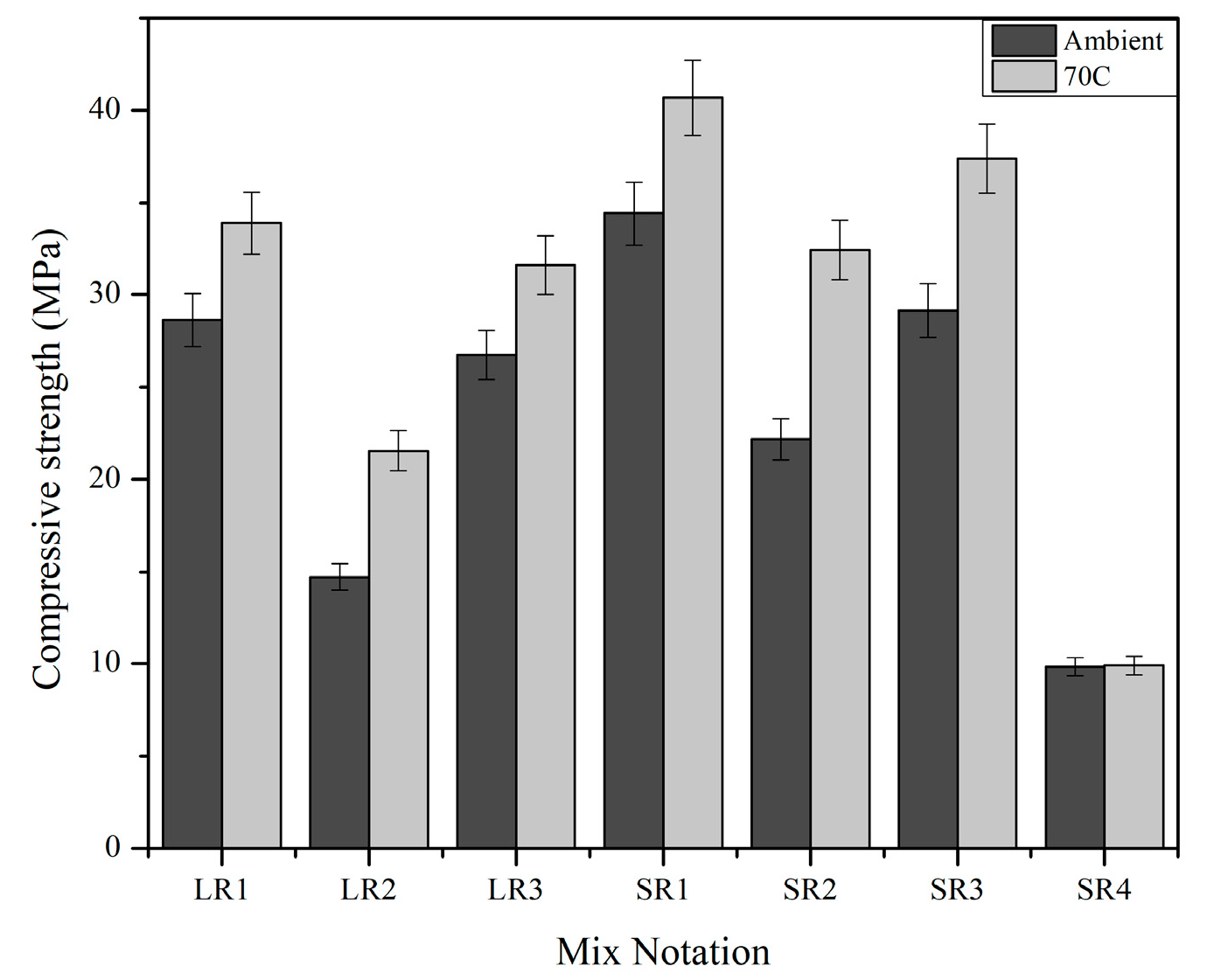
3.2. Compressive Strength
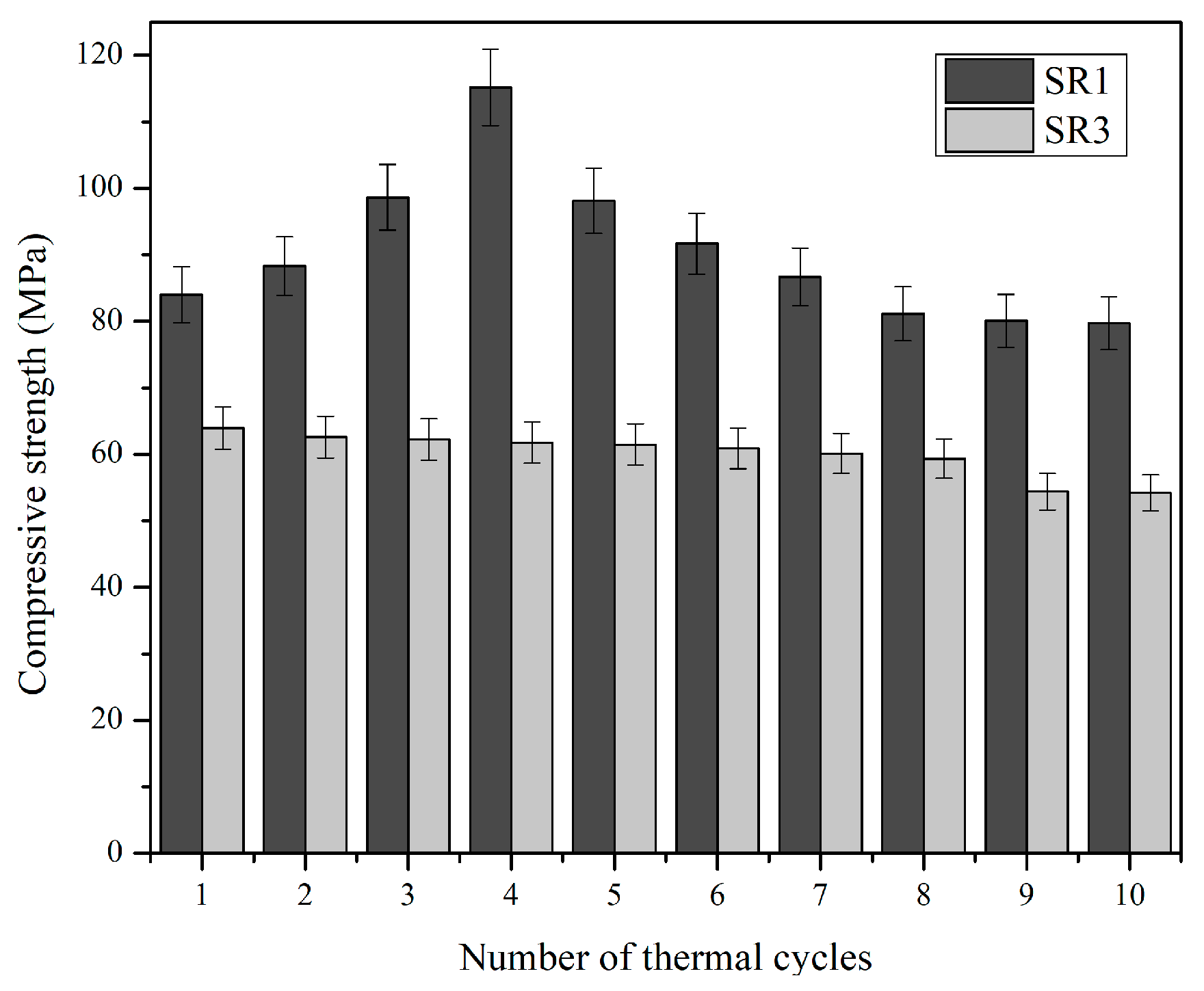
3.3. Thermal Fatigue Resistance of Geopolymers
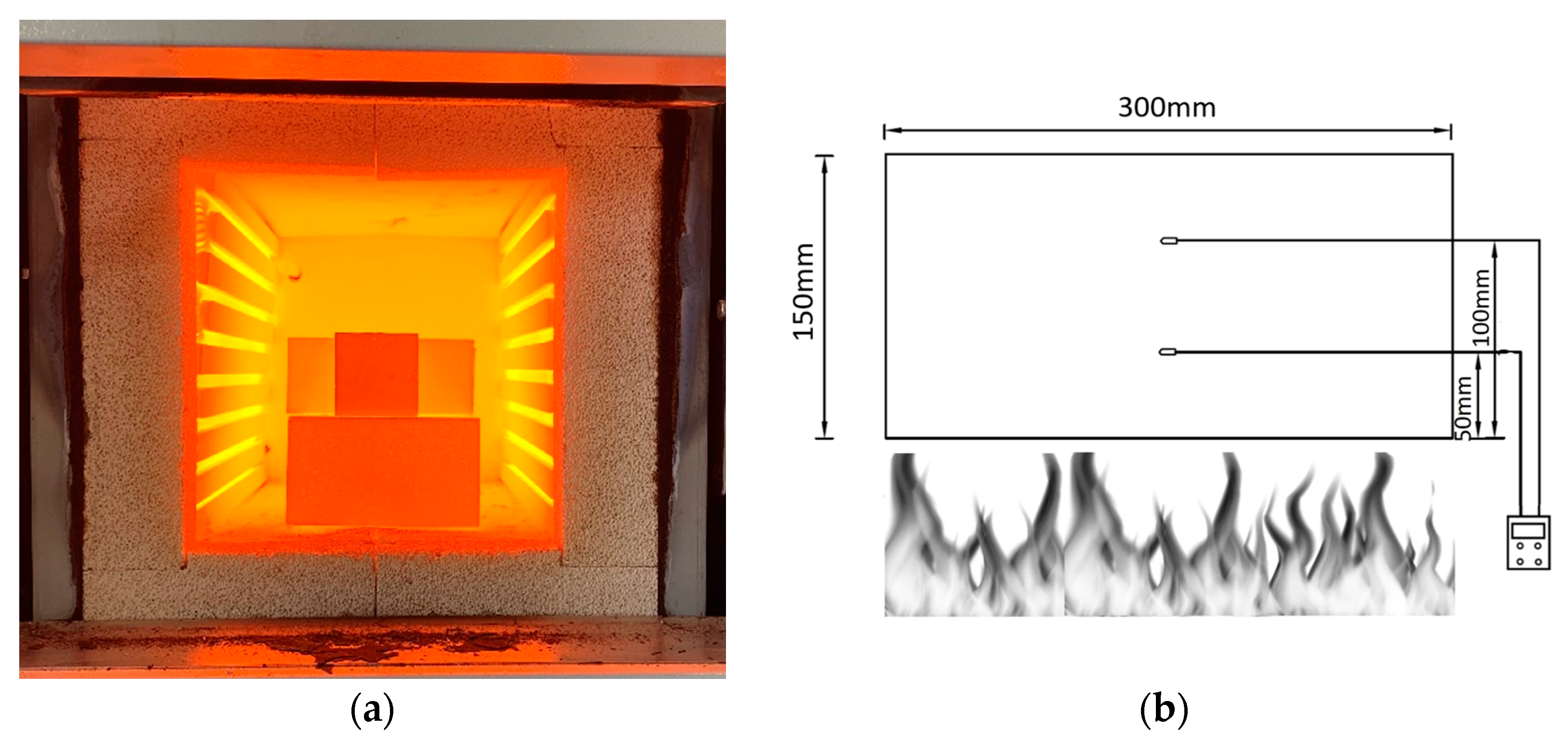
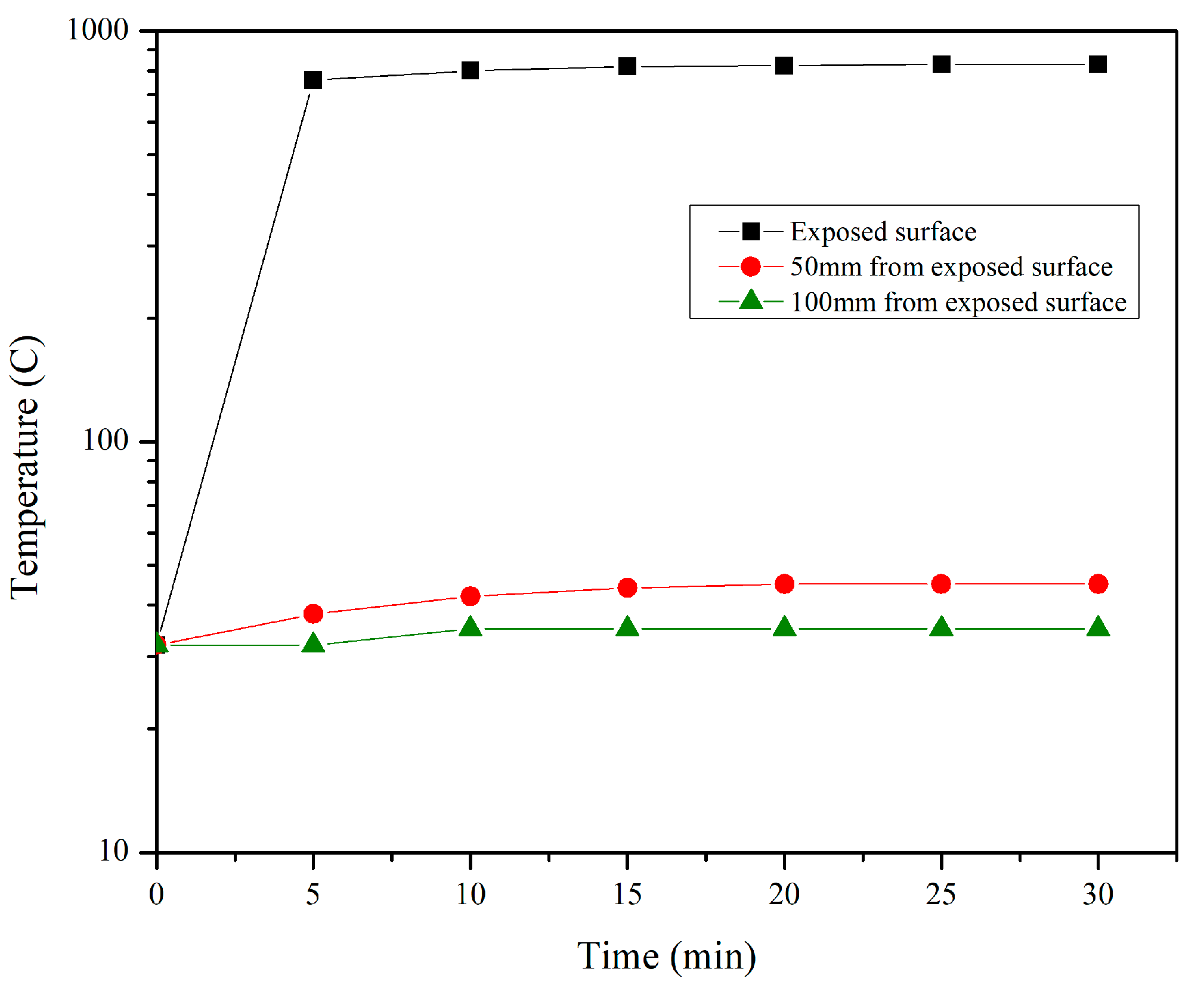
3.4. Temperature Distribution Measurement
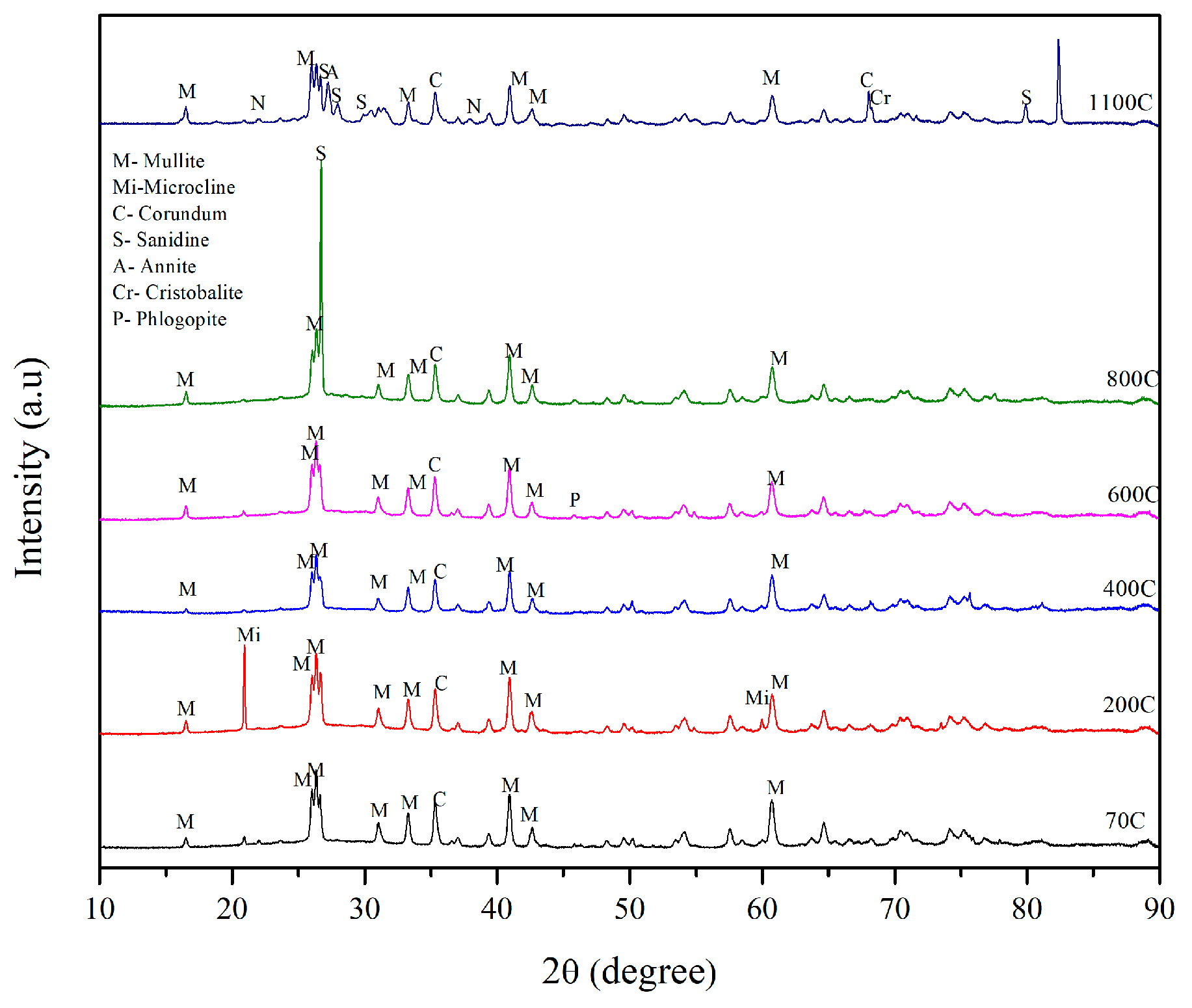
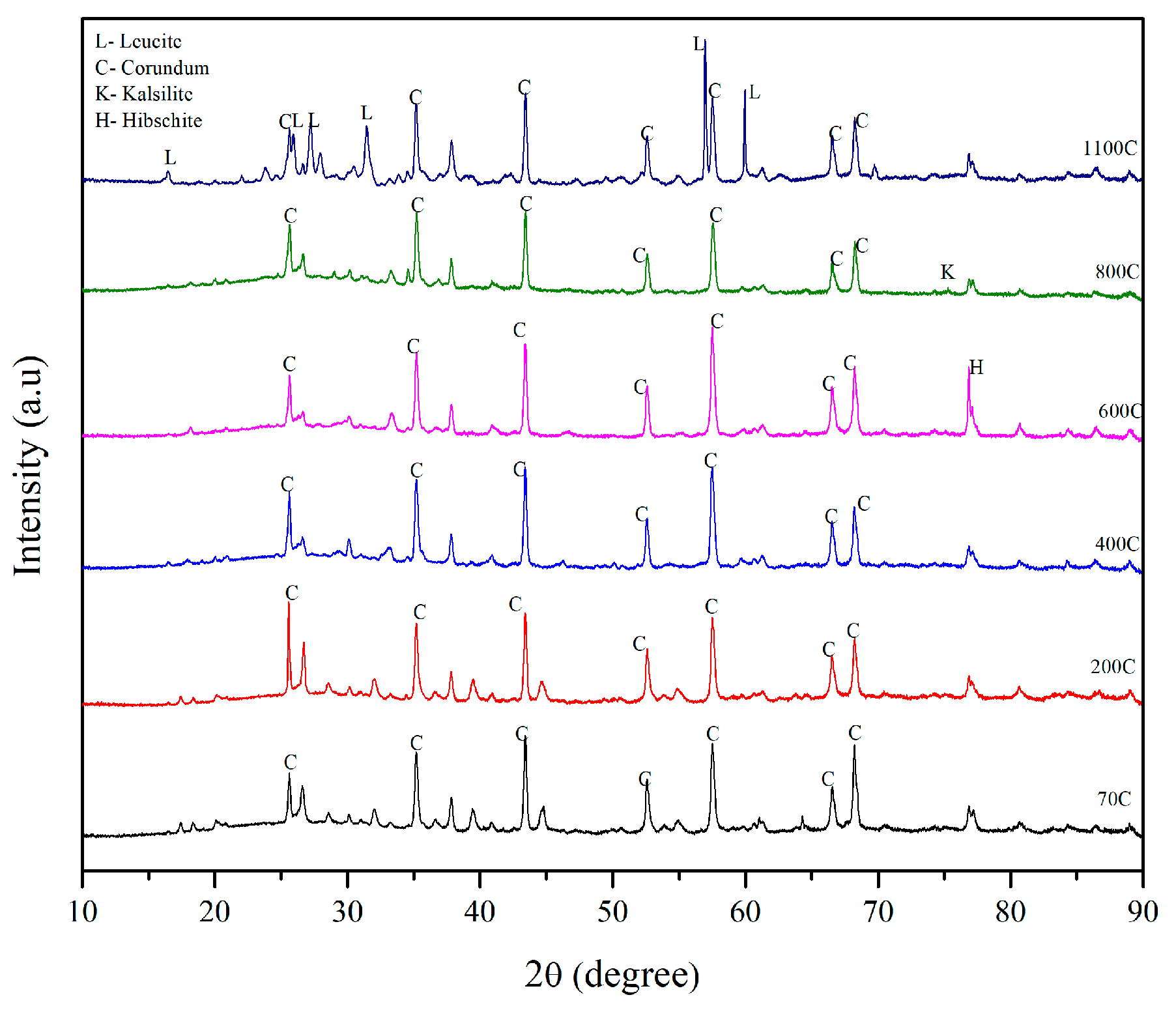
3.5. X-ray Diffraction Results
3.6. Scanning Electron Microscopy
4. Comparative Study of Refractory Potentials of Geopolymers
5. Conclusions
- All the geopolymer formulations used in this study retained their cubical shapes without cracks, spalling, or any physical disintegration post-exposure to 1100 °C for 1 h. The weight loss in the solid geopolymer formulations was more pronounced compared with their two-part liquid alkaline geopolymer counterparts.
- Advanced solid geopolymer formulations yielded better compressive strength after 1100 °C exposure compared with conventional liquid alkaline geopolymer formulations due to intensive mechano-chemical grinding in the process of making advanced solid geopolymers.
- The geopolymer mix made of 45-micron mullite, denoted as SR1, displayed its highest compressive strength, 84 MPa, after 1100 °C, and the crystalline phases of sanidine, annite, and cristobalite were identified in the sample. At 1100 °C, mullite recrystallized as a needle-like structure, densifying the matrix and increasing the compressive strength.
- The solid geopolymer mix made of alumina, denoted as SR3, had leucite as a crystalline phase at 1100 °C, which was responsible for its compressive strength of 64 MPa.
- Geopolymer mix SR1 retained its compressive strength after ten cycles of 1100 °C exposure. Mix SR1 displayed its highest compressive strength, 115.2 MPa, after four cycles, and SR3 had a gradual decrease in strength after each cycle, which further stabilized after eight cycles at 54.4 MPa.
- The temperature distribution profile of mix SR1 proves the superior thermal conductivity of the mix during direct flame exposure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poon, C.S.; Shui, Z.H.; Lam, L. Compressive Behavior of Fiber Reinforced High-Performance Concrete Subjected to Elevated Temperatures. Cem. Concr. Res. 2004, 34, 2215–2222. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yun, T.S.; Park, K.P. Evaluation of Pore Structures and Cracking in Cement Paste Exposed to Elevated Temperatures by X-Ray Computed Tomography. Cem. Concr. Res. 2013, 50, 34–40. [Google Scholar] [CrossRef]
- Zhang, H.L.; Davie, C.T. A Numerical Investigation of the Influence of Pore Pressures and Thermally Induced Stresses for Spalling of Concrete Exposed to Elevated Temperatures. Fire Saf. J. 2013, 59, 102–110. [Google Scholar] [CrossRef]
- Knyziak, P.; Kowalski, R.; Krentowski, J.R. Fire Damage of RC Slab Structure of a Shopping Center. Eng. Fail. Anal. 2019, 97, 53–60. [Google Scholar] [CrossRef]
- Bamonte, P.; Gambarova, P. Properties of Concrete Subjected to Extreme Thermal Conditions. J. Struct. Fire Eng. 2014, 5, 47–62. [Google Scholar] [CrossRef]
- Kodur, V.K.R.; Agrawal, A. An Approach for Evaluating Residual Capacity of Reinforced Concrete Beams Exposed to Fire. Eng. Struct. 2016, 110, 293–306. [Google Scholar] [CrossRef]
- Mindeguia, J.C.; Pimienta, P.; Noumowé, A.; Kanema, M. Temperature, Pore Pressure and Mass Variation of Concrete Subjected to High Temperature—Experimental and Numerical Discussion on Spalling Risk. Cem. Concr. Res. 2010, 40, 477–487. [Google Scholar] [CrossRef]
- Phan, L.T.; Lawson, J.R.; Davis, F.L. Effects of Elevated Temperature Exposure on Heating Characteristics, Spalling, and Residual Properties of High Performance Concrete. Mater. Struct. 2001, 34, 83–91. [Google Scholar] [CrossRef]
- Choinska, M.; Khelidj, A.; Chatzigeorgiou, G.; Pijaudier-Cabot, G. Effects and Interactions of Temperature and Stress-Level Related Damage on Permeability of Concrete. Cem. Concr. Res. 2007, 37, 79–88. [Google Scholar] [CrossRef]
- Khoury, G.A. Passive Fire Protection of Concrete Structures. Proc. Inst. Civ. Eng.-Struct. Build. 2008, 161, 135–145. [Google Scholar] [CrossRef]
- Hertz, K.D. Concrete Strength for Fire Safety Design. Mag. Concr. Res. 2005, 57, 445–453. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean Production and Properties of Geopolymer Concrete; A Review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Bajpai, R.; Choudhary, K.; Srivastava, A.; Sangwan, K.S.; Singh, M. Environmental Impact Assessment of Fly Ash and Silica Fume Based Geopolymer Concrete. J. Clean. Prod. 2020, 254, 120147. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative Performance of Geopolymers Made with Metakaolin and Fly Ash after Exposure to Elevated Temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Glukhovsky, V.D. Soil Sliicates; Gosstroyizdat: Kiev, Ukraine, 1959; Volume 154. [Google Scholar]
- Palomo, A.; López dela Fuente, J.I. Alkali-Activated Cementitous Materials: Alternative Matrices for the Immobilisation of Hazardous Wastes. Cem. Concr. Res. 2003, 33, 281–288. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Rivera, O.G.; Long, W.R.; Weiss, C.A., Jr.; Moser, R.D.; Williams, B.A.; Torres-Cancel, K.; Gore, E.R.; Allison, P.G. Effect of Elevated Temperature on Alkali-Activated Geopolymeric Binders Compared to Portland Cement-Based Binders. Cem. Concr. Res. 2016, 90, 43–51. [Google Scholar] [CrossRef]
- Pan, Z.; Sanjayan, J.G.; Collins, F. Effect of Transient Creep on Compressive Strength of Geopolymer Concrete for Elevated Temperature Exposure. Cem. Concr. Res. 2014, 56, 182–189. [Google Scholar] [CrossRef]
- Li, Y.; Pimienta, P.; Pinoteau, N.; Tan, K.H. Effect of Aggregate Size and Inclusion of Polypropylene and Steel Fibers on Explosive Spalling and Pore Pressure in Ultra-High-Performance Concrete (UHPC) at Elevated Temperature. Cem. Concr. Compos. 2019, 99, 62–71. [Google Scholar] [CrossRef]
- Humur, G.; Çevik, A. Mechanical Characterization of Lightweight Engineered Geopolymer Composites Exposed to Elevated Temperatures. Ceram. Int. 2022, 48, 13634–13650. [Google Scholar] [CrossRef]
- Tu, W.; Zhang, M. Behaviour of Alkali-Activated Concrete at Elevated Temperatures: A Critical Review. Cem. Concr. Compos. 2023, 138, 104961. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive Strength and Microstructure of Alkali-Activated Fly Ash/Slag Binders at High Temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Yuan, C.; Wang, Q.; Sarker, P.K.; Shi, X. Deterioration of Ambient-Cured and Heat-Cured Fly Ash Geopolymer Concrete by High Temperature Exposure and Prediction of Its Residual Compressive Strength. Constr. Build. Mater. 2020, 262, 120924. [Google Scholar] [CrossRef]
- Liang, X.; Wu, C.; Yang, Y.; Li, Z. Experimental Study on Ultra-High Performance Concrete with High Fire Resistance under Simultaneous Effect of Elevated Temperature and Impact Loading. Cem. Concr. Compos. 2019, 98, 29–38. [Google Scholar] [CrossRef]
- Peng, G.-F.; Yang, W.-W.; Zhao, J.; Liu, Y.-F.; Bian, S.-H.; Zhao, L.-H. Explosive Spalling and Residual Mechanical Properties of Fiber-Toughened High-Performance Concrete Subjected to High Temperatures. Cem. Concr. Res. 2006, 36, 723–727. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, J.; Peng, G.; van Breugel, K. A Meso-Level Investigation into the Explosive Spalling Mechanism of High-Performance Concrete under Fire Exposure. Cem. Concr. Res. 2014, 65, 64–75. [Google Scholar] [CrossRef]
- Li, Y. Material Properties and Explosive Spalling of Ultra-High Performance Concrete in Fire. Ph.D. Thesis, Nanyang Technological University, Singapore, 2018. [Google Scholar]
- Zhao, R.; Sanjayan, J.G. Geopolymer and Portland Cement Concretes in Simulated Fire. Mag. Concr. Res. 2011, 63, 163–173. [Google Scholar] [CrossRef]
- He, R.; Dai, N.; Wang, Z. Thermal and Mechanical Properties of Geopolymers Exposed to High Temperature: A Literature Review. Adv. Civ. Eng. 2020, 2020, 7532703. [Google Scholar] [CrossRef]
- Jiang, X.; Xiao, R.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A Laboratory Investigation of Steel to Fly Ash-Based Geopolymer Paste Bonding Behavior after Exposure to Elevated Temperatures. Constr. Build. Mater. 2020, 254, 119267. [Google Scholar] [CrossRef]
- Shaikh, F.U.A. Effect of Cooling on the Residual Mechanical Properties and Cracking of Plain and Fibrous Geopolymer Concretes at Elevated Temperatures. Struct. Concr. 2019, 20, 1583–1595. [Google Scholar] [CrossRef]
- Luhar, S.; Chaudhary, S.; Luhar, I. Thermal Resistance of Fly Ash Based Rubberized Geopolymer Concrete. J. Build. Eng. 2018, 19, 420–428. [Google Scholar] [CrossRef]
- Kaya, M.; Uysal, M.; Yilmaz, K.; Atis, C.D. Behaviour of Geopolymer Mortars after Exposure to Elevated Temperatures. Mater. Sci. 2018, 24, 428–436. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Heah, C.-Y.; Liew, Y.-M. Behaviour Changes of Ground Granulated Blast Furnace Slag Geopolymers at High Temperature. Adv. Cem. Res. 2020, 32, 465–475. [Google Scholar] [CrossRef]
- Türker, H.T.; Balçikanli, M.; Durmuş, İ.H.; Özbay, E.; Erdemir, M. Microstructural Alteration of Alkali Activated Slag Mortars Depend on Exposed High Temperature Level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- Topal, Ö.; Karakoç, M.B.; Özcan, A. Effects of Elevated Temperatures on the Properties of Ground Granulated Blast Furnace Slag (GGBFS) Based Geopolymer Concretes Containing Recycled Concrete Aggregate. Eur. J. Environ. Civ. Eng. 2022, 26, 4847–4862. [Google Scholar] [CrossRef]
- Behera, P.; Baheti, V.; Militky, J.; Naeem, S. Microstructure and Mechanical Properties of Carbon Microfiber Reinforced Geopolymers at Elevated Temperatures. Constr. Build. Mater. 2018, 160, 733–743. [Google Scholar] [CrossRef]
- Khater, H.M.; Gharieb, M. Synergetic Effect of Nano-Silica Fume for Enhancing Physico-Mechanical Properties and Thermal Behavior of MK-Geopolymer Composites. Constr. Build. Mater. 2022, 350, 128879. [Google Scholar] [CrossRef]
- ASTM C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2008.
- Zhang, D.; Dasari, A.; Tan, K.H. On the Mechanism of Prevention of Explosive Spalling in Ultra-High Performance Concrete with Polymer Fibers. Cem. Concr. Res. 2018, 113, 169–177. [Google Scholar] [CrossRef]
- Deshpande, A.A.; Kumar, D.; Ranade, R. Influence of High Temperatures on the Residual Mechanical Properties of a Hybrid Fiber-Reinforced Strain-Hardening Cementitious Composite. Constr. Build. Mater. 2019, 208, 283–295. [Google Scholar] [CrossRef]
- Luo, X.; Sun, W.; Chan, S.Y.N. Effect of Heating and Cooling Regimes on Residual Strength and Microstructure of Normal Strength and High-Performance Concrete. Cem. Concr. Res. 2000, 30, 379–383. [Google Scholar] [CrossRef]
- ASTM C109; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2020.
- Hager, I. Colour Change in Heated Concrete. Fire Technol. 2014, 50, 945–958. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-Ash Based Geopolymer Mortar for High-Temperature Application–Effect of Slag Addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Xiao, R.; Polaczyk, P.; Zhang, M.; Hu, W.; Bai, Y.; Huang, B. A Comparative Study on Geopolymers Synthesized by Different Classes of Fly Ash after Exposure to Elevated Temperatures. J. Clean. Prod. 2020, 270, 122500. [Google Scholar] [CrossRef]
- Hassan, A.; Arif, M.; Shariq, M. Mechanical Behaviour and Microstructural Investigation of Geopolymer Concrete After Exposure to Elevated Temperatures. Arab. J. Sci. Eng. 2020, 45, 3843–3861. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A Critical Review of Geopolymer Properties for Structural Fire-Resistance Applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Ameri, F.; Shoaei, P.; Zareei, S.A.; Behforouz, B. Geopolymers vs. Alkali-Activated Materials (AAMs): A Comparative Study on Durability, Microstructure, and Resistance to Elevated Temperatures of Lightweight Mortars. Constr. Build. Mater. 2019, 222, 49–63. [Google Scholar] [CrossRef]
- Bakharev, T. Thermal Behaviour of Geopolymers Prepared Using Class F Fly Ash and Elevated Temperature Curing. Cem. Concr. Res. 2006, 36, 1134–1147. [Google Scholar] [CrossRef]
- Wattanasiriwech, S.; Arif Nurgesang, F.; Wattanasiriwech, D.; Timakul, P. Characterisation and Properties of Geopolymer Composite Part 1: Role of Mullite Reinforcement. Ceram. Int. 2017, 43, 16055–16062. [Google Scholar] [CrossRef]
- Ascensão, G.; Faleschini, F.; Marchi, M.; Segata, M.; Van De Sande, J.; Rahier, H.; Bernardo, E.; Pontikes, Y. High-Temperature Behavior of CaO-FeOx-Al2O3-SiO2-Rich Alkali Activated Materials. Appl. Sci. 2022, 12, 2572. [Google Scholar] [CrossRef]
- Mo, B.; Zhu, H.; Cui, X.; He, Y.; Gong, S. Effect of Curing Temperature on Geopolymerization of Metakaolin-Based Geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Rendtorff, N.M.; Garrido, L.B.; Aglietti, E.F. Thermal Shock Behavior of Dense Mullite–Zirconia Composites Obtained by Two Processing Routes. Ceram. Int. 2008, 34, 2017–2024. [Google Scholar] [CrossRef]
- Rendtorff, N.M.; Garrido, L.B.; Aglietti, E.F. Effect of the Addition of Mullite–Zirconia to the Thermal Shock Behavior of Zircon Materials. Mater. Sci. Eng. A 2008, 498, 208–215. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Kovalchuk, G.Y. Directed Synthesis of Alkaline Aluminosilicate Minerals in a Geocement Matrix. J. Mater. Sci. 2007, 42, 2944–2952. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical Evolution of Na-Geopolymer Derived from Metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Perera, D.S.; Trautman, R.L. Geopolymers with the Potential for Use as Refractory Castables. Adv. Technol. Mater. Mater. Process. 2005, 7, 187–190. [Google Scholar]
- Bezerra, B.P.; Luz, A.P. Geopolymers: A Viable Binder Option for Ultra-Low-Cement and Cement-Free Refractory Castables? J. Eur. Ceram. Soc. 2024, 44, 5241–5251. [Google Scholar] [CrossRef]
- Farias, T.W.B.; Bezerra, B.P.; Luz, A.P. Metakaolin-Based Geopolymer as an Alternative Binder in Semi-Insulating Refractories. Mater. Lett. 2024, 354, 135321. [Google Scholar] [CrossRef]
- Deutou, J.G.N.; Kaze, R.C.; Kamseu, E.; Sglavo, V.M. Controlling the Thermal Stability of Kyanite-Based Refractory Geopolymers. Materials 2021, 14, 2903. [Google Scholar] [CrossRef] [PubMed]
- Kassem, N.; Ahmed, D.; Kishar, E. Effect of Elevated Temperatures on The Performance of Metakaolin Geopolymer Pastes Incorporated by Cement Kiln Dust. Egypt. J. Chem. 2021, 64, 1911–1926. [Google Scholar] [CrossRef]
- Boum, R.B.E.; Kaze, C.R.; Nemaleu, J.G.D.; Djaoyang, V.B.; Rachel, N.Y.; Ninla, P.L.; Owono, F.M.; Kamseu, E. Thermal Behaviour of Metakaolin–Bauxite Blends Geopolymer: Microstructure and Mechanical Properties. SN Appl. Sci. 2020, 2, 1358. [Google Scholar] [CrossRef]
- Yaşın, S.; Ahlatcı, H. Thermal Investigation of Fine Alumina Powder Reinforced Na-Metakaolin-Based Geopolymer Binder for Refractory Applications. J. Aust. Ceram. Soc. 2019, 55, 587–593. [Google Scholar] [CrossRef]
- Moosavi, A.; Asadi, S.; Shoraki, H.J. Microstructure and Mechanical Properties of Tabular Alumina Composites with Geopolymer Binder at Elevated Temperatures. Ceram. Int. 2019, 45, 9092–9098. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Tan, K.H.; Yang, E.-H. Effect of Alkali Cation Type on Strength Endurance of Fly Ash Geopolymers Subject to High Temperature Exposure. Mater. Des. 2018, 154, 8–19. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Factors Affecting the Performance of Metakaolin Geopolymers Exposed to Elevated Temperatures. J. Mater. Sci. 2008, 43, 824–831. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.-H.; Tan, K.H. Effects of Si/Al Molar Ratio on Strength Endurance and Volume Stability of Metakaolin Geopolymers Subject to Elevated Temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Guerrieri, M.; Sanjayan, J.G. Behavior of Combined Fly Ash/Slag-based Geopolymers When Exposed to High Temperatures. Fire Mater. 2010, 34, 163–175. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Temuujin, J.; van Riessen, A. Thermal Analysis of Geopolymer Pastes Synthesised from Five Fly Ashes of Variable Composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Williams, R.; Temuujin, J.; van Riessen, A. Assessing the Suitability of Three Australian Fly Ashes as an Aluminosilicate Source for Geopolymers in High Temperature Applications. Mater. Sci. Eng. A 2011, 528, 3390–3397. [Google Scholar] [CrossRef]
- Rickard, W.D.A.; Kealley, C.S.; van Riessen, A. Thermally Induced Microstructural Changes in Fly Ash Geopolymers: Experimental Results and Proposed Model. J. Am. Ceram. Soc. 2015, 98, 929–939. [Google Scholar] [CrossRef]


| Oxide Composition (%) | Physical Characteristics | ||||||
|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | LOI | Specific Gravity | Strength Activity |
| 2.58 | 60.70 | 23.71 | 4.73 | 1.14 | 0.49 | 2.27 | 80.77% |
| Aggregate | Al2O3 | SiO2 | Fe2O3 | CaO | TiO2 |
|---|---|---|---|---|---|
| Mullite | 57.41% | 40.62% | 0.56% | - | 1.22% |
| Alumina | >93% | - | <0.3% | <5.5% | - |
| Mix Notation | Fly Ash | Mullite | Alumina | KOH | K2SiO3 | RHS | Water |
|---|---|---|---|---|---|---|---|
| LR1 | 26 | 52 | - | 6 | 16 | - | - |
| LR2 | 26 | 52 | - | 6 | 16 | - | - |
| LR3 | 27 | - | 54 | 5 | 13 | - | - |
| SR1 | 26 | 52 | - | 3 | 8 | - | 11 |
| SR2 | 26 | 52 | - | 3 | 8 | - | 11 |
| SR3 | 27 | - | 54 | 2.5 | 6.5 | 10 | |
| SR4 | 25 | 51 | - | 7 | - | 4 | 13 |
| Author | Geopolymer Mix Details | Mechanical Performance |
|---|---|---|
| Bezerra and Luz [60] | Geopolymers based on partially or fully replaced calcium aluminate cement (CAC) in high-alumina castables with sodium silicate as an activator. | A dosage of 2.7% weight CAC and 1.3% weight geopolymer at 1400 °C attained a flexural strength of 39.1 MPa. |
| Farias et al. [61] | Metakaolin-based geopolymers with semi-insulating fused silica-containing castables. | The geopolymer-bonded castable exhibited a flexural strength of 6.24 MPa after exposure to 815 °C. |
| Deutou et al. [62] | Metakaolin-, calcined bauxite-, and calcined talc-based geopolymer with kyanites of various particle sizes as fillers. Potassium hydroxide and potassium silicate as activators. | An 80 µm kyanite-filler-based geopolymer achieved a flexural strength of 45 MPa at a temperature of 1200 °C. |
| Ahmed and Kishar [63] | Metakaolin geopolymer pastes incorporated with cement kiln dust and sodium hydroxide and sodium silicate as activators. | Geopolymer mix with 20% cement kiln dust withstood high temperatures with a strength of around 35 MPa at ambient temperature and around 22.5 MPa at 800 °C. |
| Boum et al. [64] | Metakaolin–bauxite-blended geopolymer with sodium hydroxide and sodium silicate as activators. | The mechanical strength of the samples decreased from 35.2 to 11.1 MPa at room temperature. Compressive strength of 98 MPa at 1200 °C was achieved for a mix with 20% bauxite by weight. |
| Yaşın and Ahlatcı [65] | Metakaolin-based geopolymer binder reinforced with fine alumina powder, sodium hydroxide, and sodium silicate. | The compressive strength of the sample after 1250 °C exposure was reported to be 134 MPa, whereas the unexposed sample had 30.41 MPa strength. |
| Moosavi et al. [66] | Metakaolin-based geopolymer with microsilica (25% vol) and tabular alumina aggregates (75% vol). Potassium hydroxide as activator. | Flexural strength was reduced by 23.43 MPa after exposure to 1200 °C. Similar trends were observed in compressive strength with around 40% reduction in strength. |
| Lahoti et al. [67] | Fly ash geopolymers with sodium and potassium-based activators individually and in combination. | A 30–40% increase in strength in the potassium-activator-based geopolymer, whereas the sodium-activator-based geopolymer reduced in strength (10%) after high-temperature exposure. After exposure to 500 °C, the compressive strength increased from 40 MPa to 59 MPa for the potassium-based geopolymer, and at 900 °C, it reduced to 54 MPa. |
| King et al. [68] | Metakaolin geopolymer activated by combinations of sodium–potassium silicate and sodium–potassium hydroxide. | Lesser strength losses due to elevated temperature exposures were observed in geopolymers with high Si/Al ratios (>1.5) when they were exposed to 800 °C. The 3-day compressive strength reduction was 4–6%. |
| Lahoti et al. [69] | Metakaolin-based geopolymers activated by sodium hydroxide and sodium silicate. | Compressive strength drastically decreased at 900 °C. At 25 °C, it was around 65 MPa, and at high temperatures, the strength dwindled to 6 MPa for a mix with a Si/Al ratio of 1.75. |
| Kong et al. [14] | Metakaolin- and fly ash-based geopolymers with sodium silicate and potassium hydroxide activators. | Fly ash geopolymers increased in strength after 800 °C exposure, whereas metakaolin geopolymers decreased in strength. The metakaolin geopolymer’s unexposed strength was 38.5 MPa, and after exposure, it was 25.4 MPa. Fly ash’s unexposed strength was 59 MPa, and after exposure, it was 62.8 MPa. |
| Guerrieri and Sanjayan [70] | Fly-ash–slag-based geopolymer in varying dosages with sodium activators. | Geopolymer mix with a 65%/35% (FA/Slag) ratio achieved the highest compressive strength. The residual compressive strength after 800 °C was 20 MPa. |
| Rickard et al. [71] | Fly ash geopolymers with sodium silicate and sodium aluminate activators. | The compressive strength of samples increased after 1000 °C exposure, wherein the amount of Si or Al added by the activating solution was reduced. The effect was more pronounced in the sodium-aluminate-activated samples, which exhibited strength gains of almost five times, wherein 40% of the total Al was added via activating solution. |
| Rickard et al. [72] | Fly ash geopolymers with sodium silicate and sodium aluminate activators. | After exposure to 1000 °C, the geopolymer sample exhibited an increase in compressive strength. The unexposed sample had a compressive strength of 33 MPa, whereas, after exposure, the strength increased to 132 MPa. |
| Rickard et al. [73] | Fly ash geopolymers with sodium silicate and sodium hydroxide activators. | Geopolymers made from unreacted low-strength and low-density fly ash attained better strengths after high-temperature exposure (1000 °C) compared with geopolymer samples made from highly reactive and high-strength fly ash. In the former case, the strength increased from 28 MPa to 93 MPa. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Amritphale, S.; Matthews, J.; Paul, N.; Matthews, E.; Edwards, R. Advanced Solid Geopolymer Formulations for Refractory Applications. Materials 2024, 17, 1386. https://doi.org/10.3390/ma17061386
Hussain S, Amritphale S, Matthews J, Paul N, Matthews E, Edwards R. Advanced Solid Geopolymer Formulations for Refractory Applications. Materials. 2024; 17(6):1386. https://doi.org/10.3390/ma17061386
Chicago/Turabian StyleHussain, Shaik, Sudhir Amritphale, John Matthews, Niloy Paul, Elizabeth Matthews, and Richard Edwards. 2024. "Advanced Solid Geopolymer Formulations for Refractory Applications" Materials 17, no. 6: 1386. https://doi.org/10.3390/ma17061386






