Mesoporous Carbons and Highly Cross-Linking Polymers for Removal of Cationic Dyes from Aqueous Solutions—Studies on Adsorption Equilibrium and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Adsorbents Preparation
2.2.1. Synthesis of Mesoporous Carbons
2.2.2. Synthesis of Mesoporous Polymer Materials
2.3. Adsorbate Molecules
2.4. Methods of Investigations
2.4.1. Nitrogen Adsorption/Desorption Measurement
2.4.2. X-ray Diffraction (XRD) and Small Angle X-ray Scattering (SAXS)
2.4.3. Scanning Electron Microscopy
2.4.4. X-ray Photoelectron Spectroscopy
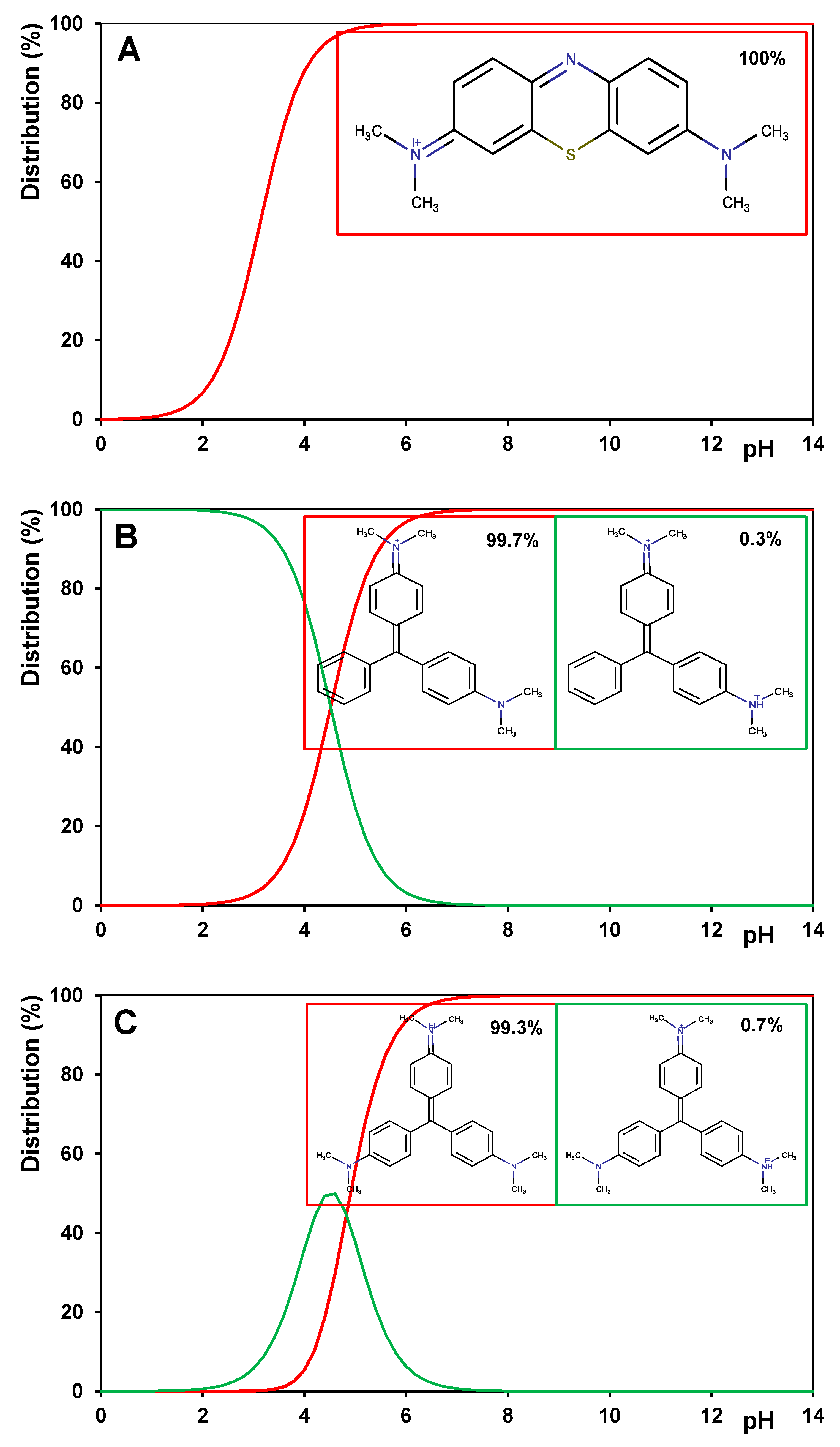
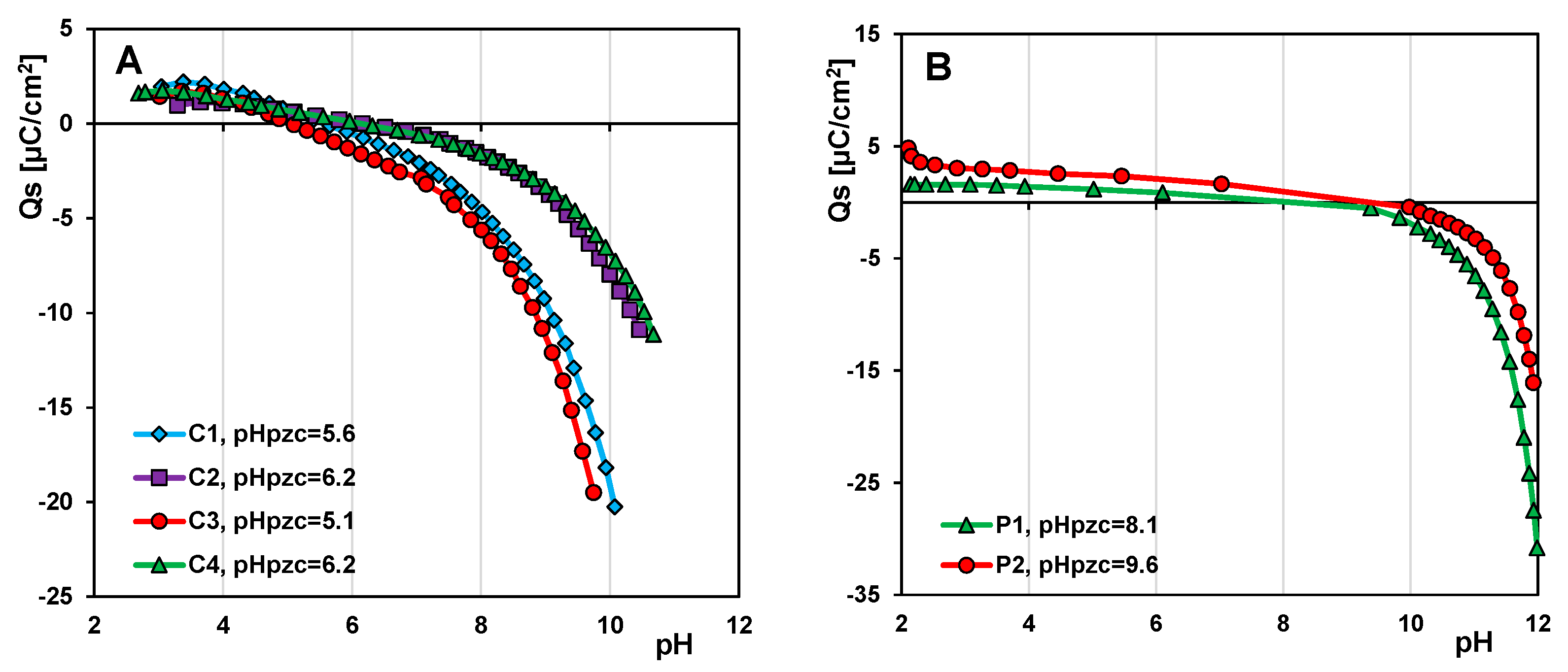
2.4.5. Potentiometric Titration Measurement
2.4.6. Adsorption Studies
- 1,2-mixed-order equation [65]where F is the relative adsorption progress in time, f2 < 1 is the normalized share of the second-order process in the kinetics. In special cases, the MOE equation may be degraded to the simple kinetic equations of the first- (f2 = 0) and the second-order (f2 = 1) type;
3. Results and Discussion
3.1. Adsorbents Characteristics
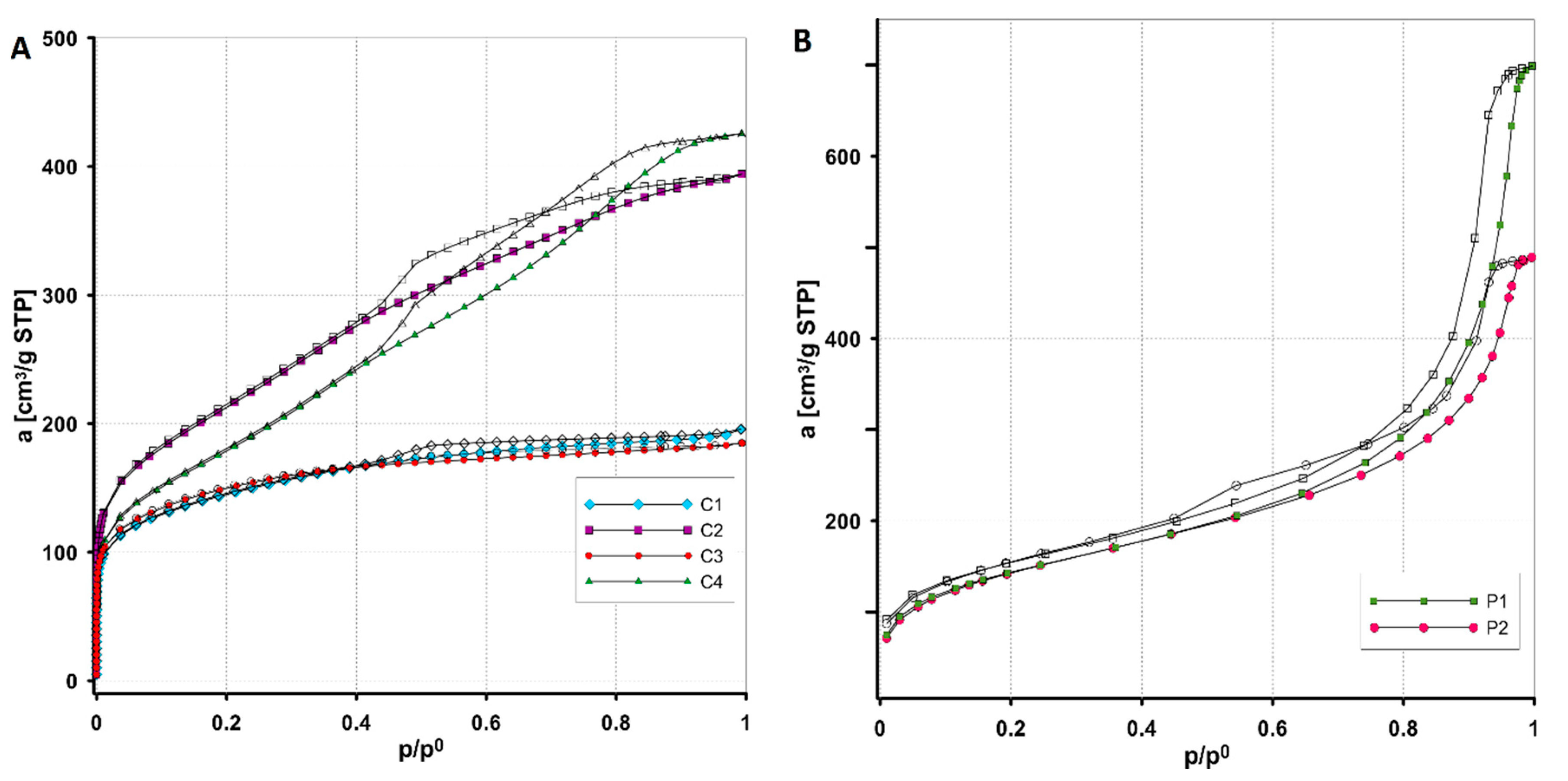
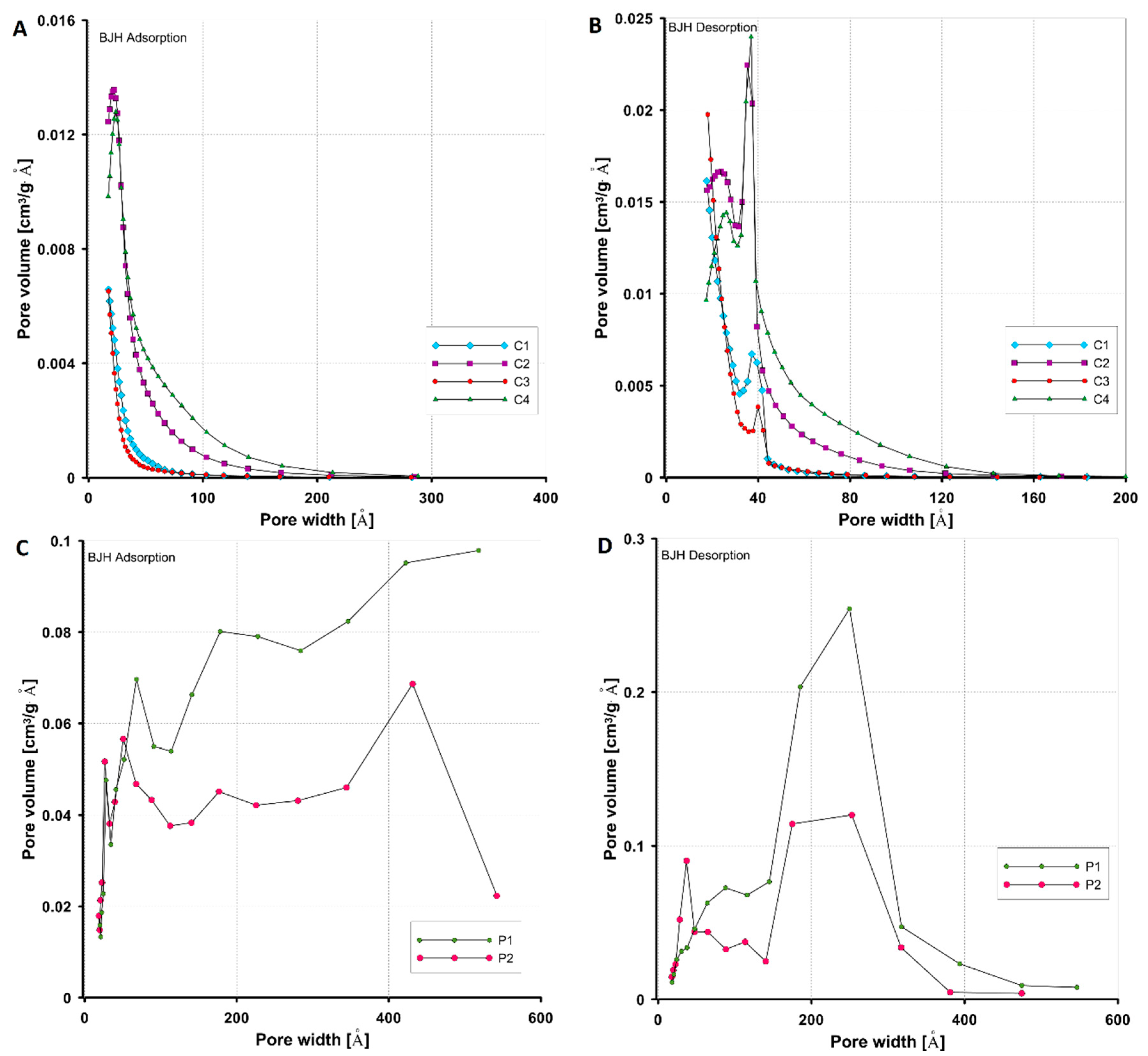
3.1.1. Porosity Characteristics by Low-Temperature N2 Adsorption/Desorption
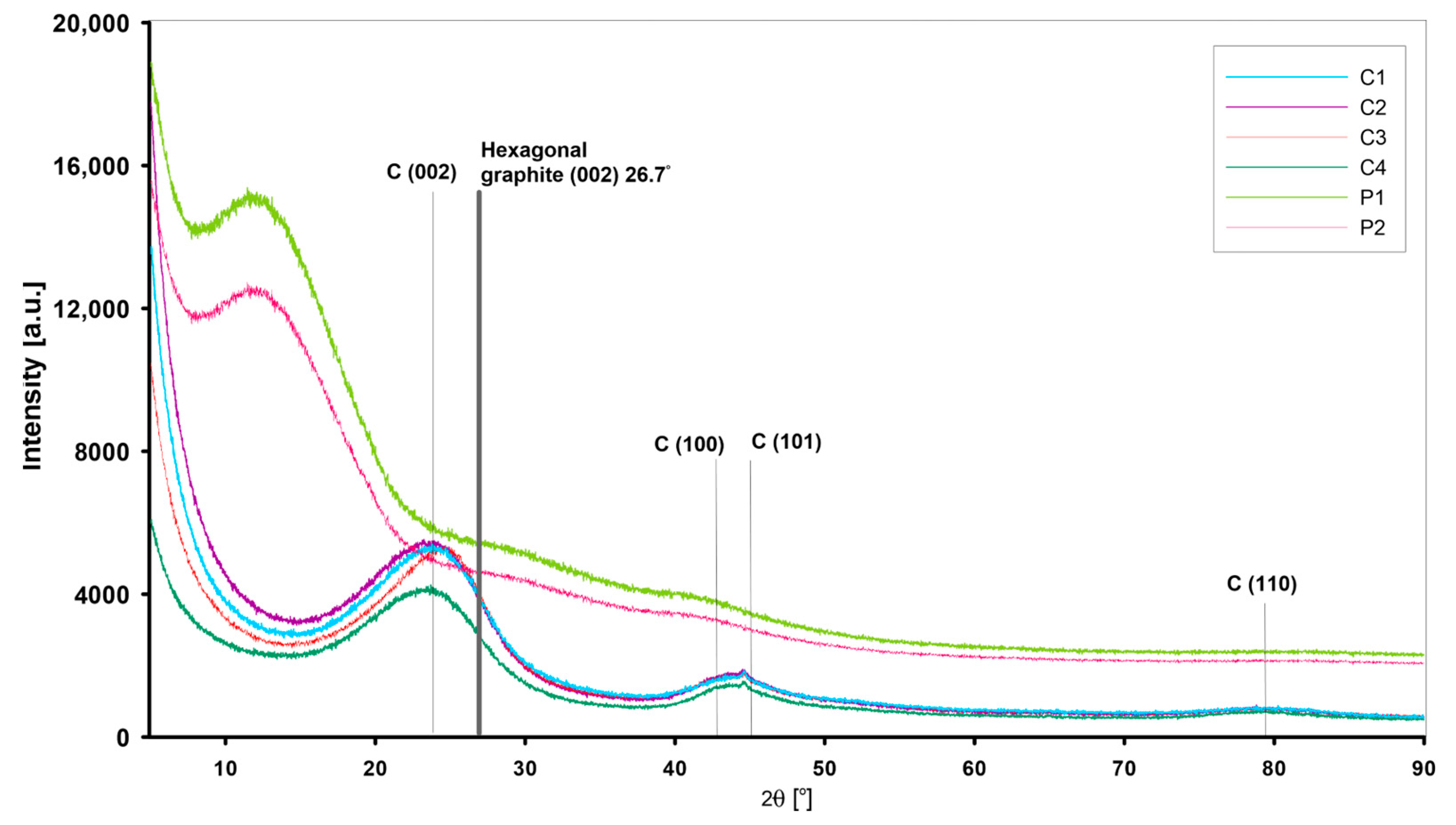
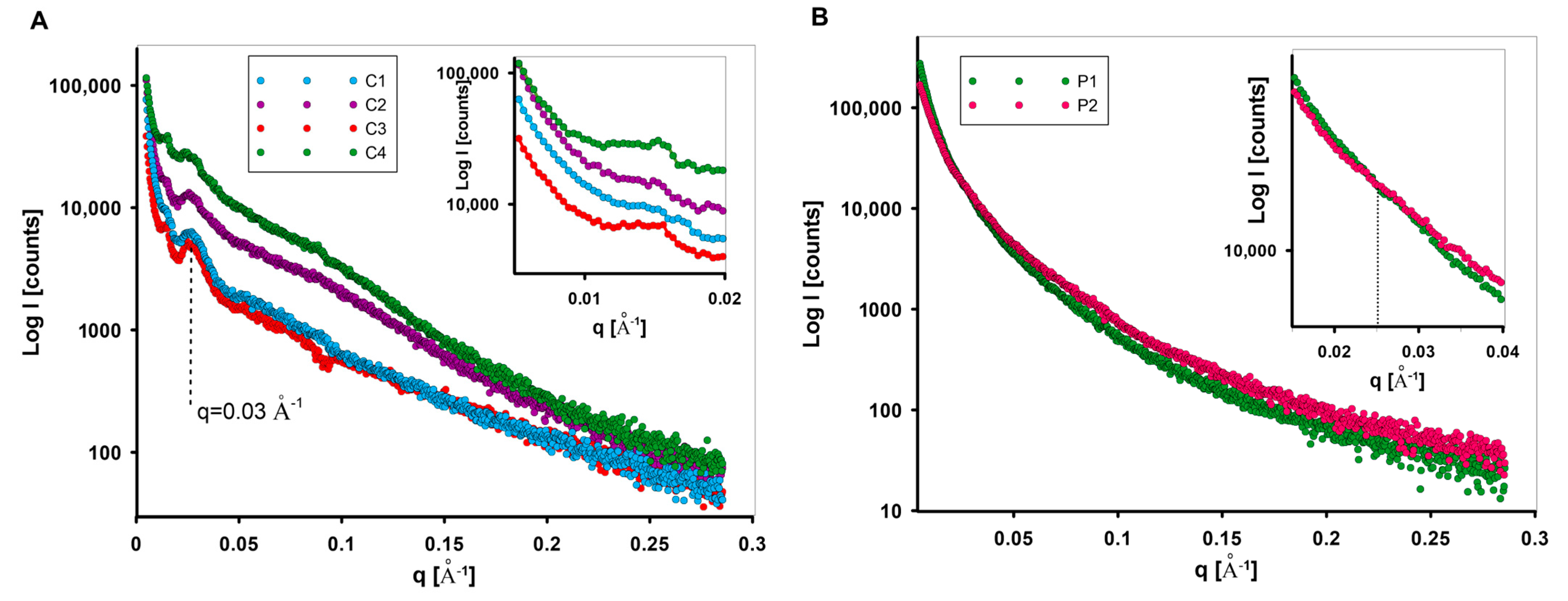
3.1.2. Structure of Nano-Sized Materials by XRD and Small-Angle X-ray Scattering
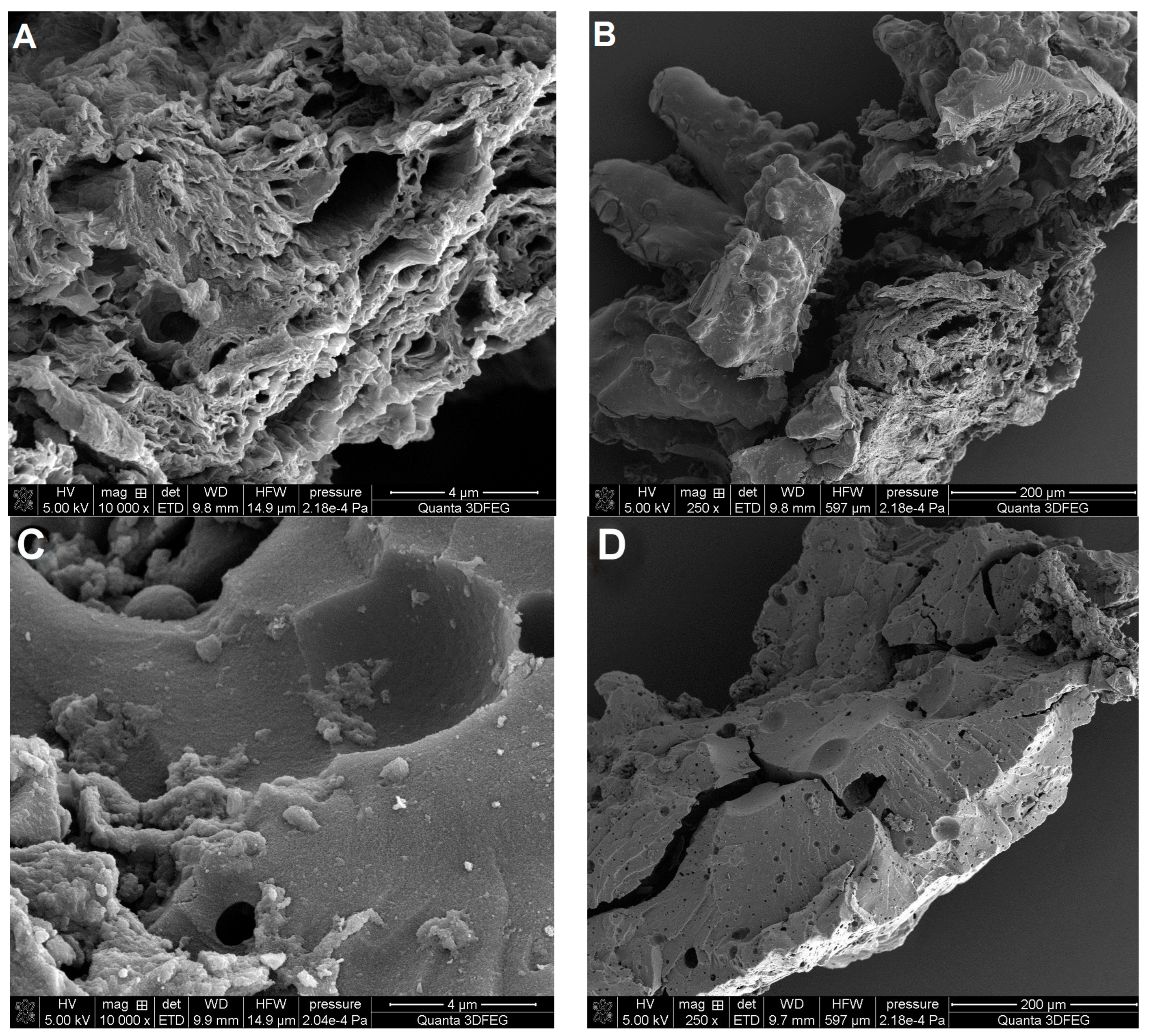
3.1.3. Surface Morphology of Adsorbents
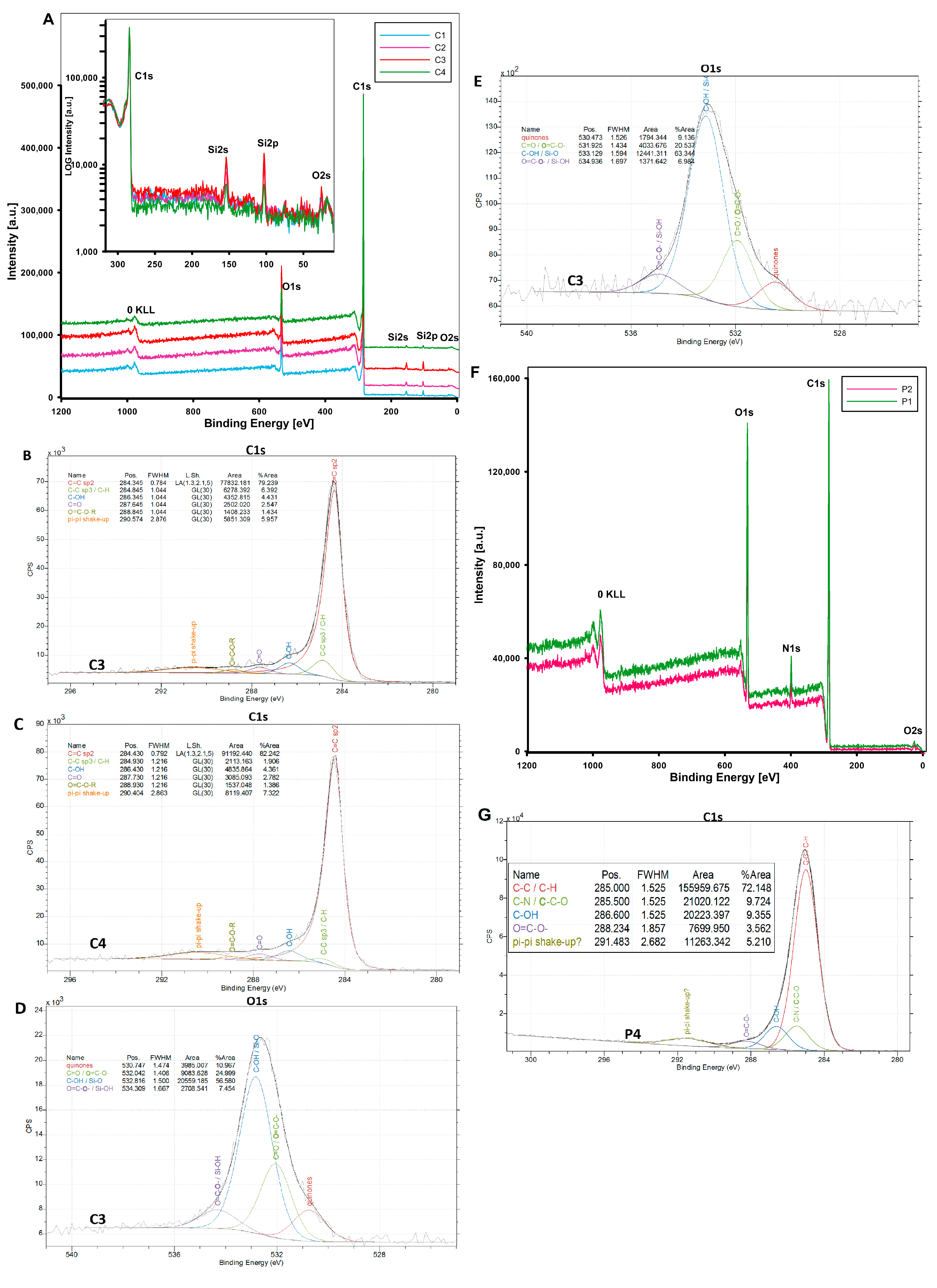
3.1.4. Surface Chemistry of Adsorbents
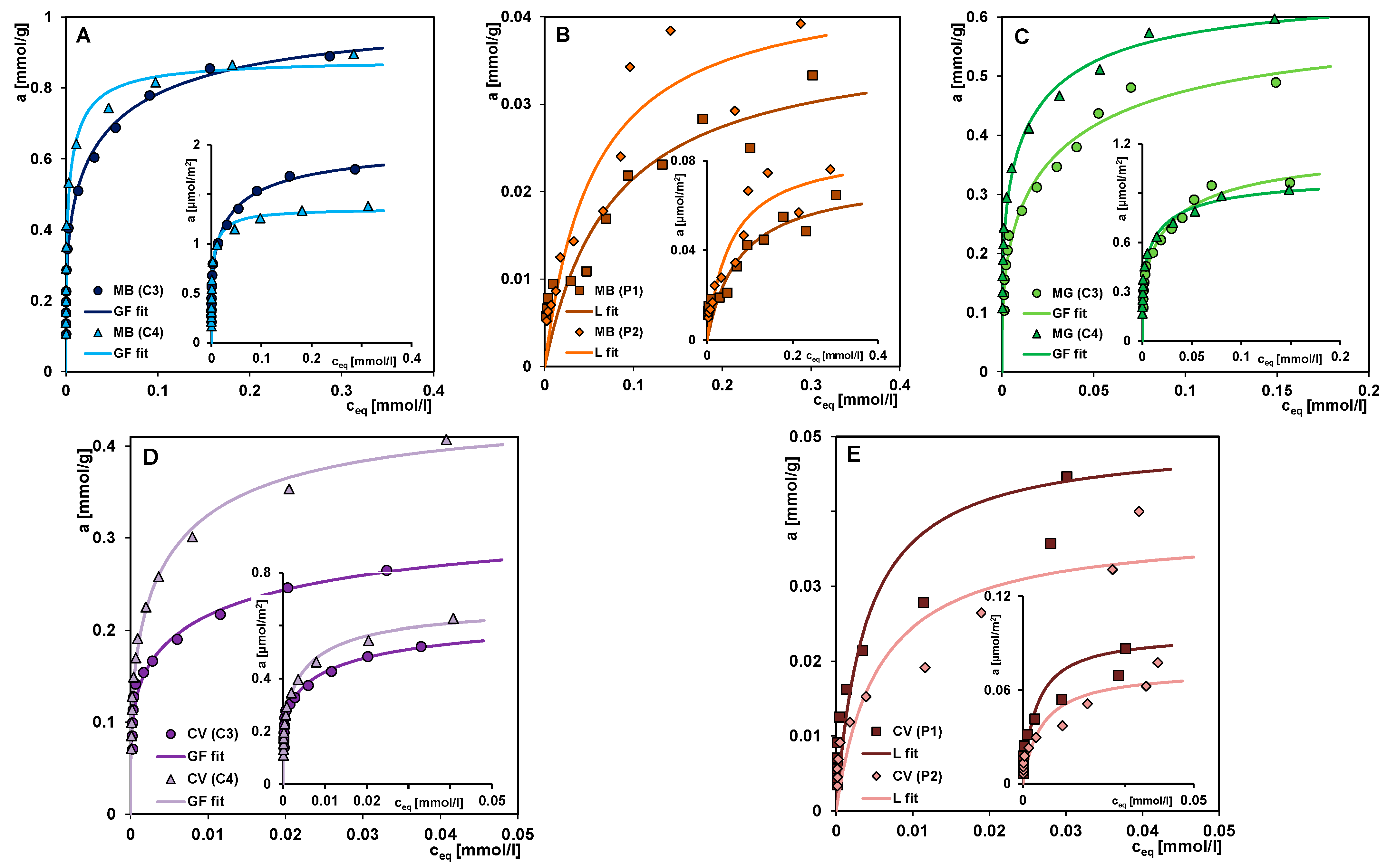
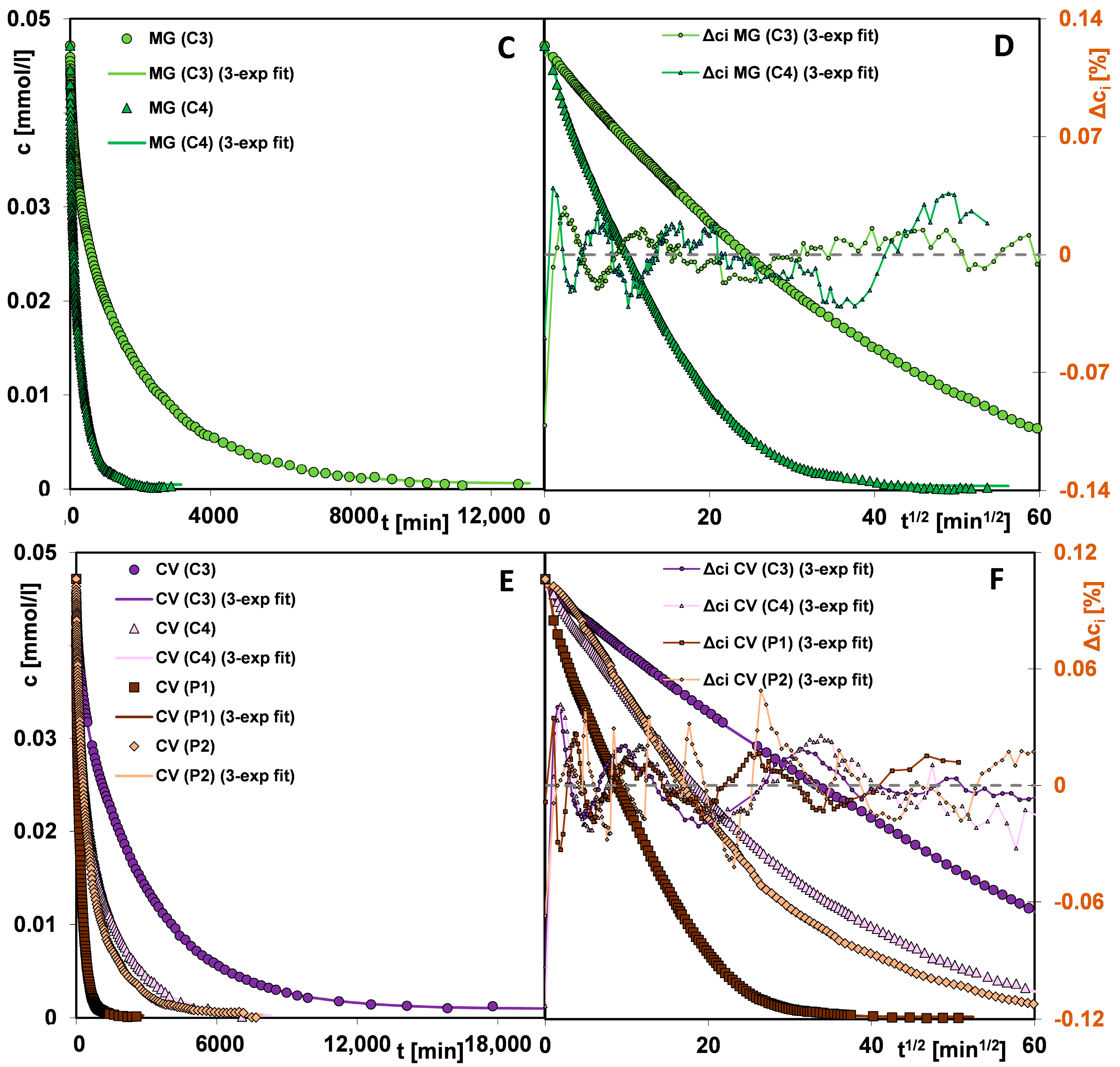
3.2. The Adsorption Properties of the Mesoporous Materials
4. Conclusions
- The proposed synthesis methods allow mesoporous carbon and polymer materials with differentiated characteristics to be obtained. Differences in the properties of the porous structure were determined by nitrogen adsorption and SAXS measurements. These differences concerned the specific surface area, the presence of micro-sized pores, the content of meso-sized pores, and levels of closed porosity, influencing adsorption capacity. The tested materials show activity in the interaction with X-ray radiation in the range of small diffraction angles. The SAXS effect allowed various proportions of the scattering objects to be determined, as well as their size distribution and the quality of the carbon/pores or polymer/pores interfacial area. The phenomenon of blurring of the interfacial area was confirmed in the case of some samples (e.g., sample C2) or their sharp boundary (as in the case of polymer samples). The content of closed porosity was determined.
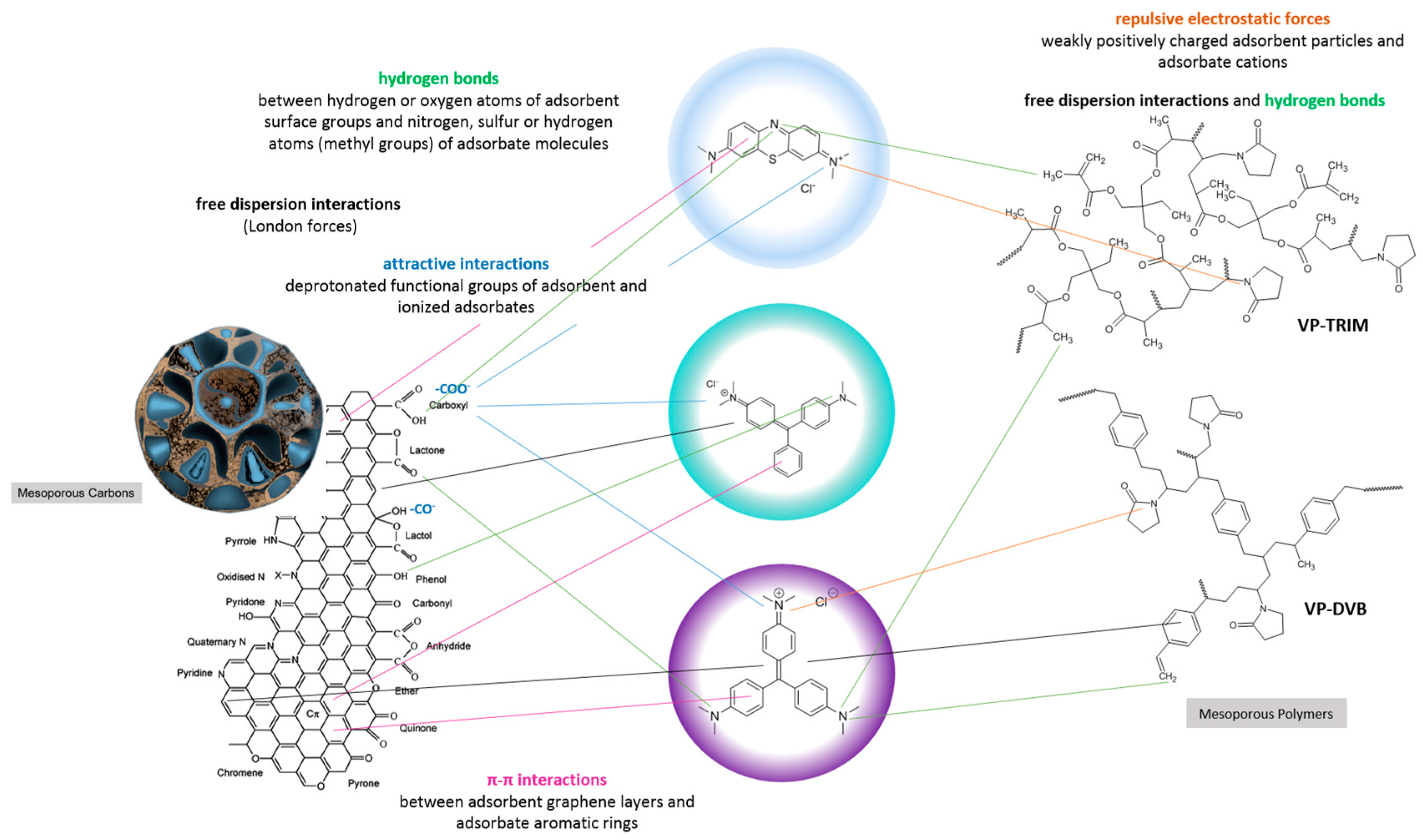
- The surface properties of mesoporous carbon and polymer materials were determined by identifying the surface functional groups and their atomic content (XPS method), and surface charge (potentiometric titration method). The acidic nature of the surface of carbon materials was confirmed due to the presence of functional groups such as C=O or O=C-O-, C-OH, Si-O-, O=C-O-, and Si-OH groups. These groups give the adsorbent a charge appropriate for the adsorption of cationic dyes. Unlike carbon materials, mesoporous polymer materials exhibit rather basic properties, which are influenced by the interactions between carbon atoms and the presence of nitrogen.
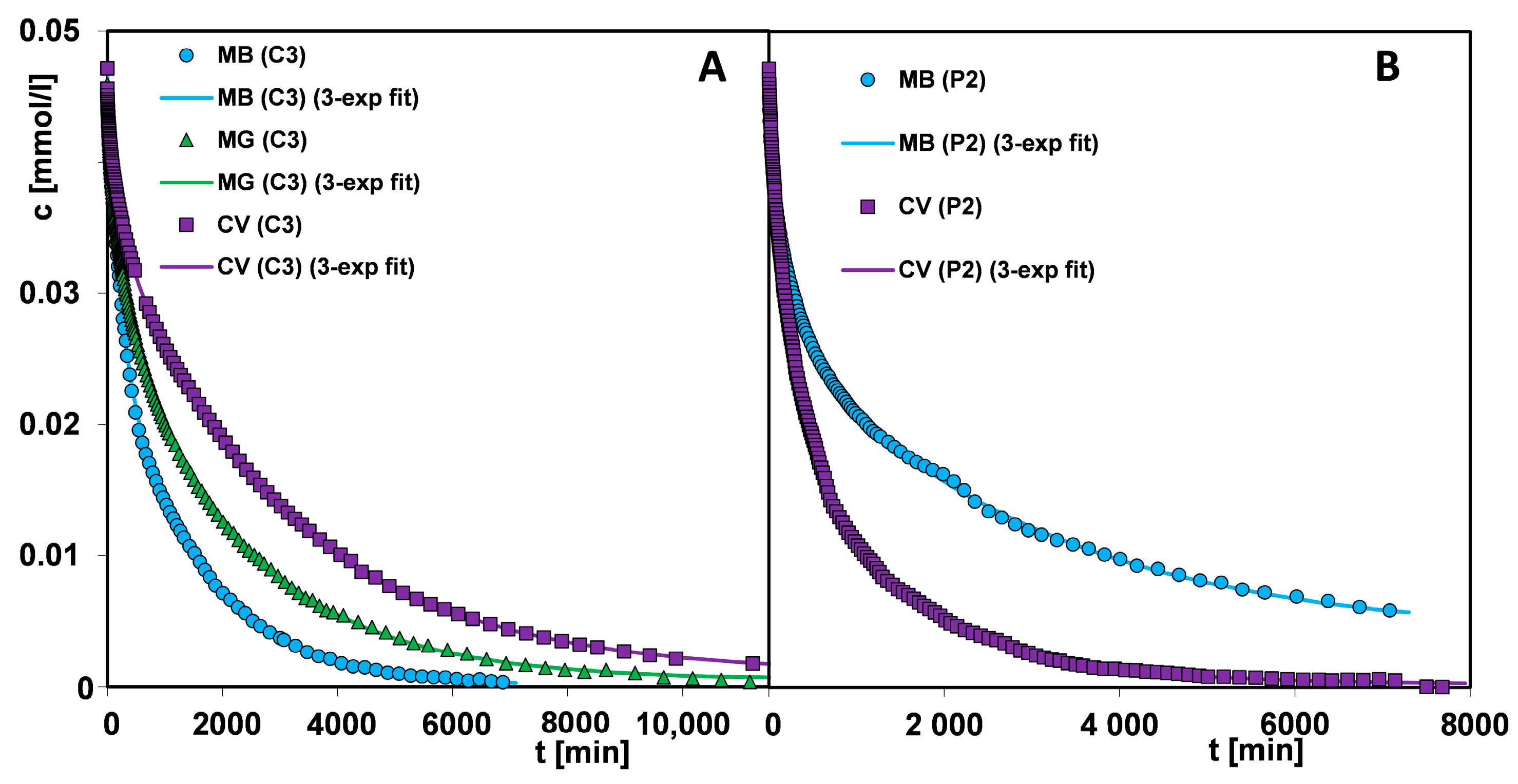
- The significantly higher affinity of carbons for cationic dyes is mainly related to strong attractive electrostatic forces, while in the polymer-dye system, there are repulsive electrostatic ones. Methylene blue, as a small molecule with a linear shape, is more effectively adsorbed on solids characterized by the presence of micropores and small mesopores. For the adsorption of malachite green and crystal violet, due to their larger particle size and spatially expanded shape, mesoporous materials showing a medium mesopores range are better suited. For adsorbents with small pores, a sieve effect is observed in the applied adsorbates. The mechanism of dye adsorption on carbon and polymer materials is based on electrostatic interactions, π-π interactions (except for systems with P1), free dispersion interactions (London forces), and hydrogen bridges. The higher affinity of the adsorbent for the adsorbate also translates into kinetics; in the case of polymers, crystal violet is adsorbed faster despite the larger size and spatially expanded structure of the molecule. Faster kinetics is observed for carbons—methylene blue systems.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Tan, X.F.; Liu, Y.G.; Liu, S.B.; Li, M.F.; Gu, Y.L.; Zhang, P.; Ye, S.J.; Yang, Z.Z.; Yang, Y.Y. Biomass-derived porous graphitic carbon materials for energy and environmental applications. J. Mater. Chem. A 2020, 8, 5773–5811. [Google Scholar] [CrossRef]
- Guo, H.; Mei, P.; Xiao, J.T.; Huang, X.S.; Ishag, A.; Sun, Y.B. Carbon materials for extraction of uranium from seawater. Chemosphere 2021, 278, 130411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q. Application of low-carbon environmental protection concept in bamboo material product design. Fresenius Environ. Bull. 2021, 30, 11332–11339. [Google Scholar]
- Mukhin, V.M.; Voropaeva, N.L.; Tkachev, A.G.; Bogdanovich, N.I.; Spiridonov, J.J. Vegetable waste as perspective raw materials for the production of carbon adsorbents. Inz. Miner. J. Pol. Miner. Eng. Soc. 2016, 17, 241–245. [Google Scholar]
- Müller, E.A. Adsorption of super greenhouse gases on microporous carbons. Environ. Sci. Technol. 2005, 39, 8736–8741. [Google Scholar] [CrossRef]
- Padron-Ramirez, I.; Torres-Figueredo, N.; Corcho-Valdes, A.L.; de Leon-Cabrera, J.P.; Chao-Mujica, F.J.; Cruz, K.V.; Antuch, M.; Deschamps, J.; Reguera, E.; Desdin-Garcia, L.F. Methane and carbon dioxide adsorption on carbon nano-onions synthesized by the submerged arc-discharge method. Adsorption 2024, 30, 25–38. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, J.M.; Yun, Y.S. Mechanisms of the removal of hexavalent chromium by biomaterials or biomaterial-based activated carbons. J. Hazard. Mater. 2006, 137, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Sabermahani, F.; Mahani, N.M.; Pourgholamrezazadeh, N. Adsorption of Pb(II) by activated carbon prepared from Melia azedarach fruit: Equilibrium and thermodynamics. Iran. Chem. Commun. 2018, 6, 148–159. [Google Scholar]
- Buchgraber, W. Carbon/carbon composite friction discs for aerospace. Mater. Werkst. 2003, 34, 317–321. [Google Scholar] [CrossRef]
- Fanter, D.L.; Strandburg, D.B.; Dry, A.C. Carbon fiber-reinforced composites: Entering a new era. In Proceedings of the 40th International SAMPE Symposium and Exhibition on Materials Challenge Diversification and the Future, Anaheim, CA, USA, 8–11 May 1995; pp. 989–993. [Google Scholar]
- Hajmohammadi, Z.; Fattahi, R.; Zarei-Behjani, Z.; Hosseinzadeh, S. Carbon nanoparticles for medicine: Current and future. Bull. Mater. Sci. 2022, 45, 8. [Google Scholar] [CrossRef]
- Shabashini, A.; Panja, S.K.; Nandi, G.C. Applications of carbon dots (CDs) in latent fingerprints imaging. Chem.-Asian J. 2021, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Juntgen, H. New applications of carbon adsorbents. Carbon 1976, 14, 291. [Google Scholar] [CrossRef]
- Tanabe, Y.; Yasuda, E. Science and application of new carbon. Nippon. Seramikkusu Kyokai Gakujutsu Ronbunshi-J. he Ceram. Soc. Jpn. 1991, 99, 1069–1077. [Google Scholar] [CrossRef]
- Van Bellingen, C.; Probst, N.; Grivei, E. Specific conductive carbon blacks in plastics applications. Polym. Polym. Compos. 2002, 10, 63–71. [Google Scholar] [CrossRef]
- Zammataro, A.; Sfrazzetto, G.T. Carbon dots as catalysts: A new class of nanozymes. Curr. Organocatalysis 2020, 7, 3–6. [Google Scholar] [CrossRef]
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.Q.; Shi, Y.F.; Yang, H.F.; Li, Z.; Yu, C.Z.; Tu, B.; Zhao, D.Y. Ordered mesoporous polymers and homologous carbon frameworks: Amphiphilic surfactant templating and direct transformation. Angew. Chem.-Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Sekar, M.; Sakthi, V.; Rengaraj, S. Kinetics and equilibrium adsorption study of lead(II) onto activated carbon prepared from coconut shell. J. Colloid Interface Sci. 2004, 279, 307–313. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.G.; Li, M.T.; Meng, X.Y.; Wang, L.; Wang, R.H.; Yin, J.; Fu, H.G. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J. Hazard. Mater. 2005, 124, 192–199. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.L.; Wang, Z.D.; Fan, X.K.; Yan, C.F. Synergistic effects of N-doping and mesoporous structures in block copolymer-derived three-dimensionally ordered mesoporous carbon for PEMFC. Int. J. Hydrogen Energy 2024, 51, 747–757. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd supported on mesoporous activated carbons with high oxidation resistance as catalysts for toluene oxidation. Appl. Catal. B-Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for friedel-crafts reaction of benzene. Angew. Chem. -Int. Ed. 2006, 45, 4467–4471. [Google Scholar] [CrossRef]
- Huang, X.; Wu, S.S.; Du, X.Z. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon 2016, 101, 135–142. [Google Scholar] [CrossRef]
- Karavasili, C.; Amanatiadou, E.P.; Sygellou, L.; Giasafaki, D.K.; Steriotis, T.A.; Charalambopoulou, G.C.; Vizirianakis, I.S.; Fatouros, D.G. Development of new drug delivery system based on ordered mesoporous carbons: Characterisation and cytocompatibility studies. J. Mater. Chem. B 2013, 1, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Warren, K.E.; Naskar, A.K. Soft-templated mesoporous carbons as potential materials for oral drug delivery. Carbon 2014, 71, 47–57. [Google Scholar] [CrossRef]
- Zhao, Q.F.; Lin, Y.Z.; Han, N.; Li, X.; Geng, H.J.; Wang, X.D.; Cui, Y.; Wang, S.L. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Wan, L.; Jiao, J.; Cui, Y.; Guo, J.W.; Han, N.; Di, D.H.; Chang, D.; Wang, P.; Jiang, T.Y.; Wang, S.L. Hyaluronic acid modified mesoporous carbon nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanotechnology 2016, 27, 135102. [Google Scholar] [CrossRef]
- Song, C.; Du, J.P.; Zhao, J.H.; Feng, S.A.; Du, G.X.; Zhu, Z.P. Hierarchical porous core-shell carbon nanoparticles. Chem. Mater. 2009, 21, 1524–1530. [Google Scholar] [CrossRef]
- Si, W.J.; Xing, W.; Zhuo, S.P. Preparation of hierarchical porous carbon and its application in EDLC. Chin. J. Inorg. Chem. 2009, 25, 1159–1164. [Google Scholar]
- Liu, X.W.; Liu, X.H.; Sun, B.F.; Zhou, H.L.; Fu, A.P.; Wang, Y.Q.; Guo, Y.G.; Guo, P.Z.; Li, H.L. Carbon materials with hierarchical porosity: Effect of template removal strategy and study on their electrochemical properties. Carbon 2018, 130, 680–691. [Google Scholar] [CrossRef]
- Xing, W.; Zhuo, S.P.; Gao, X.L. Preparation of hierarchical porous carbon by post activation. Mater. Lett. 2009, 63, 1311–1313. [Google Scholar] [CrossRef]
- Yang, M.; Wang, G. Synthesis of hierarchical porous carbon particles by hollow polymer microsphere template. Colloids Surf. A-Physicochem. Eng. Asp. 2009, 345, 121–126. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Gao, Q.M. Hierarchical porous carbons prepared by an easy one-step carbonization and activation of phenol-formaldehyde resins with high performance for supercapacitors. J. Power Sources 2011, 196, 1615–1619. [Google Scholar] [CrossRef]
- Cai, L.F.; Chen, L.Y.; Wang, C.L.; Liang, Y.R.; Fu, R.W.; Wu, D.C. Liquid-phase methylene blue adsorption of a novel hierarchical porous carbon aerogel. New Carbon Mater. 2015, 30, 560–565. [Google Scholar] [CrossRef]
- Li, S.S.; Zhang, H.J.; Hu, S.Y.; Liu, J.; Zhu, Q.; Zhang, S.W. Synthesis of hierarchical porous carbon in molten salt and its application for dye adsorption. Nanomaterials 2019, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Chen, Z.G.; Ni, C.Y.; Chen, F.; Gu, C.; Cao, Y.; Wu, Z.Y.; Li, P. Adsorption of phenol from aqueous solution by a hierarchical micro-nano porous carbon material. Rare Met. 2012, 31, 582–589. [Google Scholar] [CrossRef]
- Wei, S.C.; Li, D.T.; Huang, Z.; Huang, Y.Q.; Wang, F. High-capacity adsorption of Cr(VI) from aqueous solution using a hierarchical porous carbon obtained from pig bone. Bioresour. Technol. 2013, 134, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, F.; Mu, G.Q.; Zhou, Z.G.; Xie, Y.; Li, L.; Yang, Y.Y.; Wang, X.Z. Adsorption of toluene vapours on micro-meso hierarchical porous carbon. Micro Nano Lett. 2018, 13, 641–645. [Google Scholar] [CrossRef]
- Chen, M.Q.; Su, Z.; Jiang, K.; Pan, Y.K.; Zhang, Y.Y.; Long, D.H. Promoting sulfur immobilization by a hierarchical morphology of hollow carbon nanosphere clusters for high-stability Li-S battery. J. Mater. Chem. A 2019, 7, 6250–6258. [Google Scholar] [CrossRef]
- Lu, P.-J.; Lin, H.-C.; Yu, W.-T.; Chern, J.-M. Chemical regeneration of activated carbon used for dye adsorption. J. Taiwan Inst. Chem. Eng. 2011, 42, 305–311. [Google Scholar] [CrossRef]
- Santos, D.H.D.S.; Xiao, Y.; Chaukura, N.; Hill, J.M.; Selvasembian, R.; Zanta, C.L.P.S.; Meili, L. Regeneration of dye-saturated activated carbon through advanced oxidative processes: A review. Heliyon 2022, 8, e10205. [Google Scholar] [CrossRef]
- Mohd Nasir, M.Z.; Indiran, G.; Ahmad Zaini, M.A. Assessment of thermal regeneration of spent commercial activated carbon for methylene blue dye removal. Part. Sci. Technol. 2021, 39, 504–510. [Google Scholar] [CrossRef]
- Dai, Y.J.; Zhang, N.X.; Xing, C.M.; Cui, Q.X.; Sun, Q.Y. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Sobiesiak, M. Analysis of structure and properties of DVB–GMA based porous polymers. Adsorption 2019, 25, 257–266. [Google Scholar] [CrossRef]
- Podkościelna, B.; Wawrzkiewicz, M.; Klapiszewski, Ł. Synthesis, characterization and sorption ability of epoxy resin-based sorbents with amine groups. Polymers 2021, 13, 4139. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Józwicki, M. Polymers based on 9,10-bis(methacryloyloxymethyl)anthracene—Towards synthesis and characterization. Materials 2023, 16, 2610. [Google Scholar] [CrossRef]
- Grochowicz, M.; Szajnecki, Ł.; Rogulska, M. Crosslinked 4- vinylpyridine monodisperse functional microspheres for sorption of ibuprofen and ketoprofen. Polymers 2022, 14, 2080. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Gawdzik, B.; Rogulska, M. Polymeric microspheres with highly developed internal structure and remarkable thermal stability. Materials 2021, 14, 2240. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, K.; Hyeon, T. Fabrication of novel mesocellular carbon foams with uniform ultralarge mesopores. J. Am. Chem. Soc. 2001, 123, 5146–5147. [Google Scholar] [CrossRef]
- Blachnio, M.; Derylo-Marczewska, A.; Winter, S.; Zienkiewicz-Strzalka, M. Mesoporous carbons of well-organized structure in the removal of dyes from aqueous solutions. Molecules 2021, 26, 2159. [Google Scholar] [CrossRef]
- Burkinshaw, S.M. Dyes. In Physico-Chemical Aspects of Textile Coloration; John Wiley and Sons: New York, NY, USA, 2016; pp. 65–79. [Google Scholar]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A.; Nosach, L.V.; Voronin, E.F. Chitosan–silica composites for adsorption application in the treatment of water and wastewater from anionic dyes. Int. J. Mol. Sci. 2023, 24, 11818. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Nosach, L.V.; Voronin, E.F.; Derylo-Marczewska, A.; Wasilewska, M. Effect of geometric modification of fumed nanoscale silica for medical applications on adsorption of human serum albumin: Physicochemical and surface properties. Int. J. Biol. Macromol. 2022, 220, 1294–1308. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Jaroniec, M. A new isotherm equation for single-solute adsorption from dilute solutions on energetically heterogeneous solids. Monatshefte Für Chem. Chem. Mon. 1983, 114, 711–715. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzalka, M.; Blachnio, M. Nitrogenous bases in relation to the colloidal silver phase: Adsorption kinetic, and morphology investigation. Appl. Sci. 2023, 13, 3696. [Google Scholar] [CrossRef]
- Galaburda, M.; Zienkiewicz-Strzalka, M.; Blachnio, M.; Bogatyrov, V.; Kutkowska, J.; Choma, A.; Derylo-Marczewska, A. Ag-containing carbon nanocomposites: Physico-chemical properties and antimicrobial activity. Sustainability 2023, 15, 16817. [Google Scholar] [CrossRef]
- Guinier, A.; Fournet, G. Small-Angle Scattering of X-rays; John Wiley and Sons: New York, NY, USA, 1955. [Google Scholar]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Gülen, J.; Zorbay, F. Methylene blue adsorption on a low cost adsorbent—Carbonized peanut shell. Water Environ. Res. 2017, 89, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, X.; Xu, Q.; Dhaouadi, F.; Sellaoui, L.; Seliem, M.K.; Ben Lamine, A.; Belmabrouk, H.; Bajahzar, A.; Bonilla-Petriciolet, A.; et al. Adsorption of methylene blue from aqueous solution on activated carbons and composite prepared from an agricultural waste biomass: A comparative study by experimental and advanced modeling analysis. Chem. Eng. J. 2022, 430, 132801. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, L. Two birds with one stone: One-pot hydrothermal treatment of dragon fruit peel to simultaneously prepare efficient biochar adsorbent and fluorescent carbon quantum dots. Ind. Crops Prod. 2024, 210, 118086. [Google Scholar] [CrossRef]
- Zeng, S.; Tan, J.; Xu, X.; Huang, X.; Zhou, L. Facile synthesis of amphiphilic peach gum polysaccharide as a robust host for efficient encapsulation of methylene blue and methyl orange dyes from water. Int. J. Biol. Macromol. 2020, 154, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tan, J.; Wang, G.; Zhou, L. Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes. Carbohydr. Polym. 2018, 199, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, J.; He, B.; Zhang, F.; Li, H. Peach gum for efficient removal of methylene blue and methyl violet dyes from aqueous solution. Carbohydr. Polym. 2014, 101, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Balsamo, M.; Montagnaro, F. Liquid–solid mass transfer in adsorption systems—An overlooked resistance? Ind. Eng. Chem. Res. 2020, 59, 22007–22016. [Google Scholar] [CrossRef]
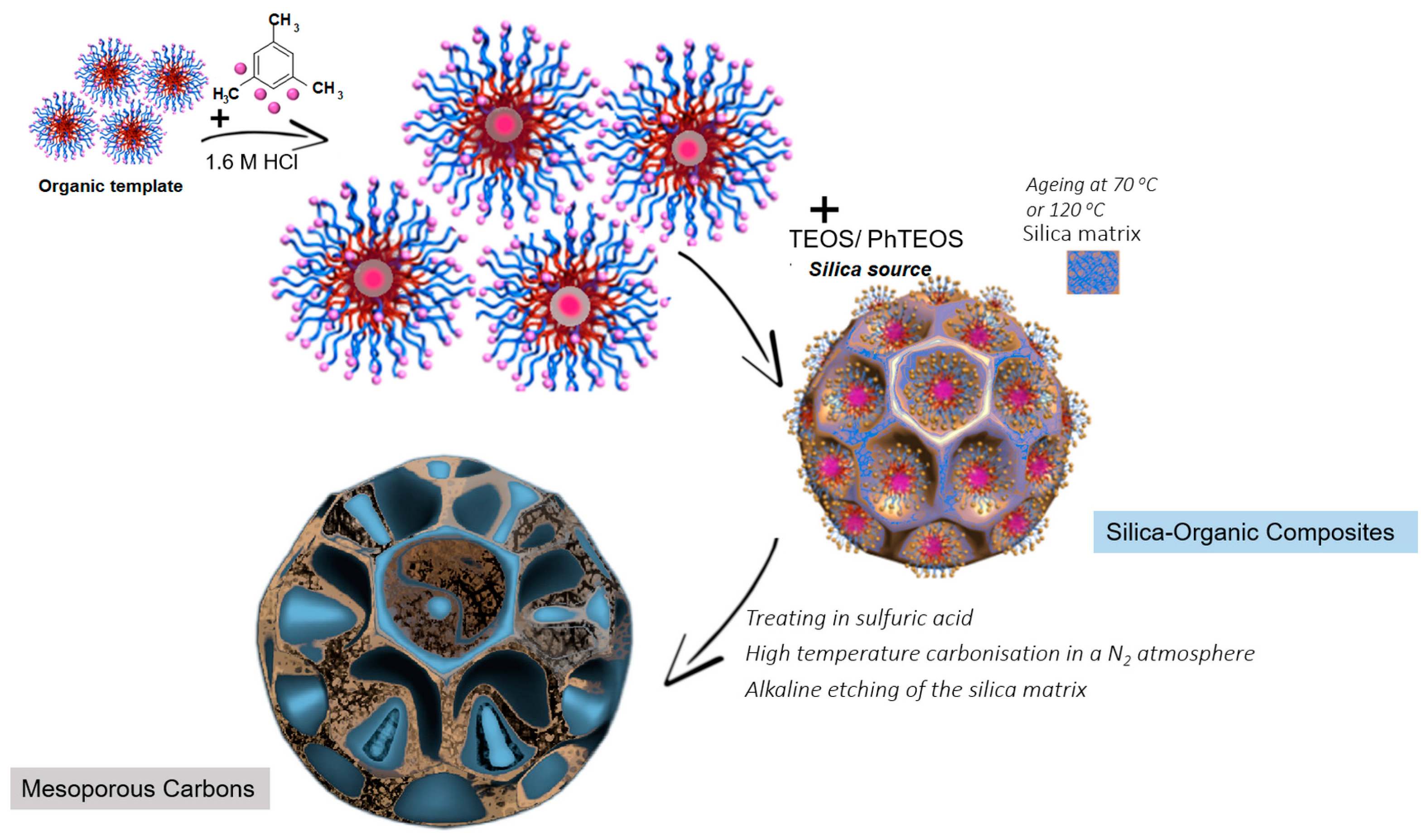





| Carbon | Pluronic/Mw 1/Formula | Temperature/Process Duration of Silica-Organic Composite Ageing | Temperature/Process Duration of Vacuum Heating of Composite + Catalyst (Stage 1) | Temperature/Process Duration of Vacuum Heating of Composite + Catalyst (Stage 2) | Temperature/Process Duration of Carbonisation |
|---|---|---|---|---|---|
| C1 | PE9400/4600 g/mol/(EO)21(PO)47(EO)21 | 70 °C/24 h | 100 °C/12 h | 160 °C/12 h | 800 °C/6 h |
| C2 | PE6400/2900 g/mol /(EO)13(PO)30(EO)13 | 70 °C/24 h | 100 °C/12 h | 160 °C/12 h | 800 °C/6 h |
| C3 | PE9400/4600 g/mol /(EO)21(PO)47(EO)21 | 120 °C/24 h | 100 °C/12 h | 160 °C/12 h | 800 °C/6 h |
| C4 | PE6400/2900 g/mol /(EO)13(PO)30(EO)13 | 120 °C/24 h | 100 °C/12 h | 160 °C/12 h | 800 °C/6 h |
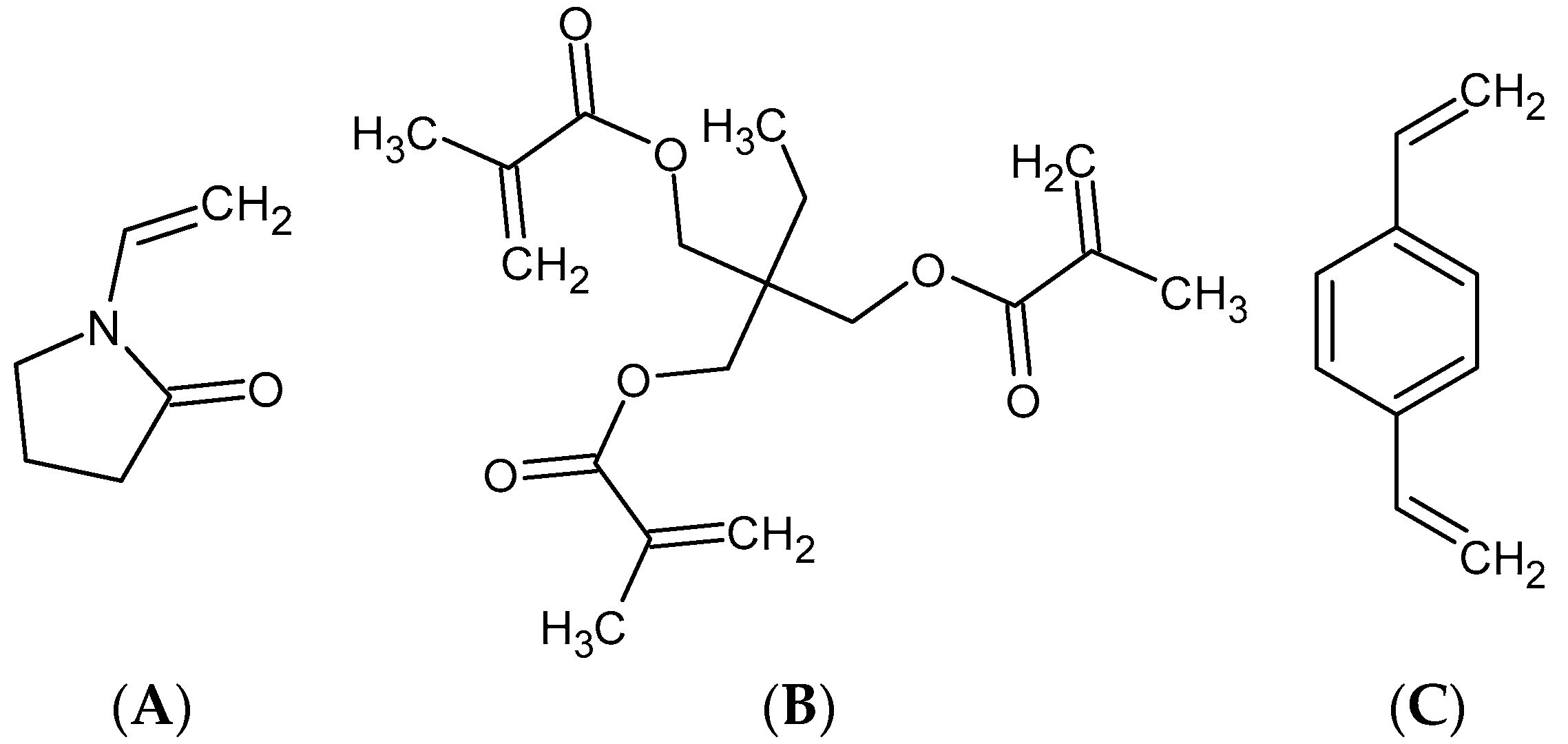
| Polymer | Monomers (g) | Diluents (mL) | Stabilizers (g) | ||||
|---|---|---|---|---|---|---|---|
| VP | TRIM | DVB | Toluene | Dodecane | PVA | CaCl2 | |
| P1 | 3.7 | 11.3 | - | 22.5 | - | 6.5 | - |
| P2 | 6.9 | - | 8.1 | 19.1 | 3.4 | 6.5 | 30 |
| Dye Code | Chemical Formula | Molecular Weight [g/mol] | Ionisation Constant, pKa | Water Solubility [%] | Dmin/Dmax1 [Å] | Ref. |
|---|---|---|---|---|---|---|
| MB | C16H18N3ClS | 319.85 | 2.6; 11.2 | 4.36; 4 | 4.6/9.9 | [60] |
| MG | C23H25ClN2 | 364.91 | 6.9 | 4 | 8.0/9.7 | [60,61] |
| CV | C25H30N3Cl | 407.99 | 0.8; 9.4 | 1.7 | 9.5/9.7 | [60] |
| Carbon | SBET [m2/g] | Smic [m2/g] | Vt [cm3/g] | Vmic (t-plot) [cm3/g] | Dh [nm] | Dmo (ads. BJH) [nm] | Dmo (des. BJH) [nm] |
|---|---|---|---|---|---|---|---|
| C1 | 502 | 243 | 0.30 | 0.11 | 2.4 | 3.14 | 2.75 |
| C2 | 760 | 40 | 0.61 | 0.01 | 3.2 | 3.43 | 3.24 |
| C3 | 508 | 215 | 0.29 | 0.10 | 2.2 | 2.95 | 2.58 |
| C4 | 649 | 37 | 0.65 | 0.01 | 2.7 | 4.05 | 3.74 |
| P1 | 515 | - | 1.06 | 0.08 | 8.3 | 9 | 9.3 |
| P2 | 515 | - | 0.75 | - | 5.8 | 6.3 | 6.1 |
| Sample Identifier | Name | Position | FWHM | %At. Conc. | % St.Dev. |
|---|---|---|---|---|---|
| C1 | C1s | 284.5 | 1.8 | 84.8 | 0.105 |
| O1s | 532.8 | 2.5 | 11.5 | 0.081 | |
| Si2p | 103.0 | 2.5 | 3.7 | 0.079 | |
| C2 | C1s | 284.5 | 1.8 | 88.4 | 0.087 |
| O1s | 532.8 | 2.4 | 9.2 | 0.066 | |
| Si2p | 103.0 | 2.5 | 2.4 | 0.064 | |
| C3 | C1s | 284.5 | 1.7 | 82.4 | 0.156 |
| O1s | 532.8 | 2.6 | 13.2 | 0.114 | |
| Si2p | 103.0 | 2.3 | 4.4 | 0.129 | |
| C4 | C1s | 284.5 | 1.7 | 91.0 | 0.153 |
| O1s | 532.8 | 2.6 | 7.3 | 0.126 | |
| Si2p | 103.0 | 2.6 | 1.7 | 0.097 | |
| P1 | C1s | 285.0 | 2.9 | 76.9 | 0.119 |
| N1s | 399.0 | 2.4 | 3.6 | 0.090 | |
| O1s | 531.8 | 3.3 | 19.6 | 0.098 | |
| P2 | C1s | 285.0 | 2.5 | 92.3 | 0.149 |
| N1s | 399.8 | 2.5 | 2.6 | 0.140 | |
| O1s | 531.8 | 3.1 | 5.1 | 0.070 |
| Adsorption System | am | m | n | log K | R2 |
|---|---|---|---|---|---|
| MB (C3) | 1.01 | 0.25 | 1 | 1.33 | 0.98 |
| MB (C4) | 0.88 | 0.26 | 1 | 1.62 | 0.97 |
| MB (P1) | 0.038 | 1 | 1 | 1.12 | 0.84 |
| MB(P2) | 0.044 | 1 | 1 | 1.26 | 0.89 |
| MG (C3) | 0.60 | 0.32 | 1 | 0.96 | 0.97 |
| MG (C4) | 0.65 | 0.26 | 1 | 1.19 | 0.98 |
| CV (C3) | 0.33 | 0.22 | 1 | 1.24 | 0.96 |
| CV (C4) | 0.44 | 0.30 | 1 | 1.35 | 0.99 |
| CV (P1) | 0.050 | 1 | 1 | 2.41 | 0.86 |
| CV (P2) | 0.038 | 1 | 1 | 2.26 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zienkiewicz-Strzalka, M.; Blachnio, M.; Derylo-Marczewska, A.; Winter, S.; Maciejewska, M. Mesoporous Carbons and Highly Cross-Linking Polymers for Removal of Cationic Dyes from Aqueous Solutions—Studies on Adsorption Equilibrium and Kinetics. Materials 2024, 17, 1374. https://doi.org/10.3390/ma17061374
Zienkiewicz-Strzalka M, Blachnio M, Derylo-Marczewska A, Winter S, Maciejewska M. Mesoporous Carbons and Highly Cross-Linking Polymers for Removal of Cationic Dyes from Aqueous Solutions—Studies on Adsorption Equilibrium and Kinetics. Materials. 2024; 17(6):1374. https://doi.org/10.3390/ma17061374
Chicago/Turabian StyleZienkiewicz-Strzalka, Malgorzata, Magdalena Blachnio, Anna Derylo-Marczewska, Szymon Winter, and Malgorzata Maciejewska. 2024. "Mesoporous Carbons and Highly Cross-Linking Polymers for Removal of Cationic Dyes from Aqueous Solutions—Studies on Adsorption Equilibrium and Kinetics" Materials 17, no. 6: 1374. https://doi.org/10.3390/ma17061374







