Effect of SF and GGBS on Pore Structure and Transport Properties of Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Preparation
- (1)
- Weighing: weighing the raw materials according to the mix proportion in Table 3, the percentage of gel material was determined based on the cement’s mass and the W/B is the ratio of the mass of water and gel material. A, B, and C are the corresponding numbers for the three concrete comparisons in the table.
- (2)
- Mixing: firstly, the cement, aggregate, and mineral admixture was poured into the mixer in turn, dragged to mix for 3 min, and then 1/2 of the water was added and mixed for 3 min; finally, we poured in the remaining 1/2 of the water and water reducing agent and mixed. The mixture was then be discharged after it had good fluidity. The mixer used in this study is a forced single-axis mixer with a speed of 45 rpm, which conforms to the national standard GB/T 10171-2016 [37].
- (3)
- Forming: wipe the molds required for subsequent performance tests clean and then evenly apply a layer of release agent to each surface inside. The use of molds was in line with the construction industry standard JG237-2008 [38]. The evenly mixed concrete mixture was poured into the mold, placed on a vibrating table for compaction, and the surface of the concrete was screeded. The surface was smoothed and then covered with a layer of plastic film to prevent the loss of free water. The sample required for this experiment is 150 mm × 150 mm × 150 mm cube.
- (4)
- Demolding and curing: the samples were demolded approximately 24 h after casting and then cured in the water at a temperature of 20 ± 2 °C
- (5)
- After the samples were cured to the specified age, we cut the 150 mm × 150 mm × 150 mm cubes into Φ 50 × 100 mm cylinders for subsequent tests.
2.3. Experimental Methods
2.3.1. Microscopic Tests
- (1)
- Nuclear Magnetic Resonance (NMR) test
- (2)
- Mercury Intrusion Porosimetry (MIP) test
2.3.2. Permeability Test
- (1)
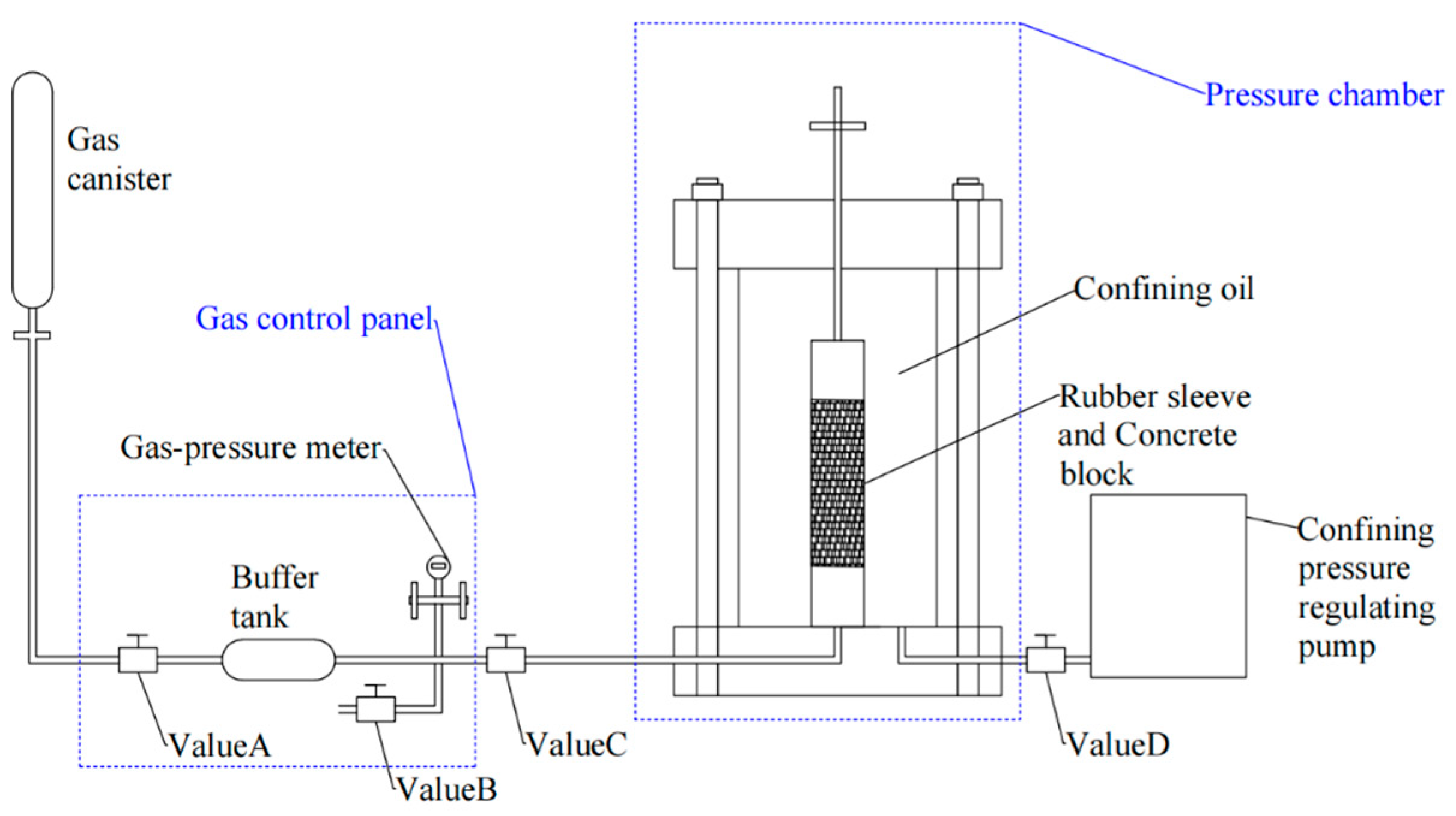
- Gas permeability test
- (2)
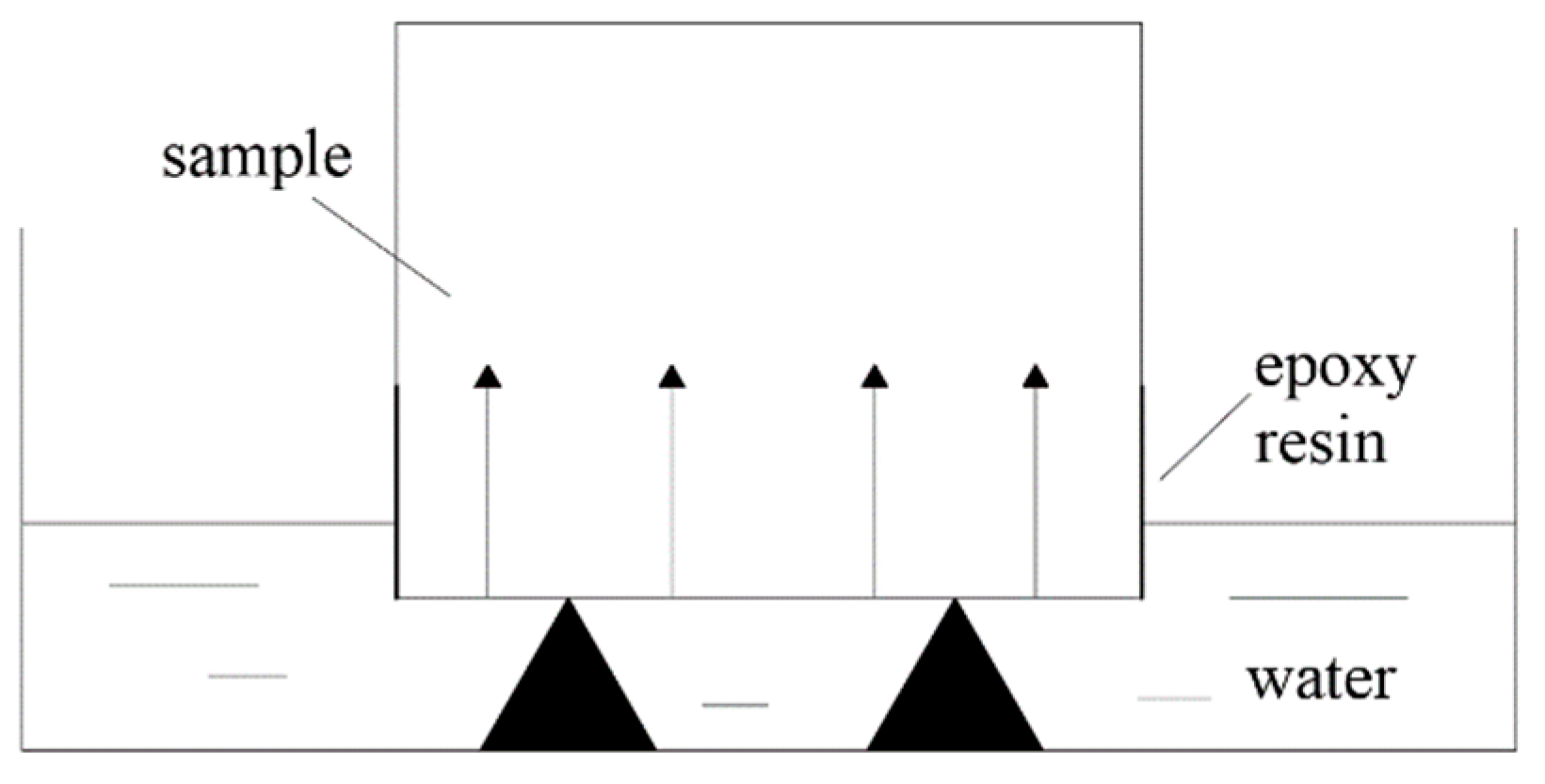
- Capillary water absorption test
3. Results and Analysis
3.1. Pore Structure
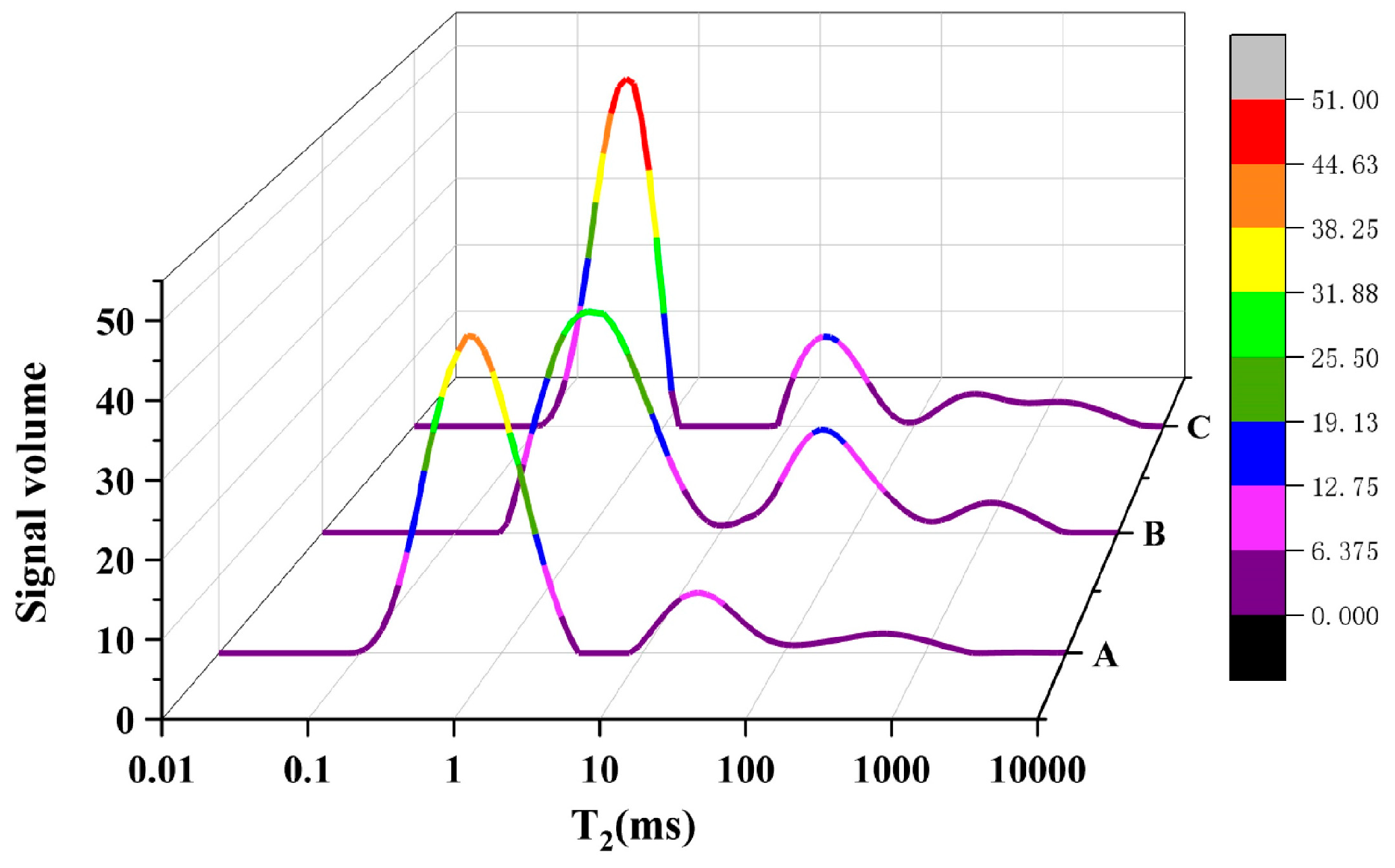
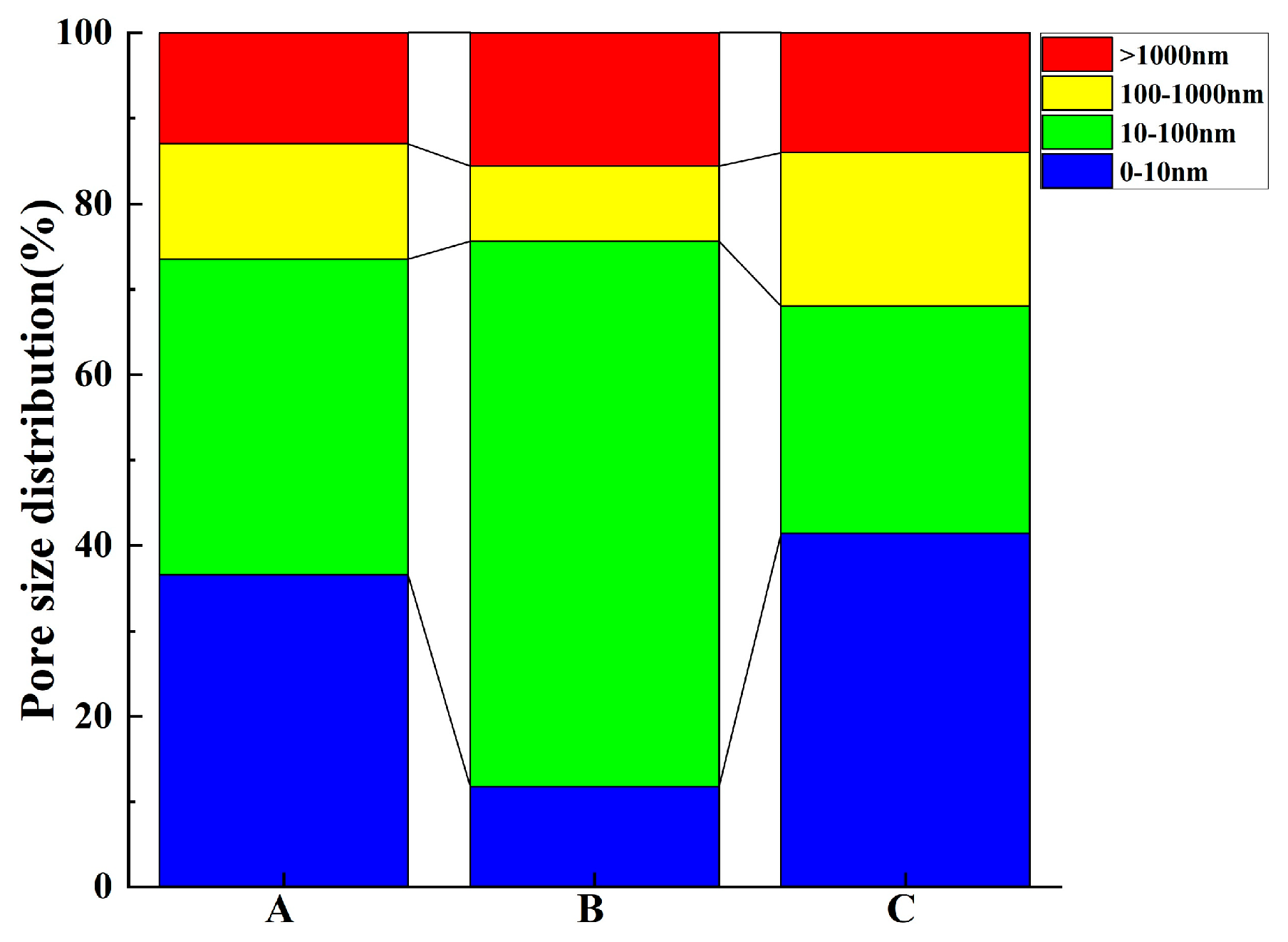
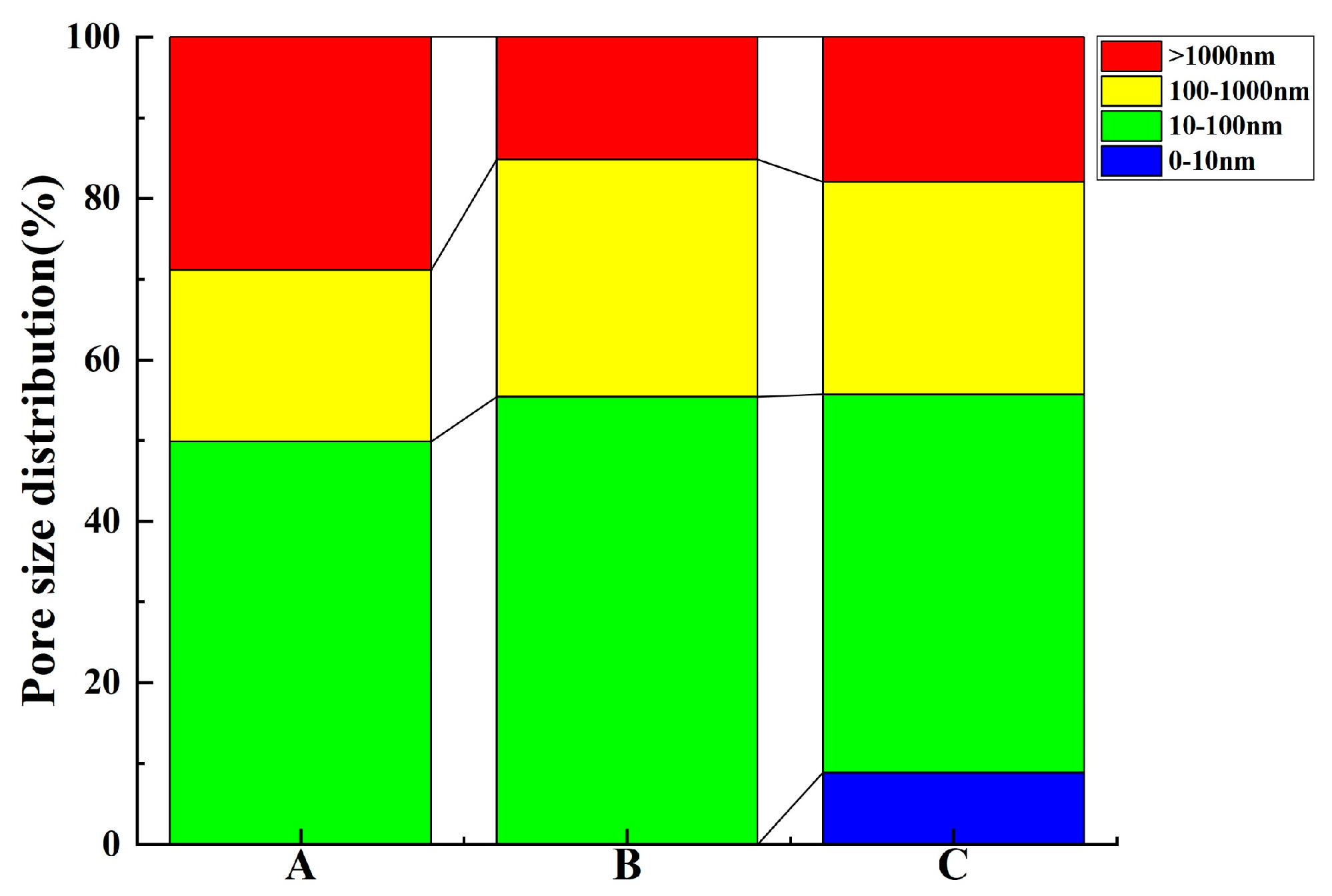
3.1.1. NMR Test
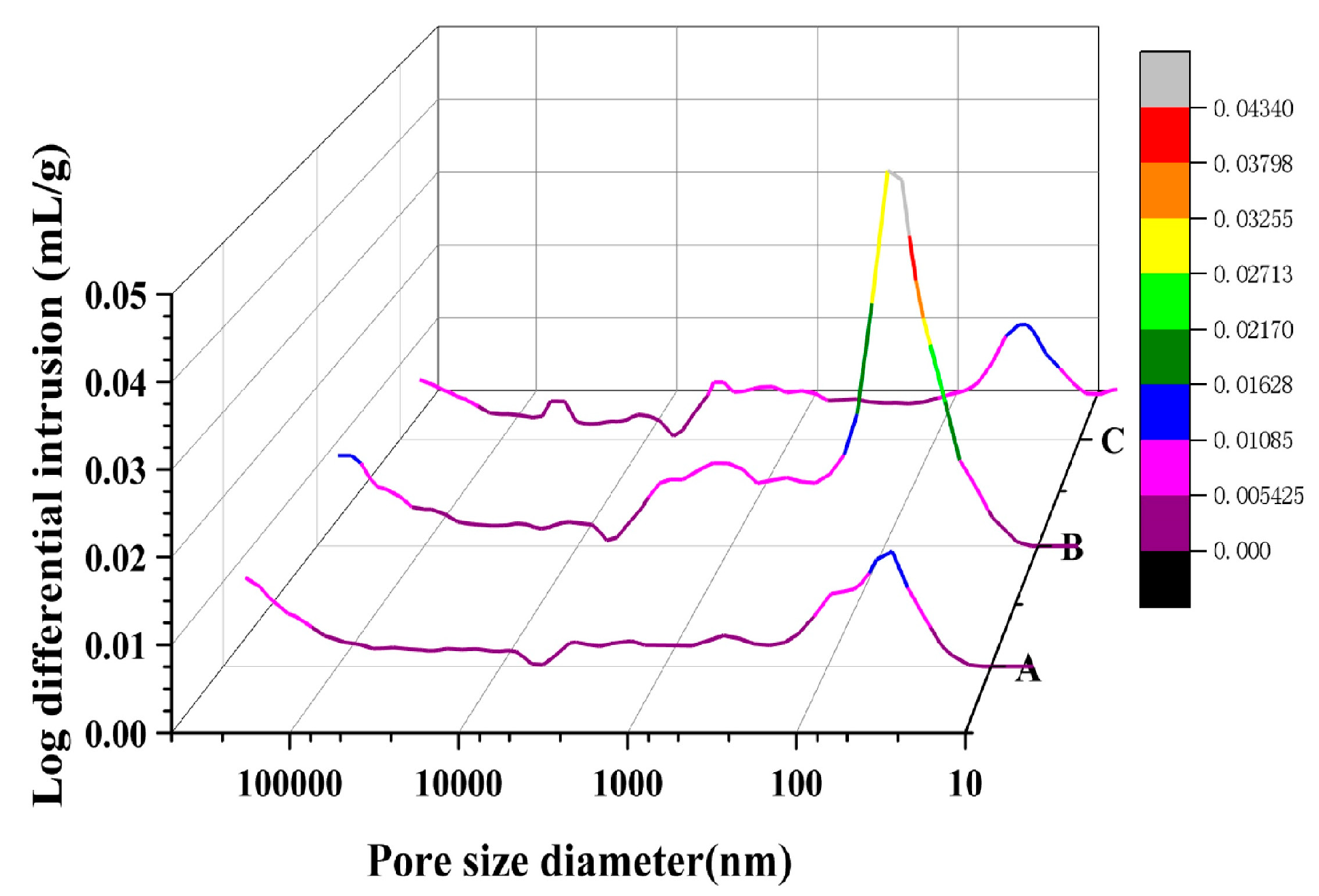
3.1.2. MIP Test
3.2. Transport Properties
3.2.1. Gas Permeability
- (1)
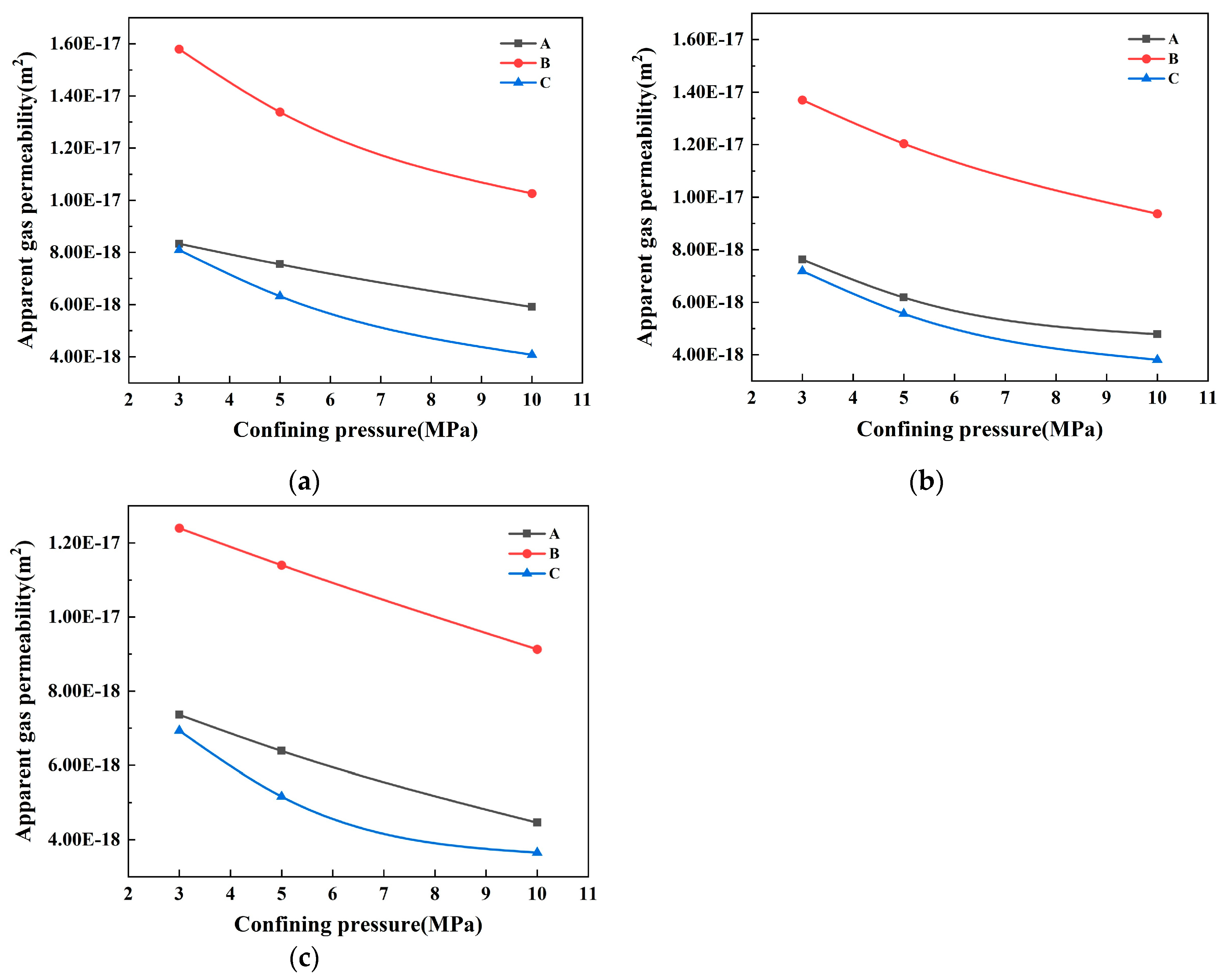
- Apparent gas permeability
- (2)
- Intrinsic gas permeability
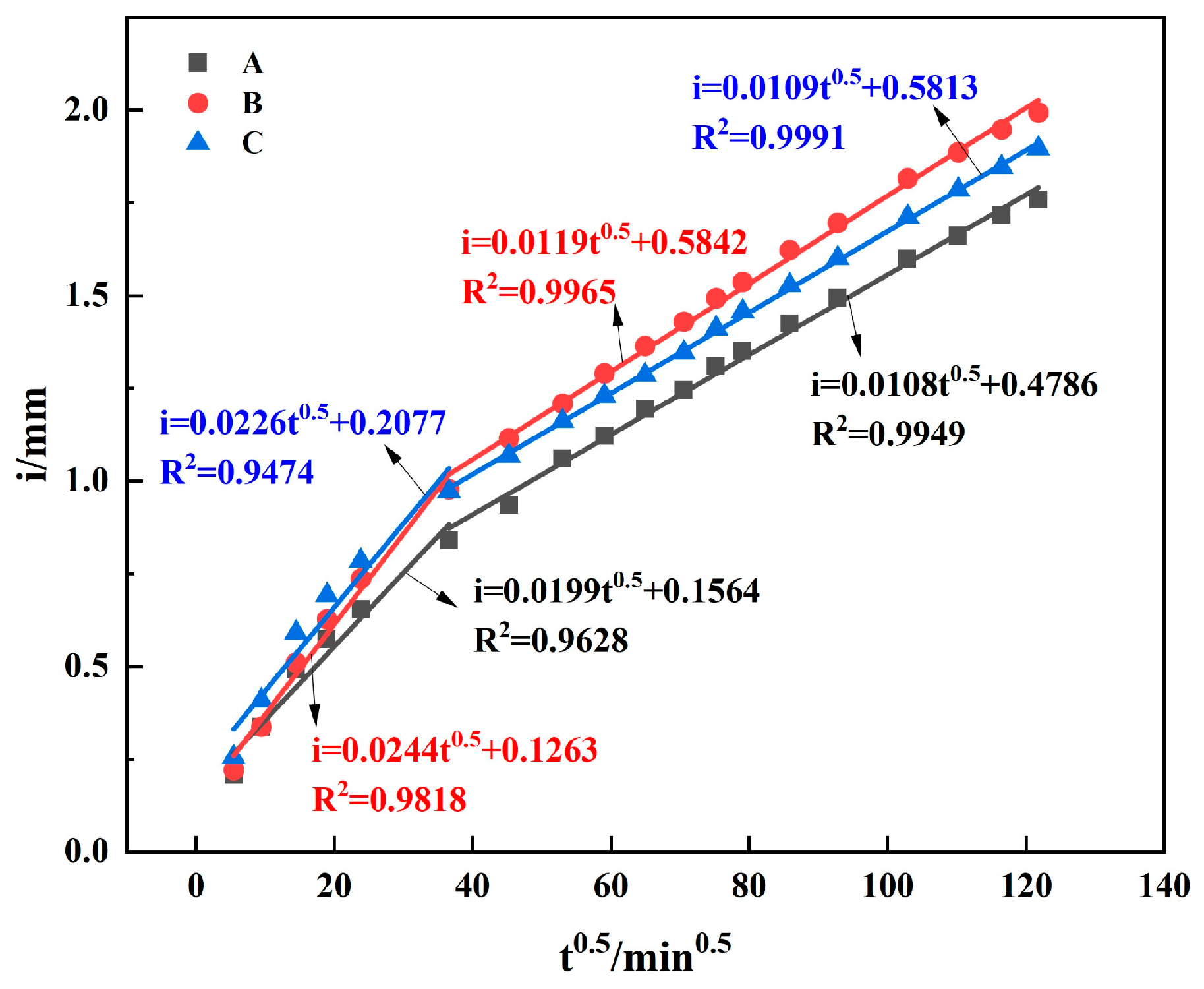
3.2.2. Capillary Water Absorption
3.3. Relationship between Pore Structure and Permeability
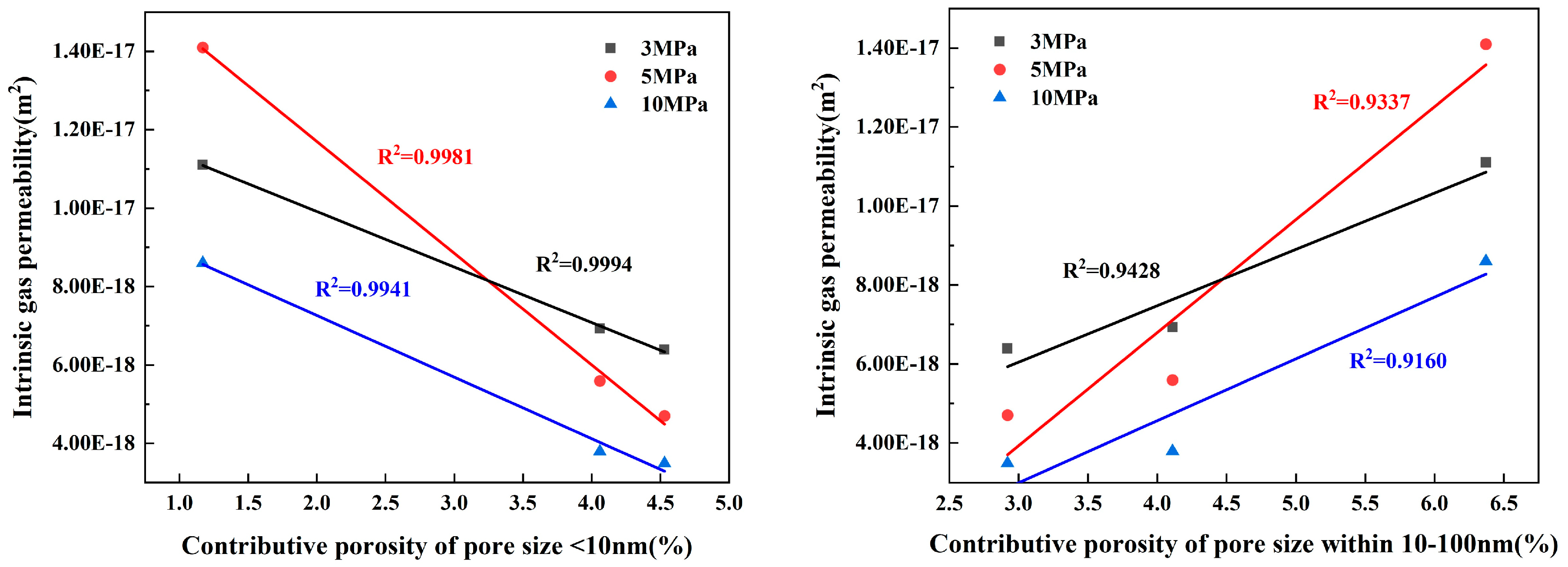
3.3.1. Relationship between Porosity and Permeability
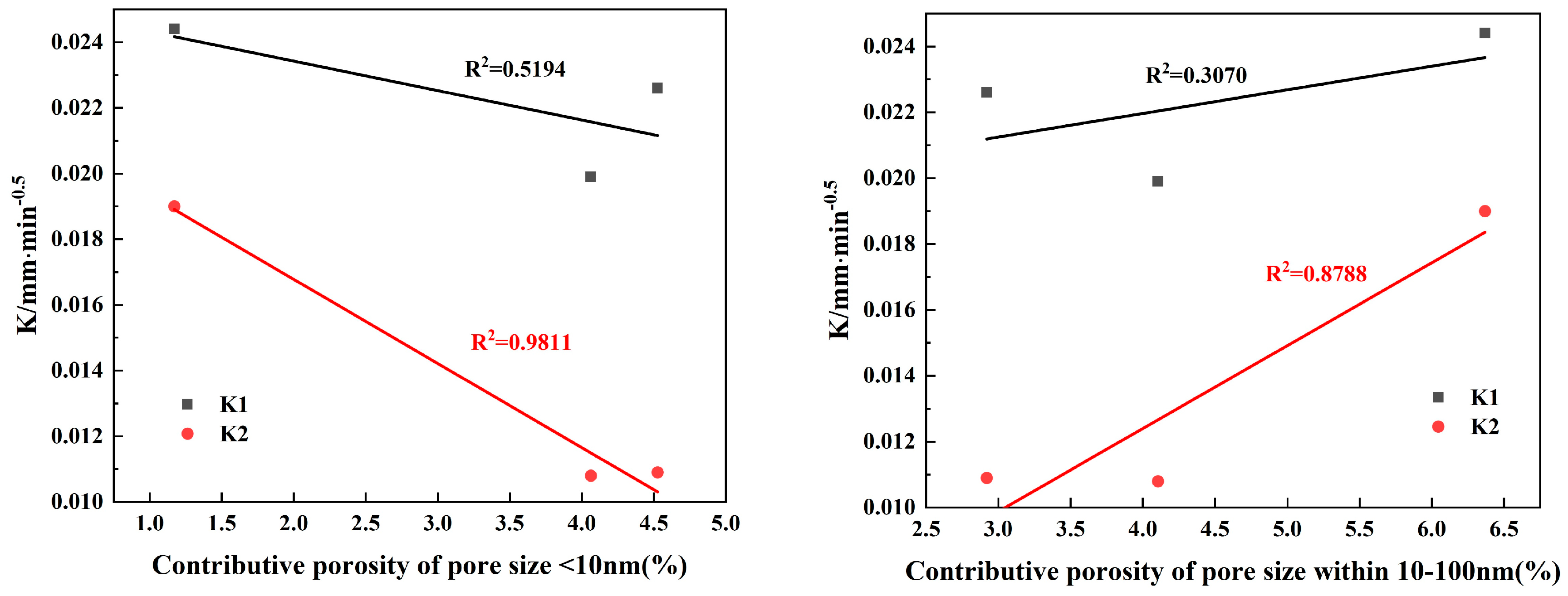
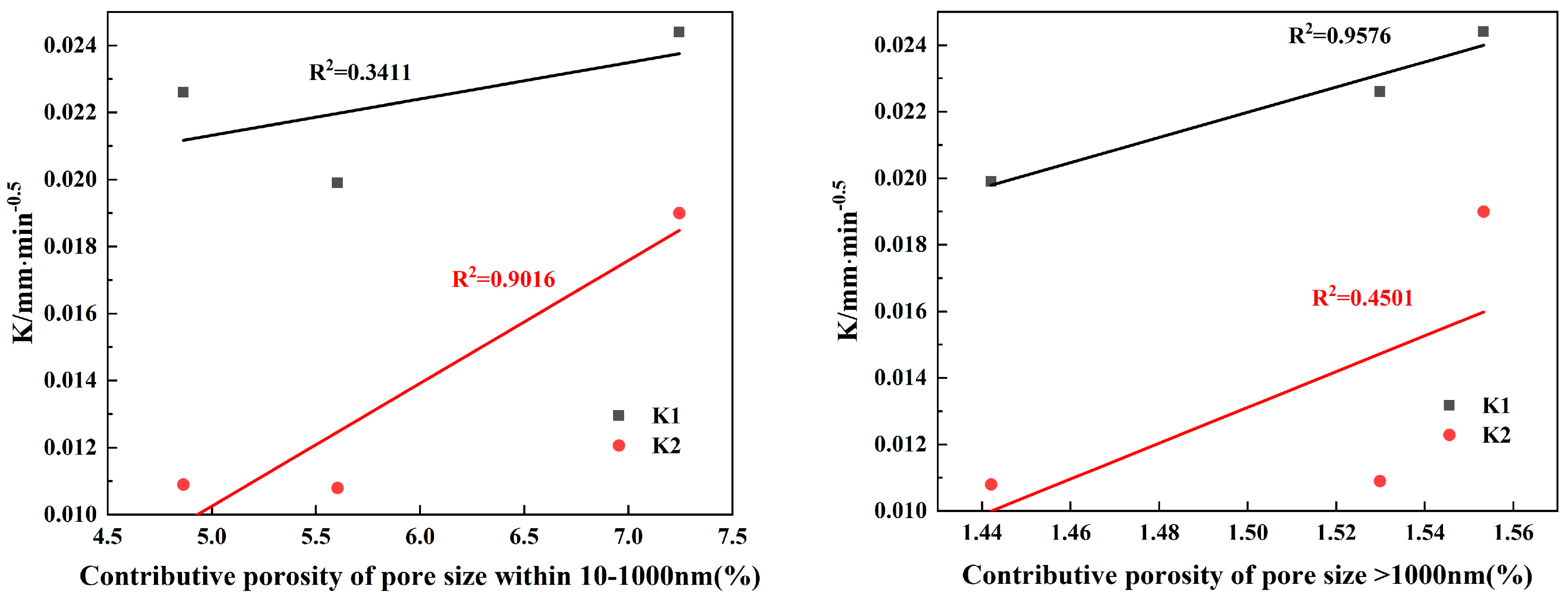
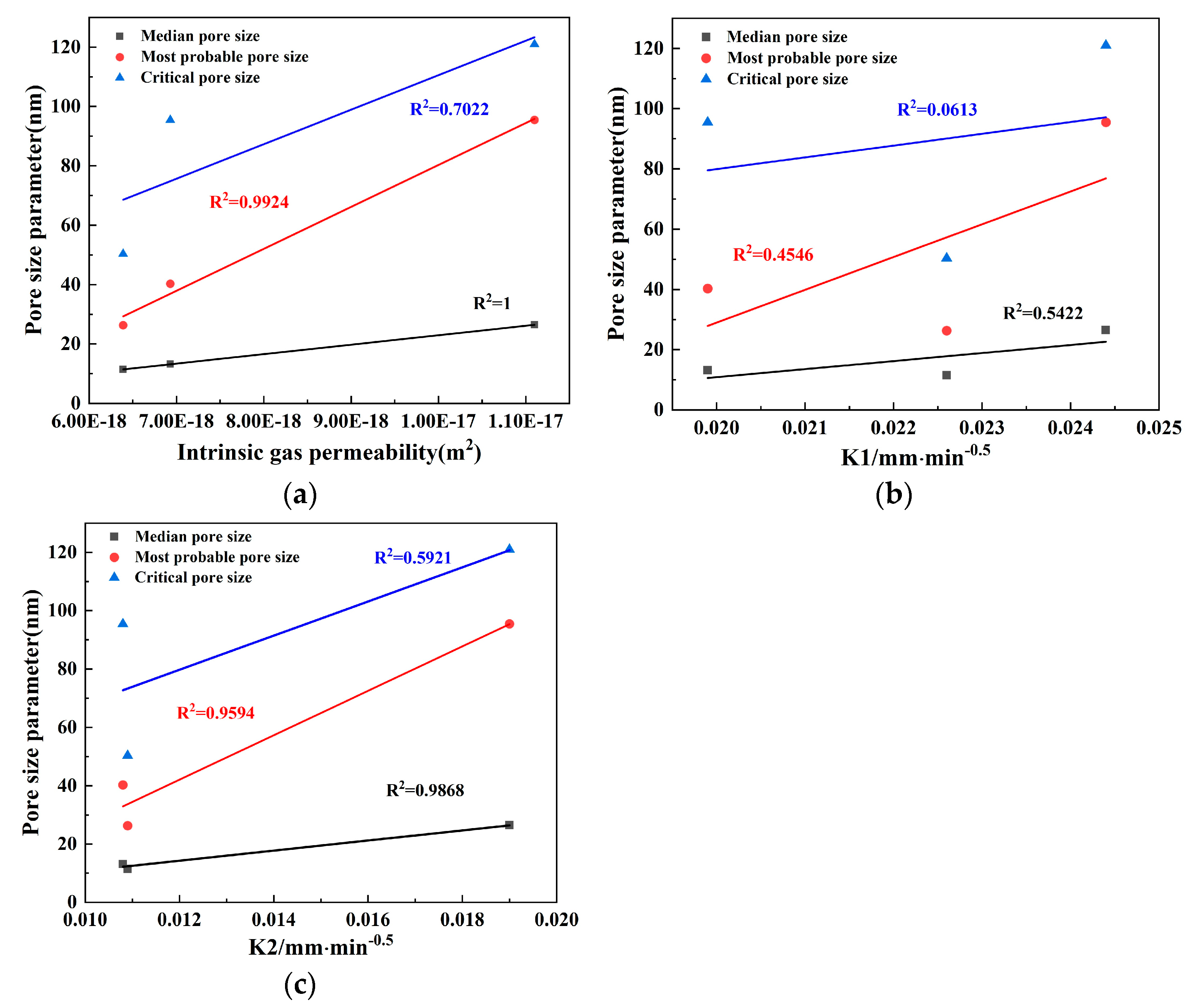
3.3.2. Relationship between Pore Size and Permeability
4. Conclusions
- Based on the NMR and MIP test results, the incorporation of SF and GGBS significantly reduced the most probable pore size and the content of harmful pores in concrete. When incorporated at lower substitution rates, silica fume had a more pronounced impact on the pore structure of concrete materials than Ground Granulated Blast-Furnace Slag. It is clear that different combinations of mineral admixtures can produce concretes with better performance, and the selection of these combinations should be based on the physical properties associated with the expected performance of the concrete.
- GGBS replacement without SF had no significant effect on the transport properties. To reduce the permeability, the incorporation of SF in concrete is very effective. In binary systems, adding SF refined the pore structure and improved the transport properties more than GGBS by decreasing the proportion of capillary pores, even with only a 15% addition. However, in ternary systems, SF and GGBS synergistically did not show a significant effect on the permeability. Furthermore, the experimental findings strongly suggest that both the median and most probable pore sizes in concrete significantly impact its transport properties.
- Distinct differences exist in the porosity and pore size distribution, as determined by Mercury Intrusion Porosimetry and Nuclear Magnetic Resonance tests. It was observed that NMR proves valuable in ascertaining microscopic porosity, while MIP exhibits proficiency in identifying macro-porosity. However, variations in specimen size and sample pretreatment introduced a degree of randomness into the MIP measurements. NMR test outcomes are potentially more representative of the real porosity of concrete, but it is also critical to understand how metallic components in concrete materials affect things.
- The results of contributing porosity for different pore sizes show the following: An increase in <10 nm pores has a positive effect on reducing the gas permeability coefficient and the capillary late absorption coefficient of concrete. In the ranges of 10–100 nm and 10–1000 nm, the gas permeability coefficient and the capillary late absorption coefficient both increase with the increase in contributing porosity, which show highly correlated positive correlations, but the correlation between gas permeability and contributing porosity correlates better than capillary water absorption. Pores > 1000 nm correlate poorly with gas permeability but show a highly correlated positive correlation with the capillary initial water absorption coefficient.
5. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Lv, Z.; Duan, Z.; Zhang, C. Pore Structure Characteristics, Modulation and Its Effect on Concrete Properties: A Review. Constr. Build. Mater. 2023, 397, 132430. [Google Scholar] [CrossRef]
- Liu, L.; He, Z.; Cai, X.; Fu, S. Application of Low-Field NMR to the Pore Structure of Concrete. Appl. Magn. Reson. 2020, 52, 15–31. [Google Scholar] [CrossRef]
- Zhang, J.; Bian, F.; Zhang, Y.; Fang, Z.; Fu, C.; Guo, J. Effect of Pore Structures on Gas Permeability and Chloride Diffusivity of Concrete. Constr. Build. Mater. 2018, 163, 402–413. [Google Scholar] [CrossRef]
- Tangadagi, R.B.; Manjunatha, M.; Seth, D.; Preethi, S. Role of Mineral Admixtures on Strength and Durability of High Strength Self Compacting Concrete: An Experimental Study. Materialia 2021, 18, 101144. [Google Scholar] [CrossRef]
- Fayaz, M.; Krishnaiah, R.V.; Raju, K.V.B.; Chauhan, M.S. Experimental Study on Mechanical Properties of Concrete Using Mineral Admixtures. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Shi, H.; Xu, B.; Zhou, X. Influence of Mineral Admixtures on Compressive Strength, Gas Permeability and Carbonation of High Performance Concrete. Constr. Build. Mater. 2009, 23, 1980–1985. [Google Scholar] [CrossRef]
- Tibbetts, C.M.; Riding, K.A.; Ferraro, C.C. A Critical Review of the Testing and Benefits of Permeability-Reducing Admixtures for Use in Concrete. Cement 2021, 6, 100016. [Google Scholar] [CrossRef]
- Zhimin, H.; Junzhe, L.; Kangwu, Z. Influence of Mineral Admixtures on the Short and Long-Term Performance of Steam-Cured Concrete. Energy Procedia 2012, 16, 836–841. [Google Scholar] [CrossRef]
- Meddah, M.S.; Tagnit-Hamou, A. Pore Structure of Concrete with Mineral Admixtures and Its Effect on Self-Desiccation Shrinkage. Aci Mater. J. 2009, 106, 241–250. [Google Scholar]
- Shen, D.; Jiao, Y.; Gao, Y.; Zhu, S.; Jiang, G. Influence of Ground Granulated Blast Furnace Slag on Cracking Potential of High Performance Concrete at Early Age. Constr. Build. Mater. 2020, 241, 117839. [Google Scholar] [CrossRef]
- Ozturk, M.; Karaaslan, M.; Akgol, O.; Sevim, U.K. Mechanical and Electromagnetic Performance of Cement Based Composites Containing Different Replacement Levels of Ground Granulated Blast Furnace Slag, Fly Ash, Silica Fume and Rice Husk Ash. Cem. Concr. Res. 2020, 136, 106177. [Google Scholar] [CrossRef]
- Öner, M.; Erdoğdu, K.; Günlü, A. Effect of Components Fineness on Strength of Blast Furnace Slag Cement. Cem. Concr. Res. 2003, 33, 463–469. [Google Scholar] [CrossRef]
- Ding, Q.; Yang, J.; Hou, D.; Zhang, G. Insight on the Mechanism of Sulfate Attacking on the Cement Paste with Granulated Blast Furnace Slag: An Experimental and Molecular Dynamics Study. Constr. Build. Mater. 2018, 169, 601–611. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Shearer, C.R.; Reza, A.N.R.; Saha, A.K.; Khan, M.N.N.; Hossain, M.M.; Sarker, P.K. Improving the Sulfate Attack Resistance of Concrete by Using Supplementary Cementitious Materials (SCMs): A Review. Constr. Build. Mater. 2021, 281, 122628. [Google Scholar] [CrossRef]
- Duan, P.; Shui, Z.; Chen, W.; Shen, C. Enhancing Microstructure and Durability of Concrete from Ground Granulated Blast Furnace Slag and Metakaolin as Cement Replacement Materials. J. Mater. Res. Technol. 2013, 2, 52–59. [Google Scholar] [CrossRef]
- Singh, P.; Kirar, D.B. Experimental Study by Use of GGBS as Partial Replacement of Cement on Compressive Strength of Concrete. Int. J. Res. Appl. Sci. Eng. Technol. 2024, 12, 970–975. [Google Scholar] [CrossRef]
- Aprianti, E.; Shafigh, P.; Zawawi, R.; Hassan, Z.F.A. Introducing an Effective Curing Method for Mortar Containing High Volume Cementitious Materials. Constr. Build. Mater. 2016, 107, 365–377. [Google Scholar] [CrossRef]
- Rostami, M.; Behfarnia, K. The Effect of Silica Fume on Durability of Alkali Activated Slag Concrete. Constr. Build. Mater. 2017, 134, 262–268. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q. Effect of Silica Fume on Durability of Concrete Composites Containing Fly Ash. Sci. Eng. Compos. Mater. 2013, 20, 57–65. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Q.-F. Durability of High Performance Concrete Composites Containing Silica Fume. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2013, 227, 343–349. [Google Scholar] [CrossRef]
- Soltani, A.; Tarighat, A.; Rostami, R.; Tavakoli, D.; Moradi, A. Investigation of Mechanical Properties of Concrete with Clinoptilolite and Silica Fume Using Taguchi Method. Innov. Infrastruct. Solut. 2024, 9, 77. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Tahmouresi, B.; Khoshkbijari, R.K. Effect of Fly Ash and Silica Fume on Transition Zone, Pore Structure and Permeability of Concrete. Mag. Concr. Res. 2018, 70, 519–532. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, J.; Zhao, Y.; Li, S.; Guo, Z.; Luo, X.; Chen, G. Promoting Utilization Rate of Ground Granulated Blast Furnace Slag (GGBS): Incorporation of Nanosilica to Improve the Properties of Blended Cement Containing High Volume GGBS. J. Clean. Prod. 2022, 332, 130096. [Google Scholar] [CrossRef]
- Zhang, M.-H.; Islam, J.; Peethamparan, S. Use of Nano-Silica to Increase Early Strength and Reduce Setting Time of Concretes with High Volumes of Slag. Cem. Concr. Compos. 2012, 34, 650–662. [Google Scholar] [CrossRef]
- Garboczi, E.J. Permeability, Diffusivity, and Microstructural Parameters: A Critical Review. Cem. Concr. Res. 1990, 20, 591–601. [Google Scholar] [CrossRef]
- Sinsiri, T.; Chindaprasirt, P.; Jaturapitakkul, C. Influence of Fly Ash Fineness and Shape on the Porosity and Permeability of Blended Cement Pastes. Int. J. Min. Met. Mater 2010, 17, 683–690. [Google Scholar] [CrossRef]
- Chen, J.J.; Kwan, A.K.H.; Jiang, Y. Adding Limestone Fines as Cement Paste Replacement to Reduce Water Permeability and Sorptivity of Concrete. Constr. Build. Mater. 2014, 56, 87–93. [Google Scholar] [CrossRef]
- Pei, Y.; Agostini, F.; Skoczylas, F. The Effects of High Temperature Heating on the Gas Permeability and Porosity of a Cementitious Material. Cem. Concr. Res. 2017, 95, 141–151. [Google Scholar] [CrossRef]
- Sakai, Y. Correlations between Air Permeability Coefficients and Pore Structure Indicators of Cementitious Materials. Constr. Build. Mater. 2019, 209, 541–547. [Google Scholar] [CrossRef]
- Tsivilis, S.; Chaniotakis, E.; Batis, G.; Meletiou, C.; Kasselouri, V.; Kakali, G.; Sakellariou, A.; Pavlakis, G.; Psimadas, C. The Effect of Clinker and Limestone Quality on the Gas Permeability, Water Absorption and Pore Structure of Limestone Cement Concrete. Cem. Concr. Compos. 1999, 21, 139–146. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Ma, Q.; Zhou, X.; Wang, Q. Study on the Correlation between SHPC Pore Structure and Air Permeability. Teh. Vjesn. 2017, 24, 1425–1430. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, T.; He, Y.; Wang, Y.; Gao, Y.; Zhang, Y. The Slippage Effect of Concrete Gas Permeability and the Influence of Its Microstructure. Constr. Build. Mater. 2022, 333, 127384. [Google Scholar] [CrossRef]
- Lockington, D.; Parlange, J.-Y.; Dux, P. Sorptivity and the Estimation of Water Penetration into Unsaturated Concrete. Mat. Struct. 1999, 32, 342–347. [Google Scholar] [CrossRef]
- Daoke Baba GB50204-2015; Code for Quality Acceptance of Concrete Structural Engineering Construction. China Academy of Building Research: Beijing, China, 2015. Available online: https://www.doc88.com/p-7857712601292.html (accessed on 8 March 2024).
- National Standards|GB/T 8074-2008; Testing Method for Specific Surface of Cement—Blaine Method. China Building Materials Federation: Beijing, China, 2008. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=CF7AC64C96F85D758B970C5786ED1879 (accessed on 8 March 2024).
- GB T 50080-2016; Standard Test Methods for Properties of Ordinary Concrete Mixes. Ministry of Housing and Urban-Rural Development: Beijing, China, 2016. Available online: https://www.doc88.com/p-9902565892794.html (accessed on 8 March 2024).
- GB/T 10171-2016; Building Construction Machinery and Equipment—Concrete Mixing Plant (Tower). China Machinery Industry Federation: Beijing, China, 2016. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=D45FA0AB8E88A2F12968E409B946799C (accessed on 8 March 2024).
- JG237-2008; Mould for Concrete Specimens. Ministry of Housing and Urban-Rural Development: Beijing, China, 2008. Available online: https://www.doc88.com/p-9753918274284.html (accessed on 8 March 2024).
- Coates, G.R.; Xiao, L.; Prammer, M.G. NMR Logging: Principles and Applications; Halliburton Energy Services: Houston, TX, USA, 1999. [Google Scholar]
- Valori, A.; McDonald, P.J.; Scrivener, K.L. The Morphology of C–S–H: Lessons from 1H Nuclear Magnetic Resonance Relaxometry. Cem. Concr. Res. 2013, 49, 65–81. [Google Scholar] [CrossRef]
- Gallé, C. Effect of Drying on Cement-Based Materials Pore Structure as Identified by Mercury Intrusion Porosimetry: A Comparative Study between Oven-, Vacuum-, and Freeze-Drying. Cem. Concr. Res. 2001, 31, 1467–1477. [Google Scholar] [CrossRef]
- Caré, S. Effect of Temperature on Porosity and on Chloride Diffusion in Cement Pastes. Constr. Build. Mater. 2008, 22, 1560–1573. [Google Scholar] [CrossRef]
- RILEM TC 116-PCD. Permeability of Concrete as a Criterion of Its Durability. Mater. Struct. 1999, 32, 163–173. Available online: https://www.scirp.org/reference/referencespapers?referenceid=1228620 (accessed on 4 March 2024).
- Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes. Available online: https://www.astm.org/c1585-13.html (accessed on 4 March 2024).
- Chen, W.; Li, K.; Wu, M.; Liu, D.; Wang, P.; Liang, Y. Influence of Pore Structure Characteristics on the Gas Permeability of Concrete. J. Build. Eng. 2023, 79, 107852. [Google Scholar] [CrossRef]
- Chen, H.; Tang, D.; Li, S.; Xu, H.; Tao, S.; Wang, J.; Liu, Y. Dynamic Evaluation of Heterogeneity in Pore-Fracture System of Different Rank Coals under Different Confining Pressure Based on Low-Field NMR. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 1620–1634. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, J.; Li, D.; Zhang, Y.; Bian, F.; Fang, Z. The Influence of Admixture on Chloride Time-Varying Diffusivity and Microstructure of Concrete by Low-Field NMR. Ocean Eng. 2017, 142, 94–101. [Google Scholar] [CrossRef]
- Hilal, A.A.; Thom, N.H.; Dawson, A.R. Pore Structure and Permeation Characteristics of Foamed Concrete. ACT 2014, 12, 535–544. [Google Scholar] [CrossRef]
- El-Dieb, A.S.; Hooton, R.D. Evaluation of the Katz-Thompson Model for Estimating the Water Permeability of Cement-Based Materials from Mercury Intrusion Porosimetry Data. Cem. Concr. Res. 1994, 24, 443–455. [Google Scholar] [CrossRef]
- Larsen, E.S.; Geiker, M. Two High Performance Concrete Bridges. Struct. Eng. Int. 1995, 5, 231. [Google Scholar] [CrossRef]
- Talah, A.; Kharchi, F. A Modified Test Procedure to Measure Gas Permeability of Hollow Cylinder Concrete Specimens. Int. J. Eng. Technol. 2013, 5, 91–94. [Google Scholar] [CrossRef]
- Kameche, Z.A.; Ghomari, F.; Choinska, M.; Khelidj, A. Assessment of Liquid Water and Gas Permeabilities of Partially Saturated Ordinary Concrete. Constr. Build. Mater. 2014, 65, 551–565. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G.; Yang, Z. Pore Size Dependent Connectivity and Ionic Transport in Saturated Cementitious Materials. Constr. Build. Mater. 2020, 238, 117680. [Google Scholar] [CrossRef]
- Hou, P.; Cheng, X.; Qian, J.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of Surface-Treatment of Nano-SiO2 on the Transport Properties of Hardened Cement Pastes with Different Water-to-Cement Ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Anez, L.; Calas-Etienne, S.; Primera, J.; Woignier, T. Gas and Liquid Permeability in Nano Composites Gels: Comparison of Knudsen and Klinkenberg Correction Factors. Microporous Mesoporous Mater. 2014, 200, 79–85. [Google Scholar] [CrossRef]
- Klinkenberg, L. The Permeability of Porous Media to Liquids And Gases. Drill. Prod. Pract. 2012, 200–213. [Google Scholar] [CrossRef]



| Screen Size (mm) | Cumulative Sieve Residue (%) | |
|---|---|---|
| Coarse Aggregate | Fine Aggregate | |
| 26.5 | 0 | 0 |
| 19.0 | 9.7 | 0 |
| 16.0 | 22.6 | 0 |
| 9.50 | 61.7 | 0 |
| 4.75 | 89.3 | 5.1 |
| 2.36 | 99.1 | 15.3 |
| 1.18 | / | 26.2 |
| 0.6 | / | 41.8 |
| 0.3 | / | 69.1 |
| 0.015 | / | 90.4 |
| Screen tray | 100.0 | 100.0 |
| Binders | Chemical Compositions (%) | Specific Surface Area (m2/kg) | Density (g/cm3) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | Fe2O3 | SO3 | Cl− | |||
| C | 59.27 | 20.86 | 9.28 | 2.6 | 3.74 | 1.85 | 0.04 | 349 | 3.10 |
| SF | - | 96.2 | - | 0.80 | 0.13 | - | 0.07 | 20,000 | 2.23 |
| GGBS | 35.30 | 34.50 | 16.70 | 5.01 | 1.50 | 1.24 | - | 628 | 2.93 |
| Group | Binder kg/m³ | Binder Composition (%) | W/B | Water kg/m³ | Superplasticizer kg/m³ | Coarse Aggregate kg/m³ | Fine Aggregate kg/m³ | ||
|---|---|---|---|---|---|---|---|---|---|
| C | SF | GGBS | |||||||
| A | 900 | 85 | 15 | 0 | 0.25 | 225 | 18 | 616 | 923 |
| B | 900 | 85 | 0 | 15 | 0.25 | 225 | 18 | 616 | 923 |
| C | 900 | 85 | 7.5 | 7.5 | 0.25 | 225 | 18 | 616 | 923 |
| Method | Porosity (%) | Contributive Porosity within Different Range of Pore Size in Concrete (%) | Median Pore Size (nm) | ||||
|---|---|---|---|---|---|---|---|
| <10 nm | 10–100 nm | 100–1000 nm | >1000 nm | ||||
| NMR | A | 2.26 | 29.44 | 42.47 | 17.18 | 10.91 | 13.16 |
| B | 3.93 | 11.75 | 63.88 | 8.79 | 15.58 | 26.44 | |
| C | 5.35 | 41.45 | 26.56 | 17.98 | 14.01 | 11.45 | |
| Method | Porosity (%) | Most Probable Pore Size (nm) | Critical Pore Size (nm) | |
|---|---|---|---|---|
| MIP | A | 4.38 | 40.277 | 95.451 |
| B | 9.82 | 95.474 | 120.991 | |
| C | 6.45 | 26.292 | 50.348 |
| Group | Confinement Pressures (MPa) | Inlet Pressures (bar) | ||
|---|---|---|---|---|
| Pi = 5 | Pi = 10 | Pi = 15 | ||
| A | 3 | 8.33 × 10−18 | 7.63 × 10−18 | 7.36 × 10−18 |
| 5 | 7.54 × 10−18 | 6.18 × 10−18 | 6.39 × 10−18 | |
| 10 | 5.91 × 10−18 | 4.78 × 10−18 | 4.46 × 10−18 | |
| B | 3 | 1.58 × 10−17 | 1.37 × 10−17 | 1.24 × 10−17 |
| 5 | 1.34 × 10−17 | 1.20 × 10−17 | 1.14 × 10−17 | |
| 10 | 1.03 × 10−17 | 9.37 × 10−18 | 9.13 × 10−18 | |
| C | 3 | 8.10 × 10−18 | 7.91 × 10−18 | 6.93 × 10−18 |
| 5 | 6.32 × 10−18 | 5.56 × 10−18 | 5.16 × 10−18 | |
| 10 | 4.08 × 10−18 | 3.81 × 10−18 | 3.65 × 10−18 | |
| Confining Pressure (MPa) | A | B | C |
|---|---|---|---|
| 3 | 6.93 × 10−18 | 1.11 × 10−17 | 6.39 × 10−18 |
| 5 | 5.59 × 10−18 | 1.06 × 10−17 | 4.70 × 10−18 |
| 10 | 3.79 × 10−18 | 8.60 × 10−18 | 3.49 × 10−18 |
| Initial Stage | Late Stage | |||
|---|---|---|---|---|
| K1 | R2 | K2 | R2 | |
| A | 0.0199 | 0.9628 | 0.0108 | 0.9949 |
| B | 0.0244 | 0.9818 | 0.0119 | 0.9965 |
| C | 0.0226 | 0.9474 | 0.0109 | 0.9991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Wu, M.; Liang, Y. Effect of SF and GGBS on Pore Structure and Transport Properties of Concrete. Materials 2024, 17, 1365. https://doi.org/10.3390/ma17061365
Chen W, Wu M, Liang Y. Effect of SF and GGBS on Pore Structure and Transport Properties of Concrete. Materials. 2024; 17(6):1365. https://doi.org/10.3390/ma17061365
Chicago/Turabian StyleChen, Wei, Mengmeng Wu, and Yue Liang. 2024. "Effect of SF and GGBS on Pore Structure and Transport Properties of Concrete" Materials 17, no. 6: 1365. https://doi.org/10.3390/ma17061365






