A Study of the Long-Term Electrochemical Stability of Thin-Film Titanium–Platinum Microelectrodes and Their Comparison to Classic, Wire-Based Platinum Microelectrodes in Selected Inorganic Electrolytes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
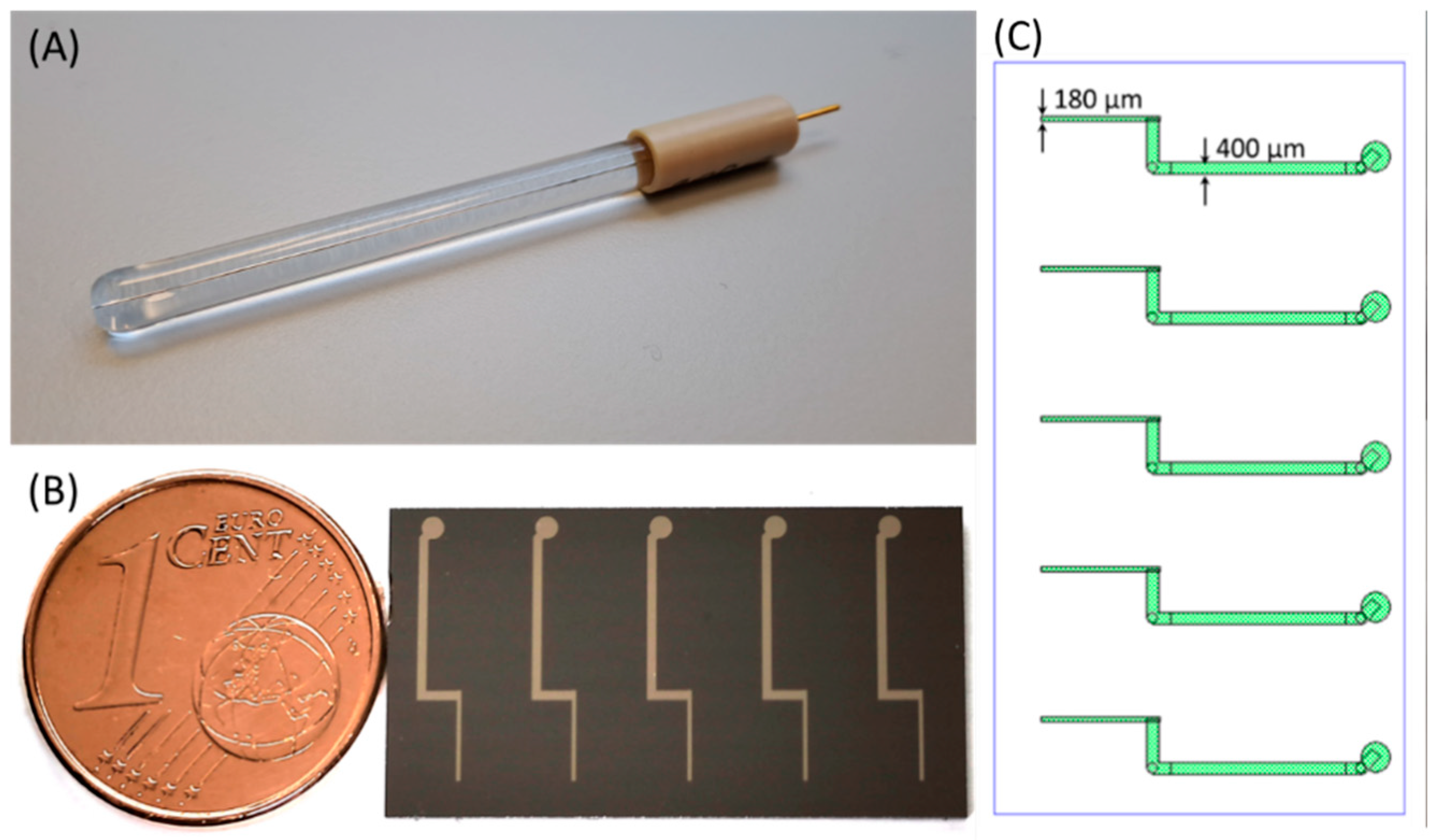
2.2. Wire-Based Pt Microelectrodes
2.3. Thin-Film Pt Microelectrodes
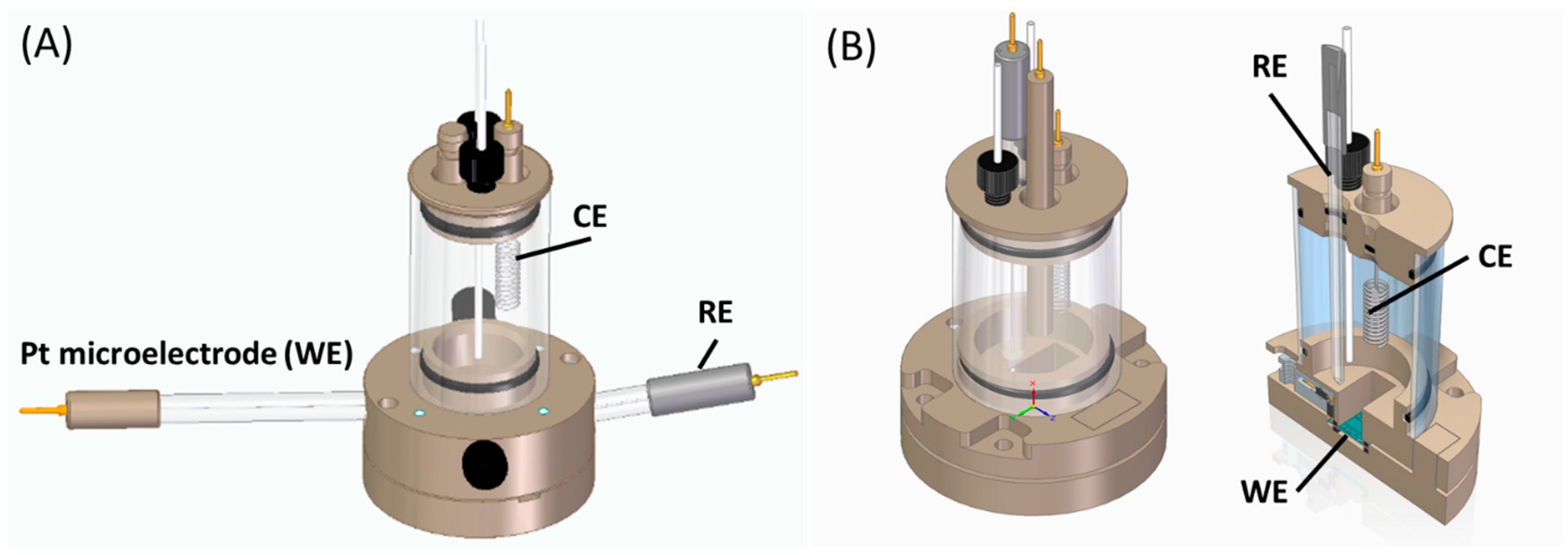
2.4. Electrochemical Measurements
2.5. Measurement Methodology
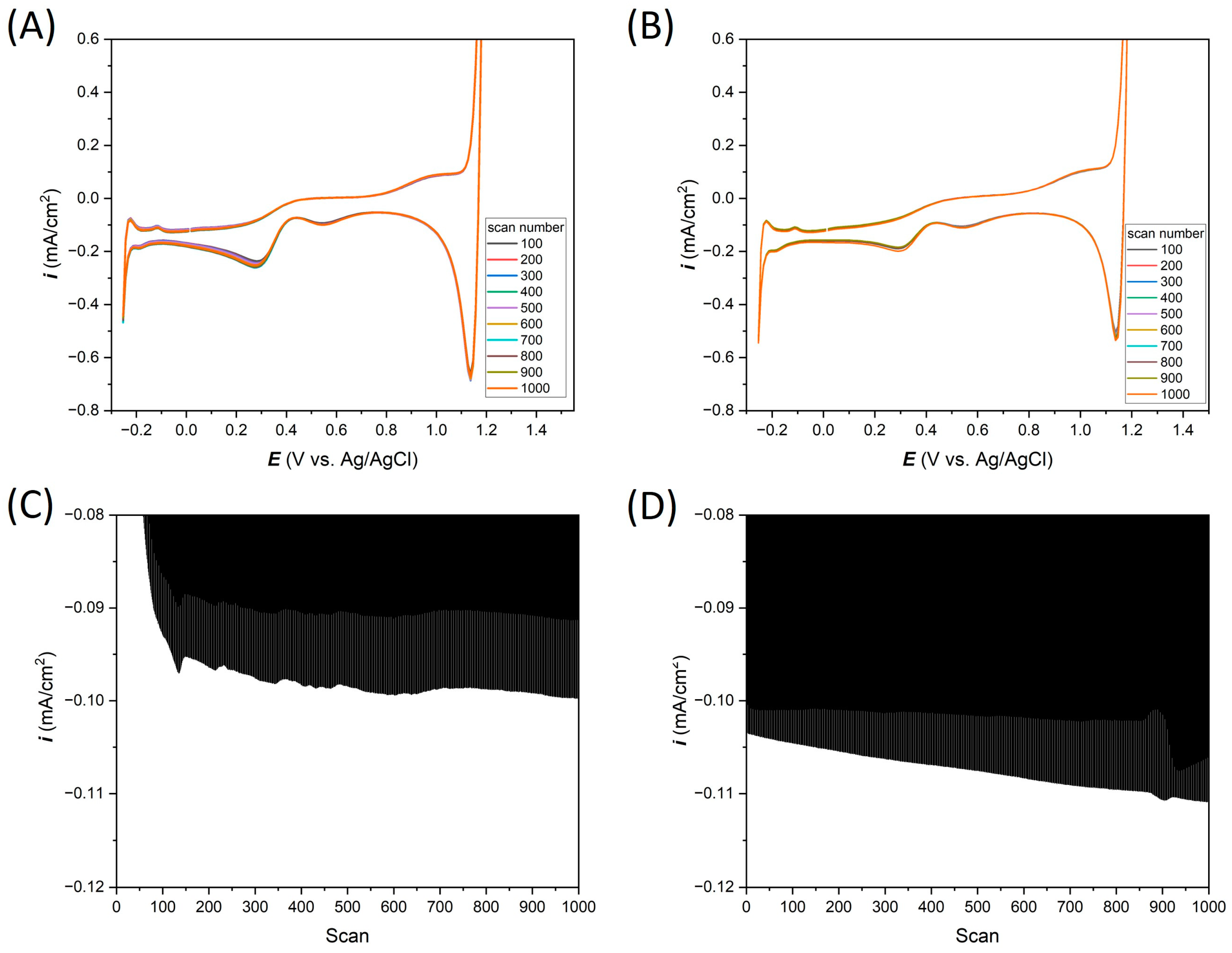
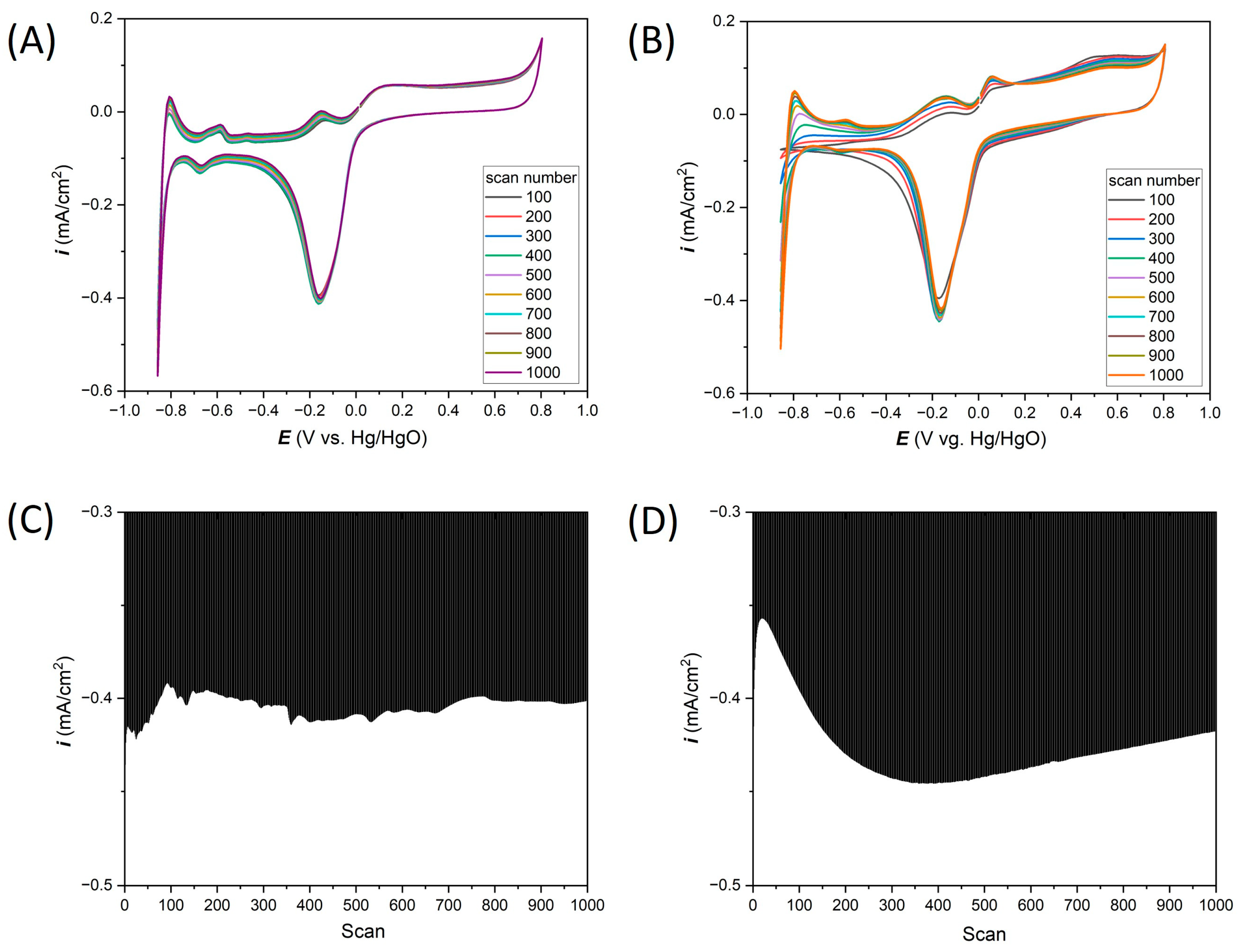
3. Results and Discussion
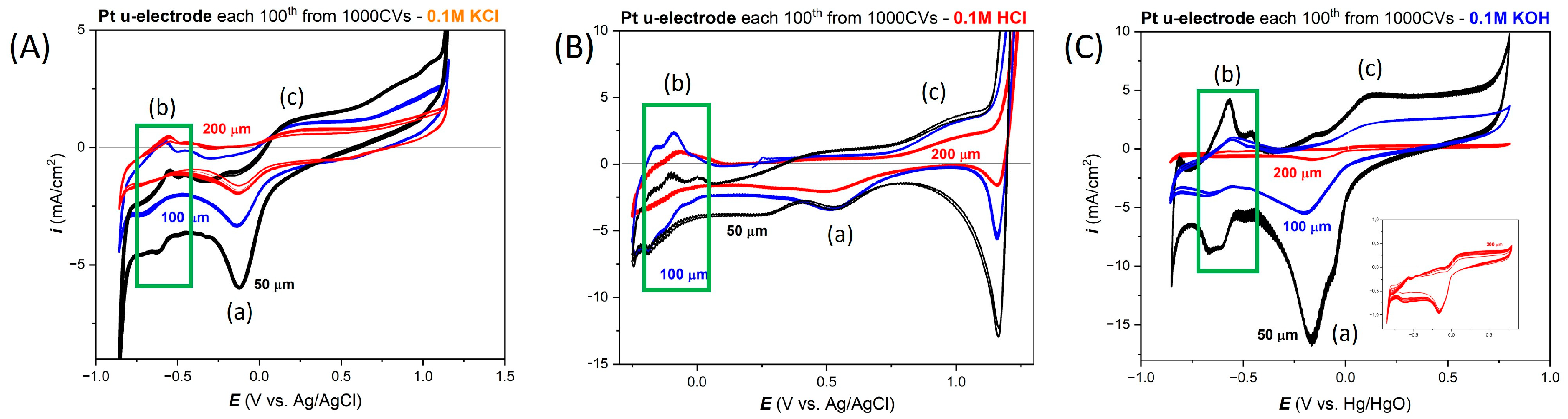
3.1. Electrochemical Response of the Wire-Based Pt Microelectrodes in Selected Electrolytes
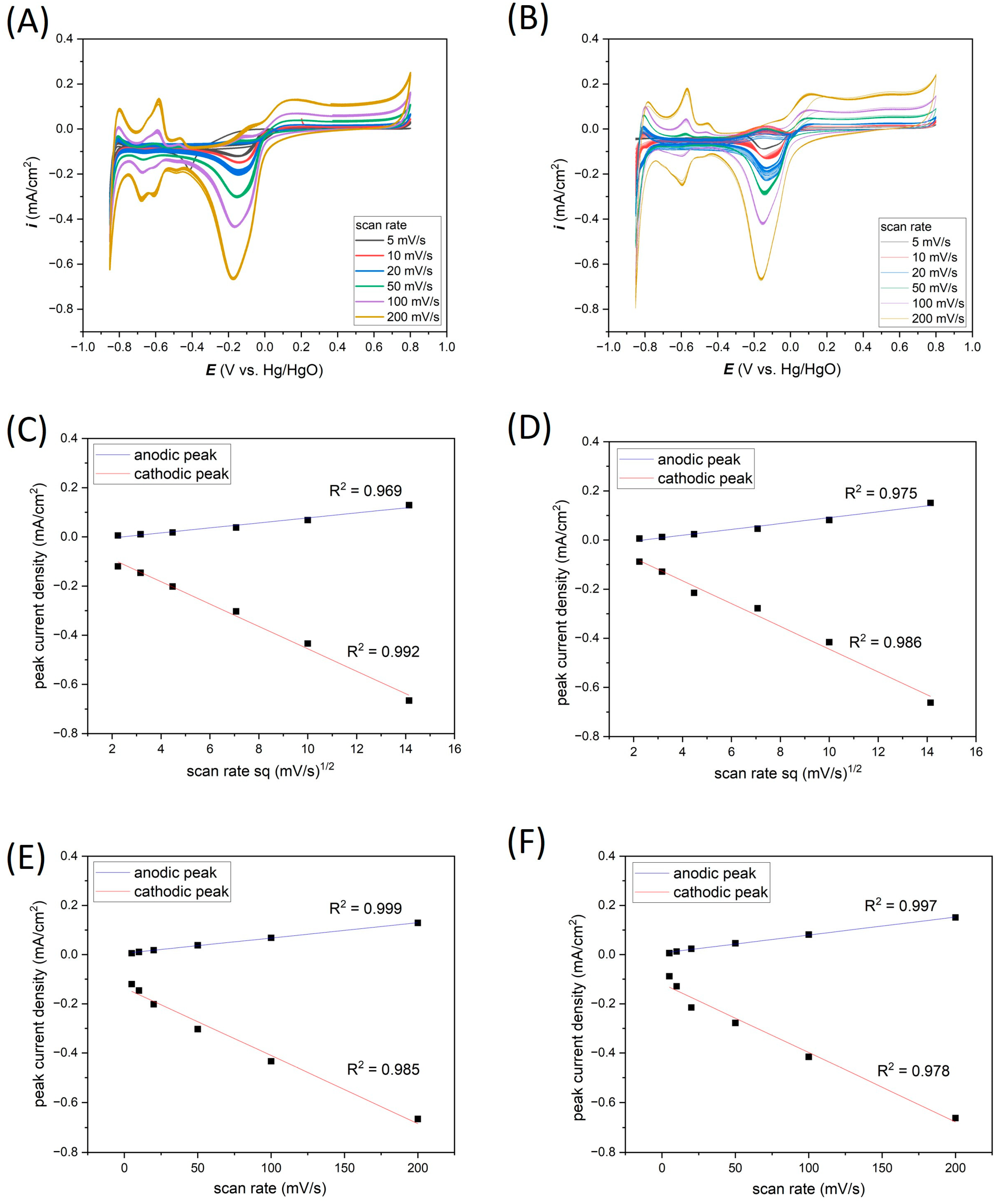
3.2. Electrochemical Response of Thin-Film Pt Microelectrodes in Neutral Salt (0.1 M KCl)
3.3. Electrochemical Response in Acid (0.1 M HCl)
3.4. Electrochemical Response in Alkaline (0.1 M KOH)
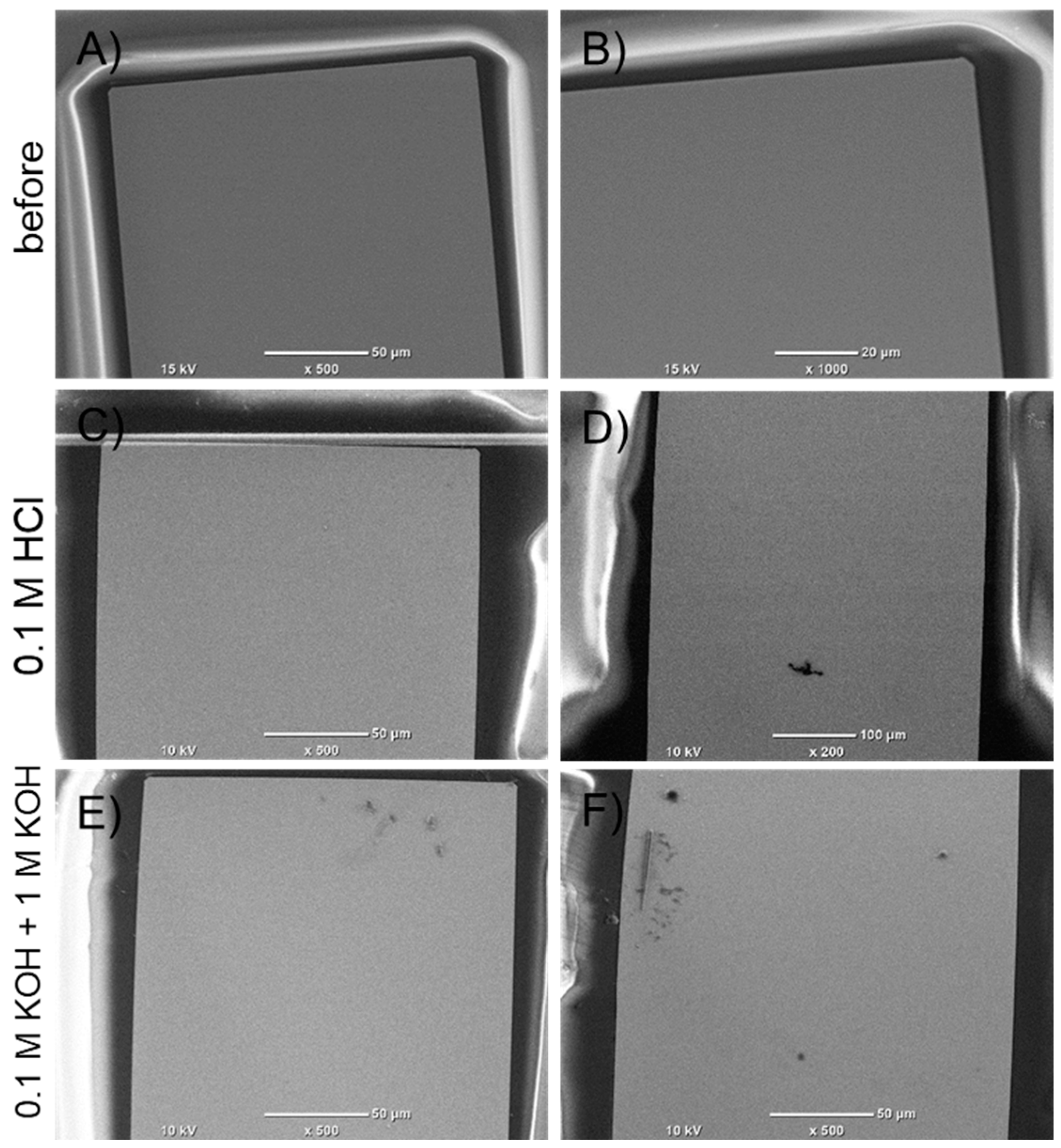
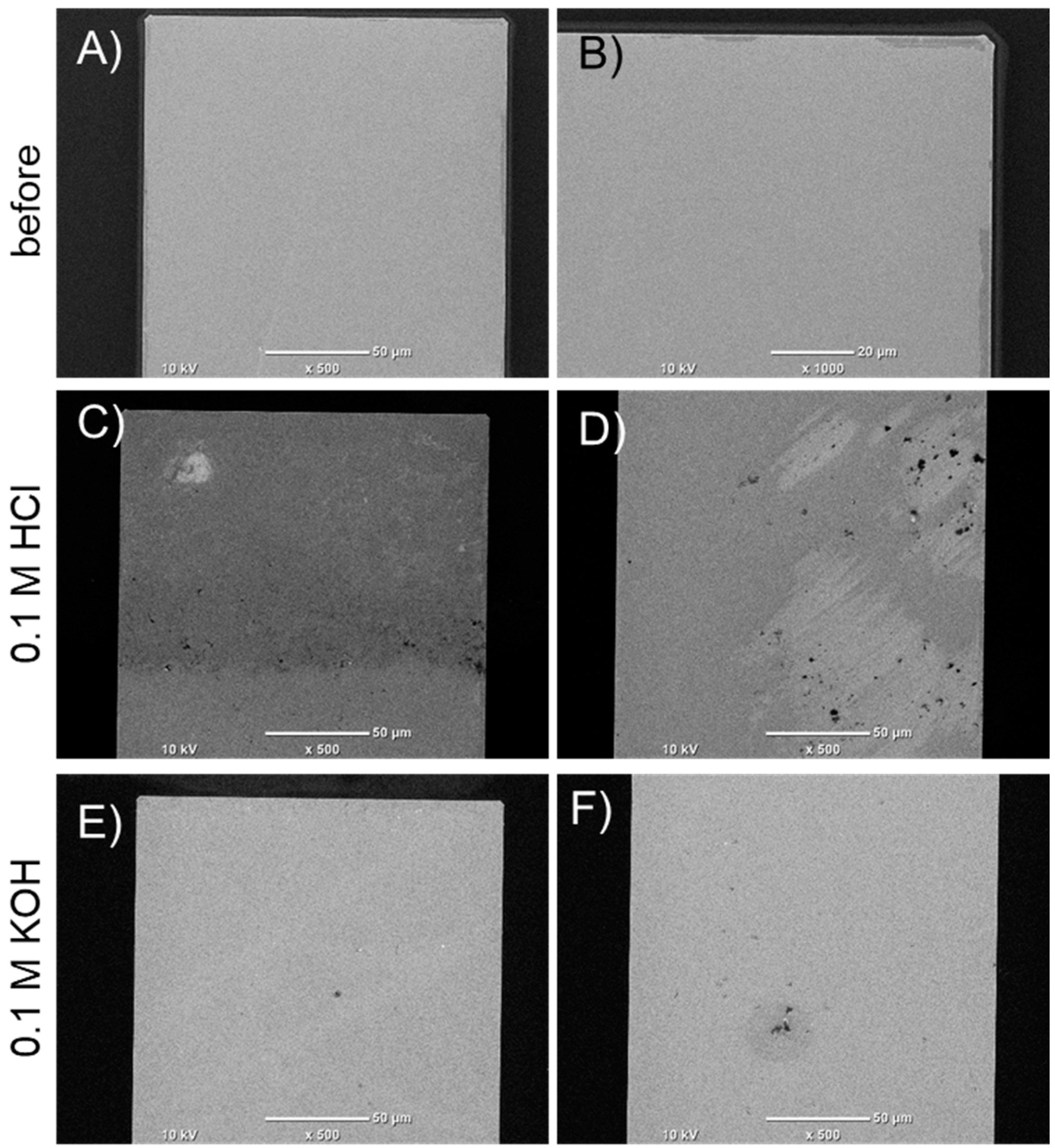
3.5. SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, Biosensors and Microfluidics: A Convergence of Fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef]
- Petrovick, J.G.; Kushner, D.I.; Tesfaye, M.; Danilovic, N.; Radke, C.J.; Weber, A.Z. Mass-Transport Resistances of Acid and Alkaline Ionomer Layers: A Microelectrode Study Part 1—Microelectrode Development. ECS Trans. 2019, 92, 77–85. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrode Substrate Innovation for Electrochemical Detection in Microchip Electrophoresis. Electrophoresis 2015, 36, 1845–1853. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Liu, Z.; Chen, A. Simultaneous Detection of Hydrazine, Sulfite, and Nitrite Based on a Nanoporous Gold Microelectrode. J. Electroanal. Chem. 2018, 819, 524–532. [Google Scholar] [CrossRef]
- Gupta, P.; Rahm, C.E.; Jiang, D.; Gupta, V.K.; Heineman, W.R.; Justin, G.; Alvarez, N.T. Parts per Trillion Detection of Heavy Metals in As-Is Tap Water Using Carbon Nanotube Microelectrodes. Anal. Chim. Acta 2021, 1155, 338353. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, D.B.; Wijesinghe, M.B.; Pichetsurnthorn, P.; Lunte, S.M. Evaluation of Dual Electrode Configurations for Microchip Electrophoresis Used for Voltammetric Characterization of Electroactive Species. Analyst 2020, 145, 865–872. [Google Scholar] [CrossRef]
- Gładysz, O.; Skibiński, P. Voltamperometric Test of Ephedrine on a Gold Disc Microelectrode. Mater. Chem. Phys. 2020, 246, 122792. [Google Scholar] [CrossRef]
- Sadrjavadi, K.; Taran, M.; Fattahi, A.; Khoshroo, A. A Microelectrode System for Simple Measurement of Neuron Specific Enolase with Photolithography Technique. Microchem. J. 2022, 182, 107889. [Google Scholar] [CrossRef]
- Moussa, S.; Mauzeroll, J. Review—Microelectrodes: An Overview of Probe Development and Bioelectrochemistry Applications from 2013 to 2018. J. Electrochem. Soc. 2019, 166, G25–G38. [Google Scholar] [CrossRef]
- Clausmeyer, J.; Schuhmann, W. Nanoelectrodes: Applications in Electrocatalysis, Single-Cell Analysis and High-Resolution Electrochemical Imaging. TrAC-Trends Anal. Chem. 2016, 79, 46–59. [Google Scholar] [CrossRef]
- Leong Tan, T.; Wang, L.-L.; Zhang, J.; Johnson, D.D.; Bai, K. Platinum Nanoparticle During Electrochemical Hydrogen Evolution: Adsorbate Distribution, Active Reaction Species and Size Effect. ACS Catal. 2015, 5, 2376–2383. [Google Scholar]
- Fan, Y.F.; Han, C.; Zhang, B. Recent Advances in Development and Application of Nanoelectrodes. Analyst 2016, 141, 5474–5487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, X.; Pan, Y.; Peng, Z. A Review of Pt-Based Electrocatalysts for Oxygen Reduction Reaction. Front. Energy 2017, 11, 268–285. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Jadali, S. A Sponge like Pd Arrays on Ni Foam Substrate: Highly Active Non-Platinum Electrocatalyst for Methanol Oxidation in Alkaline Media. Mater. Chem. Phys. 2021, 257, 123626. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Li, L.; Zhang, Y.; Xin, S.; Ni, X.; Sun, Y.; Song, K. Recent Progress of the Practical Applications of the Platinum Nanoparticle-Based Electrochemistry Biosensors. Front. Chem. 2021, 9, 677876. [Google Scholar] [CrossRef] [PubMed]
- Al-Saidi, W.S.; Al Jahdaly, B.A.; Awad, M.I. Methanol Electro-Oxidation at Platinum Electrode: In Situ Recovery of CO Poisoned Platinum Electrode. Int. J. Electrochem. Sci. 2020, 15, 1830–1839. [Google Scholar] [CrossRef]
- Rao, C.R.K.; Trivedi, D.C. Chemical and Electrochemical Depositions of Platinum Group Metals and Their Applications. Coord. Chem. Rev. 2005, 249, 613–631. [Google Scholar] [CrossRef]
- Jacobse, L.; Raaijman, S.J.; Koper, M.T.M. The Reactivity of Platinum Microelectrodes. Phys. Chem. Chem. Phys. 2016, 18, 28451–28457. [Google Scholar] [CrossRef]
- Lesch, A.; Momotenko, D.; Cortés-Salazar, F.; Wirth, I.; Tefashe, U.M.; Meiners, F.; Vaske, B.; Girault, H.H.; Wittstock, G. Fabrication of Soft Gold Microelectrode Arrays as Probes for Scanning Electrochemical Microscopy. J. Electroanal. Chem. 2012, 666, 52–61. [Google Scholar] [CrossRef]
- Mantis, I.; Hemanth, S.; Caviglia, C.; Heiskanen, A.; Keller, S.S. Suspended Highly 3D Interdigitated Carbon Microelectrodes. Carbon 2021, 179, 579–589. [Google Scholar] [CrossRef]
- Koterwa, A.; Kaczmarzyk, I.; Mania, S.; Cieslik, M.; Tylingo, R.; Ossowski, T.; Bogdanowicz, R.; Niedziałkowski, P.; Ryl, J. The Role of Electrolysis and Enzymatic Hydrolysis Treatment in the Enhancement of the Electrochemical Properties of 3D-Printed Carbon Black/Poly(Lactic Acid) Structures. Appl. Surf. Sci. 2022, 574, 151587. [Google Scholar] [CrossRef]
- Xu, L.; Hu, C.; Huang, Q.; Jin, K.; Zhao, P.; Wang, D.; Hou, W.; Dong, L.; Hu, S.; Ma, H. Trends and Recent Development of the Microelectrode Arrays (MEAs). Biosens. Bioelectron. 2021, 175, 112854. [Google Scholar] [CrossRef] [PubMed]
- Baradoke, A.; Juodkazyte, J.; Masilionis, I.; Selskis, A.; Pauliukaite, R.; Valiokas, R. Combined Soft Lithographic and Electrochemical Fabrication of Nanostructured Platinum Microelectrode Arrays for Miniaturized Sensor Applications. Microelectron. Eng. 2019, 208, 39–46. [Google Scholar] [CrossRef]
- Vereshchagina, E.; Poppe, E.; Schjølberg-Henriksen, K.; Wöhrmann, M.; Moe, S. Metal Films for MEMS Pressure Sensors: Comparison of Al, Ti, Al-Ti Alloy and Al/Ti Film Stacks. In Proceedings of the 2018 7th Electronic System-Integration Technology Conference (ESTC), Dresden, Germany, 18–21 September 2018; pp. 1–9. [Google Scholar]
- Atmaramani, R.; Chakraborty, B.; Rihani, R.T.; Usoro, J.; Hammack, A.; Abbott, J.; Nnoromele, P.; Black, B.J.; Pancrazio, J.J.; Cogan, S.F. Ruthenium Oxide Based Microelectrode Arrays for in Vitro and in Vivo Neural Recording and Stimulation. Acta Biomater. 2020, 101, 565–574. [Google Scholar] [CrossRef]
- Cui, H.; Xie, X.; Xu, S.; Chan, L.L.H.; Hu, Y. Electrochemical Characteristics of Microelectrode Designed for Electrical Stimulation. Biomed. Eng. Online 2019, 18, 86. [Google Scholar] [CrossRef]
- Donora, M.; Gonzalez-Fernandez, E.; Vásquez Quintero, A.; De Smet, H.; Underwood, I. Spatiotemporal Electrochemistry on Flexible Microelectrode Arrays: Progress towards Smart Contact Lens Integration. Sens. Actuators B Chem. 2019, 296, 126671. [Google Scholar] [CrossRef]
- Cabello, M.; Aracil, C.; Perdigones, F.; Mozo, M.; de la Cerda, B.; Quero, J.M. Gold Microelectrodes Array Embedded in PDMS for Electrical Stimulation and Signal Detection. Sens. Actuators B Chem. 2018, 257, 954–962. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Jönsson-Niedziolka, M.; Vereshchagina, E.; Dettlaff, A.; Boonkaew, S.; Pierpaoli, M.; Wittendorp, P.; Jain, S.; Tyholdt, F.; Thomas, J.; et al. Microfluidic Devices for Photo-and Pectroelectrochemical Applications. Curr. Opin. Electrochem. 2022, 36, 101138. [Google Scholar] [CrossRef]
- Moldovan, R.; Vereshchagina, E.; Milenko, K.; Iacob, B.C.; Bodoki, A.E.; Falamas, A.; Tosa, N.; Muntean, C.M.; Farcău, C.; Bodoki, E. Review on Combining Surface-Enhanced Raman Spectroscopy and Electrochemistry for Analytical Applications. Anal. Chim. Acta 2022, 1209, 339250. [Google Scholar] [CrossRef]
- Falamas, A.; Cuibus, D.; Tosa, N.; Brezestean, I.; Muntean, C.M.; Milenko, K.; Vereshchagina, E.; Moldovan, R.; Bodoki, E.; Farcau, C. Toward Microfluidic SERS and EC-SERS Applications via Tunable Gold Films over Nanospheres. Discov. Nano 2023, 18, 73. [Google Scholar] [CrossRef]
- Moldovan, R.; Milenko, K.; Vereshchagina, E.; Iacob, B.C.; Schneider, K.; Farcău, C.; Bodoki, E. EC-SERS Detection of Thiabendazole in Apple Juice Using Activated Screen-Printed Electrodes. Food Chem. 2023, 405, 134713. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Lin, T.; Xu, Y.; Zhang, W.; Asif, M.; Sun, Y.; Xiao, F. Integrated Electrochemical Microfluidic Sensor with Hierarchically Porous Nanoarrays Modified Graphene Fiber Microelectrode for Bioassay. Biosens. Bioelectron. 2022, 205, 114095. [Google Scholar] [CrossRef] [PubMed]
- Buttkewitz, M.A.; Heuer, C.; Bahnemann, J. Sensor Integration into Microfluidic Systems: Trends and Challenges. Curr. Opin. Biotechnol. 2023, 83, 102978. [Google Scholar] [CrossRef] [PubMed]
- Hawke, A.; Concilia, G.; Thurgood, P.; Ahnood, A.; Baratchi, S.; Khoshmanesh, K. A 3D Printed Flow Sensor for Microfluidic Applications. Sens. Actuators A Phys. 2023, 362, 114686. [Google Scholar] [CrossRef]
- Daubinger, P.; Kieninger, J.; Unmüssig, T.; Urban, G.A. Electrochemical Characteristics of Nanostructured Platinum Electrodes-A Cyclic Voltammetry Study. Phys. Chem. Chem. Phys. 2014, 16, 8392–8399. [Google Scholar] [CrossRef]
- El-Shafei, A.A.; Moussa, M.N.H.; El-Far, A.A. The Corrosion Inhibition Character of Thiosemicarbazide and Its Derivatives for C-Steel in Hydrochloric Acid Solution. Mater. Chem. Phys. 2001, 70, 175–180. [Google Scholar] [CrossRef]
- Forster, R.J. M Icroelectrodes: New Dimensions in Electrochemistry. Chem. Soc. Rev. 1994, 23, 289–297. [Google Scholar] [CrossRef]
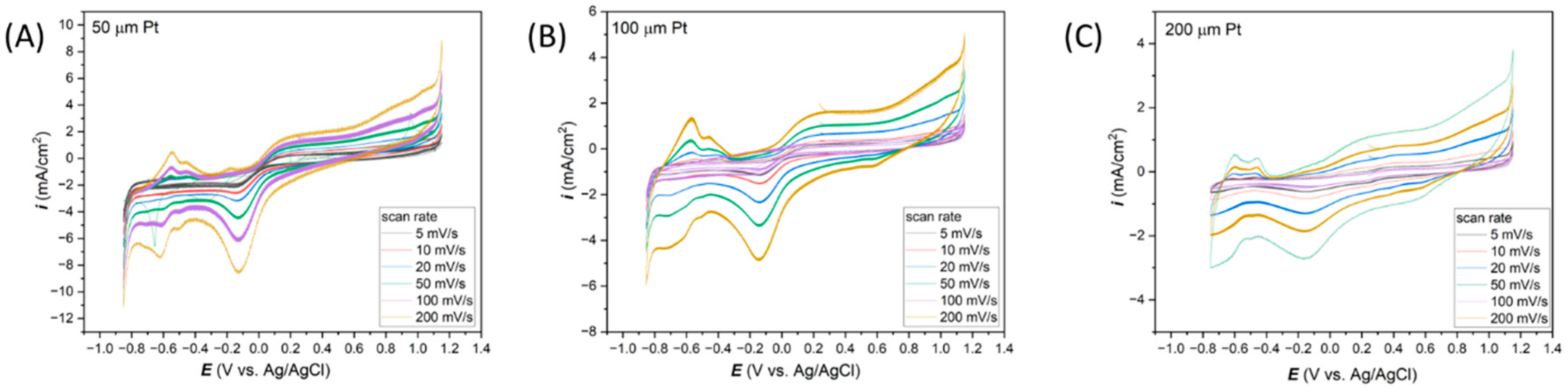

| 5 mV/s | 10 mV/s | 20 mV/s | 50 mV/s | 100 mV/s | 200 mV/s | ||
|---|---|---|---|---|---|---|---|
| 50 µm Pt | Average i (mA/cm2) | −2.02 | −2.63 | −3.18 | −4.52 | −6.23 | −8.66 |
| std. Dev (%) | 5.45 | 1.18 | 0.54 | 0.38 | 0.30 | 0.74 | |
| 100 µm Pt | Average i (mA/cm2) | −0.97 | −1.14 | −1.52 | −2.33 | −3.38 | −4.88 |
| std. Dev (%) | 11.50 | 1.69 | 0.91 | 0.70 | 0.42 | 0.29 | |
| 200 µm Pt | Average i (mA/cm2) | −0.47 | −0.62 | −0.84 | −1.31 | −1.88 | −2.74 |
| std. Dev (%) | 2.85 | 0.68 | 0.36 | 0.45 | 0.32 | 0.30 |
| 5 mV/s | 10 mV/s | 20 mV/s | 50 mV/s | 100 mV/s | 200 mV/s | ||
|---|---|---|---|---|---|---|---|
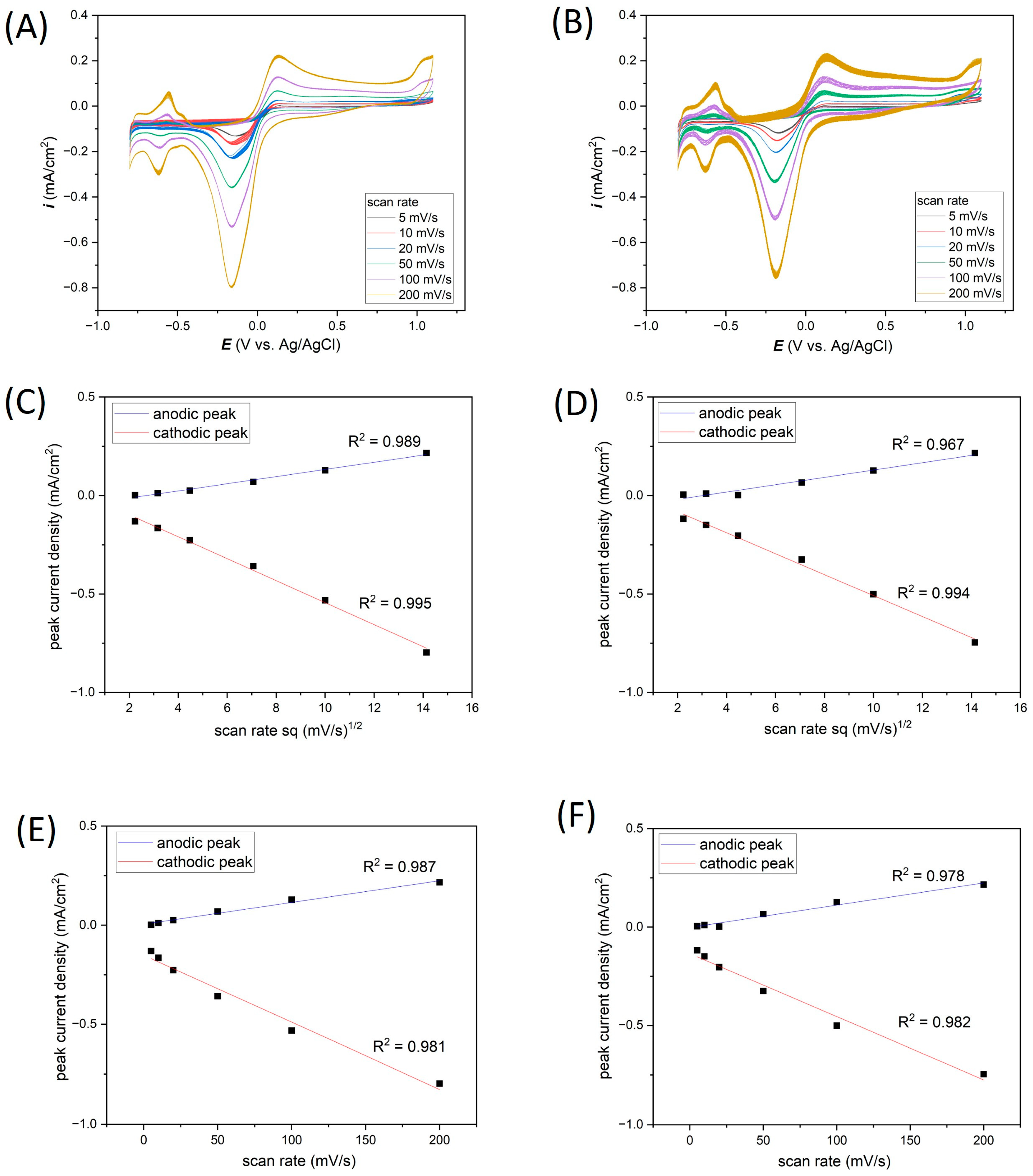
| Ti-Pt on glass | Average i (mA/cm2) | −0.13 | −0.17 | −0.23 | −0.36 | −0.53 | −0.80 |
| Standard deviation (%) | 0.38 | 2.56 | 1.74 | 0.43 | 0.30 | 0.23 | |
| Ti-Pt on Si | Average i (mA/cm2) | −0.12 | −0.15 | −0.20 | −0.33 | −0.49 | −0.75 |
| Standard deviation (%) | 1.27 | 0.95 | 0.54 | 1.30 | 1.08 | 0.65 |
| 5 mV/s | 10 mV/s | 20 mV/s | 50 mV/s | 100 mV/s | 200 mV/s | ||
|---|---|---|---|---|---|---|---|
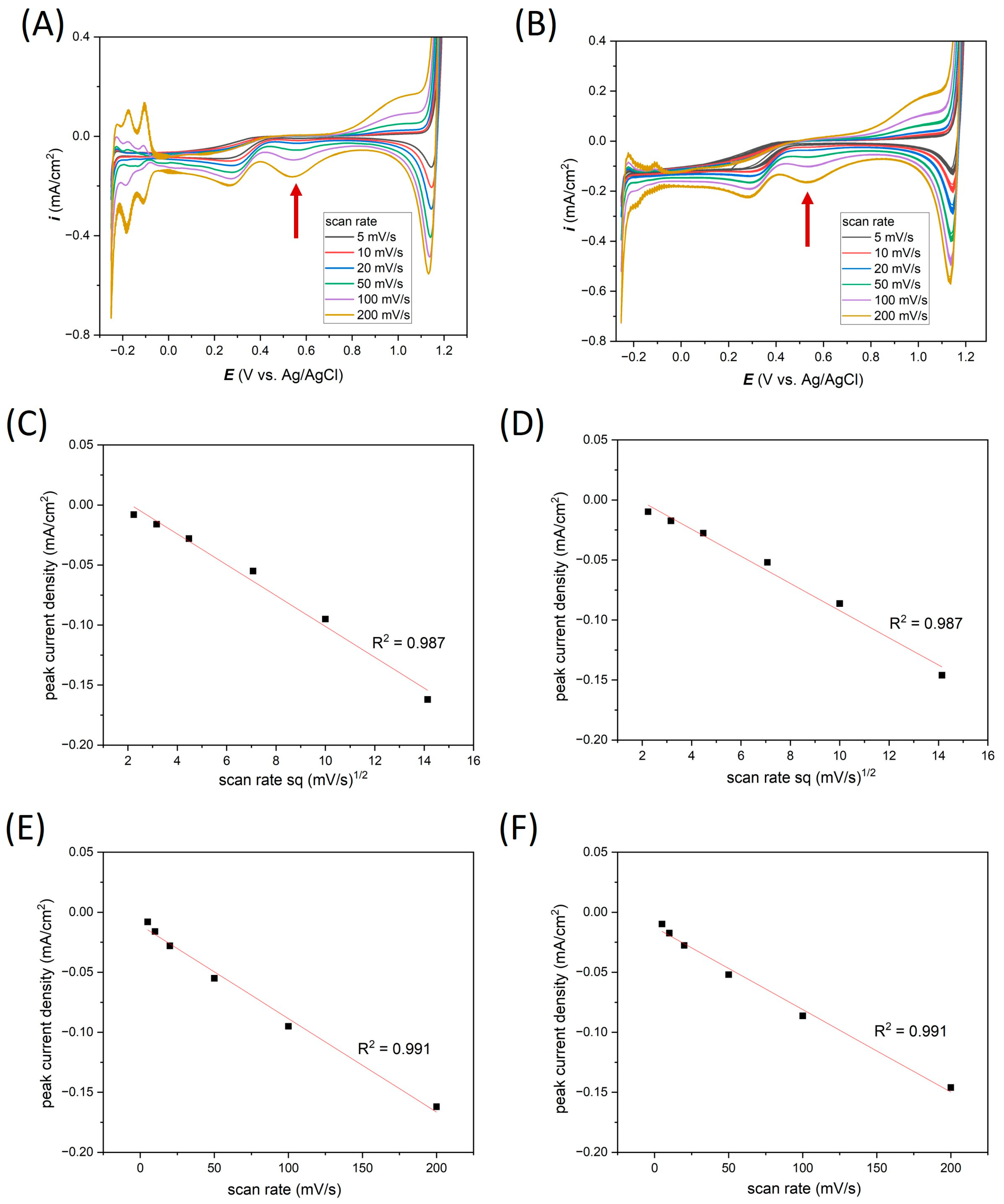
| Ti-Pt on glass | Average i (mA/cm2) | −0.02 | −0.03 | −0.04 | −0.06 | −0.09 | −0.16 |
| Standard deviation (%) | 25.24 | 5.52 | 3.75 | 0.86 | 0.76 | 0.60 | |
| Ti-Pt on Si | Average i (mA/cm2) | −0.05 | −0.06 | −0.06 | −0.07 | −0.09 | −0.15 |
| Standard deviation (%) | 12.78 | 1.24 | 0.34 | 0.71 | 0.84 | 0.86 |
| 5 mV/s | 10 mV/s | 20 mV/s | 50 mV/s | 100 mV/s | 200 mV/s | ||
|---|---|---|---|---|---|---|---|
| Ti-Pt on glass | Average i (mA/cm2) | −0.12 | −0.15 | −0.20 | −0.30 | −0.43 | −0.66 |
| Standard deviation (%) | 6.96 | 0.78 | 3.84 | 0.69 | 0.33 | 0.36 | |
| Ti-Pt on Si | Average i (mA/cm2) | −0.09 | −0.13 | −0.19 | −0.28 | −0.42 | −0.66 |
| Standard deviation (%) | 1.03 | 2.08 | 1.58 | 1.72 | 0.68 | 0.41 |
| Environment | KCl | HCl | KOH |
|---|---|---|---|
| Electrode | Standard Deviation [%] | ||
| 50 µm Pt | 1.43 | 5.67 | 4.14 |
| 100 µm Pt | 2.58 | 4.80 | 4.92 |
| 200 µm Pt | 0.83 | 2.44 | 4.02 |
| Ti-Pt on glass | 0.94 | 6.10 | 2.16 |
| Ti-Pt on Si | 0.97 | 2.79 | 2.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szklarz, Z.; Kołczyk-Siedlecka, K.; Vereshchagina, E.; Herbjørnrød, A.; Wittendorp, P.; Jain, S.; Wójcik, P.J. A Study of the Long-Term Electrochemical Stability of Thin-Film Titanium–Platinum Microelectrodes and Their Comparison to Classic, Wire-Based Platinum Microelectrodes in Selected Inorganic Electrolytes. Materials 2024, 17, 1352. https://doi.org/10.3390/ma17061352
Szklarz Z, Kołczyk-Siedlecka K, Vereshchagina E, Herbjørnrød A, Wittendorp P, Jain S, Wójcik PJ. A Study of the Long-Term Electrochemical Stability of Thin-Film Titanium–Platinum Microelectrodes and Their Comparison to Classic, Wire-Based Platinum Microelectrodes in Selected Inorganic Electrolytes. Materials. 2024; 17(6):1352. https://doi.org/10.3390/ma17061352
Chicago/Turabian StyleSzklarz, Zbigniew, Karolina Kołczyk-Siedlecka, Elizaveta Vereshchagina, Aina Herbjørnrød, Paul Wittendorp, Shruti Jain, and Pawel Jerzy Wójcik. 2024. "A Study of the Long-Term Electrochemical Stability of Thin-Film Titanium–Platinum Microelectrodes and Their Comparison to Classic, Wire-Based Platinum Microelectrodes in Selected Inorganic Electrolytes" Materials 17, no. 6: 1352. https://doi.org/10.3390/ma17061352






