Enhancing the Flame Retardancy of Polyester/Cotton Blend Fabrics Using Biobased Urea–Phytate Salt
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the PA-UR and Coated T/C Blend Fabric
2.3. Characterizations
3. Results and Discussion
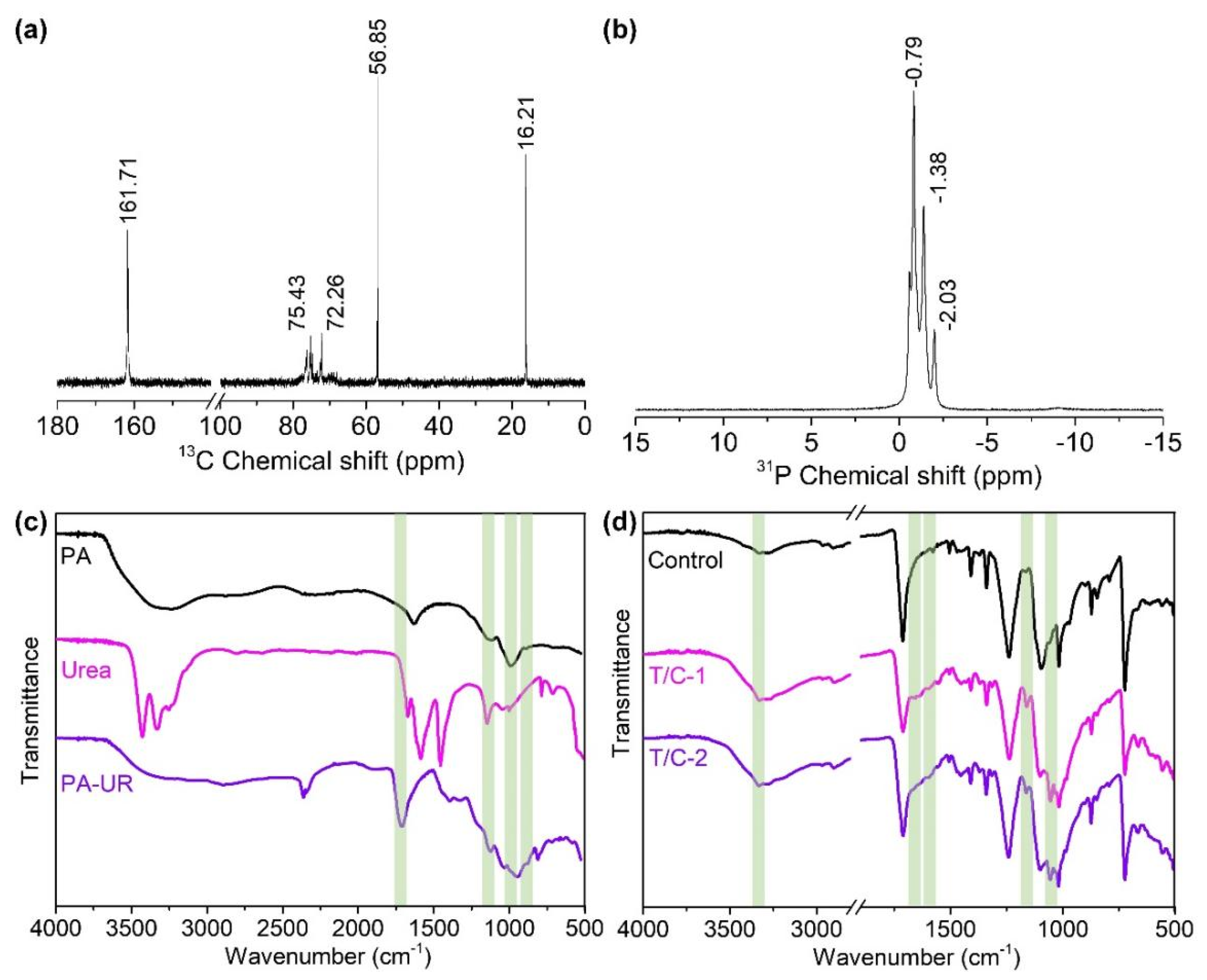
3.1. NMR and ATR/FT-IR of PA-UR
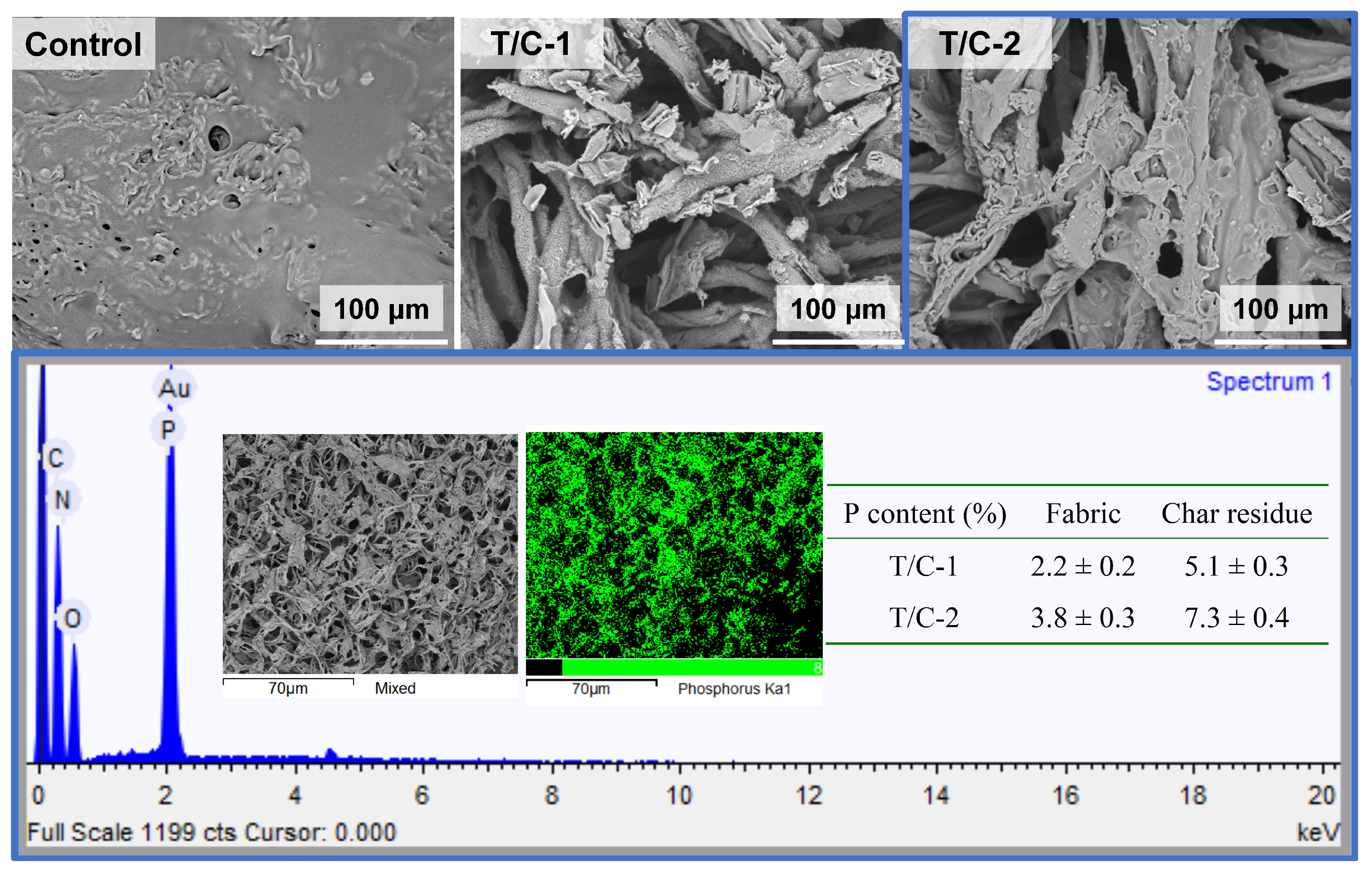
3.2. ATR/FT-IR and the Morphology of the Coated Fabric
3.3. Thermal Performance
3.4. Heat Release Capacity
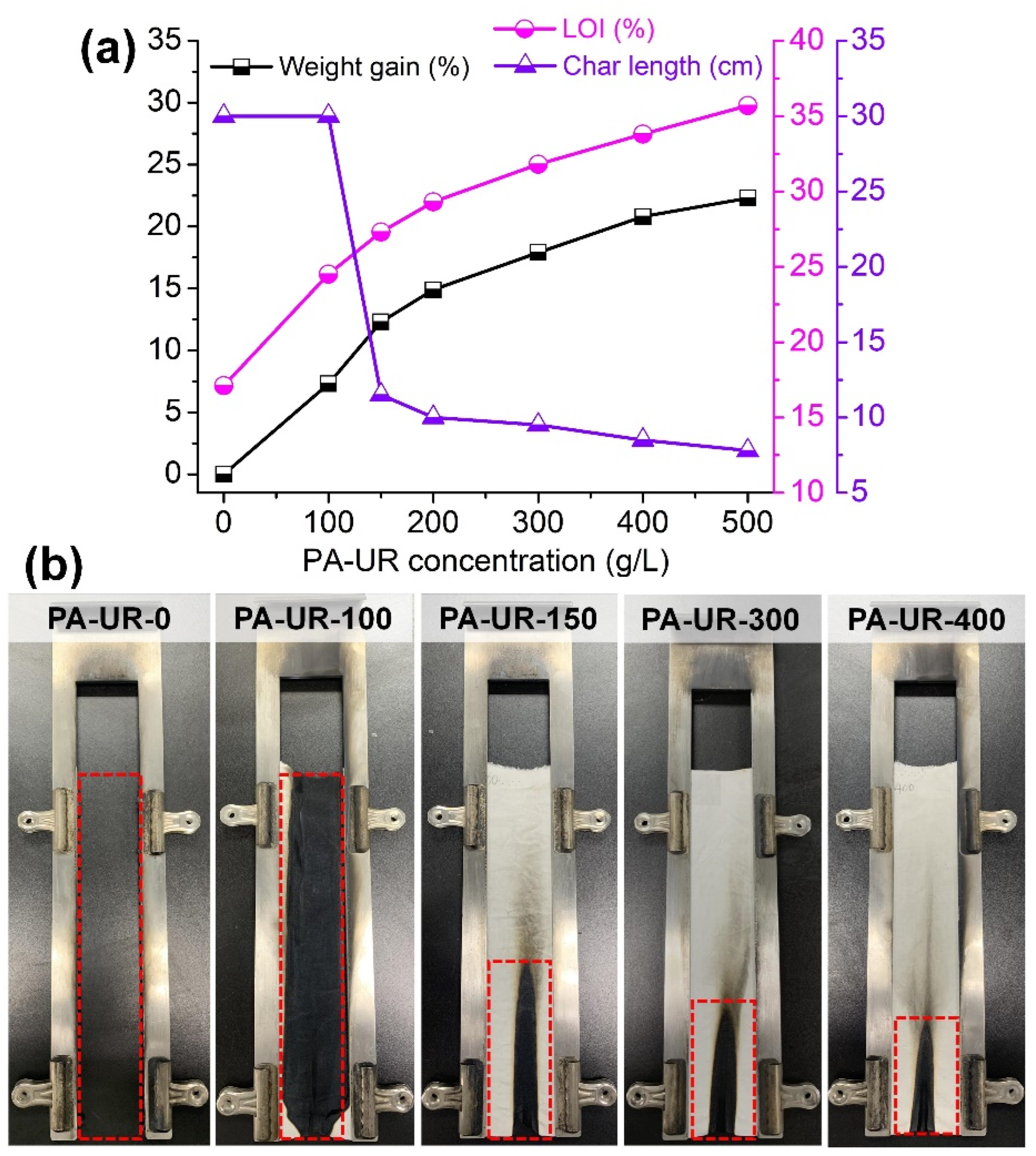
3.5. Flame Retardancy
3.6. Char Residue Analyses
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosace, G.; Migani, V.; Guido, E.; Colleoni, C. Flame retardant finishing for textiles. In Flame Retardants; Visakh, P.M., Arao, Y., Eds.; Springer: Cham, Switzerland, 2015; pp. 209–246. [Google Scholar]
- Horrocks, A.R. Flame retardant challenges for textiles and fibers: New chemistry versus innovatory solutions. Polym. Degrad. Stabil. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Brzeziński, S.; Kamińska, I. Multifunctional bioactive and improving the performance durability nanocoatings for finishing PET/CO woven fabrics by the sol-gel method. J. Alloy. Compd. 2015, 649, 387–393. [Google Scholar] [CrossRef]
- Hossain, M.S.; Islam, M.M.; Dey, S.C.; Hasan, N. An approach to improve the pilling resistance properties of three thread polyester cotton blended fleece fabric. Heliyon 2021, 7, e06921. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, W.; Haile, A. Improving hydrophilicity and comfort characteristics of polyester/cotton blend fabric through lipase enzyme treatment. Clean Technol. Environ. 2024. [Google Scholar] [CrossRef]
- Mayer-Gall, T.; Knittel, D.; Gutmann, J.S.; Opwis, K. Permanent flame retardant finishing of textiles by allyl-functionalized polyphosphazenes. ACS Appl. Mater. Inter. 2015, 7, 9349–9363. [Google Scholar] [CrossRef]
- Magovac, E.; Vončina, B.; Jordanov, I.; Grunlan, J.C.; Bischof, S. Layer-by-layer deposition: A promising environmentally benign flame-retardant treatment for cotton, polyester, polyamide and blended textiles. Materials 2022, 15, 432. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Leistner, M.; Sarwar, O.; Toler, C.M.; Henderson, R.; Grunlan, J.C. A wash-durable polyelectrolyte complex that extinguishes flames on polyester–cotton fabric. RSC Adv. 2016, 6, 33998–34004. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Flammability and combustion properties of ammonium polyphosphate-/poly (acrylic acid)-based layer by layer architectures deposited on cotton, polyester and their blends. Polym. Degrad. Stabil. 2013, 98, 1626–1637. [Google Scholar] [CrossRef]
- Wang, B.; Xu, Y.J.; Li, P.; Zhang, F.Q.; Liu, Y.; Zhu, P. Flame-retardant polyester/cotton blend with phosphorus/nitrogen/silicon-containing nano-coating by layer-by-layer assembly. Appl. Surf. Sci. 2020, 509, 145323. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by layer ammonium polyphosphate-based coatings for flame retardancy of polyester–cotton blends. Carbohydr. Polym. 2012, 88, 1460–1469. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, L.; Wang, X.; Song, L.; Hu, Y. Hypophosphorous acid cross-linked layer-by-layer assembly of green polyelectrolytes on polyester-cotton blend fabrics for durable flame-retardant treatment. Carbohydr. Polym. 2018, 201, 1–8. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mat. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, C.H.; Song, H.; Heo, J.H.; Lee, J.H. Biomolecules as green flame retardants: Recent progress, challenges, and opportunities. J. Clean. Prod. 2022, 368, 133241. [Google Scholar] [CrossRef]
- Malucelli, G. Biomacromolecules and bio-sourced products for the design of flame retarded fabrics: Current state of the art and future perspectives. Molecules 2019, 24, 3774. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Carosio, F.; Bosco, F.; Malucelli, G. DNA: A novel, green, natural flame retardant and suppressant for cotton. J. Mater. Chem. A 2013, 1, 4779–4785. [Google Scholar] [CrossRef]
- Basak, S.; Raja, A.S.M.; Saxena, S.; Patil, P.G. Tannin based polyphenolic bio-macromolecules: Creating a new era towards sustainable flame retardancy of polymers. Polym. Degrad. Stabil. 2021, 189, 109603. [Google Scholar] [CrossRef]
- Yang, H.; Yu, B.; Xu, X.; Bourbigot, S.; Wang, H.; Song, P. Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials. Green Chem. 2020, 22, 2129–2161. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restas, A.; Das, O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind. Crop. Prod. 2021, 164, 113349. [Google Scholar] [CrossRef]
- Basak, S.; Ali, S.W. Sustainable fire retardancy of textiles using bio-macromolecules. Polym. Degrad. Stabil. 2016, 133, 47–64. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, W.; Li, J.; Liu, H.; Liu, X. Eco-friendly flame retardant and dripping-resistant of polyester/cotton blend fabrics through layer-by-layer assembly fully bio-based chitosan/phytic acid coating. Int. J. Biol. Macromol. 2021, 175, 140–146. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Cheng, X.; Li, H.; Gu, X.; Sun, J.; Zhang, S. Improving the flame retardant and antibacterial performance of polyester/cotton blend fabrics with organic-inorganic hybrid coating. Polym. Degrad. Stabil. 2022, 200, 109944. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, X.; Liu, L.; Zhang, C.; Shang, X.; Yao, J. Eco-friendly, efficient and durable fireproof cotton fabric prepared by a feasible phytic acid grafting route. Cellulose 2021, 28, 3887–3899. [Google Scholar] [CrossRef]
- GB/T 5454-1997; Textiles—Burning Behavior—Oxygen index Method. Standardization Administration of China: Beijing, China, 1997.
- GB/T 5455-2014; Textiles—Burning Behaviour—Determination of Damaged Length, Afterglow Time and Afterflame Time of Vertically Oriented Specimens. Standardization Administration of China: Beijing, China, 2014.
- GB/T 17591-2006; Flame Retardant Fabrics. Standardization Administration of China: Beijing, China, 2006.
- ISO 13934-1-2013; Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. International Organization for Standardization: Geneva, Switzerland, 2013.
- Nehls, I.; Loth, F. 13C-NMR-spektroskopische Untersuchungen zur Phosphatierung von Celluloseprodukten im System H3PO4/Harnstoff. Acta Polym. 1991, 42, 233–235. [Google Scholar] [CrossRef]
- Kristensen, J.H.; Bampos, N.; Duer, M. Solid state 13C CP MAS NMR study of molecular motions and interactions of urea adsorbed on cotton cellulose. Phys. Chem. Chem. Phy. 2004, 12, 3175–3183. [Google Scholar] [CrossRef]
- Antoun, K.; Ayadi, M.; El Hage, R.; Nakhl, M.; Sonnier, R.; Gardiennet, C.; Moigne, N.L.; Besserer, A.; Brosse, N. Renewable phosphorous-based flame retardant for lignocellulosic fibers. Ind. Crop Prod. 2022, 186, 115265. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Ghilan, A.; Rusu, A.G.; Tudorachi, N.; Timpu, D. Alginate enriched with phytic acid for hydrogels preparation. Int. J. Biol. Macromol. 2021, 181, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Barbalini, M.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Phytic acid and biochar: An effective all bio-sourced flame retardant formulation for cotton fabrics. Polymers 2020, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Jin, W.J.; Guan, J.P.; Cheng, X.W.; Chen, G. Functionalization of silk fabric using phytate urea salt for durable flame retardant performance. Mater. Today Commun. 2021, 28, 102673. [Google Scholar] [CrossRef]
- Alongi, J.; Camino, G.; Malucelli, G. Heating rate effect on char yield from cotton, poly(ethylene terephthalate) and blend fabrics. Carbohydr. Polym. 2013, 92, 1327–1334. [Google Scholar] [CrossRef]
- Markwart, J.C.; Battig, A.; Zimmermann, L.; Wagner, M.; Fischer, J.; Schartel, B.; Wurm, F.R. Systematically controlled decomposition mechanism in phosphorus flame retardants by precise molecular architecture: P–O vs. P–N. ACS Appl. Polym. Mater. 2019, 1, 1118–1128. [Google Scholar] [CrossRef]


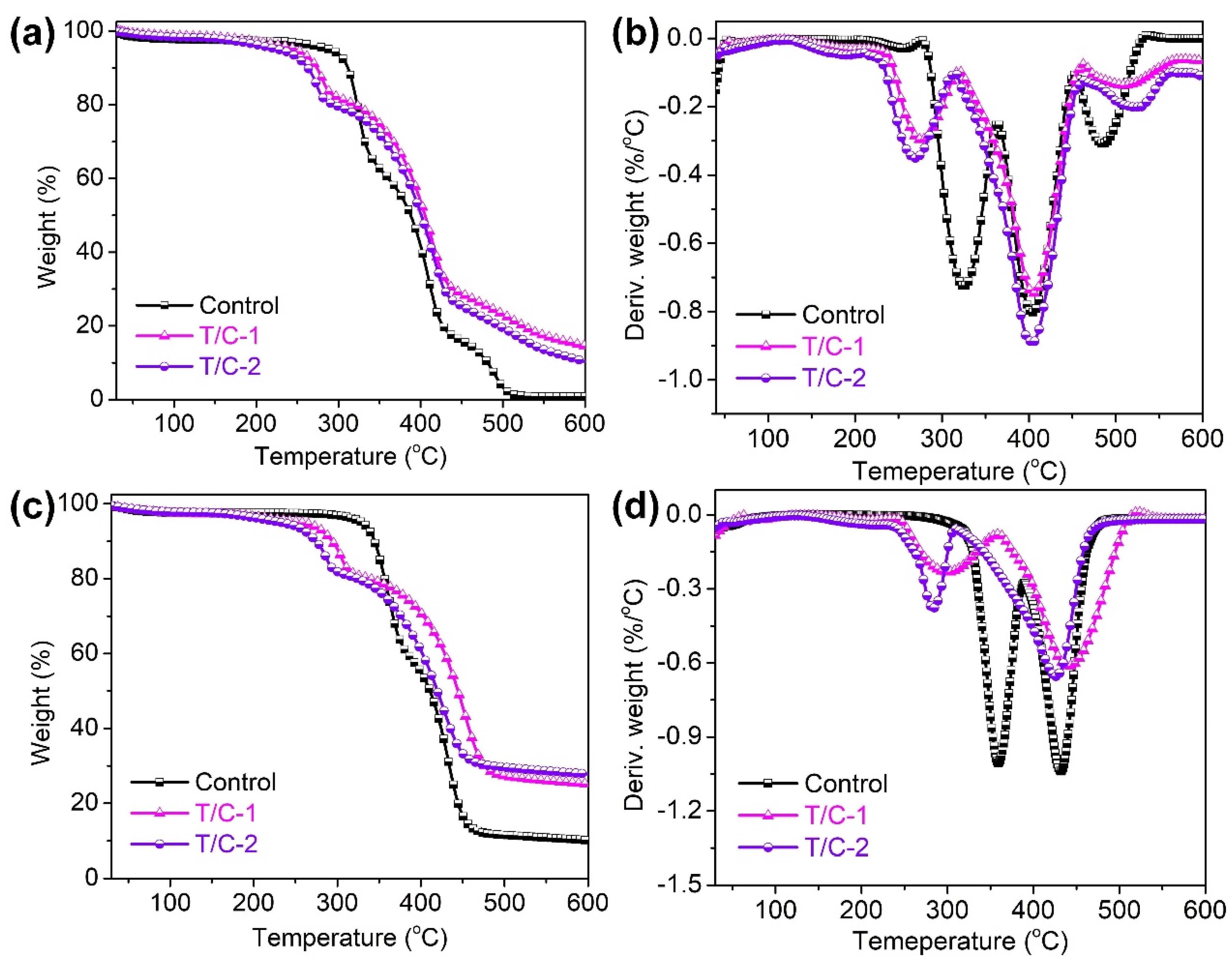



| Samples | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Residue at 600 °C (%) | |
|---|---|---|---|---|---|---|
| Air | Control | 288.7 | 324.7 | 404.0 | 485.2 | 0.8 |
| T/C-1 | 247.8 | 276.5 | 405.5 | 513.6 | 14.4 | |
| T/C-2 | 226.5 | 267.6 | 402.6 | 524.8 | 10.3 | |
| Nitrogen | Control | 329.5 | 369.8 | 434.7 | — | 10.1 |
| T/C-1 | 256.9 | 300.1 | 427.5 | — | 25.1 | |
| T/C-2 | 232.5 | 283.1 | 441.6 | — | 27.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, S.; Huang, Y.-T.; Zhang, X.; Cheng, S.-S.; Cheng, X.-W.; Guan, J.-P. Enhancing the Flame Retardancy of Polyester/Cotton Blend Fabrics Using Biobased Urea–Phytate Salt. Materials 2024, 17, 1346. https://doi.org/10.3390/ma17061346
Dong S, Huang Y-T, Zhang X, Cheng S-S, Cheng X-W, Guan J-P. Enhancing the Flame Retardancy of Polyester/Cotton Blend Fabrics Using Biobased Urea–Phytate Salt. Materials. 2024; 17(6):1346. https://doi.org/10.3390/ma17061346
Chicago/Turabian StyleDong, Shuang, Yi-Ting Huang, Xin Zhang, Shan-Shan Cheng, Xian-Wei Cheng, and Jin-Ping Guan. 2024. "Enhancing the Flame Retardancy of Polyester/Cotton Blend Fabrics Using Biobased Urea–Phytate Salt" Materials 17, no. 6: 1346. https://doi.org/10.3390/ma17061346





