Microstructures and Corrosion/Localised Corrosion of Stainless Steels, Incoloy and Their Weldments in Nitrite-Containing Chloride Environments
Abstract
:1. Introduction
1.1. Electrochemical Aspects of Pitting
1.2. Pitting of Corrosion-Resistant Alloys and Their Weldments
2. Experimental Methodologies
2.1. Test Alloys
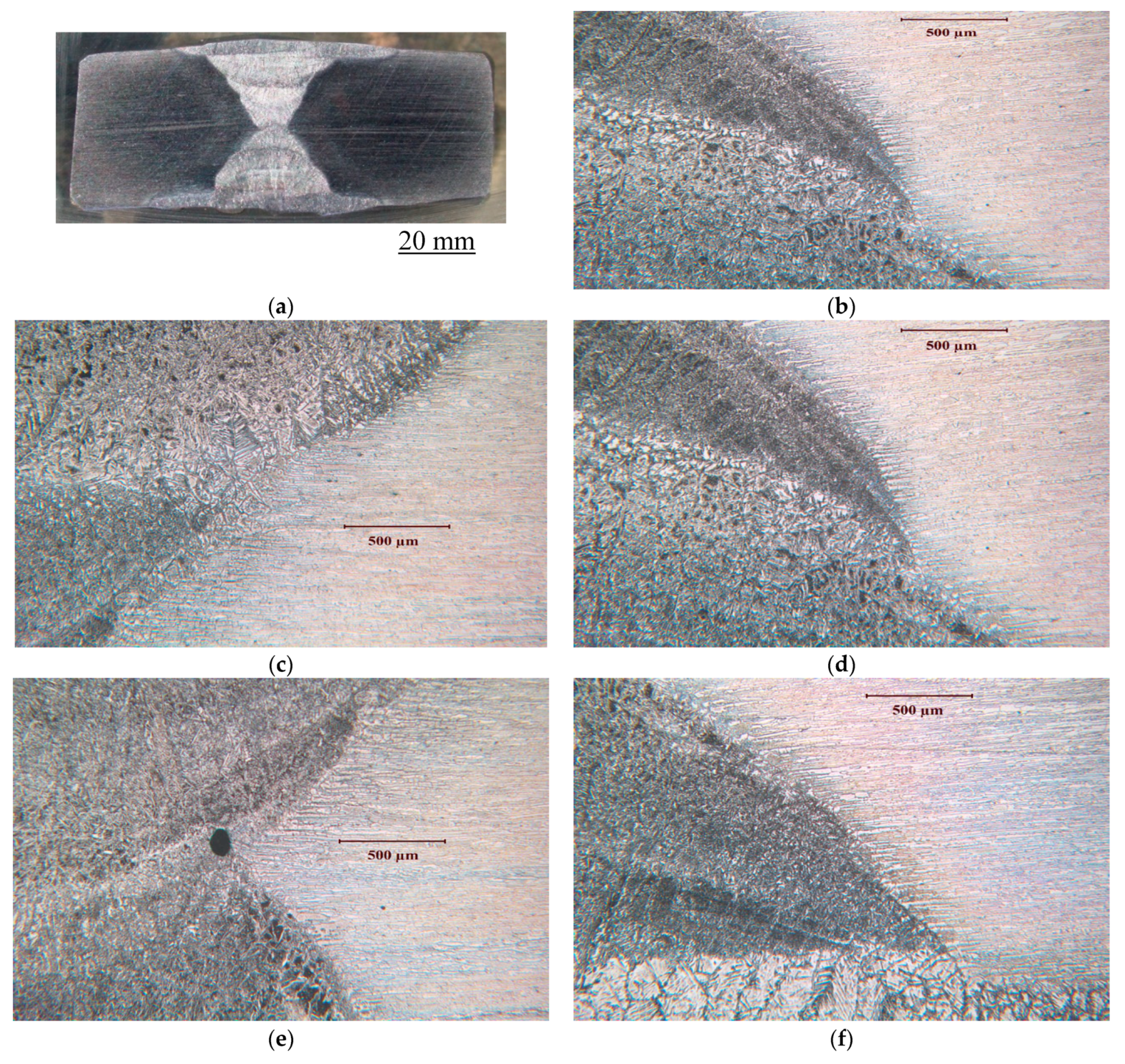
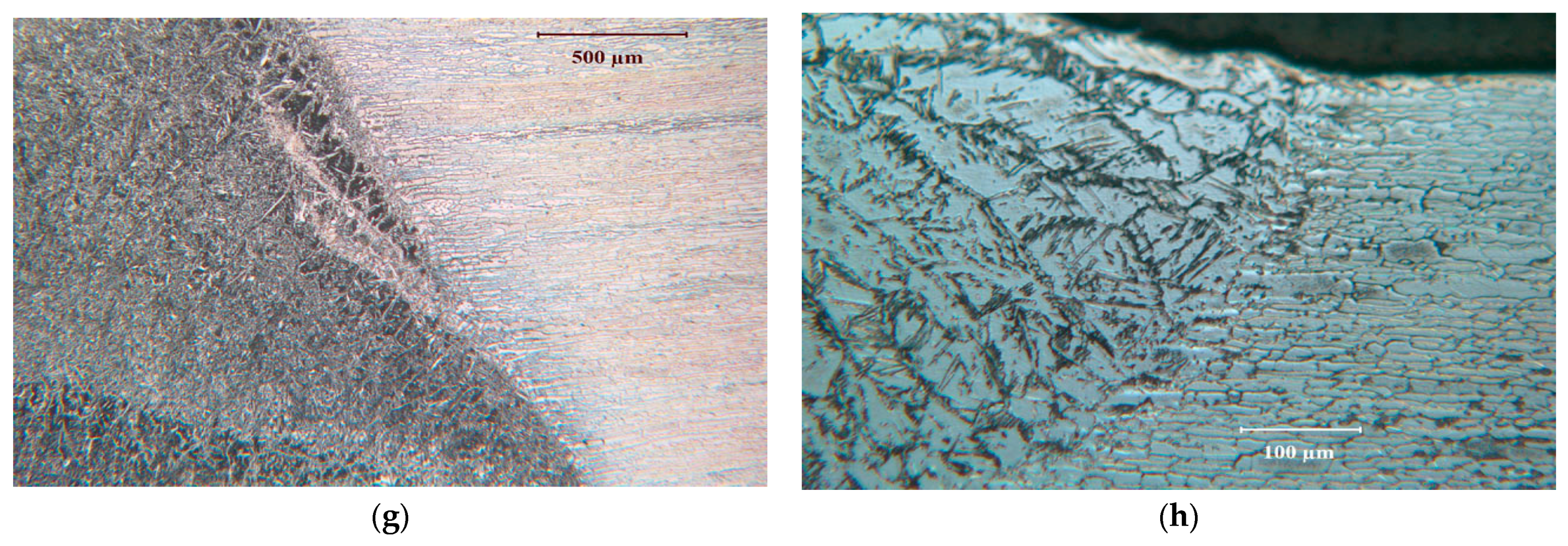
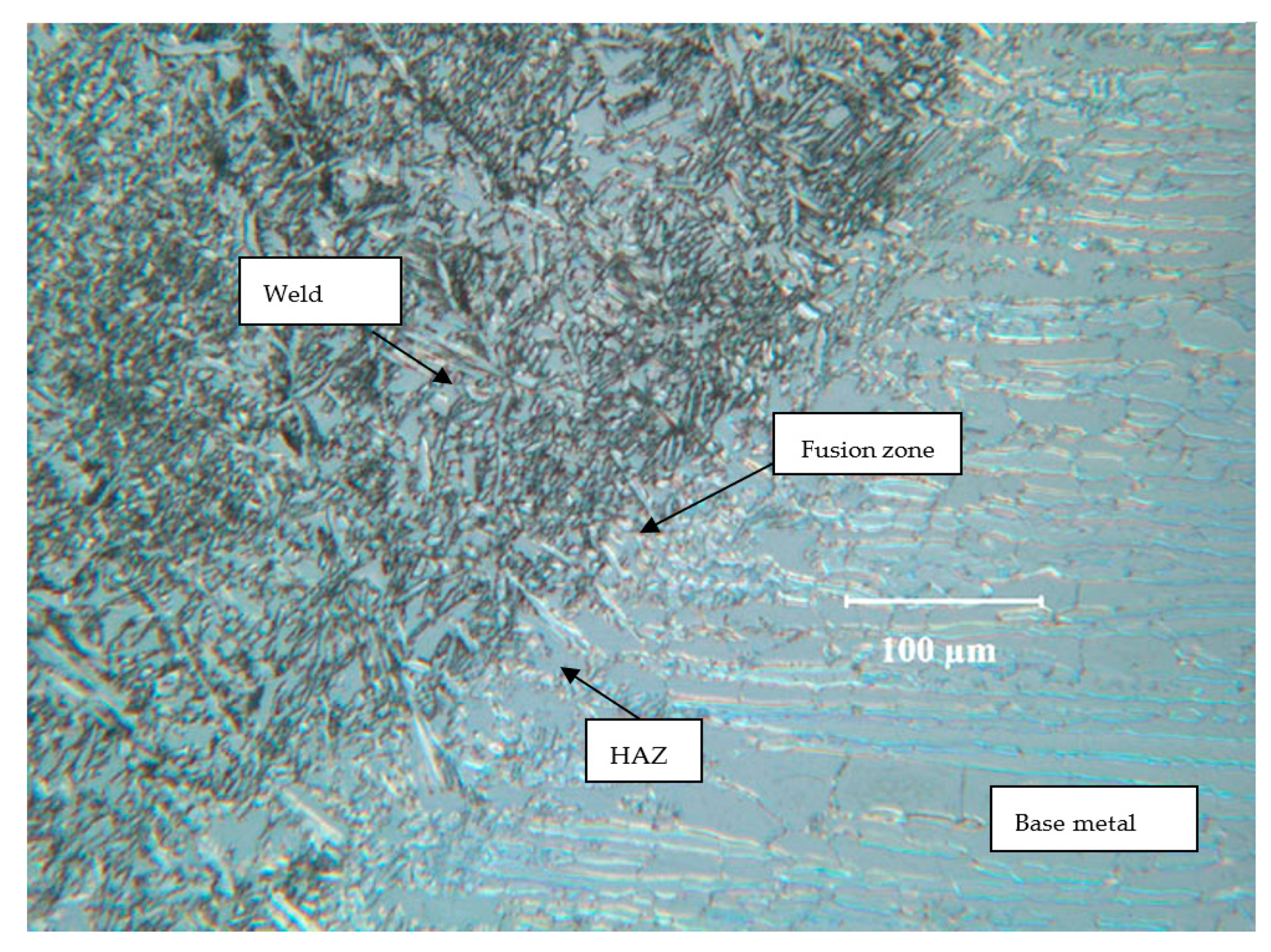
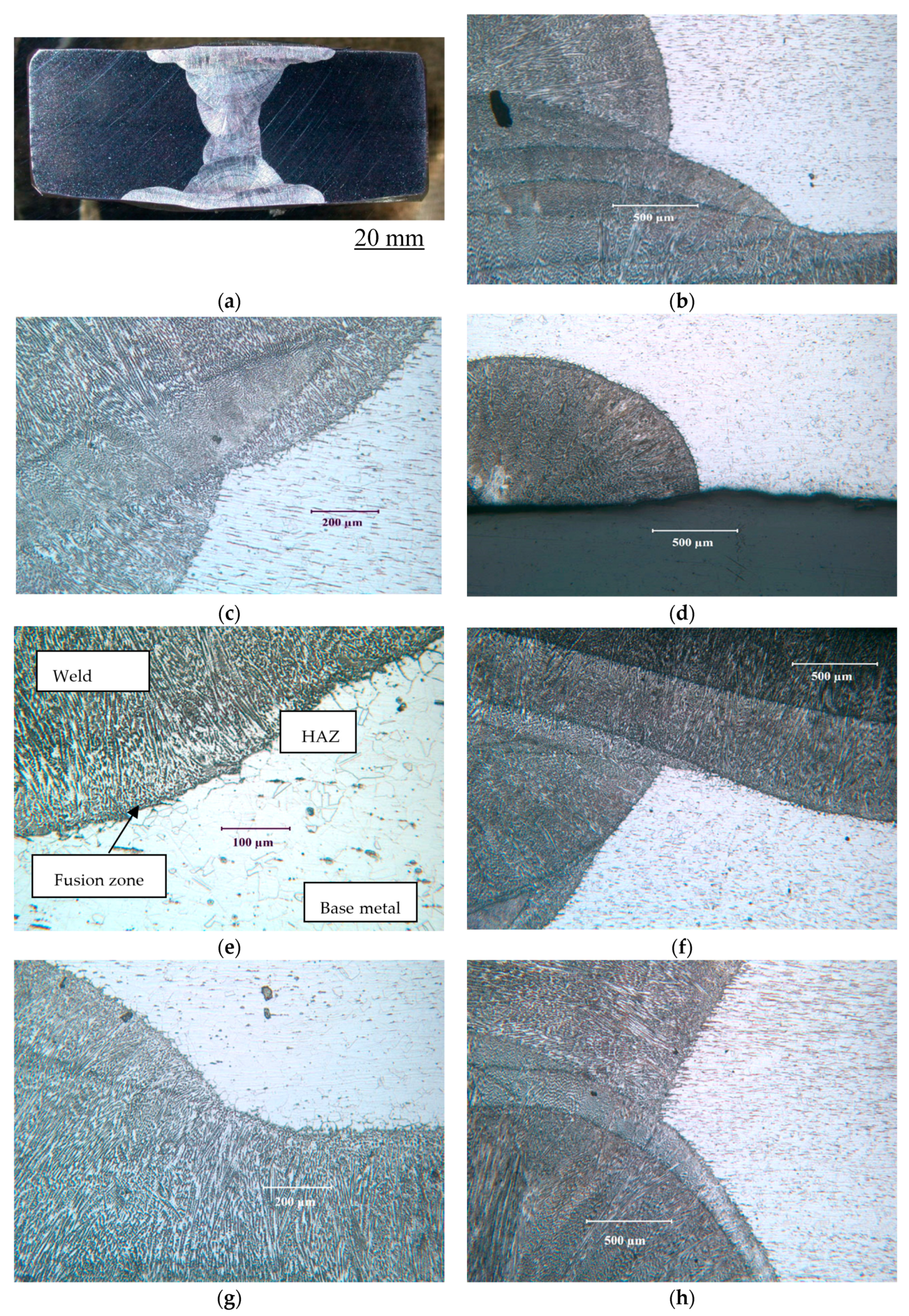
2.2. Weldments of Test Alloys and Their Microstructures
2.3. Pitting Tests
2.4. Immersion Tests and Corrosion Rates
2.5. Potentiodynamic Polarisation
3. Results and Discussion
3.1. Microstructures of Weldments
3.1.1. Duplex Stainless Steel, SAF2507 (UNS S32750)
3.1.2. Incoloy 825 (UNS N08825)
3.1.3. Stainless Steel 316L (UNS S31603)
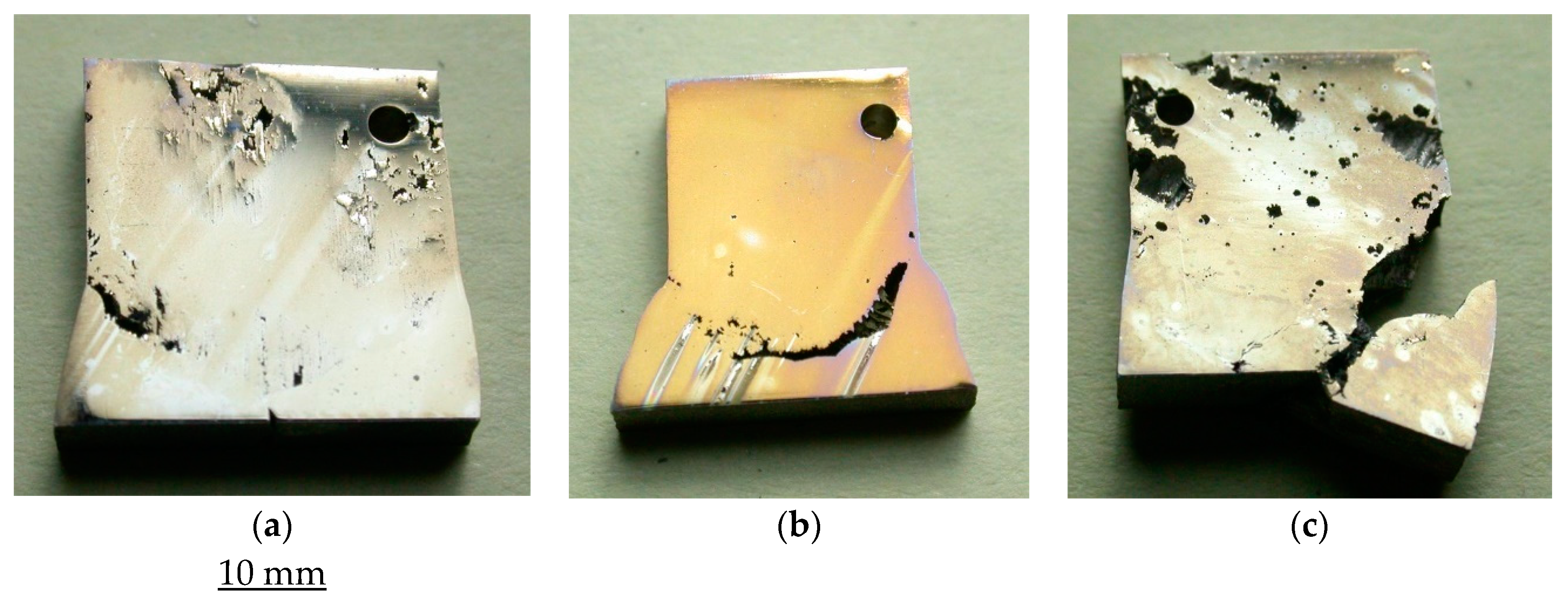
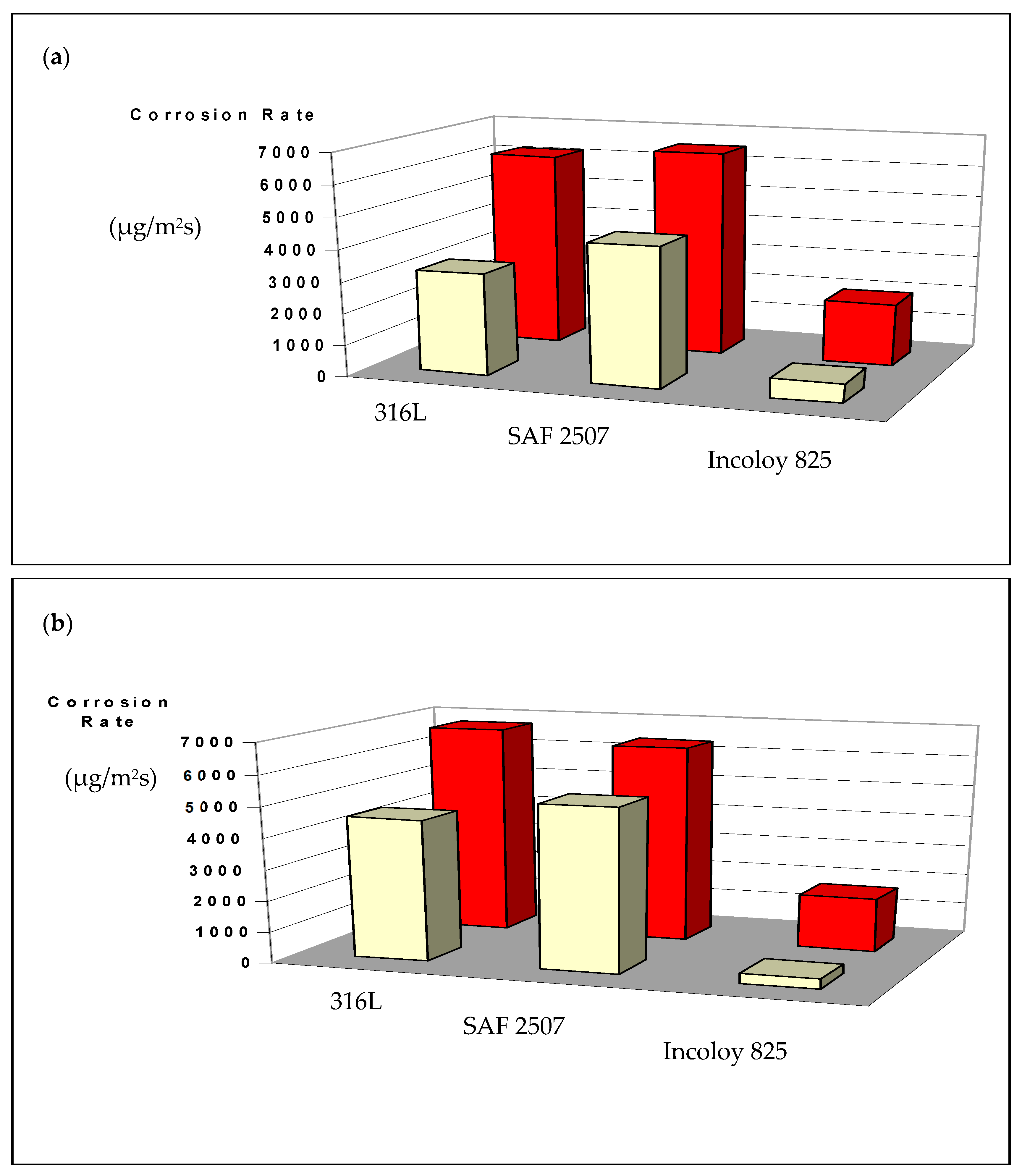
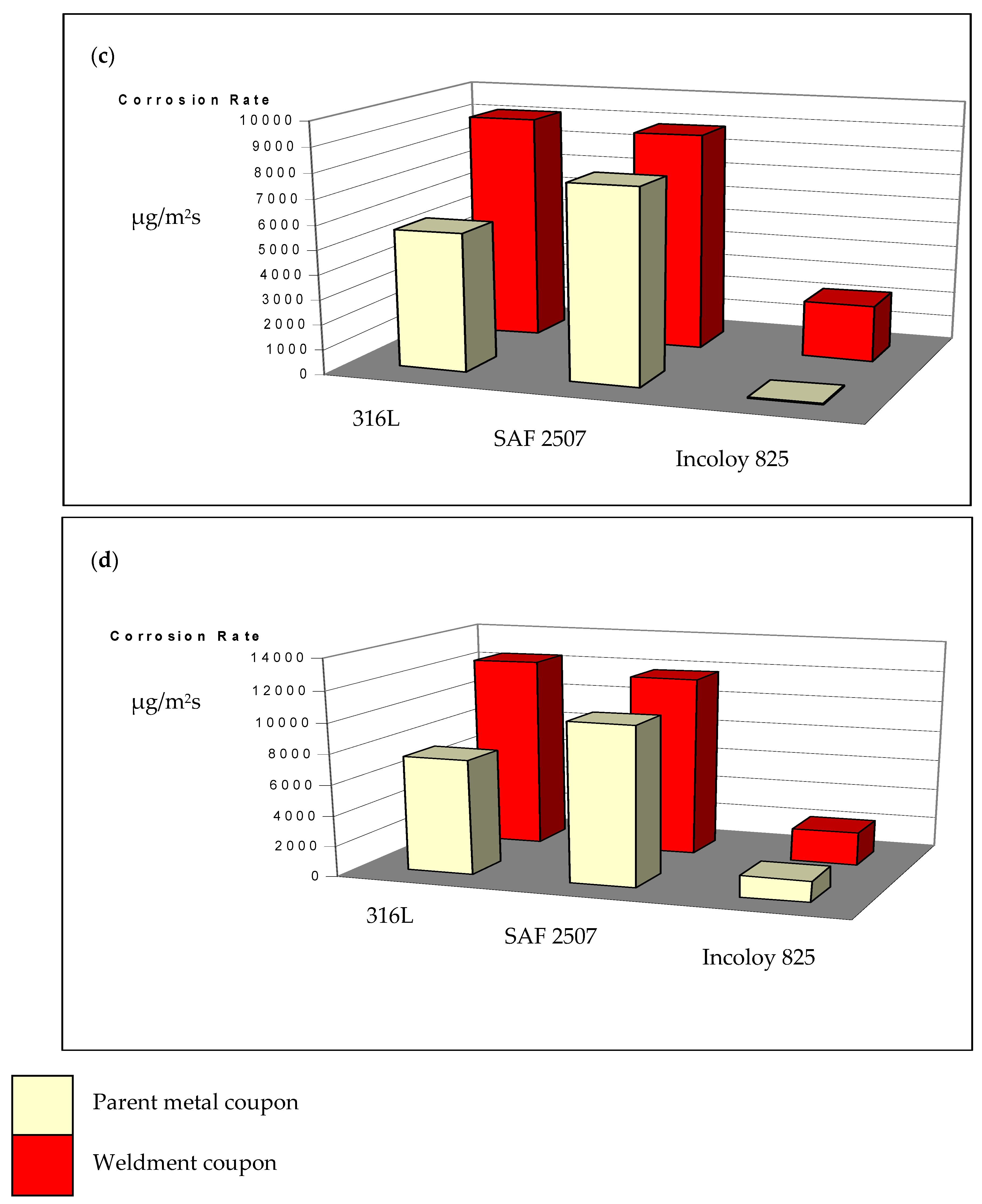
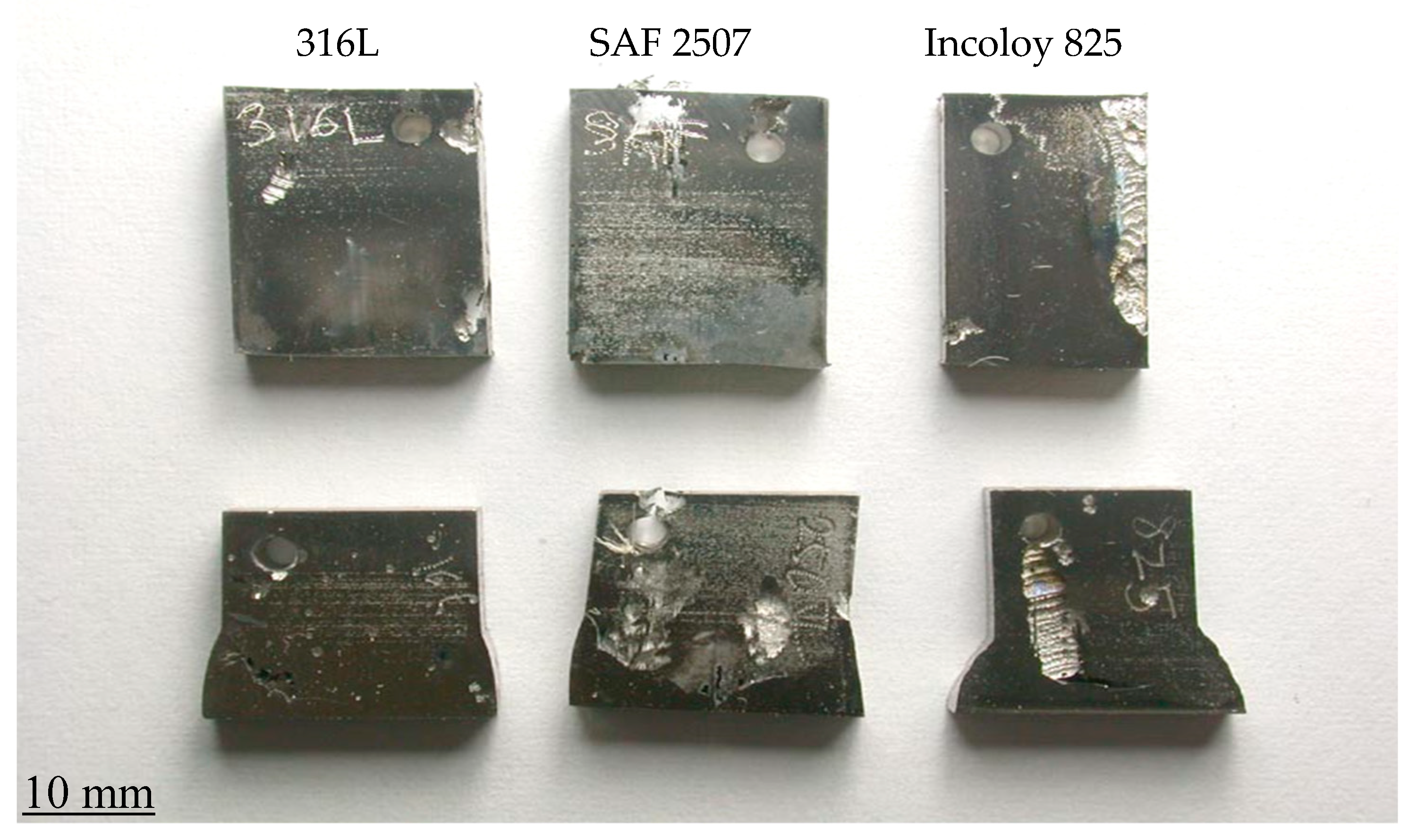
3.2. Pitting and General Corrosion Resistance of the Alloys and Their Weldments
3.2.1. Tests in Plain FeCl3
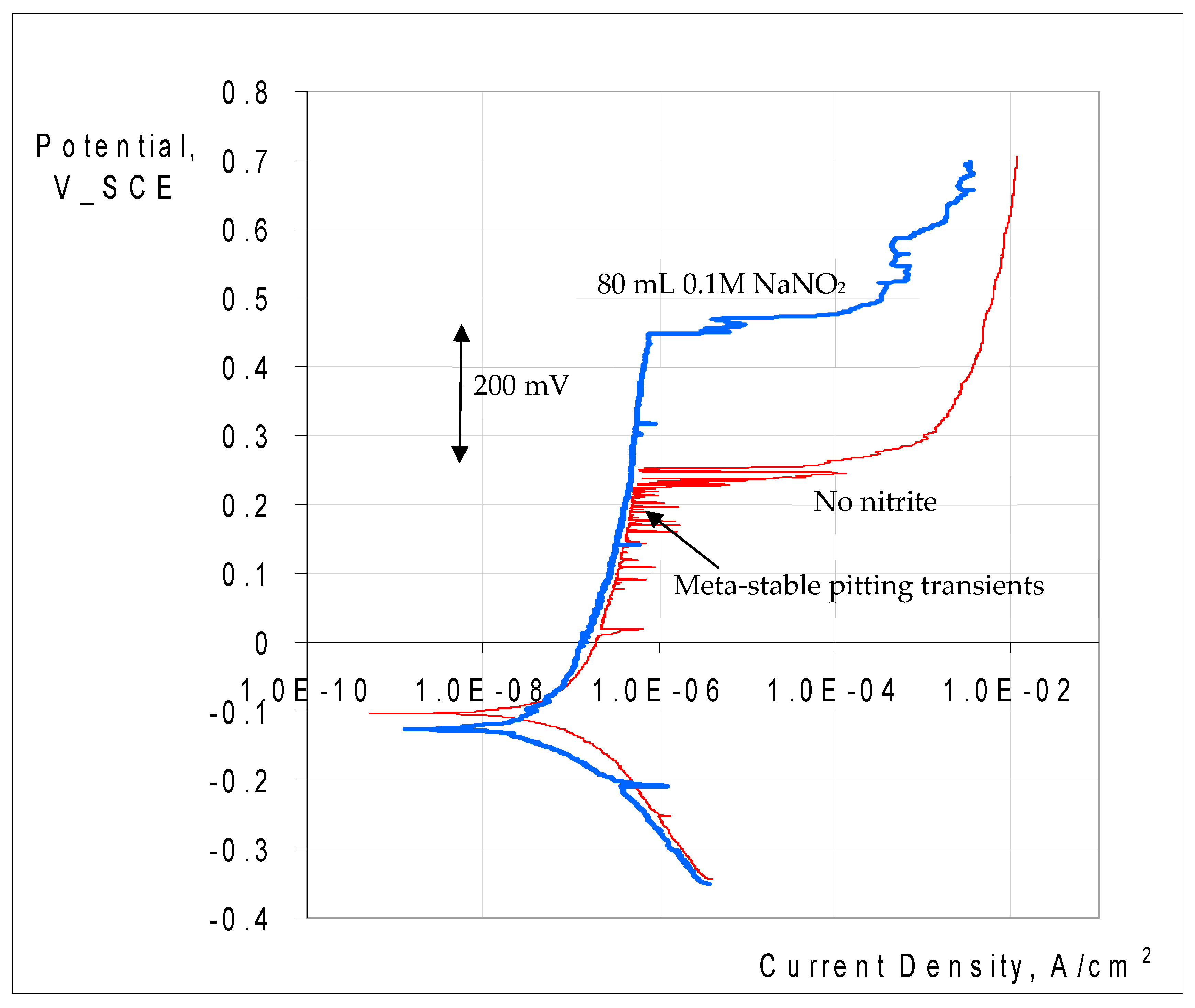
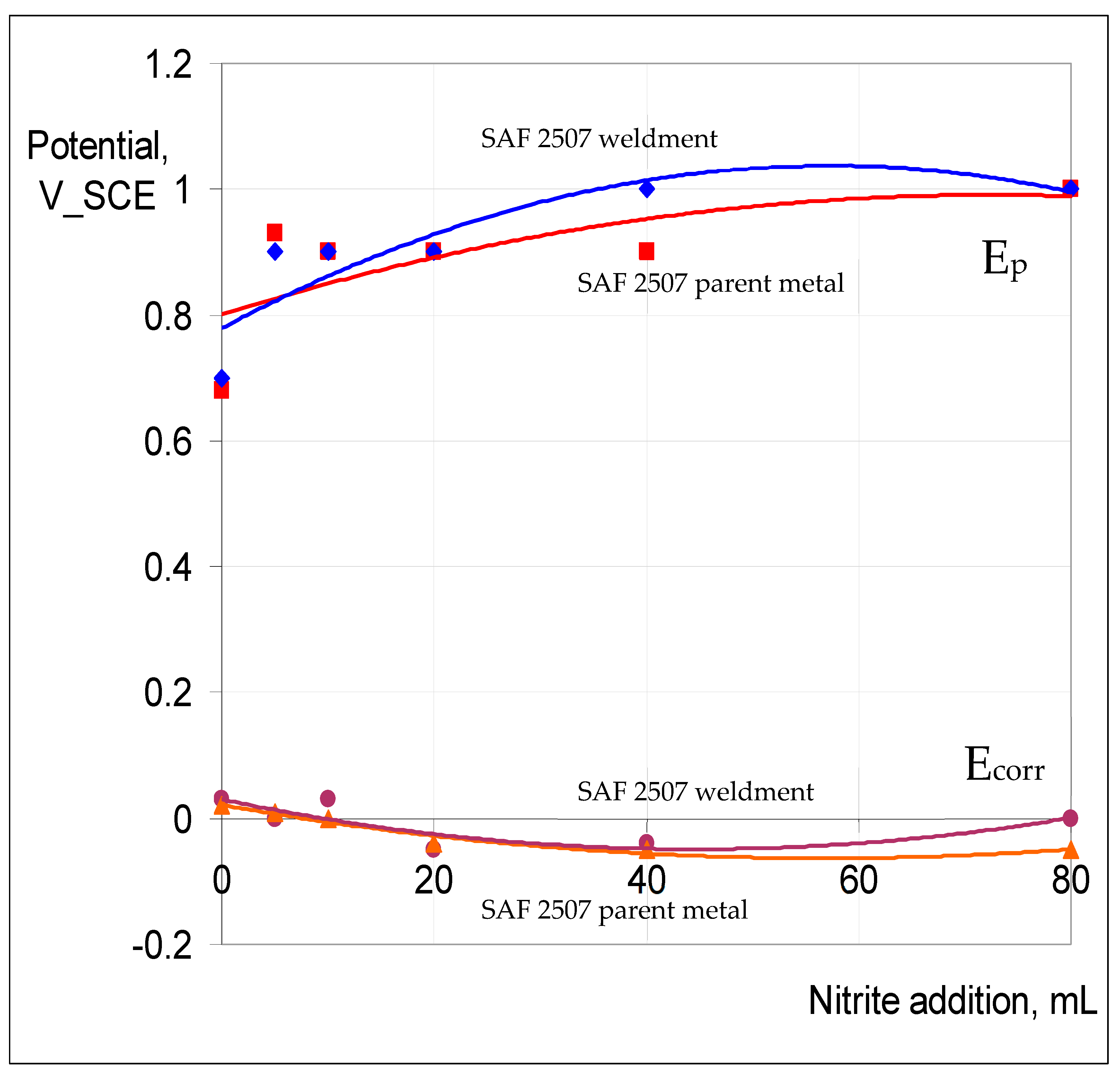
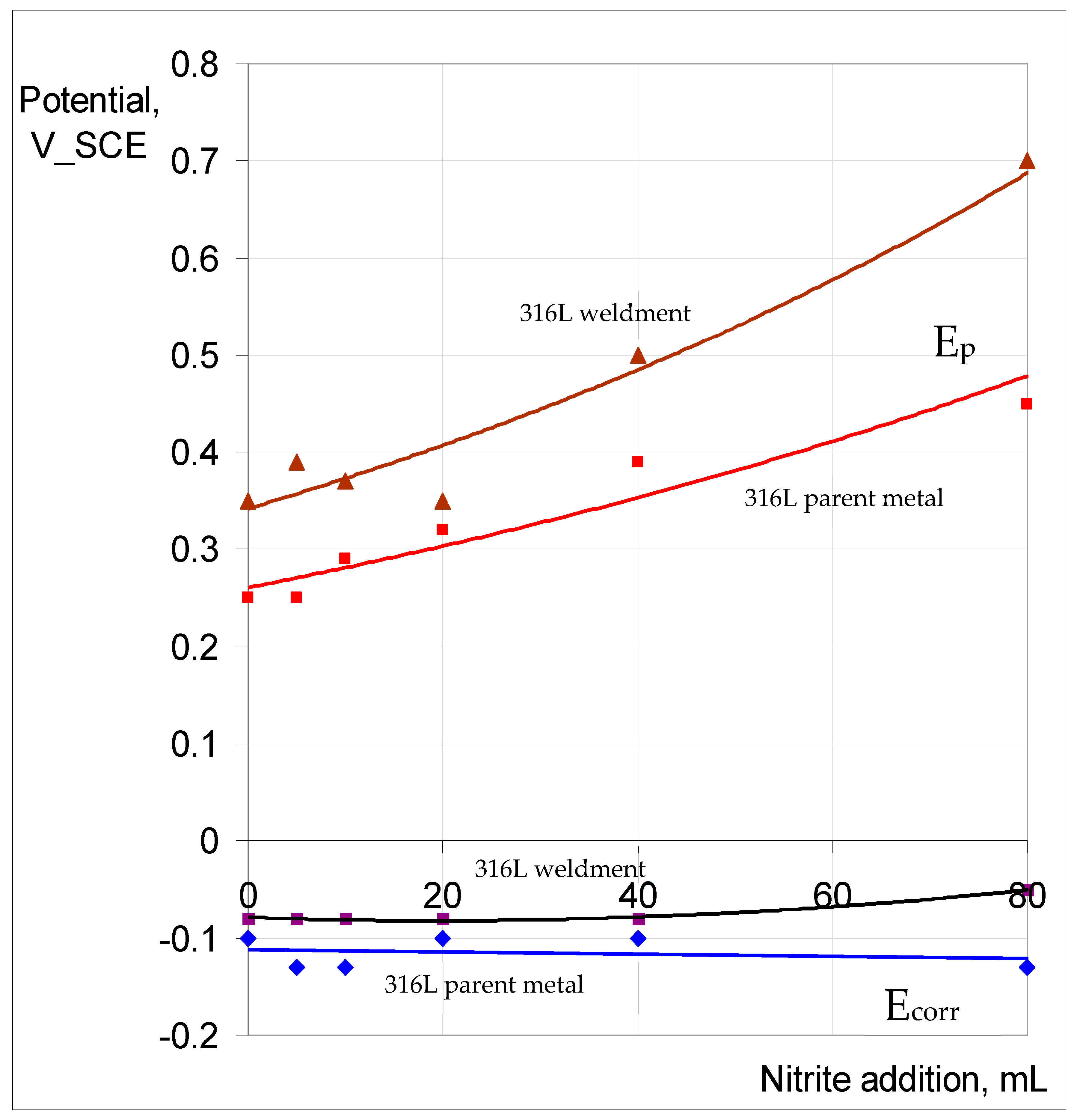
3.2.2. Role of Nitrite Addition in Corrosion and Pitting
3.2.3. Potentiodynamic Polarisation to Characterise Pitting and Corrosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSs | Stainless steels |
| DSSs | Duplex stainless steels |
| ASSs | Austenitic stainless steels |
| Ep | Pitting potential |
| SCC | Stress corrosion cracking |
| PREN | Pitting resistance equivalent number |
| IGC | Intergranular corrosion |
| PDP | Potentiodynamic polarisation |
| HAZ | Heat-affected zone |
| CPT | Critical pitting temperature |
References
- Kwon, H.-S.; Kim, H.-S. Investigation of stress corrosion susceptibility of duplex (α + γ) stainless steel in a hot chloride solution. Mater. Sci. Eng. A 1993, 172, 159–165. [Google Scholar] [CrossRef]
- Shimodaira, S.; Takano, M.; Taleizawa, Y.; Kamida, H. SCC and Hydrogen Embrittlement of Iron-Base Alloys; NACE: Houston, TX, USA, 1977; p. 1003. [Google Scholar]
- Sedriks, A.J. Corrosion of Stainless Steels; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996. [Google Scholar]
- Egan, F.; Clapp, G. In Proceedings of the Corrosion & Prevention, Hobart, Australia, 23–25 November 1998; paper#38-066, pp. 1–6.
- Notten, M.J.G. Experiences with ferritic-austenitic stainless steel in chemical process industry—Duplex are not a panacea. Stainl. Steel Eur. 1991, 24–31. [Google Scholar]
- Jones, D.A. Principles & Prevention of Corrosion, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 1992; p. 239. [Google Scholar]
- Raman, R.K.S. Role of caustic concentration and electrochemical potentials in caustic cracking of steels. Mater. Sci. Eng. A 2006, 441, 342. [Google Scholar] [CrossRef]
- Singbeil, D.; Tromans, D. Effect of Sulfide Ions on Caustic Cracking of Mild Steel. J. Electrochem. Soc. 1981, 128, 2065. [Google Scholar] [CrossRef]
- Singbeil, D.; Tromans, D. Caustic Stress Corrosion Cracking of Mild Steel. Metall. Transact. A 1982, 13, 1091. [Google Scholar] [CrossRef]
- Francis, R. Duplex Stainless Steels ’94; Gooch, T.G., Ed.; Paper KIV.; TWI: Cambridge, UK, 1994. [Google Scholar]
- Charles, J.; Bernhardsson, S. Duplex Stainless Steel ’91; Les Edition de Physique: Les Ulis, France, 1991; p. 185. [Google Scholar]
- Truman, J.E.; Kirby, H.W. The Possibility of Service Failure of Stainless Steels by Stress Corrosion Cracking. Metallurgia 1965, 72, 67–71. [Google Scholar]
- Ward, I.A.; Keys, L.H. Stainless Steels ’84; Institute of Metals: Gothenberg, Sweden, 1984. [Google Scholar]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545. [Google Scholar] [CrossRef]
- Cottis, R.A.; Newman, R.C. SCC of Duplex Stainless Steels, Health and Safety Executive—Offshore Technology Report; HMSO: Richmond, UK, 1995. [Google Scholar]
- Newman, R.C.; Shahrabi, T. The effect of alloyed nitrogen or dissolved nitrate ions on the anodic behaviour of austenitic stainless steel in hydrochloric acid. Corros. Sci. 1987, 27, 827–838. [Google Scholar] [CrossRef]
- Newman, R.C.; Ajjawi, M.A. A micro-electrode study of the nitrate effect on pitting of stainless steels. Corros. Sci. 1986, 26, 1057. [Google Scholar] [CrossRef]
- Zuo, Y.; Wang, H.; Zhao, J.; Xiong, J. The effects of some anions on metastable pitting of 316L stainless steel. Corros. Sci. 2002, 44, 13–24. [Google Scholar] [CrossRef]
- Uhlig, H.H.; Cook, J.E.W. Mechanism of Inhibiting Stress Corrosion Cracking of 18-8 Stainless Steel in MgCl2 by Acetates and Nitrates. J. Electrochem. Soc. 1969, 116, 173. [Google Scholar] [CrossRef]
- Leckie, H.P.; Uhlig, H.H. Environmental Factors Affecting the Critical Potential for Pitting in 18–8 Stainless Steel. J. Electrochem. Soc. 1975, 113, 1262. [Google Scholar] [CrossRef]
- Jones, R.L. Nitrate Effects Promoting the Stress Corrosion Cracking of AISI Type 304 Stainless Steel in MgCl2 Above 200 C. Corrosion 1975, 31, 431–434. [Google Scholar] [CrossRef]
- Illevbare, G.O.; King, K.J.; Gordon, S.R.; Elayat, H.A.; Gdowski, G.E.; Summers, T.S.E. Effect of nitrate on the repassivation potential of alloy 22 in chloride containing environments. In Proceedings of the 206th Meeting of The Electrochemical Society, Honolulu, HI, USA, 3–8 October 2004. [Google Scholar]
- Refaey, S.A.M.; El-Rehim, S.S.A.; Taha, F.; Saleh, M.B.; Ahmed, R.A. Inhibition of chloride localized corrosion of mild steel by PO43−, CrO42−, MoO42−, and NO2− anions. Appl. Surf. Sci. 2000, 158, 190. [Google Scholar] [CrossRef]
- Bardwell, J.A.; Sproule, G.I.; Mitchell, D.F.; Macdougall, B.; Graham, M.J. Nature of the passive film on Fe–Cr alloys as studied by 18O secondary ion mass spectrometry: Reduction of the prior film and stability to ex situ surface analysis. J. Chem. Soc. Faraday Trans. 1991, 87, 1011. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Siew, W.H. Stress corrosion cracking of an austenitic stainless steel in nitrite-containing chloride solutions. Materials 2014, 7, 7799–7808. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Taiwade, R.V. Effect of welding processes and conditions on the microstructure, mechanical properties and corrosion resistance of duplex stainless steel weldments—A review. J. Manuf. Process. 2017, 25, 134–152. [Google Scholar] [CrossRef]
- Baeslack, W.A.; Savage, W.F.; Duquett, D.J. The Effect of Strain Rate on Stress Corrosion Cracking in Duplex Type 304 Stainless Steel Weld Metal. Metall. Trans. 1979, 10A, 1429. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Siew, W.H. Role of nitrite addition in chloride stress corrosion cracking of a super duplex stainless steel. Corros. Sci. 2010, 52, 113–117. [Google Scholar] [CrossRef]





| Test Alloys | C | Cr | Ni | Mn | Mo | N | Cu | P | Si | Al | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAF2507 | 0.02 | 24.6 | 6.52 | 0.83 | 3.8 | 0.27 | 0.13 | 0.02 | 0.38 | <0.001 | <0.01 | Balance |
| Typical composition of SAF2505 | 0.03 | 24.00–26.00 | 6.00–8.00 | 1.20 | 3.00–5.00 | 0.24–0.32 | - | 0.035 | 0.8 | - | 0.02 | Balance |
| Incoloy 825 | 0.01 | 24.1 | 39.3 | 0.77 | 3.0 | <0.001 | 1.6 | <0.01 | 0.24 | 0.04 | <0.01 | Balance |
| Typical composition of Incoloy 825 | 0.05 | 19.50–23.50 | 38.00–46.00 | 1.00 | 2.50–3.50 | - | 1.50–3.00 | - | 0.5 | 0.2 | 0.03 | Balance |
| 316 L | 0.02 | 16.6 | 10.0 | 1.69 | 2.1 | 0.04 | 0.39 | 0.03 | 0.39 | <0.001 | 0.01 | Balance |
| Typical composition of 316L | 0.03 | 16.00–18.00 | 10.00–14.00 | 2.00 | 2.00–3.00 | 0.10 | - | 0.045 | 0.75 | - | 0.03 | Balance |
| Material | Ni | Cr | Mo | C | Cu | N | Mn | Ti | S | Si | P | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2507 | 9.5 | 25 | 4 | 0.02 | - | 0.25 | 0.4 | - | - | 0.35 | - | Bal |
| Inconel 625 | 58 (Ni+Co) | 20–23 | 8–10 | 0.1 | 0.5 | - | 0.5 | 0.4 | 0.015 | 0.5 (+0.4 Al) | - | Bal |
| 316L | 11–14 | 18–20 | 2–3 | 0.03 | - | - | 1–2.5 | - | 0.03 | - | 0.03 | Bal |
| Materials | Etchant | Voltage (V) | Current (A) | Time (s) | Observations |
|---|---|---|---|---|---|
| SAF 2507 | 10% (wt.) oxalic acid | 20 | 5 | 100 | Satisfactory microstructure |
| Incoloy 825 | 10% (wt.) oxalic acid | 15 | 5 | 20 | Over-etched, grain boundaries depleted |
| SS 316L | 10% (wt.) oxalic acid | 20 | 5 | 100 | Satisfactory microstructure |
| SAF 2507 | 10 g chromic acid in 100 mL water | 10 | 5 | 30 | Satisfactory microstructure |
| Incoloy 825 | 10 g chromic acid in 100 mL water | 10 | 5 | 30 | Satisfactory microstructure |
| SS 316L | 10 g chromic acid in 100 mL water | 6 | 5 | 50 | Satisfactory microstructure |
| Chloride:Nitrite Ratio | Nitrite Concentration (ppm) | Mass of NaNO2 Required (g) per 200 mL 6% FeCl3 * |
|---|---|---|
| 10:1 | 3963 | 1.18 |
| 6.667:1 | 5903 | 1.77 |
| 5:1 | 7872 | 2.36 |
| Elements | Cr | Mo | N | PREN |
|---|---|---|---|---|
| SAF 2507 | 24.78 | 3.78 | 0.27 | 41.6 |
| Incoloy 825 | 22.45 | 3.22 | <0.001 | 33.1 |
| 316L | 16.52 | 3.22 | 0.04 | 27.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh Raman, R.K.; Siew, W.H. Microstructures and Corrosion/Localised Corrosion of Stainless Steels, Incoloy and Their Weldments in Nitrite-Containing Chloride Environments. Materials 2024, 17, 1336. https://doi.org/10.3390/ma17061336
Singh Raman RK, Siew WH. Microstructures and Corrosion/Localised Corrosion of Stainless Steels, Incoloy and Their Weldments in Nitrite-Containing Chloride Environments. Materials. 2024; 17(6):1336. https://doi.org/10.3390/ma17061336
Chicago/Turabian StyleSingh Raman, R. K., and W. H. Siew. 2024. "Microstructures and Corrosion/Localised Corrosion of Stainless Steels, Incoloy and Their Weldments in Nitrite-Containing Chloride Environments" Materials 17, no. 6: 1336. https://doi.org/10.3390/ma17061336





