Thermal Transitions and Structural Characteristics of Poly(3,4-ethylenedioxythiophene/cucurbit[7]uril) Polypseudorotaxane and Polyrotaxane Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.2.1. Optical and Thermal Characterization
2.2.2. Structural Characterization
2.3. Synthesis and Characterization of PEDOT, PEDOT∙CB7-PS, and PEDOT∙CB7-PR
3. Results
3.1. Thin Film Preparation
3.2. Optical Properties
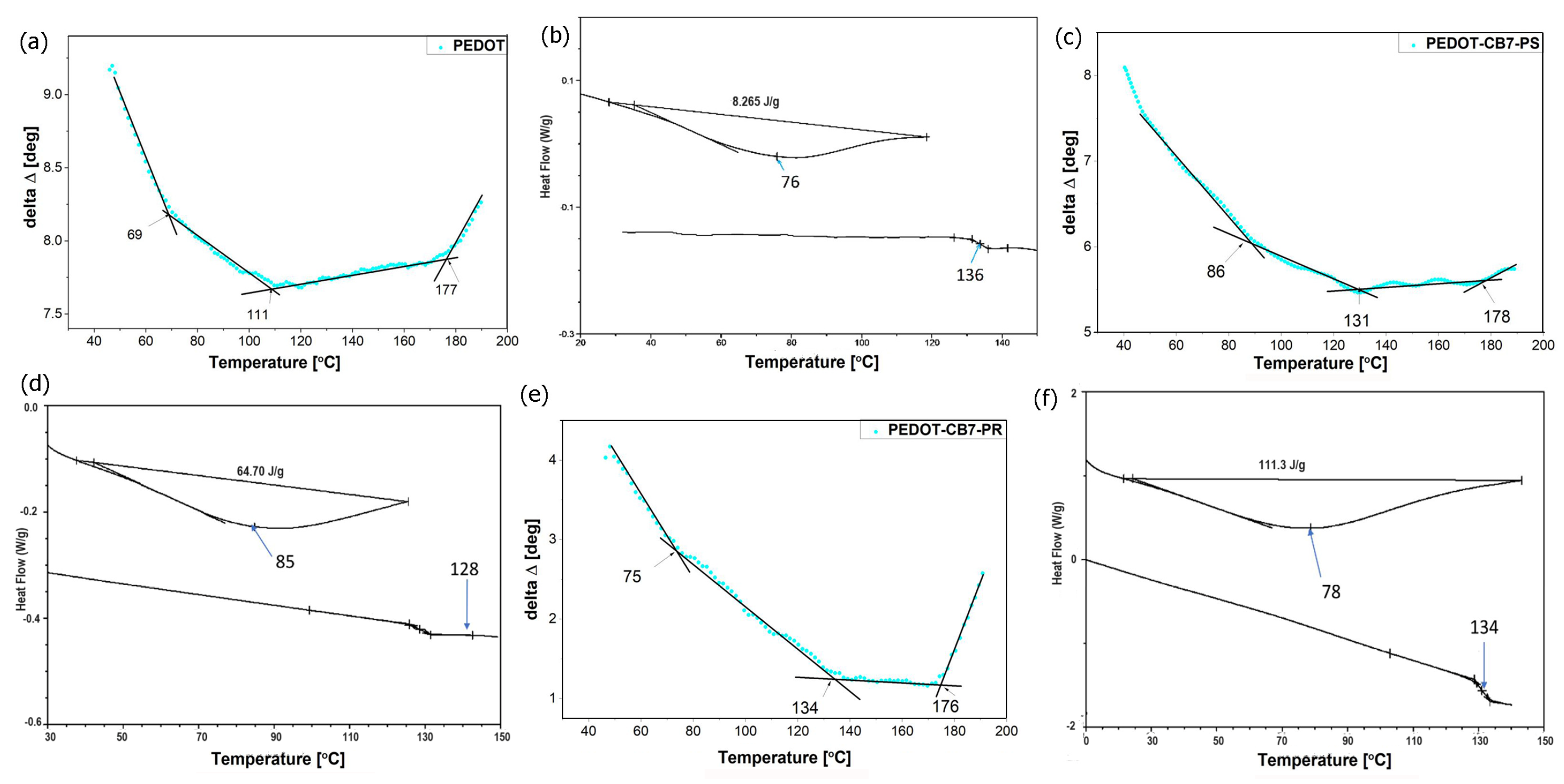
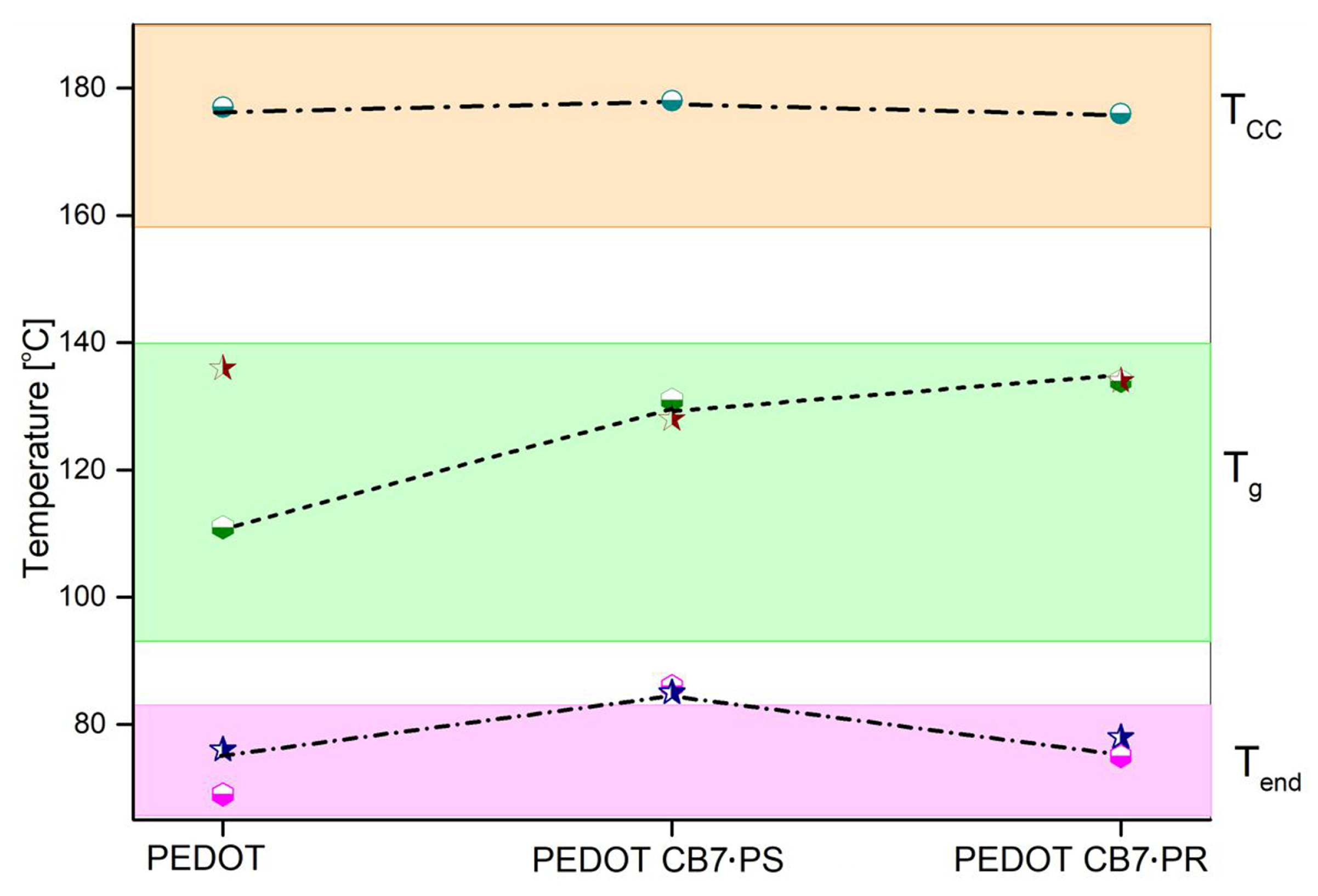
3.3. Thermal Analysis
3.4. XRD Structural Analysis
3.5. Surface Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT-PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef]
- Kang, Q.; Liao, Q.; Yang, C.; Yang, Y.; Xu, B.; Hou, J. A new PEDOT derivative for efficient organic solar cell with a fill factor of 0.80. Adv. Energy Mater. 2022, 12, 2103892. [Google Scholar] [CrossRef]
- Erazo, E.A.; Ortiz, P.; Cortés, M.T. Tailoring the PEDOT:PSS hole transport layer by electrodeposition method to improve perovskite solar cells. Electrochim. Acta 2023, 439, 141573. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Abdur, R.; Sheikh, A.K.; Singh, S.; Lee, J. Conductive MoO3–PEDOT:PSS composite loayer in MoO3/Au/MoO3–PEDOT:PSS multilayer electrode in ITO-free organic solar cells. Processes 2023, 11, 594. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Massonnet, N.; Yvenou, E.; Brenet, S.; Faure-Vincent, J.; Pouget, S.; Rieutord, F.; Okuno, H.; Benayad, A.; et al. Structure and dopant engineering in PEDOT thin films: Practical tools for a dramatic conductivity enhancement. Chem. Mater. 2016, 28, 3462–3468. [Google Scholar] [CrossRef]
- Stöcker, T.; Köhler, A.; Moos, R. Why does the electrical conductivity in PEDOT:PSS decrease with PSS content? A study combining thermoelectric measurements with impedance spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 976–983. [Google Scholar] [CrossRef]
- Shahrim, N.A.; Ahmad, Z.; Azman, A.W.; Buys, Y.F.; Sarifuddin, N. Mechanisms for doped PEDOT:PSS electrical conductivity improvement. Mater. Adv. 2021, 2, 7118–7138. [Google Scholar] [CrossRef]
- Lu, N.; Li, L.; Liu, M. Universal carrier thermoelectric-transport model based on percolation theory in organic semiconductors. Phys. Rev. B 2015, 91, 195205. [Google Scholar] [CrossRef]
- Li, L.; Lu, N.; Liu, M.; Bässler, H. General Einstein relation model in disordered organic semiconductors under quasiequilibrium. Phys. Rev. B 2014, 90, 214107. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Yu, L.; Liu, F.; Niu, J.; Yang, G.; Lu, C.; Lu, N.; Li, L.; Liu, M. Collective transport for nonlinear current-voltage characteristics of doped conducting polymer. Phys. Rev. Lett. 2023, 130, 177001. [Google Scholar] [CrossRef]
- Gutierrez-Fernandez, E.; Ezquerra, T.A.; García-Gutiérrez, M.-C. Additive effect on the structure of PEDOT:PSS dispersions and its correlation with the structure and morphology of thin films. Polymers 2022, 14, 141. [Google Scholar] [CrossRef]
- Carter, J.L.; Kelly, C.A.; Jenkins, M.J. Processing optimization of PEDOT:PSS and PEDOT:PSS/Tween 80 films. Polym. J. 2022, 55, 253–260. [Google Scholar] [CrossRef]
- Huang, J.; Miller, P.; de Mello, J.; de Mello, A.; Bradley, D. Influence of thermal treatment on the conductivity and morphology of PEDOT/PSS films. Synth. Met. 2003, 139, 569–572. [Google Scholar] [CrossRef]
- Dauzon, E.; Mansour, A.E.; Niazi, M.R.; Munir, R.; Smilgies, D.-M.; Sallenave, X.; Plesse, C.; Goubard, F.; Amassian, A. Conducting and stretchable PEDOT:PSS electrodes: Role of additives on self-assembly, morphology, and transport. ACS Appl. Mater. Interfaces 2019, 11, 17570–17582. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT:PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Song, J.; Ma, G.; Qin, F.; Hu, L.; Luo, B.; Liu, T.; Yin, X.; Su, Z.; Zeng, Z.; Jiang, Y.; et al. High-conductivity, flexible and transparent PEDOT:PSS electrodes for high performance semi-transparent supercapacitors. Polymers 2020, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, H.; Hajduk, B.; Jurusik, J.; Jarząbek, B.; Domański, M.; Łaba, K.; Wanic, A.; Łapkowski, M. The influence of PEDOT to PSS ratio on the optical properties of PEDOT:PSS thin solid films—Insight from spectroscopic ellipsometry. Acta Phys. Pol. A 2016, 130, 1242–1244. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.-P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Farcas, A.; Resmerita, A.-M. Supramolecular chemistry: Synthesis and photophysical properties of conjugated polyrotaxanes. In Encyclopedia of Physical Organic Chemistry; Wang, Z., Ed.; John Wiley& Sons: Hoboken, NJ, USA, 2017; Volume 3, pp. 1–39. [Google Scholar] [CrossRef]
- Farcas, A.; Ouldali, H.; Cojocaru, C.; Pastoriza-Gallego, M.; Resmerita, A.-M.; Oukhaled, A. Structural characteristics and the label-free detection of poly(3,4-ethylenedioxythiophene/cucurbit[7]uril) pseudorotaxane at single molecule level. Nano Res. 2023, 16, 2728–2737. [Google Scholar] [CrossRef]
- Farcas, A.; Assaf, K.I.; Resmerita, A.; Sacarescu, L.; Asandulesa, M.; Aubert, P.; Nau, W.M. Cucurbit[7]uril-threaded poly(3,4-ethylenedioxythiophene): A novel processable conjugated polyrotaxane. Eur. J. Org. Chem. 2019, 21, 3442–3450. [Google Scholar] [CrossRef]
- Bednarski, H.; Hajduk, B.; Jarka, P.; Kumari, P. Temperature coefficient of electronic polarizability in thin polymer films deposited on Si and SiO2 substrates determined via spectroscopic ellipsometry. Coatings 2024, 14, 166. [Google Scholar] [CrossRef]
- Hajduk, B.; Bednarski, H.; Jarząbek, B.; Nitschke, P.; Janeczek, H. Phase diagram of P3HT:PC70BM thin films based on variable-temperature spectroscopic ellipsometry. Polym. Test. 2020, 84, 106383. [Google Scholar] [CrossRef]
- Wang, T.; Pearson, A.J.; Dunbar, A.D.F.; Staniec, P.A.; Watters, D.C.; Coles, D.; Yi, H.; Iraqi, A.; Lidzey, D.G.; Jones, R.A.L. Competition between substrate-mediated π-π stacking and surface-mediated Tg depression in ultrathin conjugated polymer films. Eur. Phys. J. E 2012, 35, 129. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.; Zin, W.-C. Estimation of the thickness dependence of the glass transition temperature in various thin polymer films. Langmuir 2000, 16, 4064–4067. [Google Scholar] [CrossRef]
- Keddie, J.L.; Jones, R.A.; Cory, R.A. Size-dependent depression of the glass transition temperature in polymer films, Europhys. Eur. Lett. 1994, 27, 59–64. [Google Scholar] [CrossRef]
- Yamamoto, S.; Tsujii, Y.; Fukuda, T. Glass transition temperatures of high-density poly(methyl methacrylate) brushes. Macromolecules 2002, 35, 6077–6079. [Google Scholar] [CrossRef]
- Hénot, M.; Chennevière, A.; Drockenmuller, E.; Shull, K.; Léger, L.; Restagno, F. Influence of grafting on the glass transition temperature of PS thin films. Eur. Phys. J. E Soft Matter Biol. Phys. 2017, 40, 11. [Google Scholar] [CrossRef]
- Müller, C.; Andersson, L.M.; Peña-Rodríguez, O.; Garriga, M.; Inganäs, O.; Campoy-Quiles, M. Determination of thermaltransition depth profiles in polymer semiconductor films with ellipsometry. Macromolecules 2013, 46, 7325–7331. [Google Scholar] [CrossRef]
- Chandran, S.; Basu, J.K. Effect of nanoparticle dispersion on glass transition in thin films of polymer nanocomposites. Eur. Phys. J. E 2011, 34, 99. [Google Scholar] [CrossRef]
- El Ouakili, A.; Vignaud, G.; Balnois, E.; Bardeau, J.-F.; Grohens, Y. Multiple glass transition temperatures of polymer thin films as probed by multi-wavelength ellipsometry. Thin Solid Films 2011, 519, 2031–2036. [Google Scholar] [CrossRef]
- Geng, K.; Tsui, O.K.C. Effects of polymer cacticity and molecular weight on the glass transition temperature of poly(methyl methacrylate) films on silica. Macromolecules 2016, 49, 2671–2678. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Z.; Lan, Y.; Zuo, B.; Wang, X.; Yang, J.; Zhang, W.; Hu, W. Mobility gradient of poly(ethylene terephthalate) chains near a substrate scaled by the thickness of the adsorbed layer. Macromolecules 2017, 50, 6804–6812. [Google Scholar] [CrossRef]
- El Ouakili, A.; Vignaud, G.; Balnois, E.; Bardeau, J.-F.; Grohens, Y. Glass transition temperatures of isotactic poly(methymethacrylate) thin films and individual chains probed by multi wavelength ellipsometry. Eur. Phys. J. Appl. Phys. 2011, 56, 13703–13707. [Google Scholar] [CrossRef]
- Müller, C. On the glass transition of polymer semiconductors and its impact on polymer solar cell stability. Chem. Mater. 2015, 27, 2740–2754. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Etchegoin, P.; Bradley, D. Exploring the potential of ellipsometry for the characterisation of electronic, optical, morphologic and thermodynamic properties of polyfluorene thin films. Synth. Met. 2005, 155, 279–282. [Google Scholar] [CrossRef]
- Müller, C.; Bergqvist, J.; Vandewal, K.; Tvingstedt, K.; Anselmo, A.S.; Magnusson, R.; Alonso, M.I.; Moons, E.; Arwin, H.; Campoy-Quiles, M.; et al. Phase behaviour of liquid-crystalline polymer/fullerene organic photovoltaic blends: Thermal stability and miscibility. J. Mater. Chem. 2011, 21, 10676–10684. [Google Scholar] [CrossRef]
- Grohens, Y.; Brogly, M.; Labbe, C.; David, M.-O.; Schultz, J. Glass transition of stereoregular poly(methyl methacrylate) at interfaces. Langmuir 1998, 14, 2929–2932. [Google Scholar] [CrossRef]
- Clough, A.; Peng, D.; Yang, Z.; Tsui, O.K.C. Glass transition temperature of polymer films that slip. Macromolecules 2011, 44, 1649–1653. [Google Scholar] [CrossRef]
- Chandran, S.; Basu, J.K.; Mukhopadhyay, M.K. Variation in glass transition temperature of polymer nanocomposite films driven by morphological transitions. J. Chem. Phys. 2013, 138, 014902. [Google Scholar] [CrossRef]
- Bittrich, E.; Windrich, F.; Martens, D.; Bittrich, L.; Häussler, L.; Eichhorn, K.-J. Determination of the glass transition temperature in thin polymeric films used for microelectronic packaging by temperature-dependent spectroscopic ellipsometry. Polym. Test. 2017, 64, 48–54. [Google Scholar] [CrossRef]
- Christian, P.; Coclite, A.M. Vapor-phase-synthesized fluoroacrylate polymer thin films: Thermal stability and structural properties. Beilstein J. Nanotechnol. 2017, 8, 933–942. [Google Scholar] [CrossRef]
- Richter, U. Spectra Ray/3 Software Manual; Sentech Instruments GmbH: Berlin, Germany, 2011. [Google Scholar]
- Leng, J.; Opsal, J.; Chu, H.; Senko, M.; Aspnes, D. Analytic representations of the dielectric functions of materials for device and structural modeling. Thin Solid Films 1998, 313–314, 132–136. [Google Scholar] [CrossRef]
- Farcas, A.; Damoc, M.; Asandulesa, M.; Aubert, P.-H.; Tigoianu, R.I.; Ursu, E.L. The straightforward approach of tuning the photoluminescence and electrical properties of encapsulated PEDOT end-capped by pyrene. J. Mol. Liq. 2023, 376, 121461. [Google Scholar] [CrossRef]
- Henry, R.; Balar, N.; Ade, H. In-situ ellipsometry for the determination of thermal transitions and relaxations in organic photovoltaic materials. Chem. Mater. 2023, 35, 7406–7421. [Google Scholar] [CrossRef]
- Nakano, T. Synthesis, structure and function of π-stacked polymers. Polym. J. 2010, 42, 103–123. [Google Scholar] [CrossRef]
- Lai, Y.-Y.; Huang, V.-H.; Lee, H.-T.; Yang, H.-R. Stacking principles on π- and lamellar stacking for organic semiconductors evaluated by energy decomposition analysis. ACS Omega 2018, 3, 18656–18662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhou, X.; Xu, X.; Musumeci, C.; Wang, C.; Xu, W.; Meng, X.; Ma, W.; Inganäs, O. π–π Stacking distance and phase separation controlled efficiency in stable all-polymer solar cells. Polymers 2019, 11, 1665. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhu, S.; Liu, Z.; Jiang, C.; Wu, Y.; Li, H.; Wang, B.; Wang, E.; Ma, J.; Wang, C. Tailoring pi-conjugated systems: From π-π stacking to high-rate-performance organic cathodes. Chem 2018, 4, 2600–2614. [Google Scholar] [CrossRef]
- Stewart, J.J.P. MOPAC2016 Stewart Computational Chemistry; Colorado Springs, CO, USA, 2016; Available online: http://openmopac.net/index.html (accessed on 20 February 2024).
- Korth, M. Third-Generation Hydrogen-Bonding Corrections for Semiempirical QM Methods and Force Fields. J. Chem. Theory Comput. 2010, 6, 3808–3816. [Google Scholar] [CrossRef]
- Aasmundtveit, K.; Samuelsen, E.; Pettersson, L.; Inganäs, O.; Johansson, T.; Feidenhans’l, R. Structure of thin films of poly(3,4-ethylenedioxythiophene). Synth. Met. 1999, 101, 561–564. [Google Scholar] [CrossRef]
- Borghesi, A.; Tallarida, G.; Amore, G.; Cazzaniga, F.; Queirolo, G.; Alessandri, M.; Sassella, A. Influence of roughness and grain dimension on the optical functions of polycrystalline silicon films. Thin Solid Films 1998, 313–314, 243–247. [Google Scholar] [CrossRef]

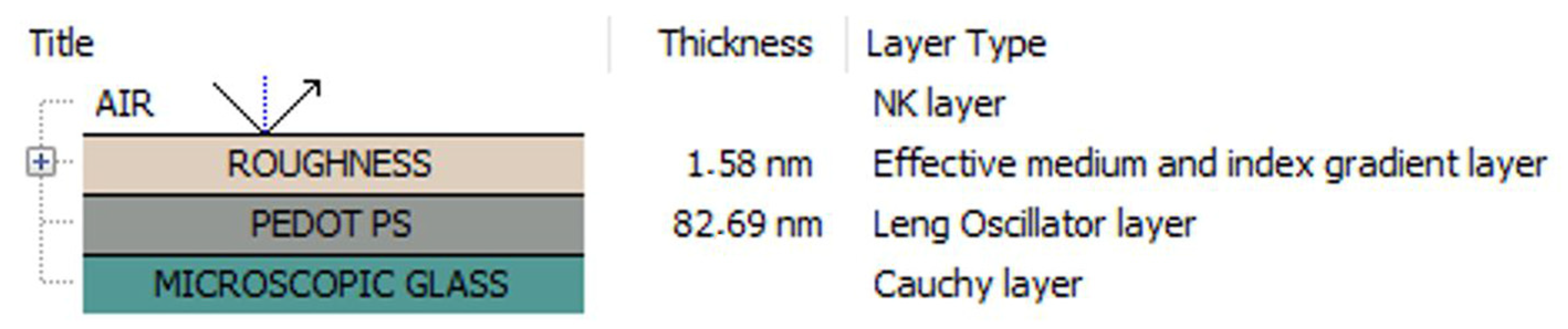
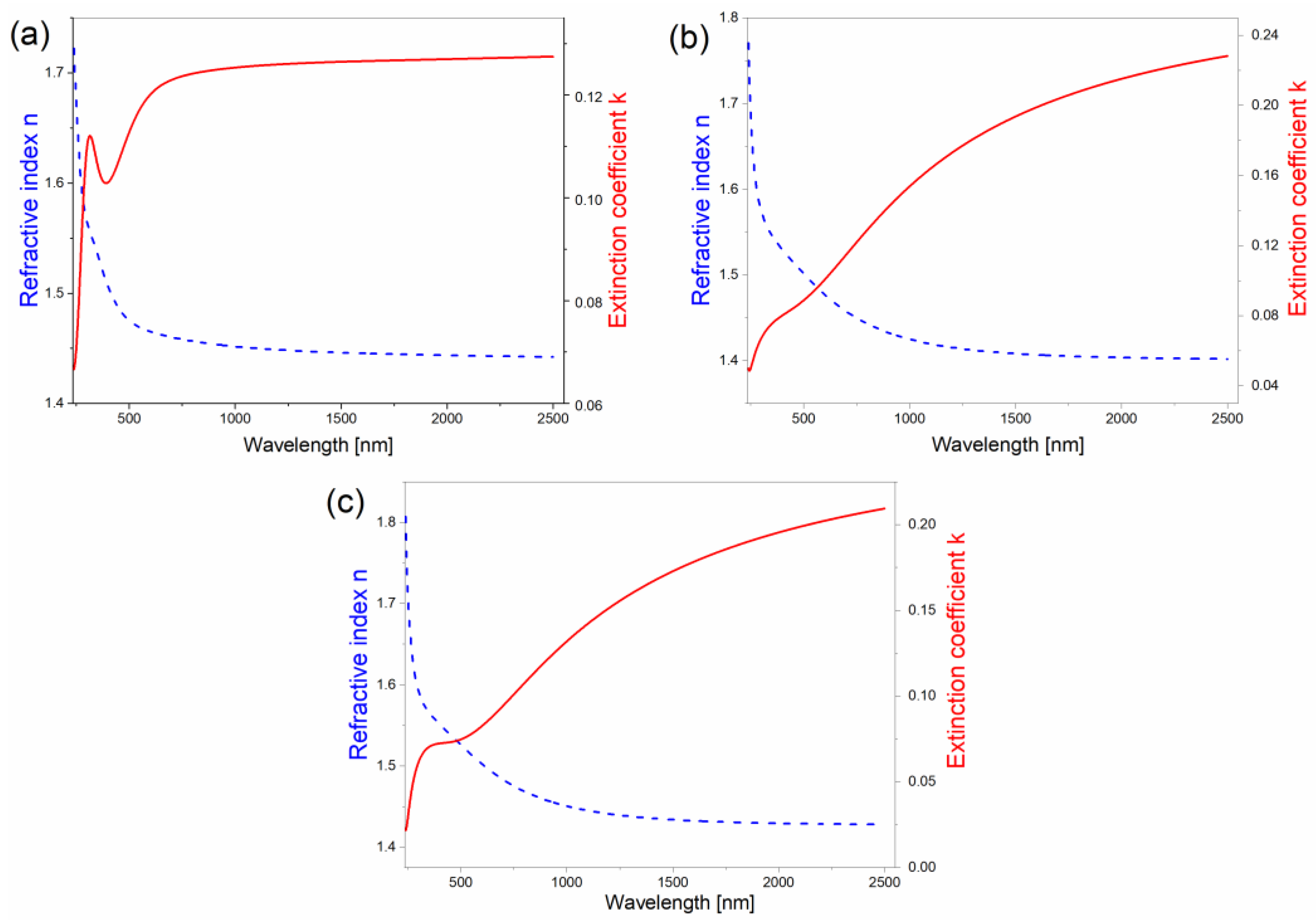


| PEDOT | PEDOT∙CB7-PS | PEDOT∙CB7-PR | |
|---|---|---|---|
| refractive index n | 1.44 | 1.40 | 1.43 |
| extinction coefficient k | 0.13 | 0.23 | 0.21 |
| PEDOT | PEDOT∙CB7-PS | PEDOT∙CB7-PR | |
|---|---|---|---|
| Roughness [nm] | 4.2 | 1.58 | 5.0 |
| Thickness [nm] | 159.4 | 82.7 | 30.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajduk, B.; Jarka, P.; Bednarski, H.; Janeczek, H.; Kumari, P.; Farcas, A. Thermal Transitions and Structural Characteristics of Poly(3,4-ethylenedioxythiophene/cucurbit[7]uril) Polypseudorotaxane and Polyrotaxane Thin Films. Materials 2024, 17, 1318. https://doi.org/10.3390/ma17061318
Hajduk B, Jarka P, Bednarski H, Janeczek H, Kumari P, Farcas A. Thermal Transitions and Structural Characteristics of Poly(3,4-ethylenedioxythiophene/cucurbit[7]uril) Polypseudorotaxane and Polyrotaxane Thin Films. Materials. 2024; 17(6):1318. https://doi.org/10.3390/ma17061318
Chicago/Turabian StyleHajduk, Barbara, Paweł Jarka, Henryk Bednarski, Henryk Janeczek, Pallavi Kumari, and Aurica Farcas. 2024. "Thermal Transitions and Structural Characteristics of Poly(3,4-ethylenedioxythiophene/cucurbit[7]uril) Polypseudorotaxane and Polyrotaxane Thin Films" Materials 17, no. 6: 1318. https://doi.org/10.3390/ma17061318





