Advances in the Applications of Clinoptilolite-Rich Tuffs
Abstract
:1. Introduction
2. Conversion of ZT
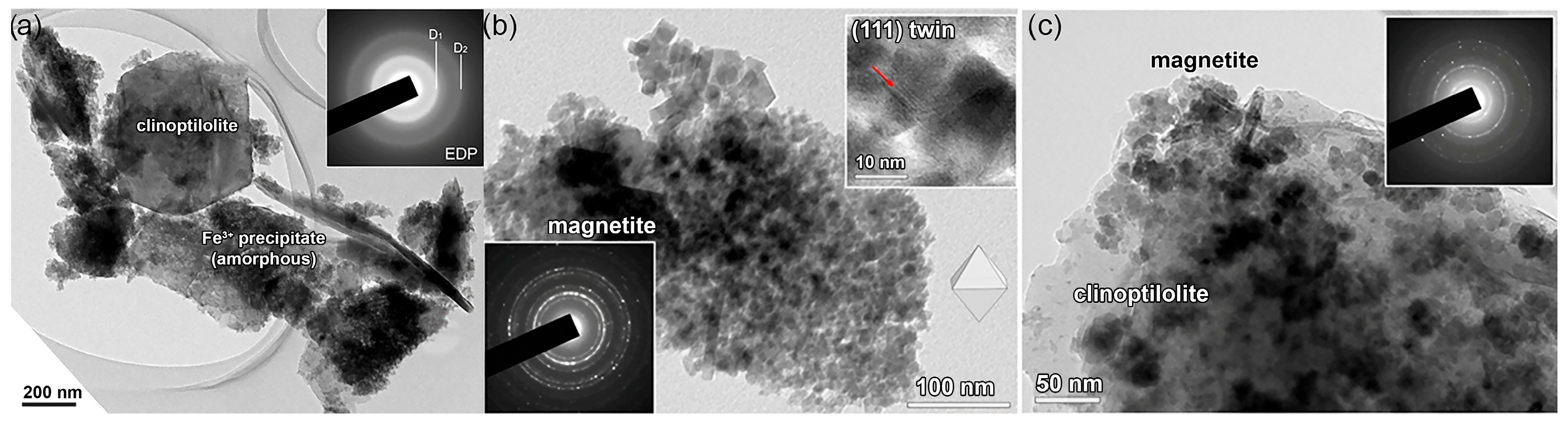
2.1. Conversion of ZT to Fe-Containing ZTs
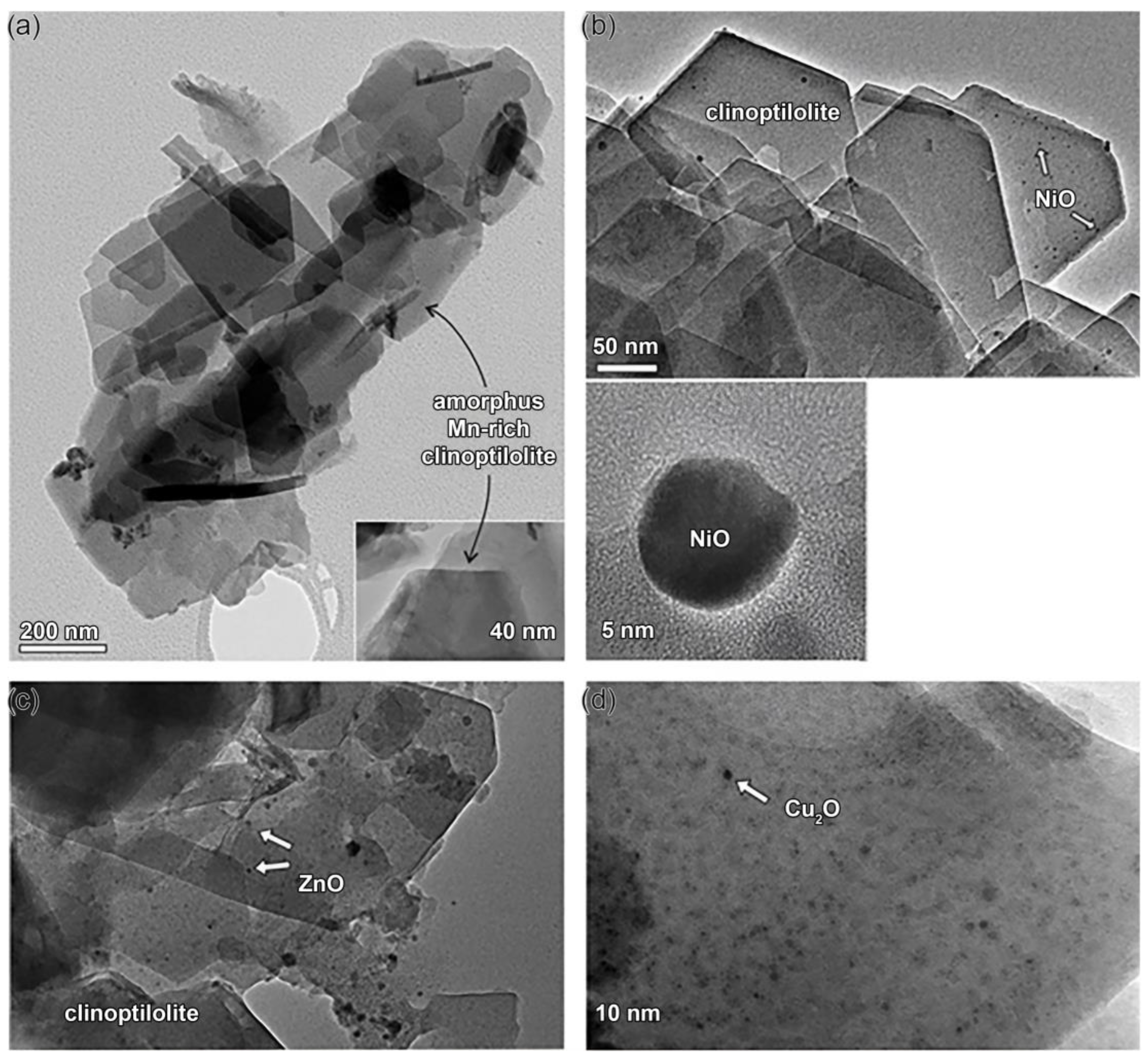
2.2. Formation of Ultrafine Nano Oxide Particles inside ZT
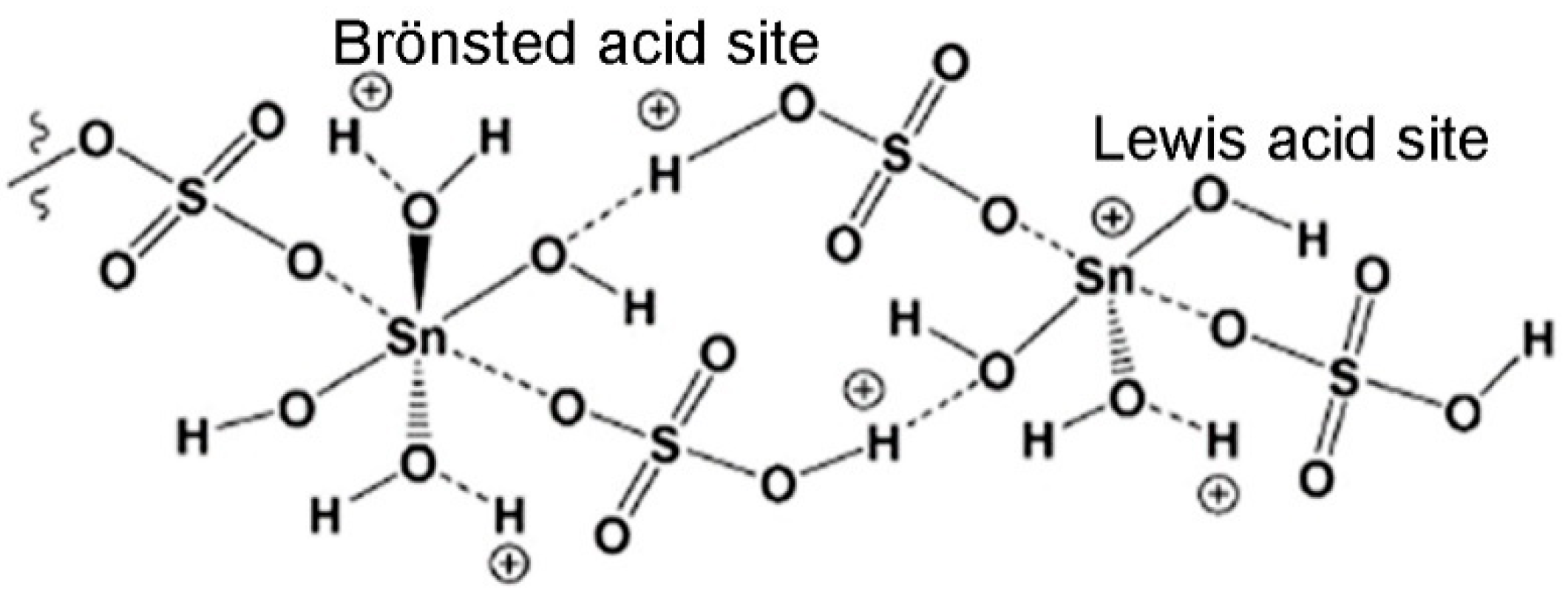
2.3. Conversion of ZT into SnO2-ZT and SO4-SnO2-ZT
3. Adsorption Studies
3.1. M(II) Adsorption (M–Mg, Mn, Ni, Cu, Zn, Pb) onto Na-ZT
3.2. Adsorption of Nitrate and Phosphate onto Fe-ZT
3.3. Adsorption of Ciprofloxacin onto Fe3O4-ZT
3.4. Adsorption of Atenolol, Acetylsalicylic Acid, and Salicylic Acid onto M-ZT
4. Use of ZT in Agriculture
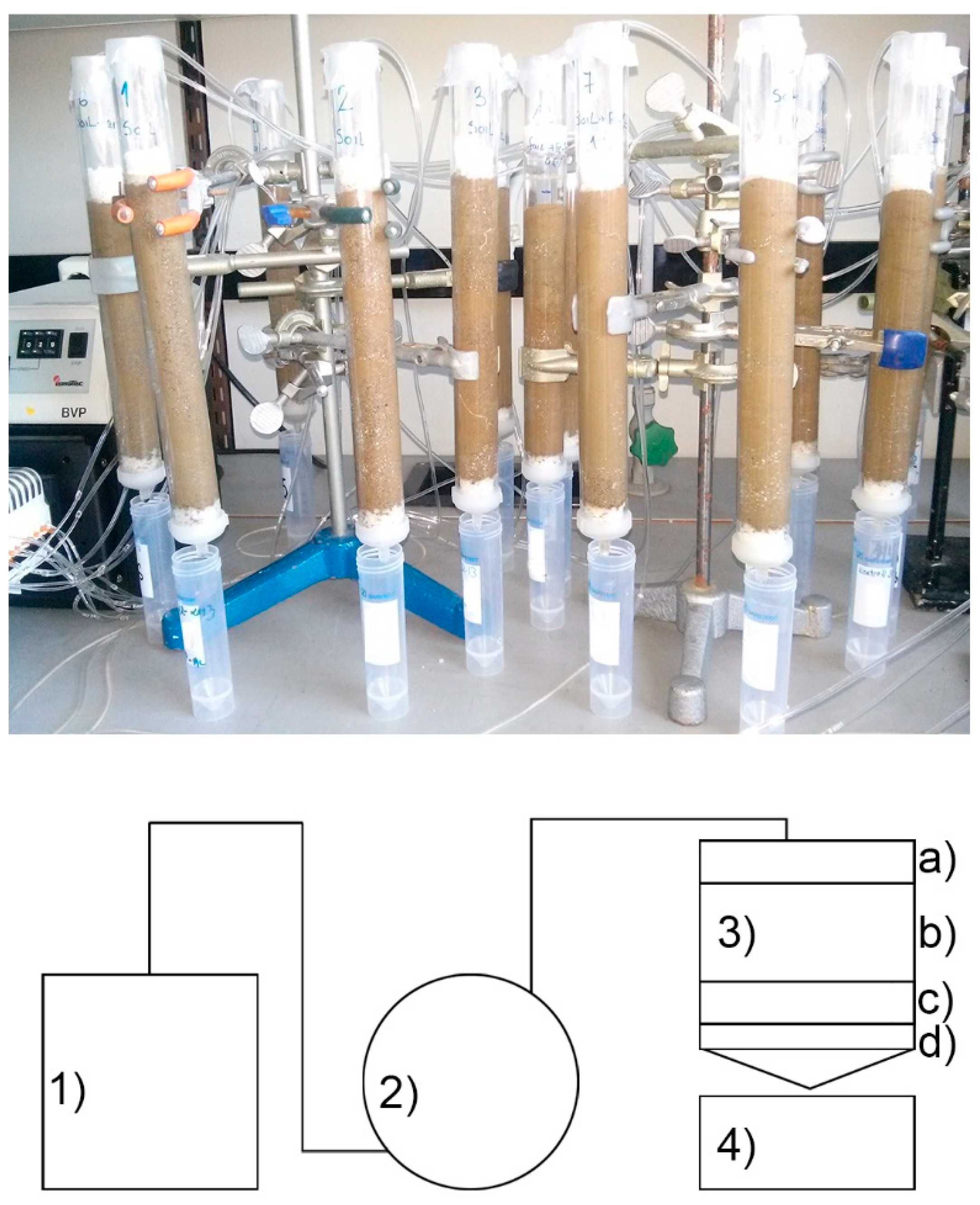
4.1. Use of ZT and Fe-ZT as Soil Supplements
4.2. Use of the Se-Containing Fe-ZT for the Growth of Pleurotus Ostreatus
4.3. Catalytic Use of ZT
Photocatalytic Activity
4.4. Interactions of Bacteria and ZT
4.4.1. Possible Applications of Bacterial Immobilization onto ZT
4.4.2. Interactions of Bacteria and M–ZT
4.4.3. Other Possible Applications of M-ZT
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yilmaz, B.; Müller, U. Catalytic applications of zeolites in chemical industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef]
- Garcia-Basabe, Y.; Rodriguez-Iznaga, I.; de Menorval, L.-C.; Llewellyn, P.; Maurin, G.; Lewis, D.W.; Binions, R.; Autie, M.; Ruiz-Salvador, A.R. Step-wise dealumination of natural clinoptilolite: Structural and physicochemical characterization. Microporous Mesoporous Mater. 2010, 135, 187–196. [Google Scholar] [CrossRef]
- Farías, T.; Ruiz-Salvador, A.R.; Velazco, L.; de Ménorval, L.C.; Rivera, A. Preparation of natural zeolitic supports for potential biomedical applications. Mater. Chem. Phys. 2009, 118, 322–328. [Google Scholar] [CrossRef]
- Godelitsas, A.; Armbruster, T. HEU-type zeolites modified by transition elements and lead. Microporous Mesoporous Mater. 2003, 61, 3–24. [Google Scholar] [CrossRef]
- Dziedzicka, A.; Sulikowski, B.; Ruggiero-Mikolajczyk, M. Catalytic and physicochemical properties of modified natural clinoptilolite. Catal. Today 2016, 259, 50–58. [Google Scholar] [CrossRef]
- Pabis-Mazgaj, E.; Gawenda, T.; Pichniarczyk, P.; Stempkowska, A. Mineral Composition and Structural Characterization of the Clinoptilolite Powders Obtained from Zeolite-Rich Tuffs. Minerals 2021, 11, 1030. [Google Scholar] [CrossRef]
- Hrenovic, J.; Rozic, M.; Sekovanic, L.; Anic-Vucinic, A. Interaction of surfactant-modified zeolites and phosphate accumulating bacteria. J. Hazard. Mater. 2008, 156, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Rajic, N.; Stojakovic, D.; Jevtic, S.; Zabukovec, N.L.; Kovac, J.; Kaucic, V. Removal of aqueous manganese using the natural zeolitic tuff from the Vranjska Banja deposit in Serbia. J. Hazard. Mater. 2009, 172, 1450–1457. [Google Scholar] [CrossRef]
- Rajic, N.; Stojakovic, D.; Daneu, N.; Recnik, A. The formation of oxide nanoparticles on the surface of natural clinoptilolite. J. Phys. Chem. Solids 2011, 72, 800–803. [Google Scholar] [CrossRef]
- Stojakovic, D.; Hrenovic, J.; Mazaj, M.; Rajic, N. On the zinc sorption by the Serbian natural clinoptilolite and the disinfecting ability and phosphate affinity of the exhausted sorbent. J. Hazard. Mater. 2011, 185, 408–415. [Google Scholar] [CrossRef]
- Stojakovic, D.; Milenkovic, J.; Daneu, N.; Rajic, N. A study of the removal of copper ions from aqueous solution using Clinoptilolite from Serbia. Clays Clay Miner. 2011, 59, 277–285. [Google Scholar] [CrossRef]
- Rajić, N.; Logar, N.Z.; Rečnik, A.; El-Roz, M.; Thibault-Starzyk, F.; Sprenger, P.; Hannevold, L.; Andersen, A.; Stöcker, M. Hardwood lignin pyrolysis in the presence of nano-oxide particles embedded onto natural clinoptilolite. Microporous Mesoporous Mater. 2013, 176, 162–167. [Google Scholar] [CrossRef]
- Pavlović, J.B.; Krogstad, T.; Rajić, N.Z. Applicability of zeolites in potassium and nitrate retention in different soil types. J. Serbian Chem. Soc. 2017, 82, 1303–1314. [Google Scholar] [CrossRef]
- Šuligoj, A.; Pavlović, J.; Arčon, I.; Rajić, N.; Tušar, N.N. SnO2-containing clinoptilolite as a composite photocatalyst for dyes removal from wastewater under solar light. Catalysts 2020, 10, 253. [Google Scholar] [CrossRef]
- Jevtić, S.; Arčon, I.; Rečnik, A.; Babić, B.; Mazaj, M.; Pavlović, J.; Matijaševic, D.; Nikšić, M.; Rajić, N. The iron(III)-modified natural zeolitic tuff as an adsorbent and carrier for selenium oxyanions. Microporous Mesoporous Mater. 2014, 197, 92–100. [Google Scholar] [CrossRef]
- Pavlović, J.; Šuligoj, A.; Opresnik, M.; Tušar, N.N.; Logar, N.Z.; Rajić, N. Studies of clinoptilolite-rich zeolitic tuffs from different regions and their activity in photodegradation of methylene blue. Catalysts 2022, 12, 224. [Google Scholar] [CrossRef]
- Kalebić, B.; Pavlović, J.; Dikić, J.; Rečnik, A.; Gyergyek, S.; Škoro, N.; Rajić, N. Use of natural clinoptilolite in the preparation of an efficient adsorbent for ciprofloxacin removal from aqueous media. Minerals 2021, 11, 518. [Google Scholar] [CrossRef]
- Pavlovic, J.; Popova, M.; Mihalyi, R.M.; Mazaj, M.; Mali, G.; Kovač, J.; Lazarova, H.; Rajic, N. Catalytic activity of SnO2- and SO4/SnO2-containing clinoptilolite in the esterification of levulinic acid. Microporous Mesoporous Mater. 2019, 279, 10–18. [Google Scholar] [CrossRef]
- Kaplanec, I.; Rečnik, A.; Mali, G.; Rajić, N. Study of the iron(III)-modified clinoptilolite in the adsorption of phosphate from aqueous medium: Mechanism and kinetics. Desalin. Water Treat. 2017, 78, 231–240. [Google Scholar] [CrossRef]
- Tomić, S.; Rajić, N.; Hrenović, J.; Povrenović, D. Removal of Mg from spring water using natural clinoptilolite. Clay Miner. 2012, 47, 81–92. [Google Scholar] [CrossRef]
- Tomic, S.; Knezevic, M.; Rajic, N.; Povrenovic, D. Removal of Magnesium in Spring Water Using the Natural Zeolite in a Continuous Flow System. Hem. Ind. 2014, 68, 475–482. [Google Scholar] [CrossRef]
- Jovanovic, M.; Rajic, N.; Obradovic, B. Novel kinetic model of the removal of divalent heavy metal ions from aqueous solutions by natural clinoptilolite. J. Hazard. Mater. 2012, 233–234, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stojakovic, D.; Milenkovic, J.; Stupar, S.; Velickovic, Z.; Rajic, N. Binary adsorption of nickel and zinc from aqueous solutions onto the Serbian natural clinoptilolite. Desalin. Water Treat. 2016, 57, 18748–18754. [Google Scholar] [CrossRef]
- Pavlović, J.B.; Milenković, J.K.; Rajić, N.Z. Modification of natural clinoptilolite for nitrate removal from aqueous media. J. Serbian Chem. Soc. 2014, 79, 1309–1322. [Google Scholar] [CrossRef]
- Kalebić, B.; Skoro, N.; Kovac, J.; Rajic, N. Regeneration of the ciprofloxacin-loaded clinoptilolite by non-thermal atmospheric plasma. Appl. Surf. Sci. 2022, 593, 153379. [Google Scholar] [CrossRef]
- Rakić, V.; Rajić, N.; Daković, A.; Auroux, A. The adsorption of salicylic acid, acetylsalicylic acid and atenolol from aqueous solutions onto natural zeolites and clays: Clinoptilolite, bentonite and kaolin. Microporous Mesoporous Mater. 2013, 166, 185–194. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T.; Tibljas, D. The effect of mineral carrier composition on phosphate-accumulating bacteria immobilization. J. Hazard. Mater. 2009, 166, 1377–1382. [Google Scholar] [CrossRef]
- Hrenović, J.; Tibljaš, D.; Dekić, S.; Rozman, T.I. Does bacterial surface hydrophobicity level influence their immobilization onto natural zeolite? In Proceedings of the 9th Croatian-Slovenian-Serbian Symposium on Zeolites, Split, Croatia, 23–25 September 2021. [Google Scholar]
- Hrenović, J.; Tibljaš, D.; Dekić, S.; Venter, C. Mode of Acinetobacter baumannii immobilization onto natural zeolite in nutrient-poor and nutrient-rich water. In Proceedings of the 8th Serbian-Croatian-Slovenian Symposium on Zeolites, Belgrade, Serbia, 3–5 October 2019. [Google Scholar]
- Hrenović, J.; Tibljaš, D.; Orhan, Y.; Büyükgüngör, H. Immobilisation of Acinetobacter calcoaceticus using natural carriers. Water SA 2005, 31, 261–266. [Google Scholar] [CrossRef]
- Hrenović, J.; Büyükgüngör, H.; Orhan, Y. Use of natural zeolite to upgrade activated sludge process. Food Technol. Biotechnol. 2003, 41, 157–165. [Google Scholar]
- Hrenovic, J.; Milenkovic, J.; Ivankovic, T.; Rajic, N. Antibacterial activity of heavy metal-loaded natural zeolite. J. Hazard. Mater. 2012, 201–202, 260–264. [Google Scholar] [CrossRef]
- Hrenovic, J.; Milenkovic, J.; Daneu, N.; Kepcija, R.M.; Rajic, N. Antimicrobial activity of metal oxide nanoparticles supported onto natural clinoptilolite. Chemosphere 2012, 88, 1103–1107. [Google Scholar] [CrossRef]
- Hrenovic, J.; Milenkovic, J.; Goic-Barisic, I.; Rajic, N. Antibacterial activity of modified natural clinoptilolite against clinical isolates of Acinetobacter baumannii. Microporous Mesoporous Mater. 2013, 169, 148–152. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Matijasevic, D.; Niksic, M.; Rajic, N. Bactericidal activity of Cu-, Zn-, and Ag-containing zeolites toward Escherichia coli isolates. Environ. Sci. Pollut. Res. 2017, 24, 20273–20281. [Google Scholar] [CrossRef] [PubMed]
- Hrenović, J.; Dekić, S.; Dikić, J.; Kazazić, S.; Durn, G.; Rajić, N. Metal-loaded zeolite remediation of soils contaminated with pandrug-resistant Acinetobacter baumannii. Arh. Hig. Rada Toksikol. 2020, 71, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Jevtić, S.; Grujić, S.; Hrenović, J.; Rajić, N. Surfactant-modified clinoptilolite as a salicylate carrier, salicylate kinetic release and its antibacterial activity. Microporous Mesoporous Mater. 2012, 159, 30–35. [Google Scholar] [CrossRef]
- Milenkovic, J.; Hrenovic, J.; Goic-Barisic, I.; Tomic, M.; Djonlagic, J.; Rajic, N. Synergistic anti-biofouling effect of Ag-exchanged zeolite and D-Tyrosine on PVC composite against the clinical isolate of Acinetobacter baumannii. Biofouling 2014, 30, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Korkuna, O.; Leboda, R.; Skubiszewska-Zięba, J.; Vrublevs’ka, T.; Ryczkowski, J. Structural and physicochemical properties of natural zeolites: Clinoptilolite and mordenite. Microporous Mesoporous Mater. 2006, 87, 243–254. [Google Scholar] [CrossRef]
- Šiljeg, M.; Cerjan Stefanović, Š.; Mazaj, M.; Novak Tušar, N.; Arčon, I.; Kovač, J.; Margeta, K.; Kaučić, V.; Zabukovec Logar, N. Structure investigation of As(III)- and As(V)-species bound to Fe-modified clinoptilolite tuffs. Microporous Mesoporous Mater. 2009, 118, 408–415. [Google Scholar] [CrossRef]
- Dávila-Jiménez, M.M.; Elizalde-González, M.P.; Mattusch, J.; Morgenstern, P.; Pérez-Cruz, M.A.; Reyes-Ortega, Y.; Wennrich, R.; Yee-Madeira, H. In situ and ex situ study of the enhanced modification with iron of clinoptilolite-rich zeolitic tuff for arsenic sorption from aqueous solutions. J. Colloid Interf. Sci. 2008, 322, 527–536. [Google Scholar] [CrossRef]
- Jiménez-Cedillo, M.J.; Olguína, M.T.; Fall, C. Adsorption kinetic of arsenates as water pollutant on iron, manganese and iron–manganese-modified clinoptilolite-rich tuffs. J. Hazard. Mater. 2009, 163, 939–945. [Google Scholar] [CrossRef]
- Sanaei, L.; Tahmasebpoor, M. Physical appearance and arsenate removal efficiency of Fe(III)-modified clinoptilolite beads affected by alginate-wet-granulation process parameters. Mater. Chem. Phys. 2021, 259, 124009. [Google Scholar] [CrossRef]
- Bilici Baskan, M.; Hadimlioglu, S. Removal of arsenate using graphene oxide-iron modified clinoptilolite-based composites: Adsorption kinetic and column study. J. Anal. Sci. Technol. 2021, 12, 22. [Google Scholar] [CrossRef]
- Jeon, C.S.; Baek, K.; Park, J.K.; Oh, Y.K.; Lee, S.D. Adsorption characteristics of As(V) on iron-coated zeolite. J. Hazard. Mater. 2009, 163, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Mohseni-Bandpi, A.; Al-Musawi, T.J.; Ghahramani, E.; Zarrabi, M.; Mohebi, S.; Vahed, S.A. Improvement of zeolite adsorption capacity for cephalexin by coating with magnetic Fe3O4 nanoparticles. J. Mol. Liq. 2016, 218, 615–624. [Google Scholar] [CrossRef]
- Mersin, G.; Açikel, Ü.; Levent, M. Efficient adsorption of Basic Blue 41 from textile wastewaters by natural and magnetically modified Manisa-Gördes clinoptilolite. Chem. Eng. Process 2021, 169, 108632. [Google Scholar] [CrossRef]
- Noori, M.; Maryam Tahmasebpoor, M.; Foroutan, R. Enhanced adsorption capacity of low-cost magnetic clinoptilolite powders/beads for the effective removal of methylene blue: Adsorption and desorption studies. Mater. Chem. Phys. 2022, 278, 125655. [Google Scholar] [CrossRef]
- Rouhani, M.; Ashrafi, S.D.; Taghavi, K.; Joubani, M.N.; Jaafari, J. Evaluation of tetracycline removal by adsorption method using magnetic iron oxide nanoparticles (Fe3O4) and clinoptilolite from aqueous solutions. J. Mol. Liq. 2022, 356, 119040. [Google Scholar] [CrossRef]
- Chmielewská, E.; Tylus, W.; Drábik, M.; Majzlan, J.; Kravčak, J.; Williams, C.; Čaplovičová, M.; Čaplovič, L. Structure investigation of nano-FeO(OH) modified clinoptilolite tuff for antimony removal. Microporous Mesoporous Mater. 2017, 248, 222–233. [Google Scholar] [CrossRef]
- Dalmaz, A.; Sivrikaya, S.Ö. Development of clinoptilolite zeolite-coated magnetic nanocomposite-based solid phase microextraction method for the determination of Rhodamine B in cosmetic products. J. Chromatogr. A 2022, 1680, 463433. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Shelyapina, M.G.; Petranovskii, V. Ion exchange in natural clinoptilolite: Aspects related to its structure and applications. Minerals 2022, 12, 1628. [Google Scholar] [CrossRef]
- Dimowa, L.T.; Petrov, O.E.; Djourelov, N.I.; Shivachev, B.L. Structural study of Zn-exchanged natural clinoptilolite using powder XRD and positron annihilation data. Clay Miner. 2015, 50, 41–54. [Google Scholar] [CrossRef]
- Dakdareh, A.M.; Falamaki, C.; Ghasemian, N. Hydrothermally grown nano-manganese oxide on clinoptilolite for low-temperature propane-selective catalytic reduction of NOx. J. Nanopart. Res. 2018, 20, 309. [Google Scholar] [CrossRef]
- Matsuhashi, H.; Miyazaki, H.; Kawamura, Y.; Nakamura, H.; Arata, K. Preparation of a solid superacid of sulfated tin oxide with acidity higher than that of sulfated zirconia and its applications to aldol aondensation and benzoylation. Chem. Mater. 2001, 13, 3038–3042. [Google Scholar] [CrossRef]
- Arata, K.; Matsuhashi, H.; Hino, M.; Nakamura, H. Synthesis of solid superacids and their activities for reactions of alkanes. Catal. Today 2003, 81, 17–30. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Jahani, F.; Sadeghi, R.; Shakeri, M. Ultrasonic-assisted chemical modification of a natural clinoptilolite zeolite: Enhanced ammonium adsorption rate and resistance to disturbing ions. J. Environ. Chem. Eng. 2023, 11, 110354. [Google Scholar] [CrossRef]
- Kragović, M.; Daković, A.; Sekulić, Ž.; Trgo, M.; Ugrina, M.; Perić, J.; Diego Gatta, G. Removal of lead from aqueous solutions by using the natural and Fe(III)-modified zeolite. Appl. Surf. Sci. 2012, 258, 3667–3673. [Google Scholar] [CrossRef]
- Ugrina, M.; Čeru, T.; Nuić, I.; Trgo, M. Comparative study of mercury(II) removal from aqueous solutions onto natural and iron-modified clinoptilolite rich zeolite. Processes 2020, 8, 1523. [Google Scholar] [CrossRef]
- Dimirkou, A. Uptake of Zn2+ ions by a fully iron-exchanged clinoptilolite. Case study of heavily contaminated drinking water samples. Water Res. 2007, 41, 2763–2773. [Google Scholar] [CrossRef]
- Velarde, L.; Sadegh, M.N.; Escalera, E.; Antii, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Vocciante, M.; De Folly D’Auris, A.; Finocchi, A.; Tagliabue, M.; Bellettato, M.; Ferrucci, A.; Reverberi, A.P.; Ferro, S. Adsorption of ammonium on clinoptilolite in presence of competing cations: Investigation on groundwater remediation. J. Clean. Prod. 2018, 198, 480–487. [Google Scholar] [CrossRef]
- Wasieleweski, S.; Rott, E.; Minke, R.; Steinmetz, H. Application of natural clinoptilolite for ammonium removal from sludge water. Molecules 2021, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Shibata, W.; Steff, K. Pb2+ exchange isotherms for zeolite Na-X at pH 5, 6, and 7. Zeolites 1997, 19, 87–89. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.H.; An, B.; Choi, S.; Park, J.H.; Jurng, J.S.; Lee, S.H.; Choi, J.W. Simultaneous removal of phosphate and nitrate in u high-capacity anion-exchange resin. Water Air Soil Pollut. 2012, 223, 5959–5966. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Devlin, M.; Brodie, J. Nutrients and eutrophication. In Marine Pollution—Monitoring, Management and Mitigation; Reichelt-Brushett, A., Ed.; Springer Textbooks in Earth Sciences, Geography and Environment; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Guan, H.; Bestland, E.; Zhu, C.; Zhu, H.; Albertsdottir, D.; Hutson, J.; Simmons, C.T.; Ginic-Markovic, M.; Tao, X.; Ellis, A.V. Variation in performance of surfactant loading and resulting nitrate removal among four selected natural zeolites. J. Hazard. Mater. 2010, 183, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Schick, J.; Caullet, P.; Paillaud, J.L.; Patarin, J.; Mangold-Callarec, C. Batch-wise nitrate removal from water on a surfactant-modified zeolite. Microporous Mesoporous Mater. 2010, 132, 39–400. [Google Scholar] [CrossRef]
- Schick, J.; Caullet, P.; Paillaud, J.L.; Patarin, J.; Mangold-Callarec, C. Nitrate sorption from water on a surfactant-modified zeolite. Fixed-bed column experiments. Microporous Mesoporous Mater. 2011, 142, 549–556. [Google Scholar] [CrossRef]
- Zhan, Y.; Lin, J.; Zhu, Z. Removal of nitrate from aqueous solution using cetylpyridinium bromide (CPB) modified zeolite as adsorbent. J. Hazard. Mater. 2011, 186, 1972–1978. [Google Scholar] [CrossRef]
- Reeve, P.; Fallowfield, H. The toxicity of cationic surfactant HDTMA-Br, desorbed from surfactant modified zeolite, towards faecal indicator and environmental microorganisms. J. Hazard. Mater. 2017, 339, 208–215. [Google Scholar] [CrossRef]
- Du, G.; Li, Z.; Liao, L.; Hanson, R.; Leick, S.; Hoeppner, N.; Jiang, W.T. Cr(VI) retention and transport through Fe(III)-coated natural zeolite. J. Hazard. Mater. 2012, 221–222, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gaval’ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Muhamad, N.; Majid, A. Enhancing nitrogen availability from urea using clinoptilolite zeolite. Geoderma 2017, 306, 152–159. [Google Scholar] [CrossRef]
- Souza, I.M.S.; Gurgel, G.C.S.; Medeiros, A.M.; Zonta, E.; Ruiz, J.A.C.; Paskocimas, C.A.; Motta, F.V.; Bomio, M.R.D. The use of clinoptilolite as carrier of nitrogened fertilizer with controlled release. J. Environ. Chem. Eng. 2018, 6, 4171–4177. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Kuzyakov, Y.; Lorenz, K.; Stahr, K. Remediation of a soil contaminated with heavy metals by immobilizing compounds. J. Plant Nutr. Soil Sci. 2006, 169, 205–212. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Hajabbasi, M.A.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 2007, 137, 388–393. [Google Scholar] [CrossRef]
- Yi, N.; Wu, Y.; Fan, L.; Hu, S. Remediating Cd-contaminated soils using natural and chitosan-introduced zeolite, bentonite, and activated carbon. Pol. J. Environ. Stud. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Colombani, N.; Mastrocicco, M.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Batch and column experiments on nutrient leaching in soils amended with Italian natural zeolitites. Catena 2015, 127, 64–71. [Google Scholar] [CrossRef]
- Moraetis, D.; Papagiannidou, S.; Pratikakis, A.; Pentari, D.; Komnitsas, K. Effect of zeolite application on potassium release in sandy soils amended with municipal compost. Desalin. Water Treat. 2015, 57, 13273–13284. [Google Scholar] [CrossRef]
- Eslami, M.; Khorassani, R.; Coltorti, M.; Malferrari, D.; Faccini, B.; Ferretti, G.; Di Giuseppe, D.; Fotovat, A.; Halajnia, A. Leaching behaviour of a sandy soil amended with natural and NH4+ and K+ saturated clinoptilolite and chabazite. Arch. Agron. Soil Sci. 2018, 64, 1142–1151. [Google Scholar] [CrossRef]
- Moreno-Tost, R.; Santamaría-González, J.; Rodríguez-Castellón, E.; Jiménez-López, A.; Autié, M.A.; González, E.; Carreras Glacial, M.; De las Pozas, C. Selective catalytic reduction of nitric oxide by ammonia over Cu-exchanged Cuban natural zeolites. Appl. Catal. B Environ. 2004, 50, 279–288. [Google Scholar] [CrossRef]
- Ghasemian, N.; Falamaki, C. Zn2+, Fe2+, Cu2+, Mn2+, H+ Ion-exchanged and raw clinoptilolite zeolite catalytic performance in the propane-SCR-NOx Process: A comparative study. Int. J. Chem. React. Eng. 2018, 16, 20160192. [Google Scholar] [CrossRef]
- Yordanova, I.; Hristov, S.; Kolev, H.; Todorova, S.; Naydenov, A. Cobalt-manganese ion-exchanged clinoptilolite supported catalysts for n-hexane oxidation. Catal. Today 2023, 423, 114267. [Google Scholar] [CrossRef]
- Sobuś, N.; Król, M.; Piotrowski, M.; Michorczyk, B.; Czekaj, I.; Kornaus, K.; Trenczek-Zając, A.; Komarek, S. Conversion of dihydroxyacetone to carboxylic acids on pretreated clinoptilolite modified with iron, copper, and cobalt. Catal. Commun. 2022, 171, 106509. [Google Scholar] [CrossRef]
- Fajdek-Bieda, A.; Wróblewska, A.; Miądlicki, P.; Tolpa, J.; Michalkiewicz, B. Clinoptilolite as a natural, active zeolite catalyst for the chemical transformations of geraniol. React. Kinet. Mech. Catal. 2021, 133, 997–1011. [Google Scholar] [CrossRef]
- Özyağcı, B.; Şahin, V.; Karabakan, A. Esterification of 1-Octanol on clinoptilolite-supported TiO2 catalysts. Silicon 2019, 11, 339–344. [Google Scholar] [CrossRef]
- Popova, M.; Shestakova, P.; Lazarova, H.; Dimitrov, M.; Kovacheva, D.; Szegedi, A.; Mali, G.; Dasireddy, V.; Likozar, B.; Wilde, N.; et al. Efficient solid acid catalysts based on sulfated tin oxides for liquid phase esterification of levulinic acid with ethanol. Appl. Catal. A Gen. 2018, 560, 119–131. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, A.; Lazarova, H.; Ristić, A.; Kalvachev, Y.; Atanasova, G.; Wilde, N.; Novak Tušar, N.; Gläser, R. Synthesis of biomass derived levulinate esters on novel sulfated Zr/KIL-2 composite catalysts. Microporous Mesoporous Mater. 2016, 235, 50–58. [Google Scholar] [CrossRef]
- Bizani, E.; Fytianos, K.; Poulios, I.; Tsiridis, V. Photocatalytic decolorization and degradation of dye solutions and wastewaters in the presence of titanium dioxide. J. Hazard. Mater. 2006, 136, 85–94. [Google Scholar] [CrossRef]
- Ajoudanian, N.; Nezamzadeh-Ejhieh, A. Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater. Sci. Semicond. Process. 2015, 36, 162–169. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Khorsandi, S. Photocatalytic degradation of 4-nitrophenol with ZnO supported nano-clinoptilolite zeolite. J. Ind. Eng. Chem. 2014, 20, 937–946. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Amiri, M. CuO supported clinoptilolite towards solar photocatalytic degradation of p-aminophenol. Powder Technol. 2013, 235, 279–288. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Moeinirad, S. Heterogeneous photocatalytic degradation of furfural using NiS-clinoptilolite zeolite. Desalination 2011, 273, 248–257. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F. Photocatalytic performance of TiO2/Clinoptilolite: Comparison study in suspension and hybrid photocatalytic membrane reactor. Chemosphere 2019, 228, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Nezamzadeh-Ejhieh, A.; Zabihi-Mobarakeh, H. Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite. J. Ind. Eng. Chem. 2014, 20, 1421–1431. [Google Scholar] [CrossRef]
- Bahrami, M.; Nezamzadeh-Ejhieh, A. Effect of the supported ZnO on clinoptilolite nano-particles in the photodecolorization of semi-real sample bromothymol blue aqueous solution. Mater. Sci. Semicond. Process. 2015, 30, 275–284. [Google Scholar] [CrossRef]
- Abdollahi, B.; Shakeri, A.; Aber, S.; Bonab, M.S. Simultaneous photodegradation of acid orange 7 and removal of Pb2+ from polluted water using reusable clinoptilolite–TiO2 nanocomposite. Res. Chem. Intermed. 2018, 44, 1505–1521. [Google Scholar] [CrossRef]
- Ullah, R.; Liu, C.; Panezai, H.; Gul, A.; Sun, J.; Wu, X. Controlled crystal phase and particle size of loaded-TiO2 using clinoptilolite as support via hydrothermal method for degradation of crystal violet dye in aqueous solution. Arab. J. Chem. 2020, 13, 4092–4101. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Vasylechko, V.; Khyzhun, O.; Gryshchouk, G.; Khalameida, S.; Vasylechko, L. Effect of high-energy milling on the structure, some physicochemical and photocatalytic properties of clinoptilolite. Appl. Catal. A Gen. 2021, 610, 117930. [Google Scholar] [CrossRef]
- Durham, D.R.; Marshall, L.C.; Miller, J.G.; Chmurny, A.B. Characterization of inorganic biocarriers that moderate system upsets during fixed-film biotreatment processes. Appl. Environ. Microbiol. 1994, 60, 3329–3335. [Google Scholar] [CrossRef]

| M(II) | Mg | Mn | Ni | Cu | Zn | Pb |
|---|---|---|---|---|---|---|
| Removal, % | 60 | 47 | 15 | 84 | 50 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlović, J.; Hrenović, J.; Povrenović, D.; Rajić, N. Advances in the Applications of Clinoptilolite-Rich Tuffs. Materials 2024, 17, 1306. https://doi.org/10.3390/ma17061306
Pavlović J, Hrenović J, Povrenović D, Rajić N. Advances in the Applications of Clinoptilolite-Rich Tuffs. Materials. 2024; 17(6):1306. https://doi.org/10.3390/ma17061306
Chicago/Turabian StylePavlović, Jelena, Jasna Hrenović, Dragan Povrenović, and Nevenka Rajić. 2024. "Advances in the Applications of Clinoptilolite-Rich Tuffs" Materials 17, no. 6: 1306. https://doi.org/10.3390/ma17061306







