Thermal-Induced Microstructure Deterioration of Egyptian Granodiorite and Associated Physico-Mechanical Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rock Description
2.2. Experimental Procedures
3. Results
3.1. Physical Analyses
3.1.1. Connection between Porosity and Mass Loss
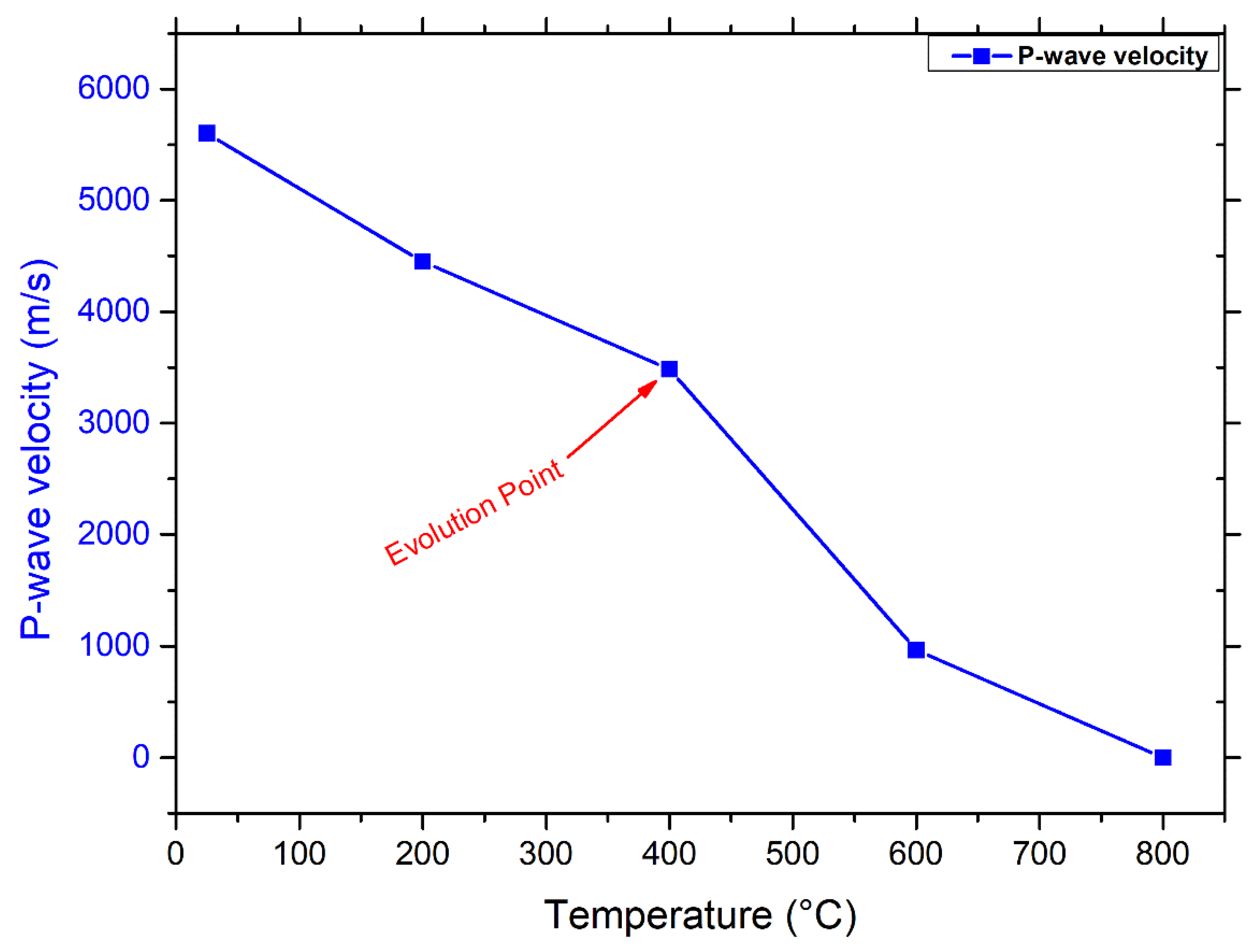
3.1.2. Connection between Porosity and P-Wave Velocity
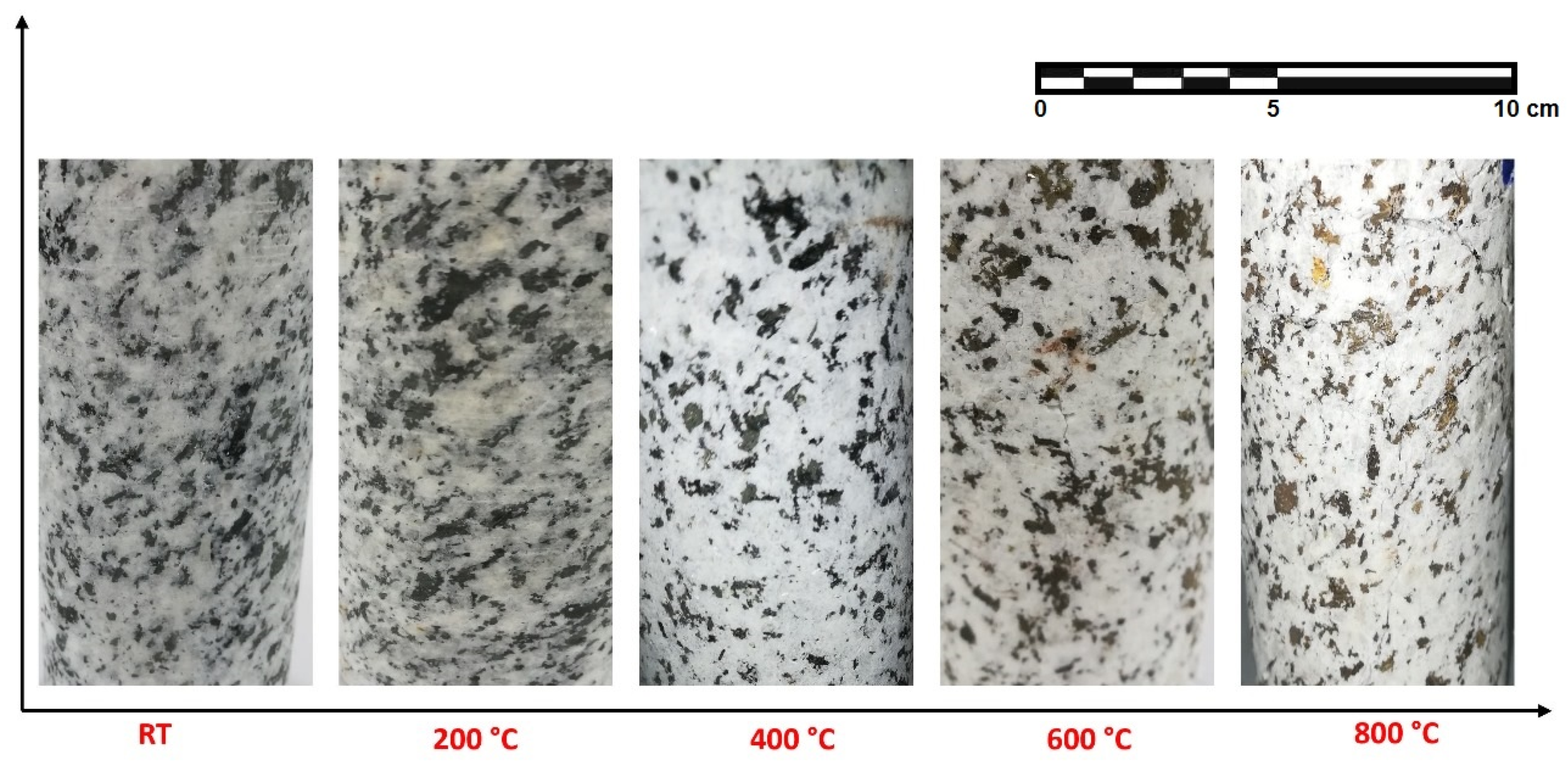
3.1.3. Appearance
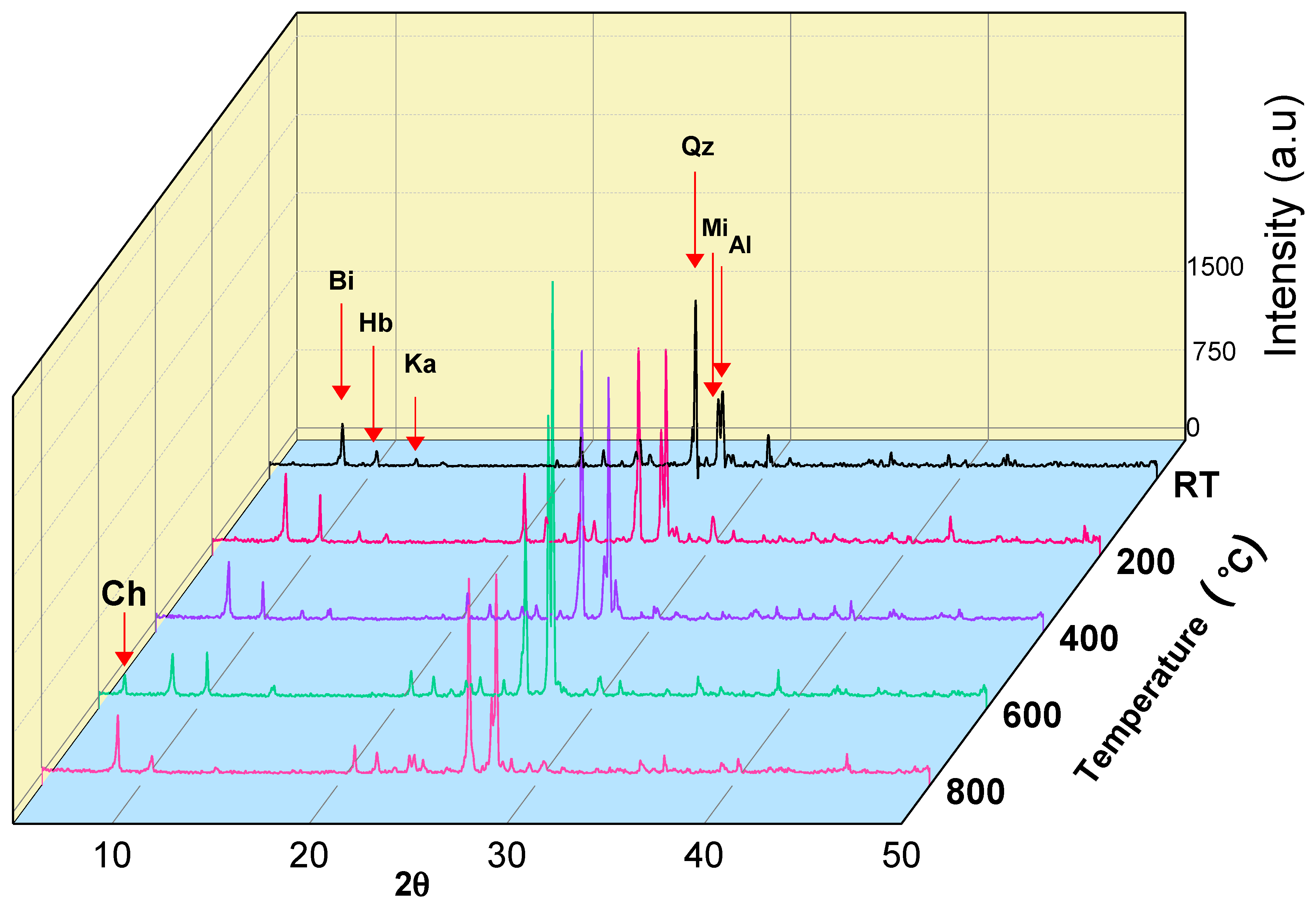
3.2. Mineralogical Analyses
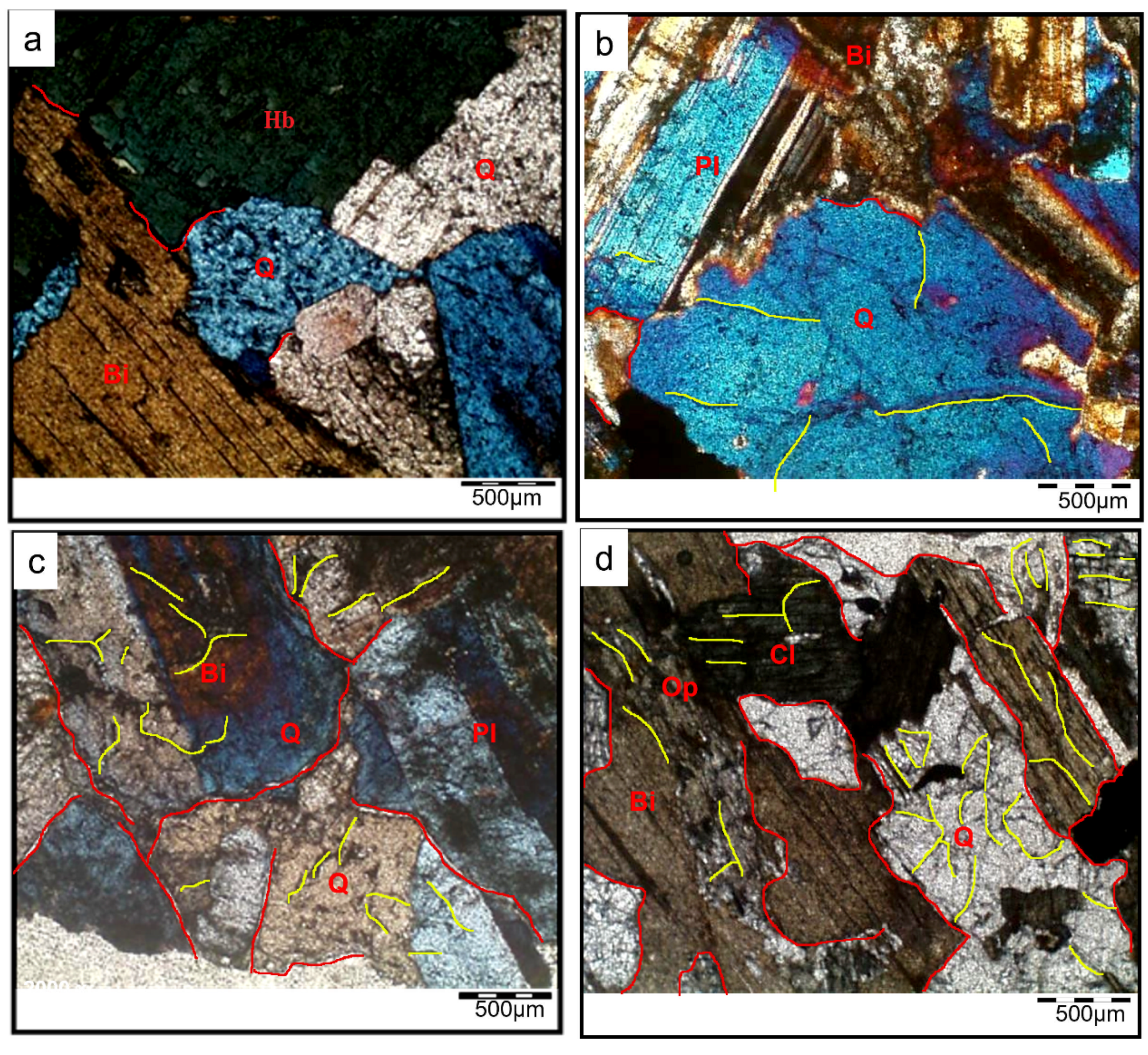
3.3. Petrographic Analyses
3.4. Mechanical Analyses
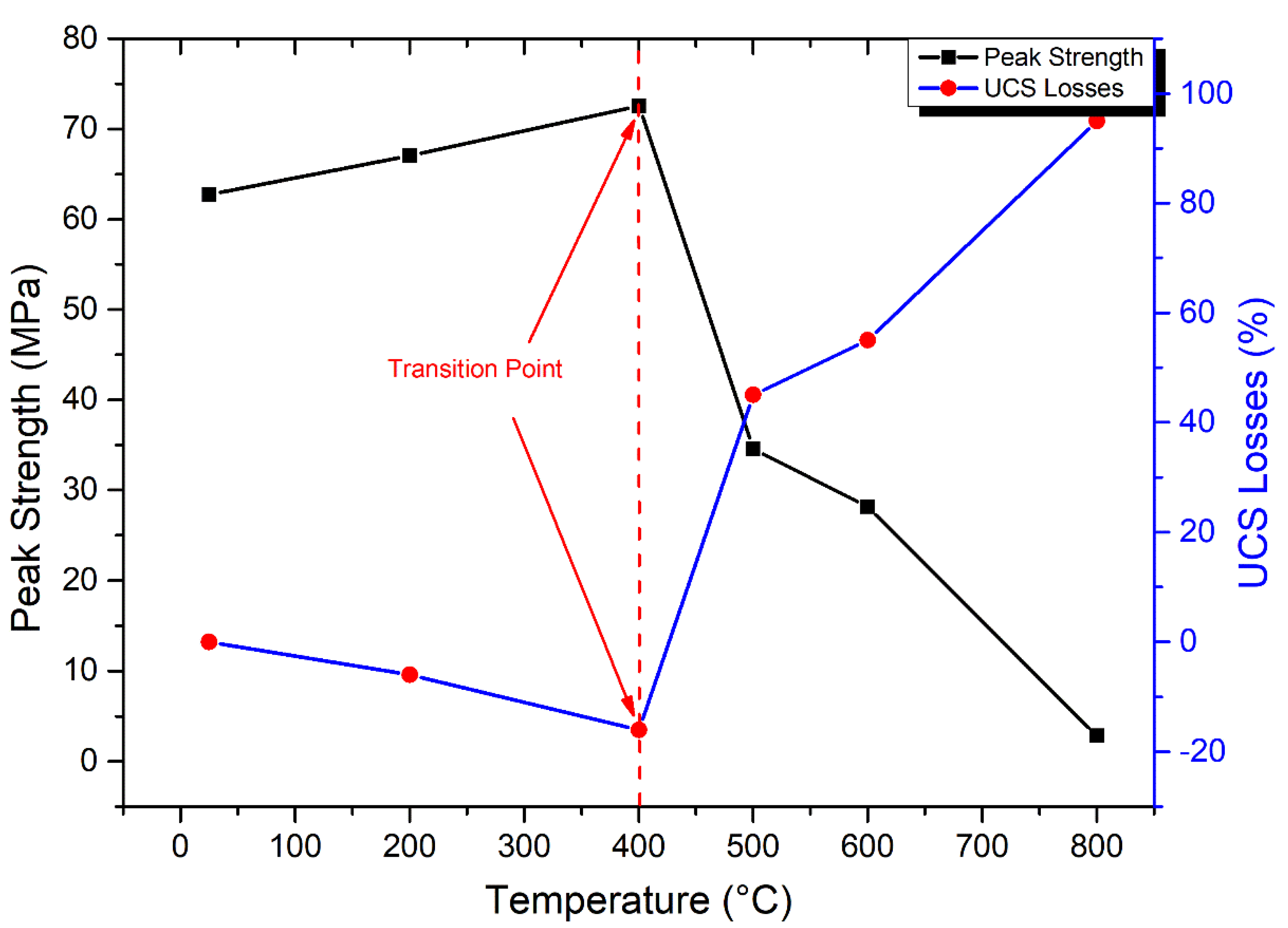
3.4.1. Uniaxial Compressive Strength
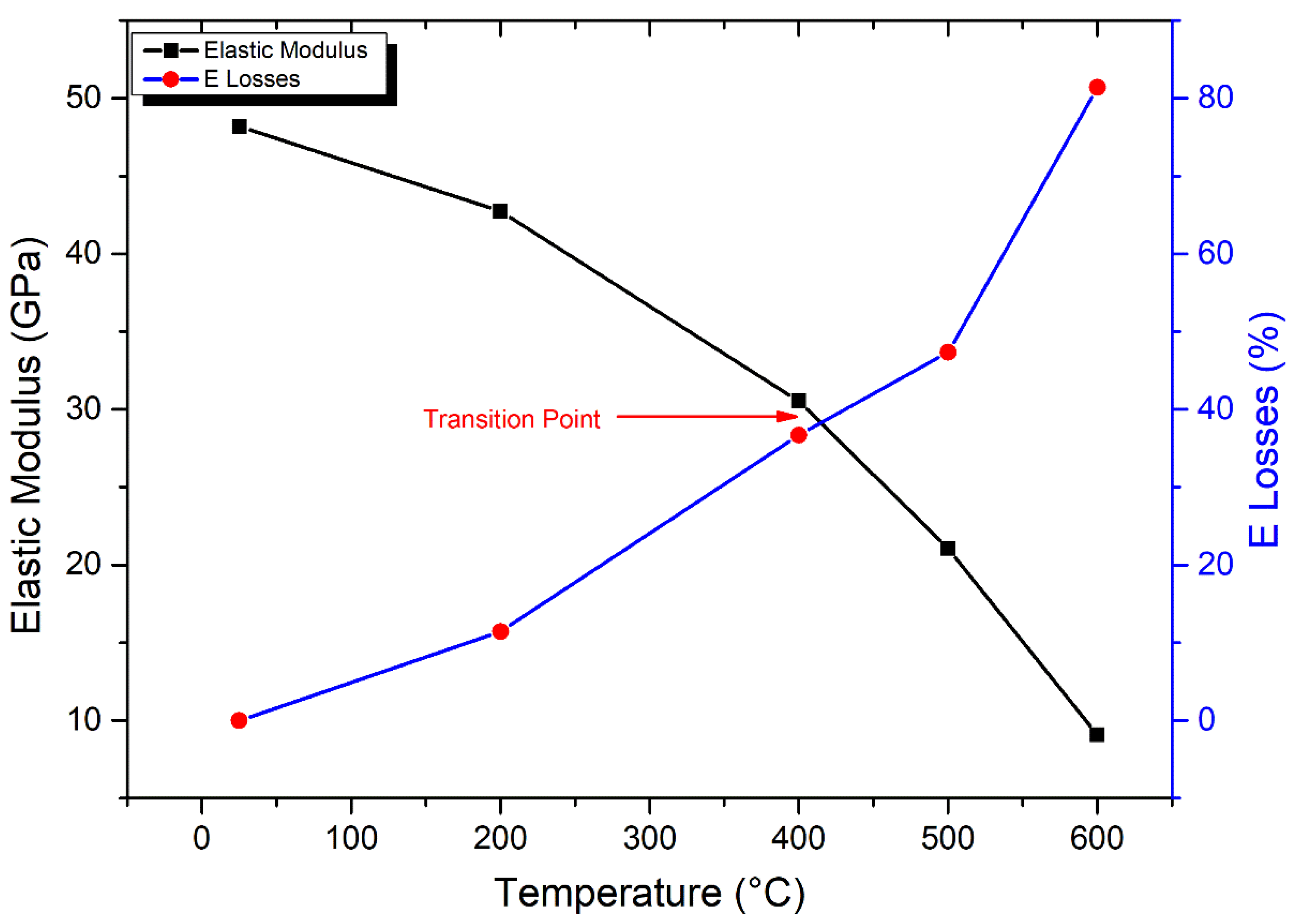
3.4.2. Elastic Modulus
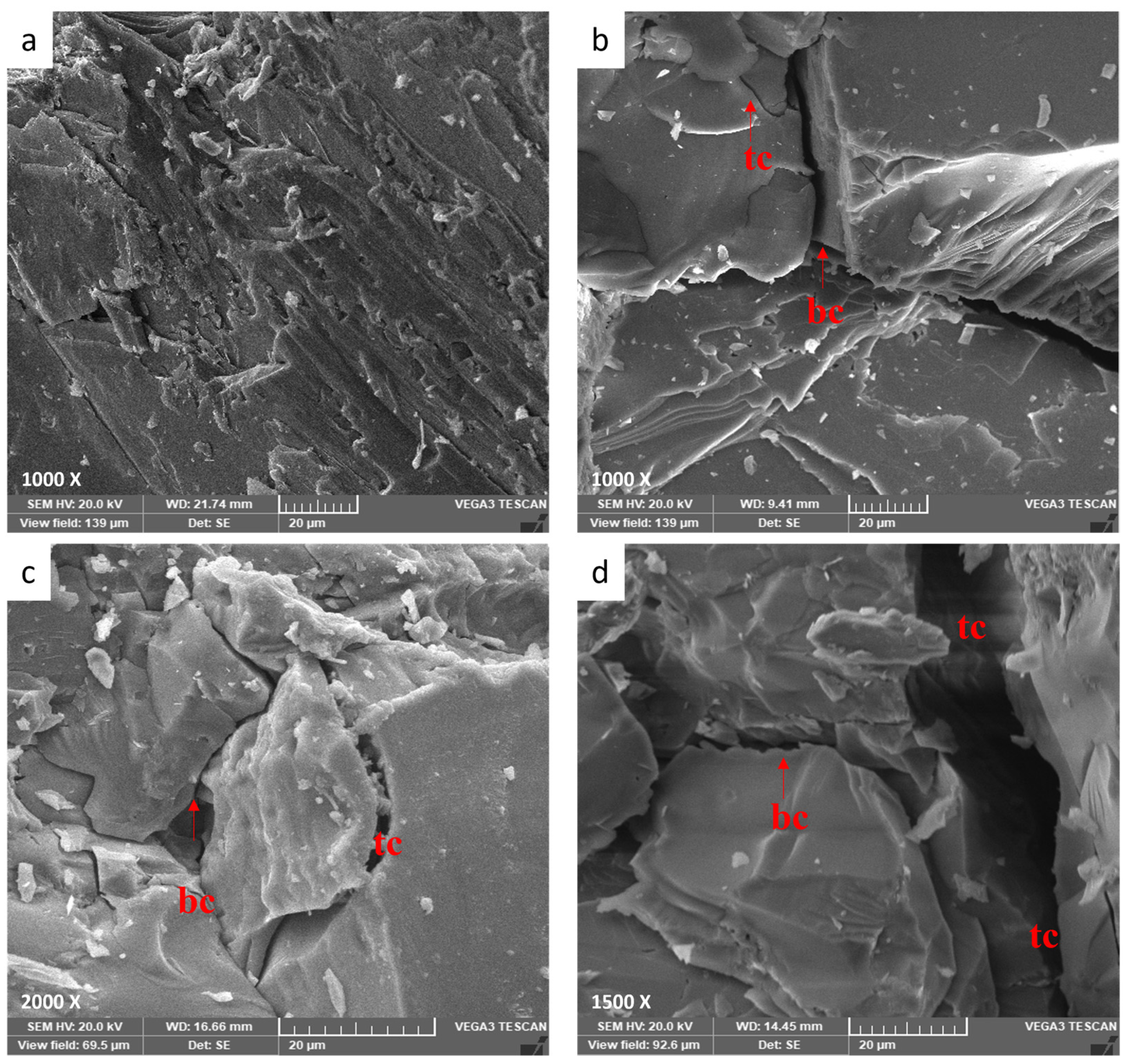
3.4.3. Thermal Crack Mechanism of Granodiorite
3.5. Thermal Damage Factor
4. Discussion
5. Conclusions
- (1)
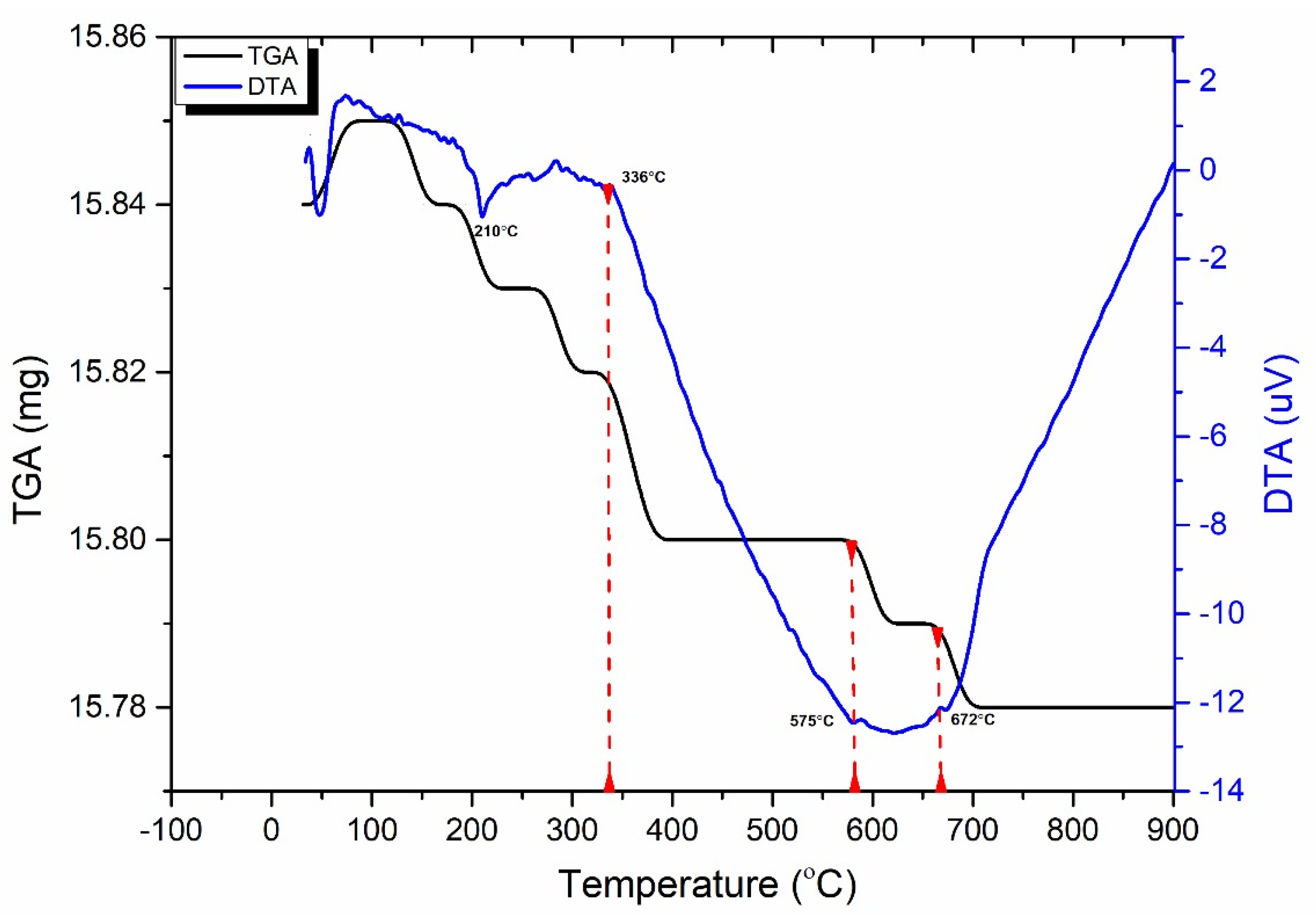
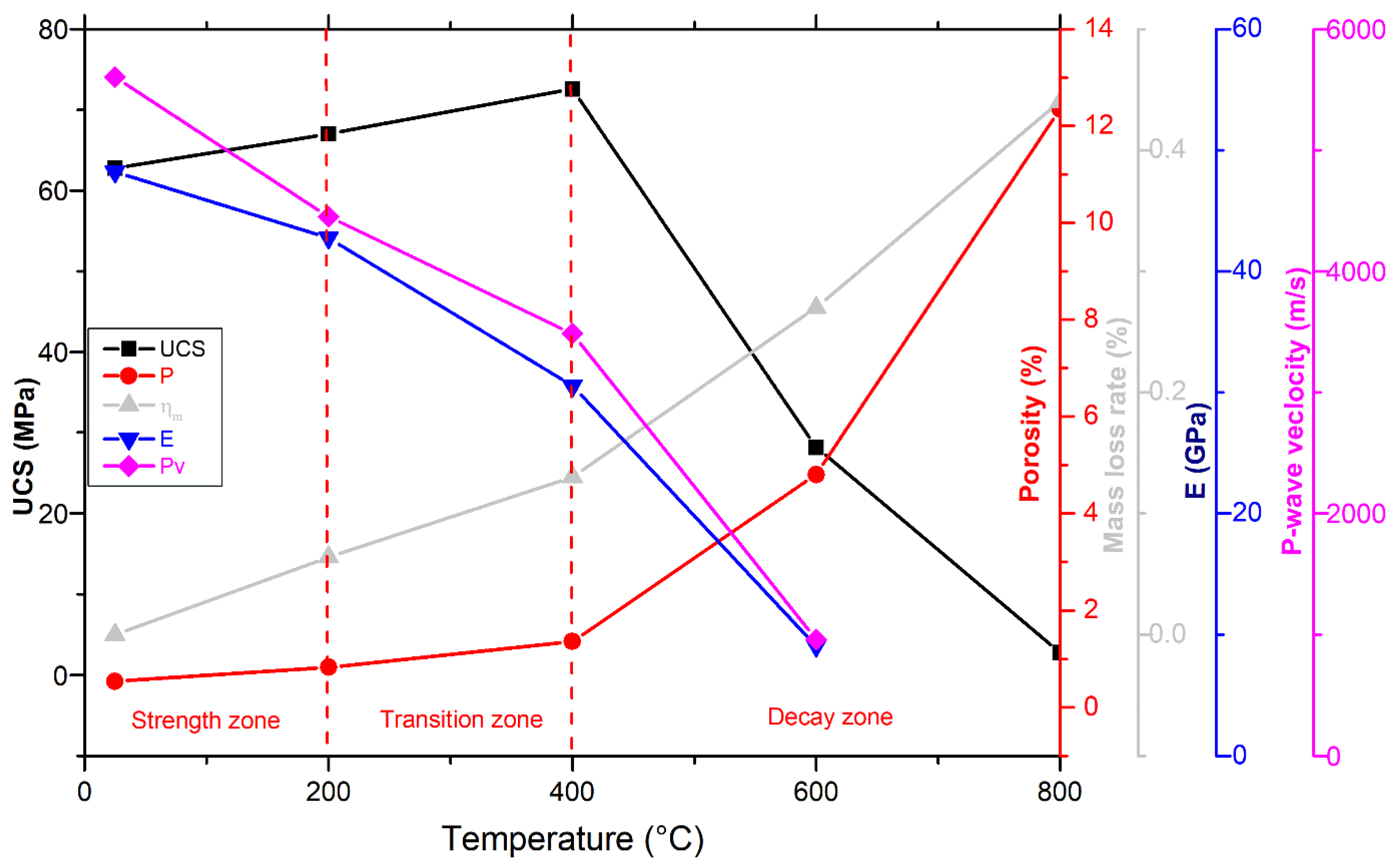
- Microscopy analyses revealed intergranular microcracks at 200 °C and some transgranular microcracks at 400 °C ascribed to attached-water evaporation and thermal stress divergences between minerals. Hence, moderate deterioration in porosity, mass loss, and P-wave velocities were observed at this temperature range because the water content of granodiorite was limited, as approved by TGA. On the other hand, trans-crystalline cracks rose by the thermal deformation of crystal grains at temperatures above 400 °C. As a result, a sharp reduction of granodiorite physical properties after the transition temperature (400 °C).
- (2)
- Beyond 400 °C, the thermochemical processes accelerated, and structural and crystal water evaporated, increasing the porosity exponentially. Hence, granodiorite characteristics deteriorate significantly, presumably due to various microcracks, the quartz α-β transition at 575 °C, according to the DTA examination. Furthermore, biotite reactions with oxygen-induced color change from lightening to pale grey with a reddish hue, and the quartz α-β transition at 575 °C, according to the DTA examination.
- (3)
- The UCS of granodiorite samples climbed first due to thermal hardening up to 400 °C; in contrast, Young’s modulus decreased when the heat treatment temperature increased. Meanwhile, UCS and E declined exponentially between 400 °C and 600 °C because of crack expansion, rising differential thermal expansion of minerals, and dehydration. Consequently, at 800 °C, the UCS dramatically reduced due to the interactions and coalescences of the intergranular and transgranular fractures, explaining why E and Vp could not be measured.
- (4)
- Three main failure modes of granodiorite can be linked to the initiation and propagation of thermal microcracks. At temperatures below 400 °C, intergranular microcracks were visible, while some transgranular microcracks appeared, and the axial splitting mode occurred. At the same time, the growth of existing micro-cracks at 600 °C led to shear mode. Forming a fracture network of intergranular and transgranular microcracks at 800 °C contributed to the complex failure mode.
- (5)
- Egyptian granodiorite has a thermal transition zone from strengthening to decay zone between 200 and 400 °C, which means 400 °C is the temperature threshold. Following this temperature, P-wave velocity and rock mass decreased significantly, porosity doubled, and the evolution of transgranular cracks was revealed via SEM and OM, resulting in a substantial decline in the UCS and E.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomez-Heras, M.; McCabe, S.; Smith, B.J.; Fort, R. Impacts of Fire on Stone-Built Heritage: An Overview. J. Archit. Conserv. 2009, 15, 47–58. [Google Scholar] [CrossRef]
- Kourkoulis, S.K. Fracture and Failure of Natural Building Stones: Applications in the Restoration of Ancient Monuments; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 1402050771. [Google Scholar]
- Ferrero, A.M.; Marini, P. Experimental Studies on the Mechanical Behaviour of Two Thermal Cracked Marbles. Rock Mech. Rock Eng. 2001, 34, 57–66. [Google Scholar] [CrossRef]
- Hajpál, M. Changes in Sandstones of Historical Monuments Exposed to Fire or High Temperature. Fire Technol. 2002, 38, 373–382. [Google Scholar] [CrossRef]
- De Bresser, J.H.P.; Urai, J.L.; Olgaard, D.L. Effect of Water on the Strength and Microstructure of Carrara Marble Axially Compressed at High Temperature. J. Struct. Geol. 2005, 27, 265–281. [Google Scholar] [CrossRef]
- Géraud, Y.; Mazerolle, F.; Raynaud, S. Comparison between Connected and Overall Porosity of Thermally Stressed Granites. J. Struct. Geol. 1992, 14, 981–990. [Google Scholar] [CrossRef]
- Den’gina, N.I.; Kazak, V.N.; Pristash, V.V. Changes in Rocks at High Temperatures. J. Min. Sci. 1993, 29, 472–477. [Google Scholar] [CrossRef]
- Torok, A.; Hajpál, M. Effect of Temperature Changes on the Mineralogy and Physical Properties of Sandstones. A Laboratory Study. Int. J. Restor. Build. Monum. 2005, 11, 211. [Google Scholar]
- Ozguven, A.; Ozcelik, Y. Effects of High Temperature on Physico-Mechanical Properties of Turkish Natural Building Stones. Eng. Geol. 2014, 183, 127–136. [Google Scholar] [CrossRef]
- Freire-Lista, D.M.M.; Fort, R.; Varas-Muriel, M.J. Thermal Stress-Induced Microcracking in Building Granite. Eng. Geol. 2016, 206, 83–93. [Google Scholar] [CrossRef]
- Koca, M.Y.; Ozden, G.; Yavuz, A.B.; Kincal, C.; Onargan, T.; Kucuk, K. Changes in the Engineering Properties of Marble in Fire-Exposed Columns. Int. J. Rock Mech. Min. Sci. 2006, 43, 520–530. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Khan, N.M.; Sun, C.; Xu, J.; Omar, A.A.; Mousa, B.G.; Abdelhamid, M.M.A.; Zaki, M.M. Prediction of Strength Parameters of Thermally Treated Egyptian Granodiorite Using Multivariate Statistics and Machine Learning Techniques. Mathematics 2022, 10, 4523. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Xu, J.; Omar, A.A.; Haoran, H.; Zaki, M.M. Micro to Macro-Cracking Mechanism in Thermally Treated Granodiorite Followed by Different Cooling Techniques. Int. J. Fract. 2023. [Google Scholar] [CrossRef]
- Smith, A.G.; Pells, P.J.N. Impact of Fire on Tunnels in Hawkesbury Sandstone. Tunn. Undergr. Space Technol. 2008, 23, 65–74. [Google Scholar] [CrossRef]
- Nordlund, E.; Zhang, P.; Dineva, S.; Saiang, C.; Mainali Ganesh, M.G. Impact of Fire on the Stability of Hard Rock Tunnels in Sweden; BeFo Rapport; Stiftelsen Bergteknisk Forskning (BeFo): Stockholm, Sweden, 2014. [Google Scholar]
- Luo, Y.-J.; Qin, B.-D.; He, J. Experimental Study of the Longitudinal Wave Velocity in Sandstone under Three Different Constraints. Zhongguo Kuangye Daxue Xuebao (J. China Univ. Min. Technol.) 2012, 41, 153–158. [Google Scholar]
- Huang, Z.; Zeng, W.; Wu, Y.; Li, S.; Gu, Q.; Zhao, K. Effects of Temperature and Acid Solution on the Physical and Tensile Mechanical Properties of Red Sandstones. Environ. Sci. Pollut. Res. 2021, 28, 20608–20623. [Google Scholar] [CrossRef]
- Sha, S.; Rong, G.; Peng, J.; Li, B.; Wu, Z. Effect of Open-Fire-Induced Damage on Brazilian Tensile Strength and Microstructure of Granite. Rock Mech. Rock Eng. 2019, 52, 4189–4202. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Bader, S.; Elkarmoty, M.; Ismael, M. Damage Evolution of Granodiorite after Heating and Cooling Treatments. Minerals 2021, 11, 779. [Google Scholar] [CrossRef]
- Ram, B.K.; Gupta, V. Physico-Mechanical Characterization of Higher Himalayan Granite under the Thermal Treatments of Different Heating–Cooling Conditions. Acta Geotech. 2024. [Google Scholar] [CrossRef]
- Vidana Pathiranagei, S.; Gratchev, I.; Kong, R. Engineering Properties of Four Different Rocks after Heat Treatment. Geomech. Geophys. Geo-Energy Geo-Resour. 2021, 7, 16. [Google Scholar] [CrossRef]
- Alzo’ubi, A.K.; Alneasan, M.; Ibrahim, F.; Okasha, N.M.; Mirrashid, M. Thermal Treatment Effects on Rock Fracture Behaviour Under Three Loading Modes: A Comparative Analysis of Granite and Mudstone. Eng. Fail. Anal. 2024, 159, 108092. [Google Scholar]
- Tomás, R.; Cano, M.; Pulgarín, L.F.; Brotóns, V.; Benavente, D.; Miranda, T.; Vasconcelos, G. Thermal Effect of High Temperatures on the Physical and Mechanical Properties of a Granite Used in UNESCO World Heritage Sites in North Portugal. J. Build. Eng. 2021, 43, 102823. [Google Scholar] [CrossRef]
- Gautam, P.K.; Verma, A.K.; Jha, M.K.; Sharma, P.; Singh, T.N. Effect of High Temperature on Physical and Mechanical Properties of Jalore Granite. J. Appl. Geophys. 2018, 159, 460–474. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Sun, C.; Xu, J.; Yang, S.; Li, J. On the Physical and Mechanical Responses of Egyptian Granodiorite after High-Temperature Treatments. Sustainability 2022, 14, 4632. [Google Scholar] [CrossRef]
- Gomah, M.E.; Li, G.; Sun, C.; Xu, J.; Sen, Y.; Jinghua, L.; Ismael, M.; Elkarmoty, M. Macroscopic and Microscopic Research on Egyptian Granodiorite Behavior Exposed to the Various Heating and Cooling Strategies. Geomech. Geophys. Geo-Energy Geo-Resour. 2022, 8, 158. [Google Scholar] [CrossRef]
- Yavuz, H.; Demirdag, S.; Caran, S. Thermal Effect on the Physical Properties of Carbonate Rocks. Int. J. Rock Mech. Min. Sci. 2010, 47, 94–103. [Google Scholar] [CrossRef]
- Różański, A.; Różańska, A.; Sobótka, M.; Pachnicz, M.; Bukowska, M. Identification of Changes in Mechanical Properties of Sandstone Subjected to High Temperature: Meso-and Micro-Scale Testing and Analysis. Arch. Civ. Mech. Eng. 2021, 21, 28. [Google Scholar] [CrossRef]
- Mahanta, B.; Ranjith, P.G.; Vishal, V.; Singh, T.N. Temperature-Induced Deformational Responses and Microstructural Alteration of Sandstone. J. Pet. Sci. Eng. 2020, 192, 107239. [Google Scholar] [CrossRef]
- Murru, A.; Freire-Lista, D.M.; Fort, R.; Varas-Muriel, M.J.; Meloni, P. Evaluation of Post-Thermal Shock Effects in Carrara Marble and Santa Caterina Di Pittinuri Limestone. Constr. Build. Mater. 2018, 186, 1200–1211. [Google Scholar] [CrossRef]
- Daoudi, A.; Eslami, J.; Beaucour, A.-L.; Vigroux, M.; Noumowé, A. High-Temperature Behaviour of Various Limestones at the Block Scale. Constr. Build. Mater. 2024, 416, 135109. [Google Scholar] [CrossRef]
- Tian, H.; Ziegler, M.; Kempka, T. Physical and Mechanical Behavior of Claystone Exposed to Temperatures up to 1000 °C. Int. J. Rock Mech. Min. Sci. 2014, 70, 144–153. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, H.; Mei, G.; Jiang, G.; Dou, B.; Xiao, P. Experimental Investigation on Mechanical Behaviors of Nanan Granite after Thermal Treatment under Conventional Triaxial Compression. Environ. Earth Sci. 2021, 80, 46. [Google Scholar] [CrossRef]
- Kumari, W.G.P.; Ranjith, P.G.; Perera, M.S.A.; Chen, B.K.; Abdulagatov, I.M. Temperature-Dependent Mechanical Behaviour of Australian Strathbogie Granite with Different Cooling Treatments. Eng. Geol. 2017, 229, 31–44. [Google Scholar] [CrossRef]
- Luc Leroy, M.N.; Marius, F.W.; François, N. Experimental and Theoretical Investigations of Hard Rocks at High Temperature: Applications in Civil Engineering. Adv. Civ. Eng. 2021, 2021, 8893944. [Google Scholar] [CrossRef]
- Alneasan, M.; Alzo’ubi, A.K. Extensive Experimental Investigation on the Effect of Thermal Treatment and Lateral Pressure on the Shear Behavior of Intact Mudstone. Sci. Rep. 2023, 13, 6820. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Wang, C. Thermally Induced Acoustic Emission in Westerly Granite. Geophys. Res. Lett. 1980, 7, 1089–1092. [Google Scholar] [CrossRef]
- Homand-Etienne, F.; Houpert, R. Thermally Induced Microcracking in Granites: Characterization and Analysis. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1989, 26, 125–134. [Google Scholar] [CrossRef]
- Ranjith, P.G.; Viete, D.R.; Chen, B.J.; Perera, M.S.A. Transformation Plasticity and the Effect of Temperature on the Mechanical Behaviour of Hawkesbury Sandstone at Atmospheric Pressure. Eng. Geol. 2012, 151, 120–127. [Google Scholar] [CrossRef]
- Wang, H.F.; Heard, H.C. Prediction of Elastic Moduli via Crack Density in Pressurized and Thermally Stressed Rock. J. Geophys. Res. Solid Earth 1985, 90, 10342–10350. [Google Scholar] [CrossRef]
- Peng, J.; Yang, S.Q. Comparison of Mechanical Behavior and Acoustic Emission Characteristics of Three Thermally-Damaged Rocks. Energies 2018, 11, 2350. [Google Scholar] [CrossRef]
- Sammaljärvi, J.; Rama, M.S.; Ikonen, J.; Muuri, E.; Hellmuth, K.-H.; Siitari-Kauppi, M. Free Radical Polymerisation of Methacrylates with Thermal Initiator in Clay Rock. Eng. Geol. 2016, 210, 70–83. [Google Scholar] [CrossRef]
- Shen, Y.-J.J.; Zhang, Y.-L.L.; Gao, F.; Yang, G.-S.S.; Lai, X.-P.P. Influence of Temperature on the Microstructure Deterioration of Sandstone. Energies 2018, 11, 1753. [Google Scholar] [CrossRef]
- Tripathi, A.; Gupta, N.; Singh, A.K.; Mohanty, S.P.; Rai, N.; Pain, A. Effects of Elevated Temperatures on the Microstructural, Physico-Mechanical and Elastic Properties of Barakar Sandstone: A Study from One of the World’s Largest Underground Coalmine Fire Region, Jharia, India. Rock Mech. Rock Eng. 2021, 54, 1293–1314. [Google Scholar] [CrossRef]
- Helmy, H.M.; Ahmed, A.F.; El Mahallawi, M.M.; Ali, S.M. Pressure, Temperature and Oxygen Fugacity Conditions of Calc-Alkaline Granitoids, Eastern Desert of Egypt, and Tectonic Implications. J. Afr. Earth Sci. 2004, 38, 255–268. [Google Scholar] [CrossRef]
- El-Ramly, M.F.; Akaad, M.K. The Basement Complex in the Central Eastern Desert, between Latitudes 24 30’and 25 40’. Geol. Surv. Cairo 1960, 8, 35. [Google Scholar]
- Nowier, A.M.; Sewifi, B.M.; El Ela, A.M. Geology, Petrography, Geochemistry and Petrogenesis of the Egyptian Younger Granites. Qatar Univ. Sci. J. 1990, 10, 363–393. [Google Scholar]
- Emam, A.; Radwan, A. Geochemistry and Petrogenesis of I-Type Granitoid Rocks around Nasb-Zurar Intrusion, West Wadi Allaqi, South Eastern Desert, Egypt. Arab. J. Geosci. 2021, 14, 581. [Google Scholar] [CrossRef]
- Chandrasekharam, D.; Pabasara Kumari, W.G.; Avanthi Isaka, B.L.; Gamage, R.P.; Rathnaweera, T.D.; Anne Perera, M.S.; Isaka, B.L.A.; Gamage, R.P.; Rathnaweera, T.D.; Perera, M.S.A.; et al. An Influence of Thermally-Induced Micro-Cracking under Cooling Treatments: Mechanical Characteristics of Australian Granite. Energies 2018, 11, 1338. [Google Scholar] [CrossRef]
- Siegesmund, S.; Menningen, J.; Shushakova, V. Marble Decay: Towards a Measure of Marble Degradation Based on Ultrasonic Wave Velocities and Thermal Expansion Data. Environ. Earth Sci. 2021, 80, 395. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Hao, S.; Geng, J.; Lv, C. Experimental Study on the Variation of Physical and Mechanical Properties of Rock after High Temperature Treatment. Appl. Therm. Eng. 2016, 98, 1297–1304. [Google Scholar] [CrossRef]
- Costa, K.O.B.; Xavier, G.C.; Marvila, M.T.; Alexandre, J.; Azevedo, A.R.G.; Monteiro, S.N. Influence of High Temperatures on Physical Properties and Microstructure of Gneiss. Bull. Eng. Geol. Environ. 2021, 80, 7069–7081. [Google Scholar] [CrossRef]
- Reuschlé, T.; Haore, S.G.; Darot, M. The Effect of Heating on the Microstructural Evolution of La Peyratte Granite Deduced from Acoustic Velocity Measurements. Earth Planet. Sci. Lett. 2006, 243, 692–700. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Geng, J. Microstructural Characterization of Limestone Exposed to Heat with XRD, SEM and TG-DSC. Mater. Charact. 2017, 134, 285–295. [Google Scholar] [CrossRef]
- Vazquez, P.; Benavente, D.; Montiel, D.; Gomez-Heras, M. Mineralogical Transformations in Granitoids during Heating at Fire-Related Temperatures. Appl. Sci. 2021, 12, 188. [Google Scholar] [CrossRef]
- Vazquez, P.; Acuña, M.; Benavente, D.; Gibeaux, S.; Navarro, I.; Gomez-Heras, M. Evolution of Surface Properties of Ornamental Granitoids Exposed to High Temperatures. Constr. Build. Mater. 2016, 104, 263–275. [Google Scholar] [CrossRef]
- Richter, D.; Simmons, G. Thermal Expansion Behavior of Igneous Rocks. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1974, 11, 403–411. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, W.; Xue, L.; Zhang, Z.; Su, T. Thermal Damage Pattern and Thresholds of Granite. Environ. Earth Sci. 2015, 74, 2341–2349. [Google Scholar] [CrossRef]
- Gautam, P.K.; Jha, M.K.; Verma, A.K.; Singh, T.N. Evolution of Absorption Energy per Unit Thickness of Damaged Sandstone. J. Therm. Anal. Calorim. 2019, 136, 2305–2318. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Hao, S.; Wang, B. Experimental Study on the Thermal Damage Characteristics of Limestone and Underlying Mechanism. Rock Mech. Rock Eng. 2016, 49, 2999–3008. [Google Scholar] [CrossRef]
- Sun, Q.; Lü, C.; Cao, L.; Li, W.; Geng, J.; Zhang, W. Thermal Properties of Sandstone after Treatment at High Temperature. Int. J. Rock Mech. Min. Sci. 2016, 85, 60–66. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Gao, B.; Wang, W.; Liu, C. Study on Pore Characteristics and Microstructure of Sandstones with Different Grain Sizes. J. Appl. Geophys. 2017, 136, 364–371. [Google Scholar] [CrossRef]
- Zhu, Z.; Kempka, T.; Ranjith, P.G.; Tian, H.; Jiang, G.; Dou, B.; Mei, G. Changes in Thermomechanical Properties Due to Air and Water Cooling of Hot Dry Granite Rocks under Unconfined Compression. Renew. Energy 2021, 170, 562–573. [Google Scholar] [CrossRef]
- Géraud, Y. Variations of Connected Porosity and Inferred Permeability in a Thermally Cracked Granite. Geophys. Res. Lett. 1994, 21, 979–982. [Google Scholar] [CrossRef]
- Pavese, A.; Curetti, N.; Diella, V.; Levy, D.; Dapiaggi, M.; Russo, U. P-V and T-V Equations of State of Natural Biotite: An in-Situ High-Pressure and High-Temperature Powder Diffraction Study, Combined with Mössbauer Spectroscopy. Am. Mineral. 2007, 92, 1158–1164. [Google Scholar] [CrossRef]
- Ohno, I.; Harada, K.; Yoshitomi, C. Temperature Variation of Elastic Constants of Quartz across the α-β Transition. Phys. Chem. Miner. 2006, 33, 1–9. [Google Scholar] [CrossRef]
- Somerton, W.H. Thermal Properties and Temperature-Related Behavior of Rock/Fluid Systems; Elsevier: Amsterdam, The Netherlands, 1992; ISBN 0444890017. [Google Scholar]






| Oxide (%)/Temp (°C) | RT | 200 | 400 | 600 | 800 |
|---|---|---|---|---|---|
| SiO2 | 61.60 | 61.20 | 59.76 | 60.48 | 61.20 |
| Al2O3 | 16.80 | 16.60 | 15.90 | 16.30 | 16.70 |
| Fe2O3 | 6.50 | 7.54 | 9.94 | 8.88 | 6.32 |
| CaO | 6.71 | 6.61 | 6.44 | 6.72 | 6.97 |
| Na2O | 3.09 | 2.96 | 2.99 | 3.17 | 3.11 |
| MgO | 2.37 | 2.31 | 2.18 | 2.04 | 2.62 |
| K2O | 1.28 | 1.15 | 1.13 | 1.39 | 1.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomah, M.E.; Li, G.; Omar, A.A.; Abdel Latif, M.L.; Sun, C.; Xu, J. Thermal-Induced Microstructure Deterioration of Egyptian Granodiorite and Associated Physico-Mechanical Responses. Materials 2024, 17, 1305. https://doi.org/10.3390/ma17061305
Gomah ME, Li G, Omar AA, Abdel Latif ML, Sun C, Xu J. Thermal-Induced Microstructure Deterioration of Egyptian Granodiorite and Associated Physico-Mechanical Responses. Materials. 2024; 17(6):1305. https://doi.org/10.3390/ma17061305
Chicago/Turabian StyleGomah, Mohamed Elgharib, Guichen Li, Ahmed A. Omar, Mahmoud L. Abdel Latif, Changlun Sun, and Jiahui Xu. 2024. "Thermal-Induced Microstructure Deterioration of Egyptian Granodiorite and Associated Physico-Mechanical Responses" Materials 17, no. 6: 1305. https://doi.org/10.3390/ma17061305






