Effect of Extrusion on Mechanical Property, Corrosion Behavior, and In Vitro Biocompatibility of the As-Cast Mg-Zn-Y-Sr Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization and Mechanical Tests
2.3. Mechanical Tests
2.4. Electrochemical Measurements
2.5. Immersion Tests
2.6. Cytotoxicity Tests
2.7. Cell Morphology
2.8. Cell Adhesion
2.9. Statistical Analysis
3. Results
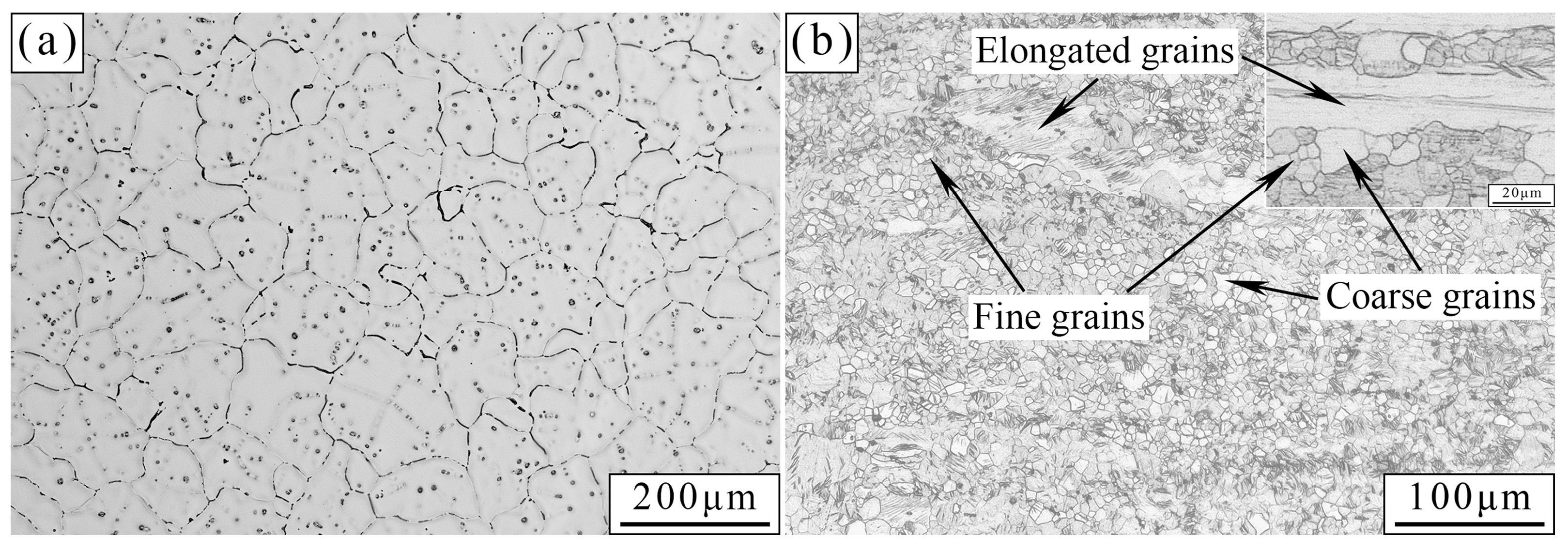
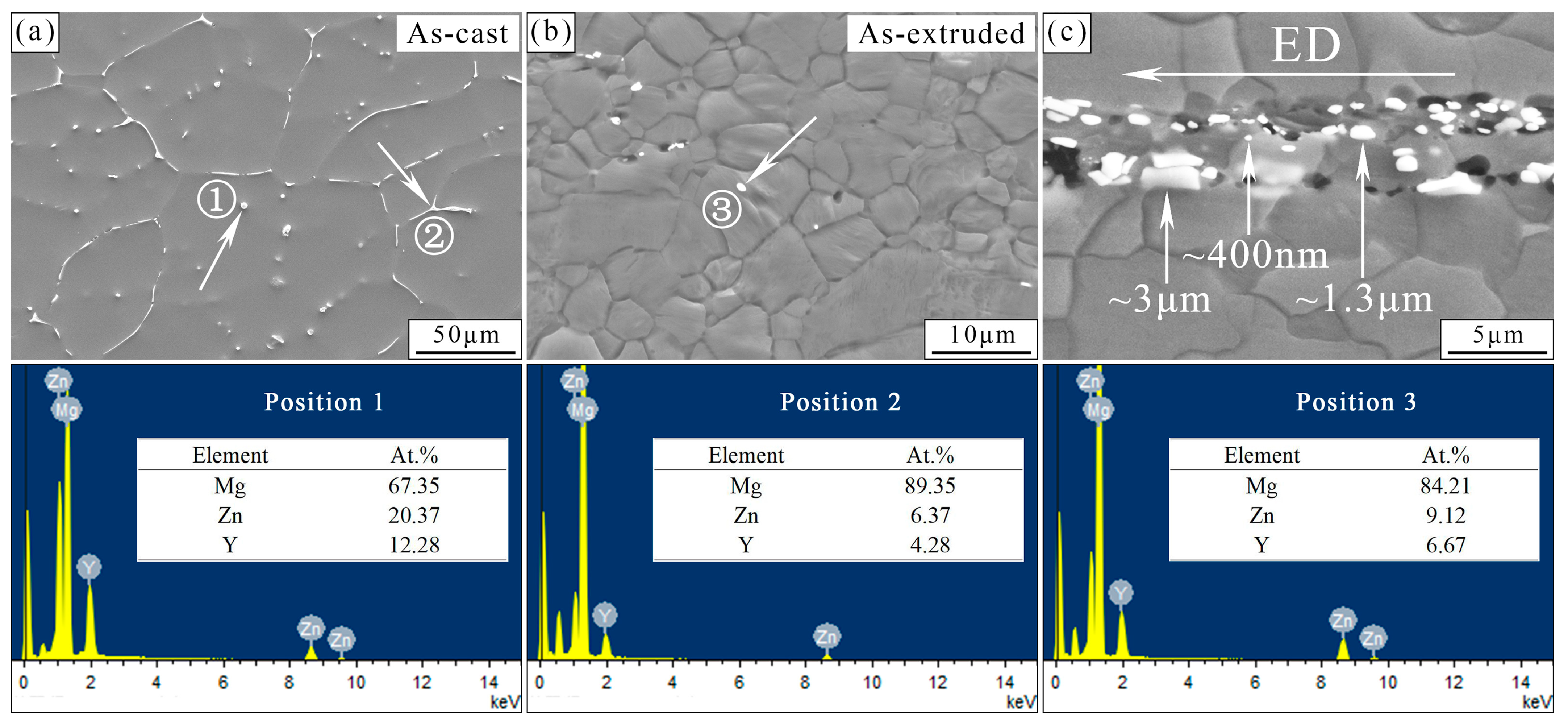
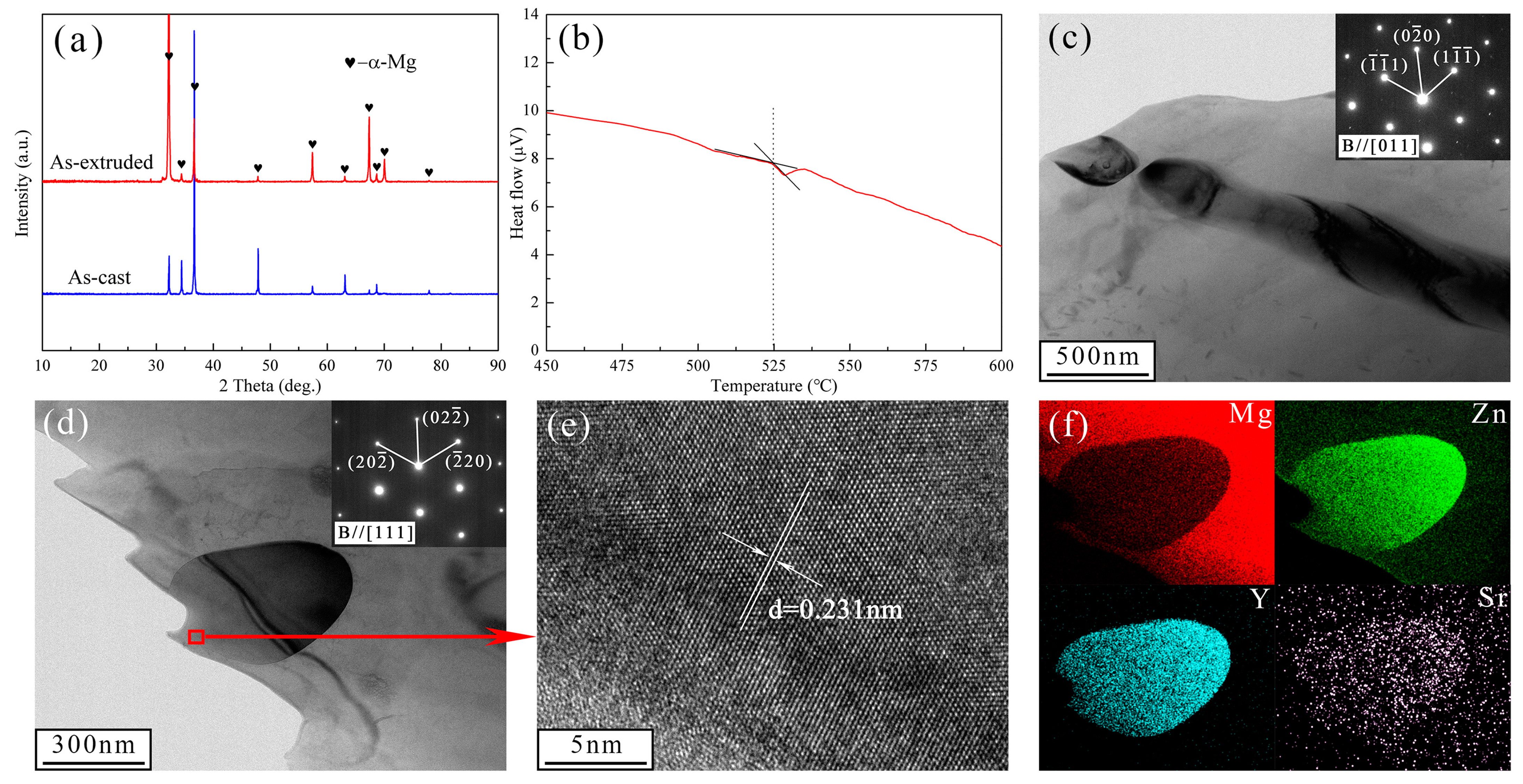
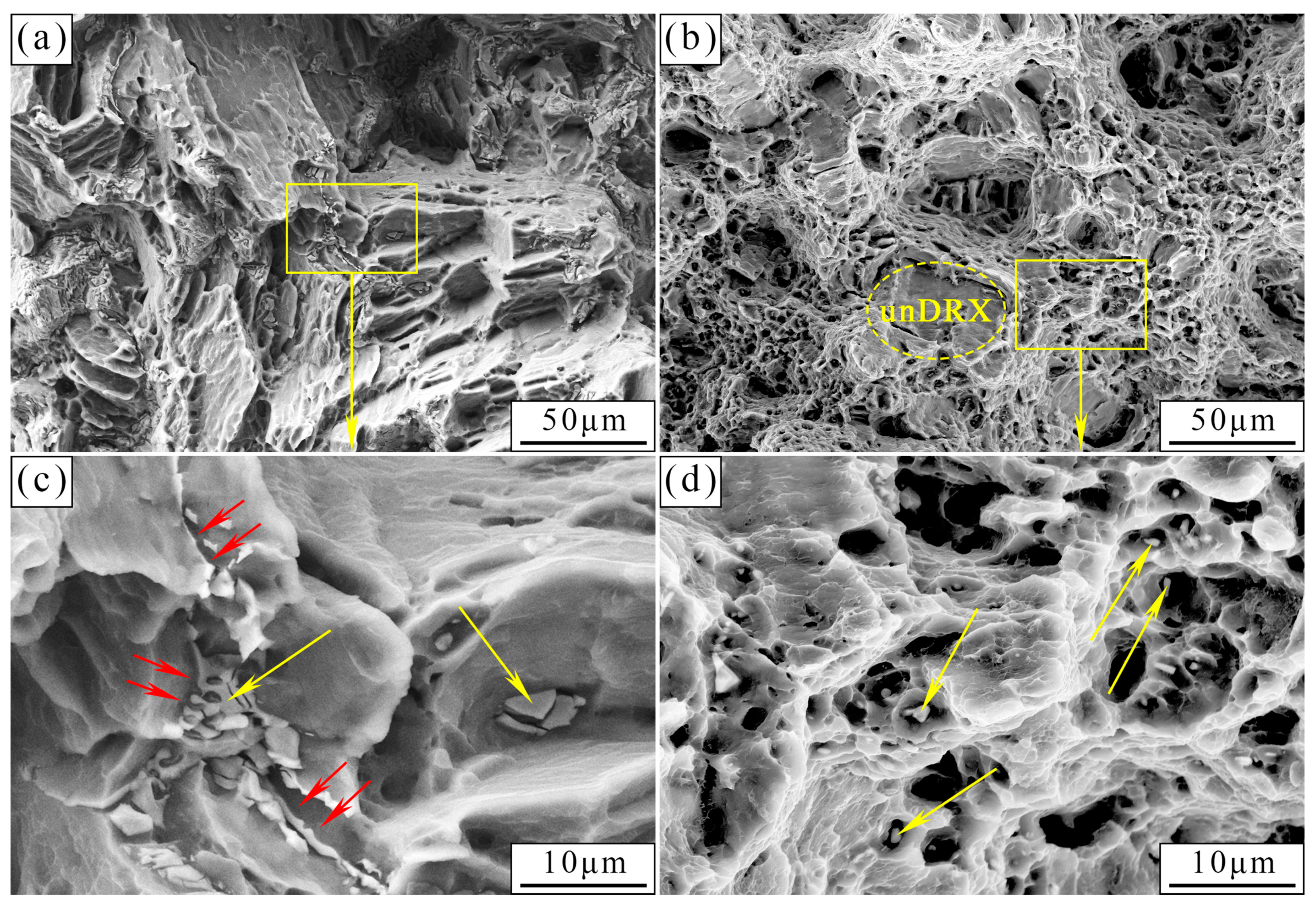
3.1. Microstructural Analysis
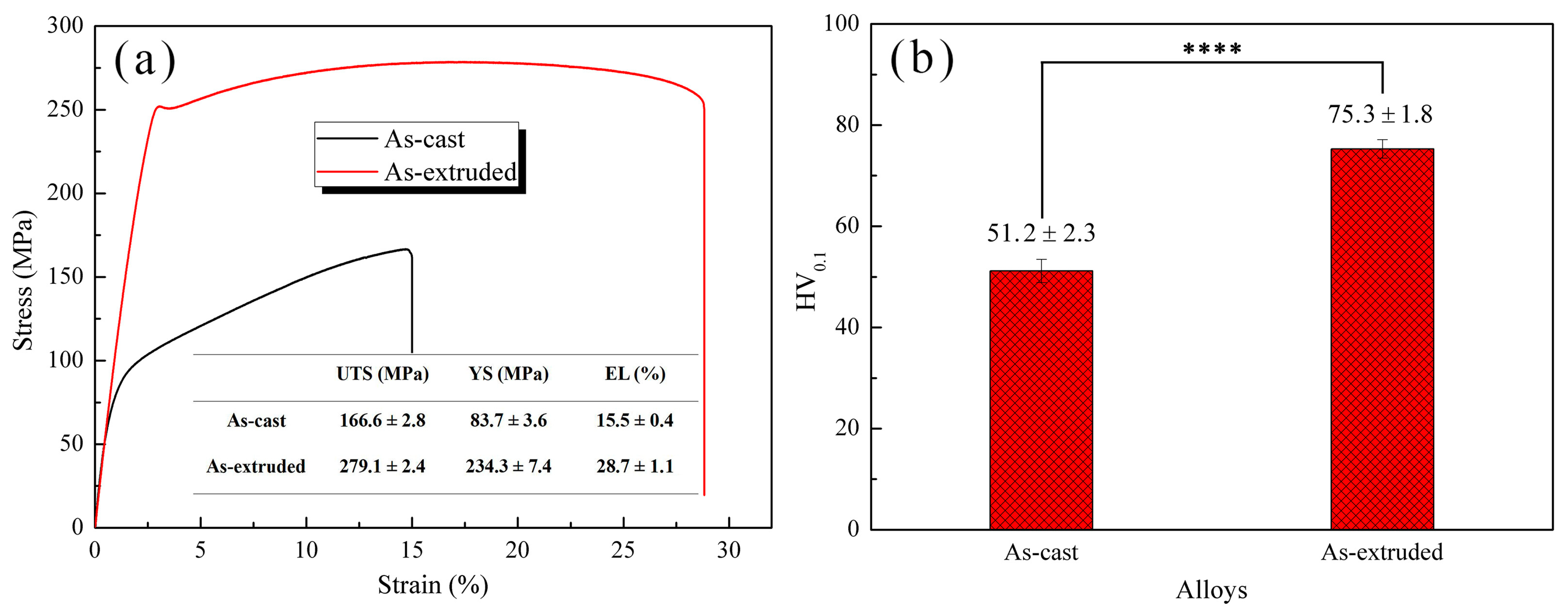
3.2. Mechanical Properties
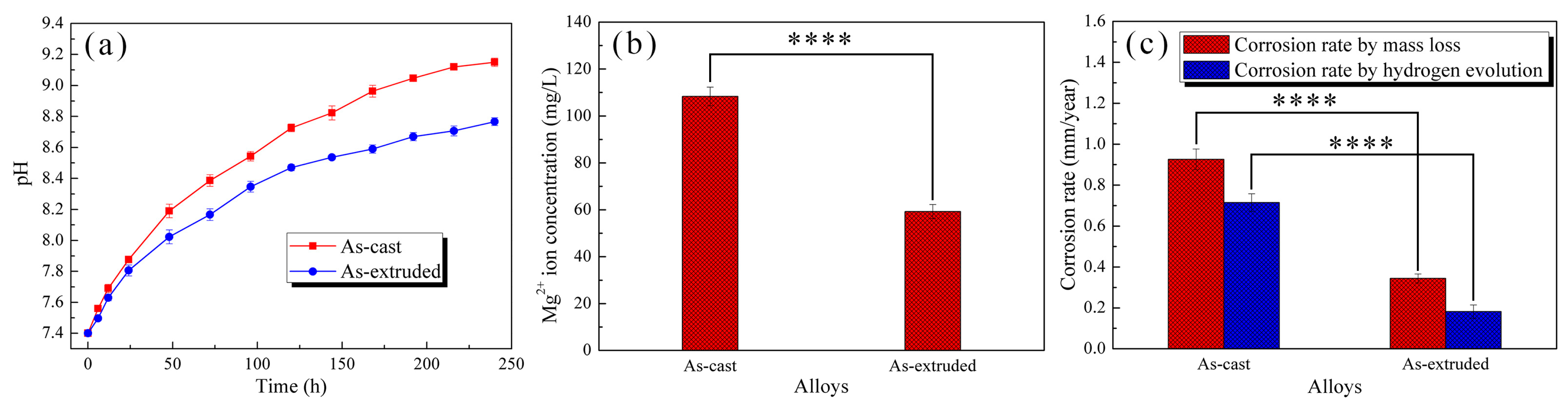
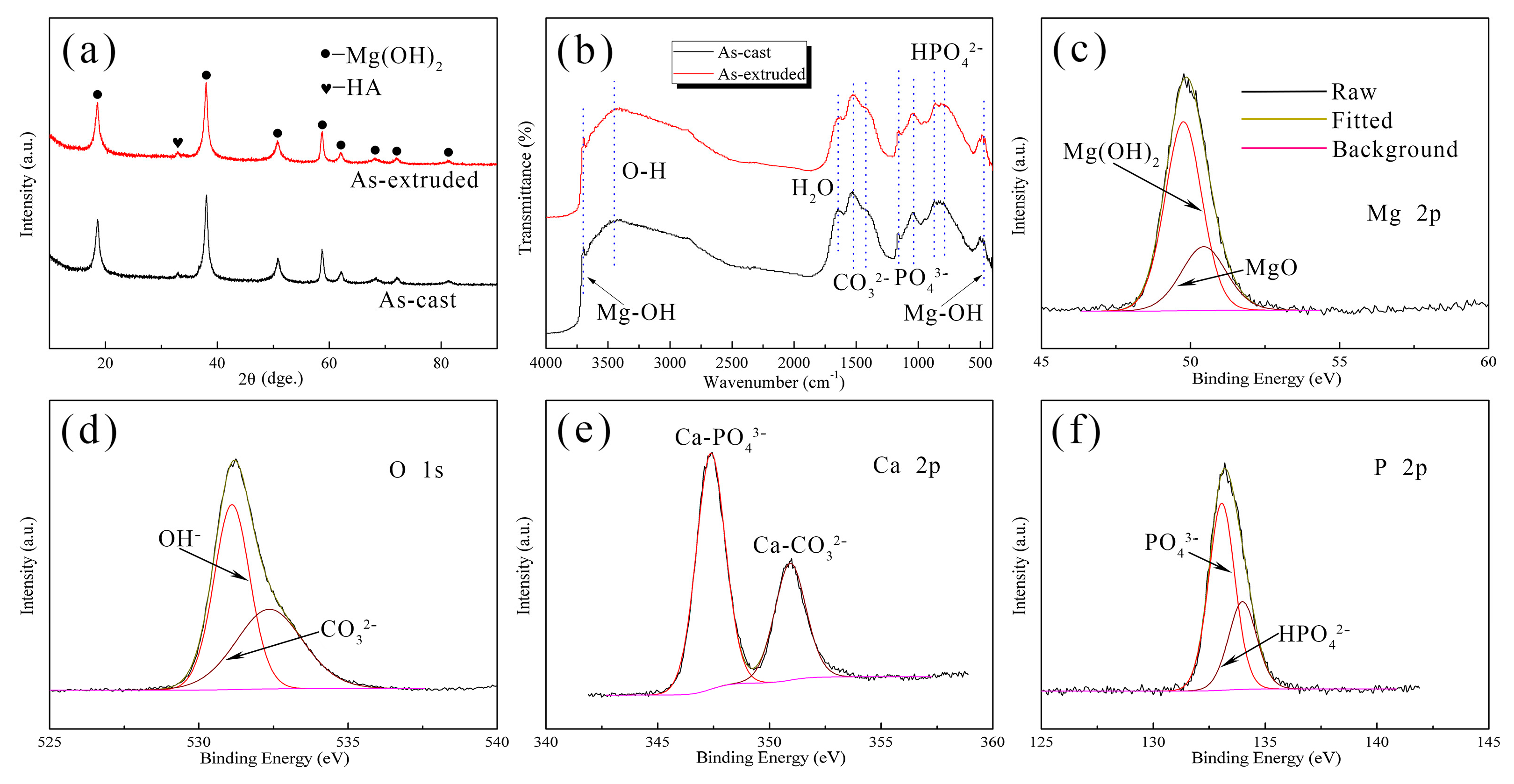
3.3. Degradation Behavior
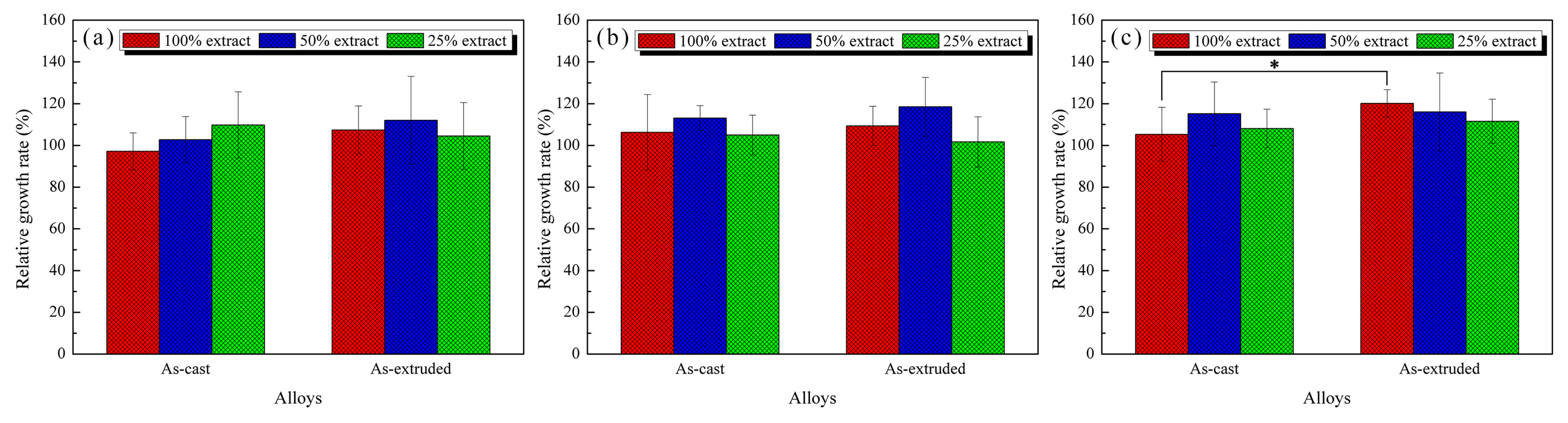
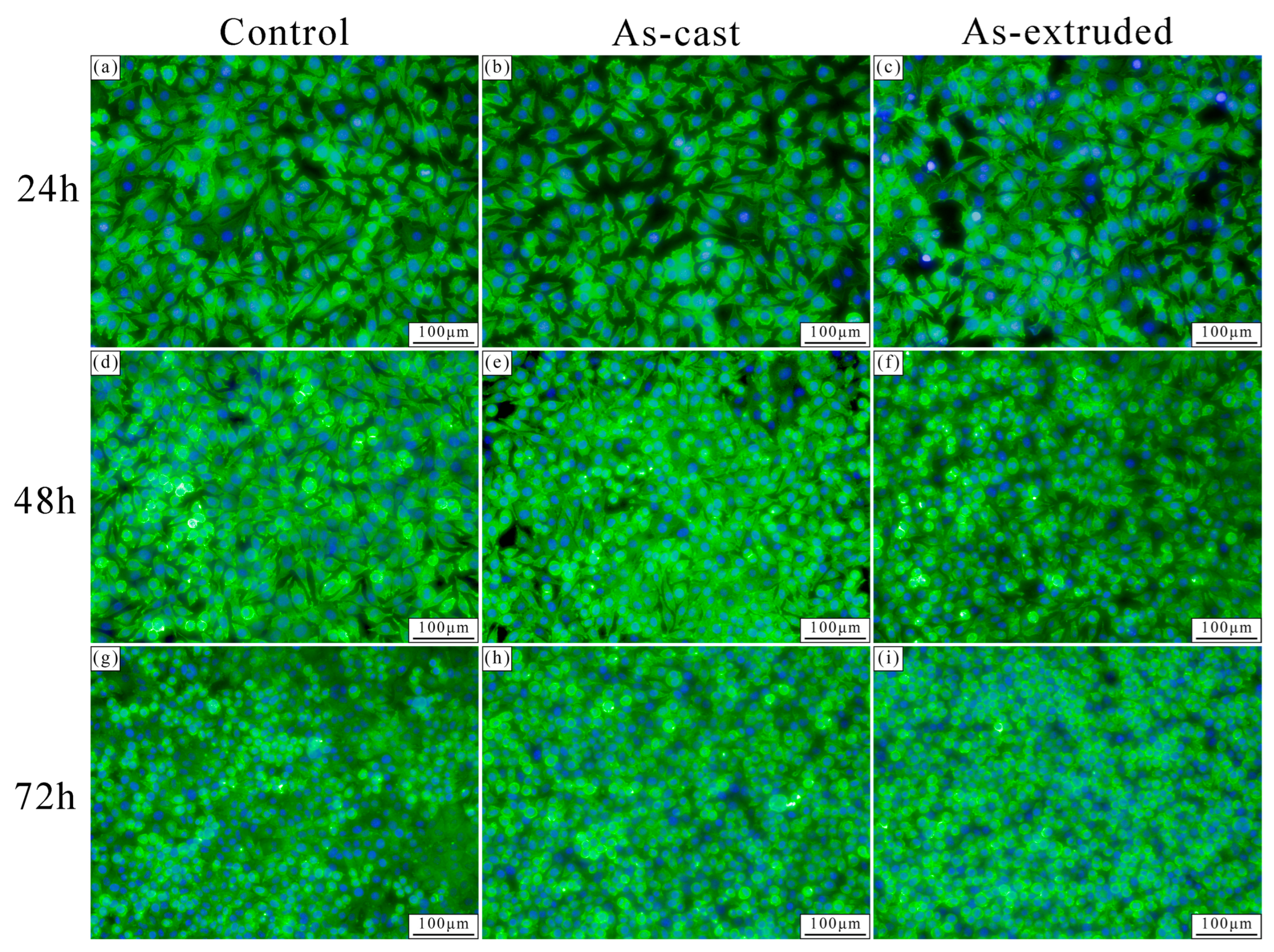
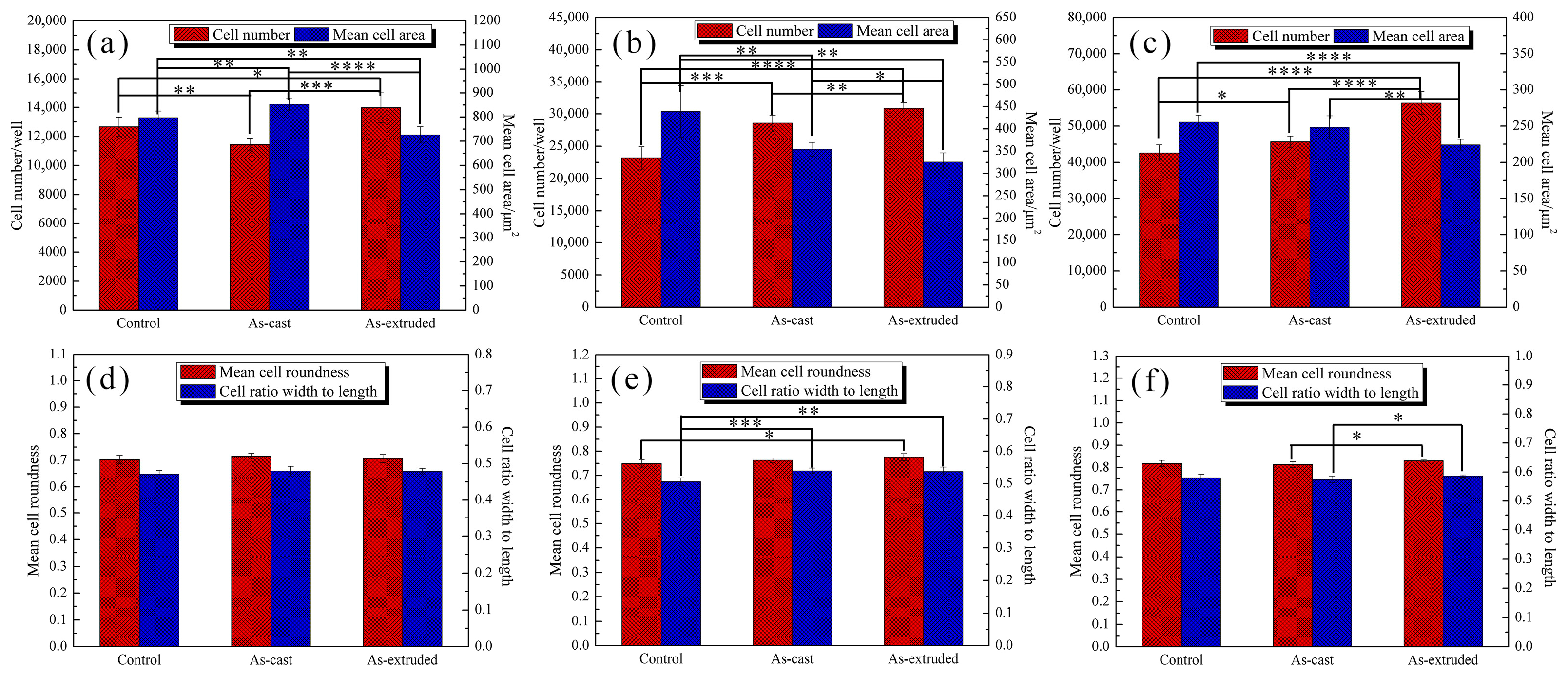
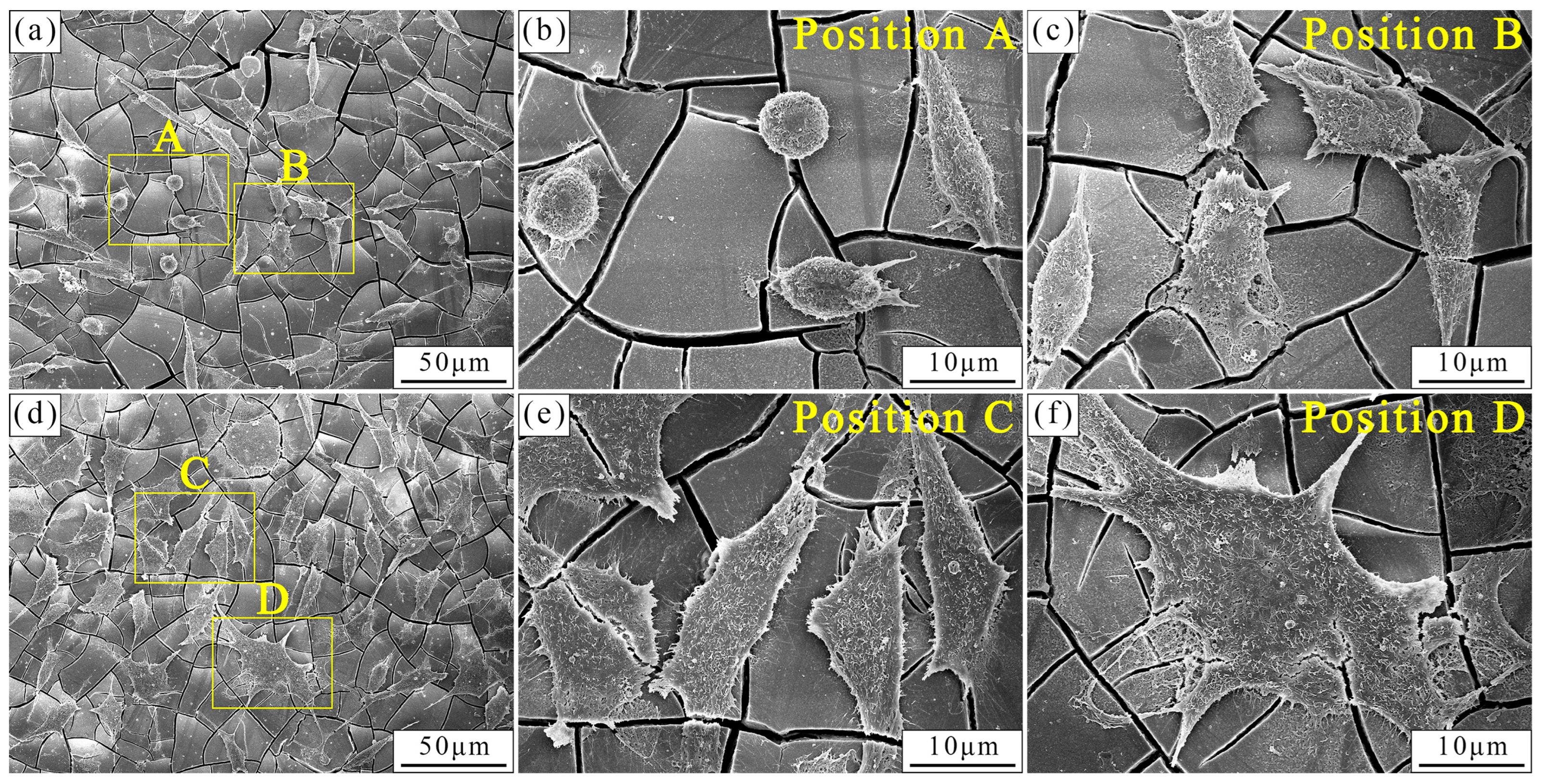
3.4. In Vitro Cytocompatibility
4. Discussion
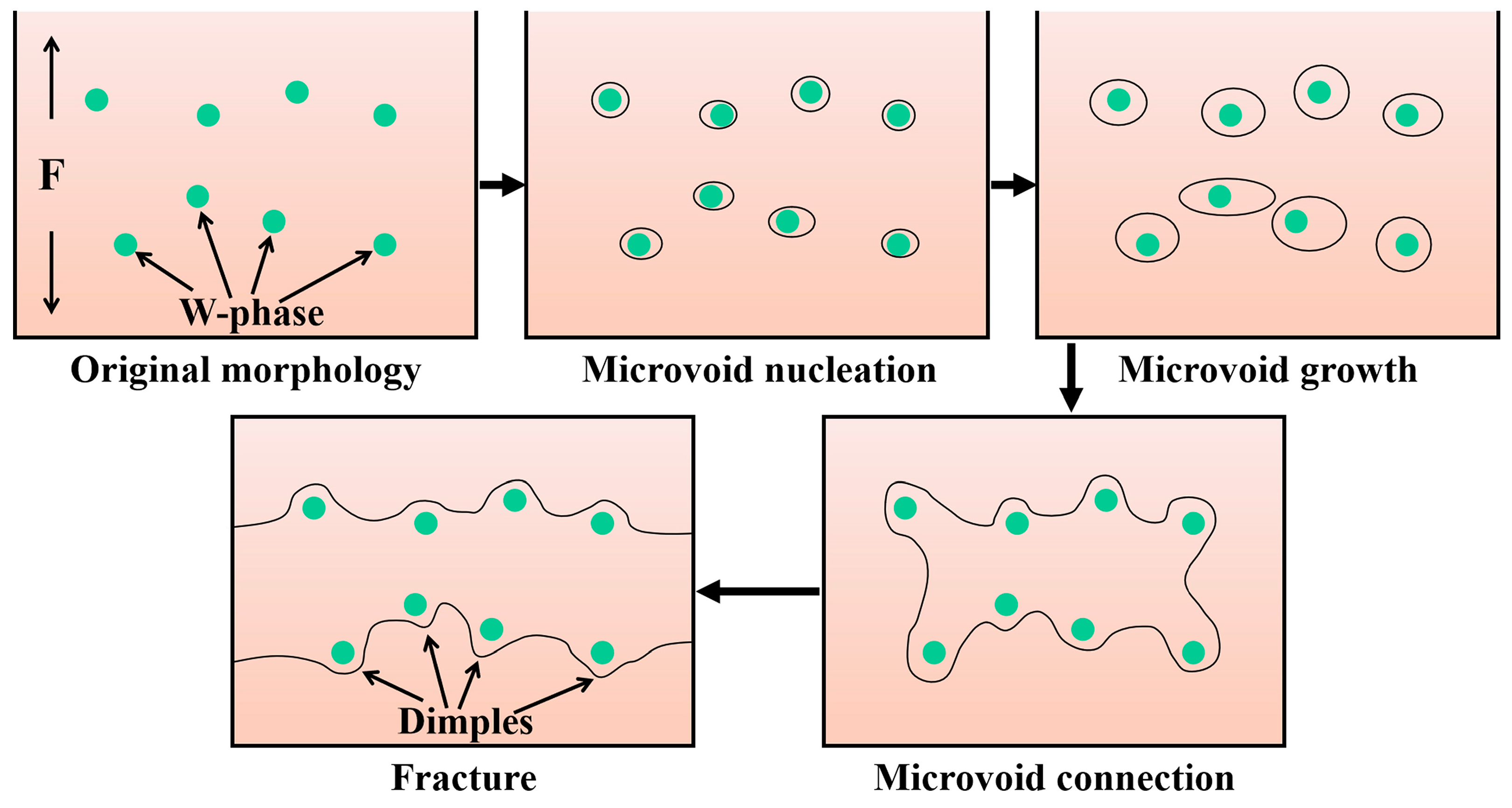
4.1. Evolution of Mechanical Properties of the As-Cast and As-Extruded Mg-1.5Zn-1.2Y-0.1Sr Alloys
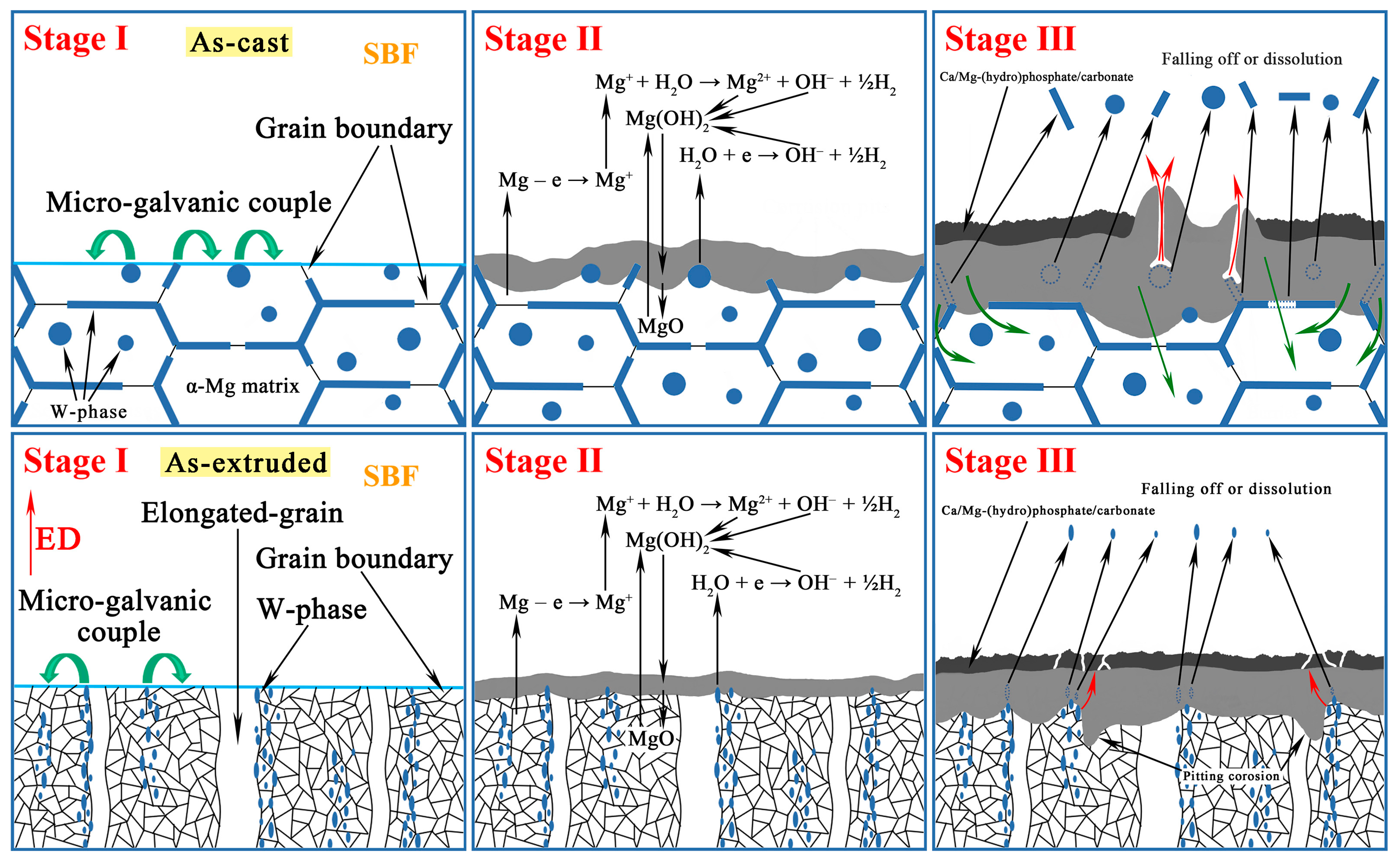
4.2. Corrosion Evolution of the As-Cast and As-Extruded Mg-1.5Zn-1.2Y-0.1Sr Alloys
4.3. Evolution of In Vitro Biocompatibility of the As-Cast and As-Extruded Mg-1.5Zn-1.2Y-0.1Sr Alloys
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bairagi, D.; Mandal, S. A comprehensive review on biocompatible Mg-based alloys as temporary orthopaedic implants: Current status, challenges, and future prospects. J. Magn. Alloys 2022, 10, 627–669. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloys Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Chen, X.B. Additive manufacturing of biodegradable magnesium-based materials: Design strategies, properties, and biomedical applications. J. Magn. Alloys 2023, 11, 801–839. [Google Scholar] [CrossRef]
- Dryhval, B.; Husak, Y.; Sulaieva, O.; Deineka, V.; Pernakov, M.; Lyndin, M.; Romaniuk, A.; Simka, W.; Pogorielov, M. In Vivo Safety of New Coating for Biodegradable Magnesium Implants. Materials 2023, 16, 5807. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Savaedi, Z.; Mirzadeh, H.; Aghdam, R.M.; Mahmudi, R. Thermal stability, grain growth kinetics, mechanical properties, and bio-corrosion resistance of pure Mg, ZK30, and ZEK300 alloys: A comparative study. Mater. Today Commun. 2022, 33, 104825. [Google Scholar] [CrossRef]
- Shafyra, S.; Nazim, E.M.; Ngadiman, N.H.A.; Sudin, I. Comparative Study on the Microstructure and Biodegradation Behavior of Commercialized Pure Mg and Mg-1.0Ca-0.5Sr Alloy in 27 mM HCO3−-SBF: The Influence of the pH Regulation Treatments. Metals 2023, 13, 136. [Google Scholar] [CrossRef]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef]
- Erinc, M.; Sillekens, W.H.; Mannens, R.G.T.M.; Werkhoven, R.J. Applicability of existing magnesium alloys as biomedical implant materials. In Proceedings of the Magnesium Technology Symposium, San Francisco, CA, USA, 15–19 February 2009; pp. 209–214. [Google Scholar]
- Seetharaman, S.; Sankaranarayanan, D.; Gupta, M. Magnesium-Based Temporary Implants: Potential, Current Status, Applications, and Challenges. J. Funct. Biomater. 2023, 14, 324. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xu, H.J.; Song, F.; Wen, P.; Tian, Y.; Zheng, Y.F. Additive manufacturing of porous magnesium alloys for biodegradable orthopedic implants: Process, design, and modification. J. Mater. Sci. Technol. 2023, 182, 79–110. [Google Scholar] [CrossRef]
- Gu, X.N.; Zheng, Y.F.; Cheng, Y.; Zhong, S.P.; Xi, T.F. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Dong, J.; Tümer, N.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zadpoor, A.A.; Zhou, J. Extrusion-based additive manufacturing of Mg-Zn alloy scaffolds. J. Magn. Alloys 2022, 10, 2491–2509. [Google Scholar] [CrossRef]
- Ci, W.J.; Chen, X.H.; Sun, Y.; Dai, X.; Zhu, G.Z.; Zhao, D.; Pan, F.S. Effect of Zn on mechanical and corrosion properties of Mg-Sc-Zn alloys. J. Mater. Sci. Technol. 2023, 158, 31–42. [Google Scholar] [CrossRef]
- Ghorbani, F.; Mirzadeh, H.; Dehghanian, C.; Emamy, M. Mechanical Properties and Corrosion Behavior of Biodegradable Mg–0.5Zr–0.5Ca–xZn Magnesium Alloys. Adv. Eng. Mater. 2023, 25, 2201778. [Google Scholar] [CrossRef]
- Jiang, H.S.; Qiao, X.G.; Xu, C.; Kamado, S.; Wu, K.; Zheng, M.Y. Influence of size and distribution of W phase on strength and ductility of high strength Mg-5.1Zn-3.2Y-0.4Zr-0.4Ca alloy processed by indirect extrusion. J. Mater. Sci. Technol. 2018, 34, 277–283. [Google Scholar] [CrossRef]
- Panemangalore, D.B.; Shabadi, R.; Gupta, M.; Lesven, L. Microstructure and Corrosion Behavior of Extruded Mg-Sn-Y Alloys. Metals 2021, 11, 1095. [Google Scholar] [CrossRef]
- Cheng, M.X.; Chen, J.H.; Yan, H.G.; Su, B.; Yu, Z.H.; Xia, W.J.; Gong, X.L. Effects of minor Sr addition on microstructure, mechanical and bio-corrosion properties of the Mg-5Zn based alloy system. J. Alloys Compd. 2017, 691, 95–102. [Google Scholar] [CrossRef]
- Kiani, F.; Lin, J.X.; Vahid, A.; Munir, K.; Wen, C.E.; Li, Y.C. Mechanical and corrosion properties of extruded Mg–Zr–Sr alloys for biodegradable implant applications. Mat. Sci. Eng. A-Struct. 2021, 831, 142192. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Cipriano, A.F.; Guan, R.G.; Zhao, Z.Y.; Liu, H.N. In vitro interactions of blood, platelet, and fibroblast with biodegradable magnesium-zinc-strontium alloys. J. Biomed. Mater. Res. A 2015, 103, 2974–2986. [Google Scholar] [CrossRef] [PubMed]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg–Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.Z.; Kang, Z.X.; Li, Y.Y. Evolution of the microstructure and fracture characteristics of a Mg-Nd-Zn-Zr-Mn alloy through heat treatment and extrusion. J. Alloys Compd. 2018, 765, 470–479. [Google Scholar] [CrossRef]
- Shi, Q.F.; Wu, H.S.; Gao, Z.S.; Wang, D.S.; Wang, J.W.; Yang, Y.W.; Li, R.X. The Synergistic Effect of Trace Ag and Hot Extruding on the Microstructure and Properties of a Biodegradable Mg-Zn-Sr-Ag Alloy. Materials 2023, 16, 6423. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Tehrani, A.H.M.; Tehrani, M.; Heydari, M. Effect of extrusion process on microstructure and mechanical and corrosion properties of biodegradable Mg-5Zn-1.5Y magnesium alloy. Int. J. Min. Met. Mater. 2022, 29, 490–502. [Google Scholar] [CrossRef]
- Wu, X.F.; Xu, C.X.; Kuan, J.; Zhang, Z.W.; Zhang, J.S.; Yang, W.F. Effects of Hot Extrusion Temperature on Mechanical and Corrosion Properties of Mg-Y-Zn-Zr Biological Magnesium Alloy Containing W Phase and I Phase. Materials 2020, 13, 1147. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xu, C.X.; Zhang, Z.W.; Yang, W.F.; Zhang, J.S. Effect of I-phase morphology and microstructure transformation in biomedical Mg-3Zn-1Mn-1Y alloys on vitro degradation behavior in dynamic simulated body fluid. J. Mater. Sci. 2021, 56, 12394–12411. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Understanding Magnesium Corrosion, A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2010, 5, 837–858. [Google Scholar] [CrossRef]
- Yin, S.Q.; Duan, W.C.; Liu, W.H.; Wu, L.; Yu, J.M.; Zhao, Z.L.; Liu, M.; Wang, P.; Cui, J.Z.; Zhang, Z.Q. Influence of specific second phases on corrosion behaviors of Mg-Zn-Gd-Zr alloys. Corros. Sci. 2019, 166, 108419. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Lotfpour, M.; Dehghanian, C.; Emamy, M.; Bahmani, A.; Malekan, M.; Saadati, A.; Taghizadeh, M.; Shokouhimehr, M. In-vitro corrosion behavior of the cast and extruded biodegradable Mg-Zn-Cu alloys in simulated body fluid (SBF). J. Magn. Alloys 2021, 9, 2078–2096. [Google Scholar] [CrossRef]
- Ayer, O. Effect of die parameters on the grain size, mechanical properties and fracture mechanism of extruded AZ31 magnesium alloys. Mat. Sci. Eng. A-Struct. 2020, 793, 139887. [Google Scholar] [CrossRef]
- El-Mahallawy, N.; Palkowski, H.; Breitinger, H.G.; Klingner, A.; Shoeib, M.; Diaa, A. Microstructure, mechanical properties, cytotoxicity, and bio-corrosion of micro-alloyed Mg–xSn–0.04Mn alloys for biodegradable orthopedic applications: Effect of processing techniques. J. Mater. Res. 2021, 36, 1456–1474. [Google Scholar] [CrossRef]
- Zhang, X.B.; Yuan, G.Y.; Wang, Z.Z. Mechanical properties and biocorrosion resistance of Mg-Nd-Zn-Zr alloy improved by cyclic extrusion and compression. Mater. Lett. 2012, 74, 128–131. [Google Scholar] [CrossRef]
- Liu, J.W.; Peng, X.D.; Li, M.L.; Wei, G.B.; Xie, W.D.; Yang, Y. Effect of Sr addition on microstructure and elevated temperature mechanical properties of Mg–3Zn–1Y alloy. Mat. Sci. Eng. A-Struct. 2016, 655, 331–338. [Google Scholar] [CrossRef]
- Chen, X.H.; Liu, L.Z.; Liu, J.; Pan, F.S. Microstructure, electromagnetic shielding effectiveness and mechanical properties of Mg–Zn–Y–Zr alloys. Mater. Design 2015, 65, 360–369. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1999, 41, 249–273. [Google Scholar] [CrossRef]
- Jamalpour, M.; Alizadeh, R. Experimental insights toward understanding how the morphology of Mg2Si particles affects degradation behavior of the biodegradable as-cast Mg–Si alloys. Intermetallics 2024, 165, 108133. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, R.C.; Peng, C.Q.; Cai, Z.Y. Microstructure and corrosion behavior of as-extruded Mg-xLi-3Al-2Zn-0.2Zr alloys (x = 5, 8, 11wt.%). Corros. Sci. 2020, 167, 108487. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Liu, X.; Hu, W.Y.; Li, J.H.; Le, Q.C.; Bao, L.; Zhu, Z.J.; Cui, J.Z. Microstructures, mechanical properties and corrosion behaviors of Mg–Y–Zn–Zr alloys with specific Y/Zn mole ratios. J. Alloys Compd. 2015, 624, 116–125. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Design 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Ma, Y.L.; Xiong, H.W.; Chen, B.Y. Effect of heat treatment on microstructure and corrosion behavior of Mg-5Al-1Zn-1Sn magnesium alloy. Corros. Sci. 2021, 191, 109759. [Google Scholar] [CrossRef]
- Kumar, R.; Mehrotra, N.; Pal, K. Effect of friction stir processing on mechanical, in vitro degradation, and biocompatibility behaviour of stir casted Mg-Zn-rare earth oxide composites for biodegradable implant applications. J. Alloys Compd. 2024, 972, 172767. [Google Scholar] [CrossRef]
- Pebere, N.; Riera, C.; Dabosi, F. Investigation of magnesium corrosion in aerated sodium sulfate solution by electrochemical impedance spectroscopy. Electrochim. Acta 1990, 35, 555–561. [Google Scholar] [CrossRef]
- Udhayan, R.; Bhatt, D.P. On the corrosion behaviour of magnesium and its alloys using electrochemical techniques. J. Power Sources 1996, 63, 103–107. [Google Scholar] [CrossRef]
- Li, J.R.; Jiang, Q.T.; Sun, H.Y.; Li, Y.T. Effect of heat treatment on corrosion behavior of AZ63 magnesium alloy in 3.5wt.% sodium chloride solution. Corros. Sci. 2016, 111, 288–301. [Google Scholar] [CrossRef]
- Cao, K.Y.; Yu, Z.X.; Zhu, L.J.; Yin, D.; Chen, L.G.; Jiang, Y.; Wang, J. Fabrication of superhydrophobic layered double hydroxide composites to enhance the corrosion-resistant performances of epoxy coatings on Mg alloy. Surf. Coat. Technol. 2021, 407, 126763. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, G.M.; Zhang, Y.H.; Zhao, Q. Growth and characterization of Mg(OH)2 film on magnesium alloy AZ31. Appl. Surf. Sci. 2011, 257, 6129–6137. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.C.; Lin, J.L.; Zhou, M.; Yu, F.; Huang, Y.C.; Kang, B.; Wang, D.L.; Wang, T.B.; Zeng, H. Alloying and brushite coating improve corrosion resistance of magnesium in a simulated physiological environment. Mater. Today Commun. 2021, 26, 101750. [Google Scholar] [CrossRef]
- Guo, Y.T.; Su, Y.C.; Gu, R.; Zhang, Z.H.; Li, G.Y.; Lian, J.S.; Ren, L.Q. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Trommer, R.M.; Santos, L.A.; Bergmann, C.P. Nanostructured hydroxyapatite powders produced by a flame-based technique. Mat. Sci. Eng. C 2009, 29, 1770–1775. [Google Scholar] [CrossRef]
- Bairagi, D.; Duley, P.; Paliwal, M.; Mandal, S. Influence of second phase precipitates on mechanical and in-vitro corrosion behaviour of Mg-4Zn-0.5Ca-0.8Mn alloy in optimum homogenized conditions. J. Magn. Alloys 2023, 11, 1343–1366. [Google Scholar] [CrossRef]
- Baril, G.; Galicia, G.; Deslouis, C.; Pébère, N.; Tribollet, B.; Vivier, V. An impedance investigation of the mechanism of pure magnesium corrosion in sodium sulfate solutions. J. Electrochem. Soc. 2007, 154, C108–C113. [Google Scholar] [CrossRef]
- Aswal, D.K.; Muthe, K.P.; Tawde, S.; Chodhury, S.; Bagkar, N.; Singh, A.; Gupta, S.K.; Yakhmi, J.V. XPS and AFM investigations of annealing induced surface modifications of MgO single crystals. J. Cryst. Growth 2002, 236, 661–666. [Google Scholar] [CrossRef]
- Pan, H.; Pang, K.; Cui, F.Z.; Ge, F.; Man, C.; Wang, X.; Cui, Z.Y. Effect of alloyed Sr on the microstructure and corrosion behavior of biodegradable Mg-Zn-Mn alloy in Hanks’ solution. Corros. Sci. 2019, 157, 420–437. [Google Scholar] [CrossRef]
- Hiromoto, S.; Tsai, A.P.; Sumita, M.; Hanawa, T. Effect of chloride ion on the anodic polarization behavior of the Zr65Al7.5Ni10Cu17.5 amorphous alloy in phosphate buffered solution. Corros. Sci. 2000, 42, 1651–1660. [Google Scholar] [CrossRef]
- Cipriano, A.F.; Sallee, A.; Tayoba, M.; Alcaraz, M.C.C.; Lin, A.; Guan, R.G.; Zhao, Z.Y.; Liu, H.N. Cytocompatibility and early inflammatory response of human endothelial cells in direct culture with Mg-Zn-Sr alloys. Acta Biomater. 2017, 48, 499–520. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, J.W. Enhancement of mechanical properties and corrosion resistance of Mg–Ca alloys through microstructural refinement by indirect extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. Effect of heat treatment on microstructure, mechanical, corrosion and biocompatibility of Mg-Zn-Zr-Gd-Nd alloy. J. Alloys Compd. 2020, 821, 153462. [Google Scholar] [CrossRef]
- Zhen, Z.; Liu, X.L.; Huang, T.; Xi, T.F.; Zheng, Y.F. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mat. Sci. Eng. C 2015, 46, 202–206. [Google Scholar] [CrossRef]
- Schmalz, G.; Langer, H.; Schweikl, H. Cytotoxicity of dental alloy extracts and corresponding metal salt solutions. J. Dent. Res. 1998, 77, 1772–1778. [Google Scholar] [CrossRef]
- Yamamoto, A.; Honma, R.; Sumita, M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998, 39, 331–340. [Google Scholar] [CrossRef]


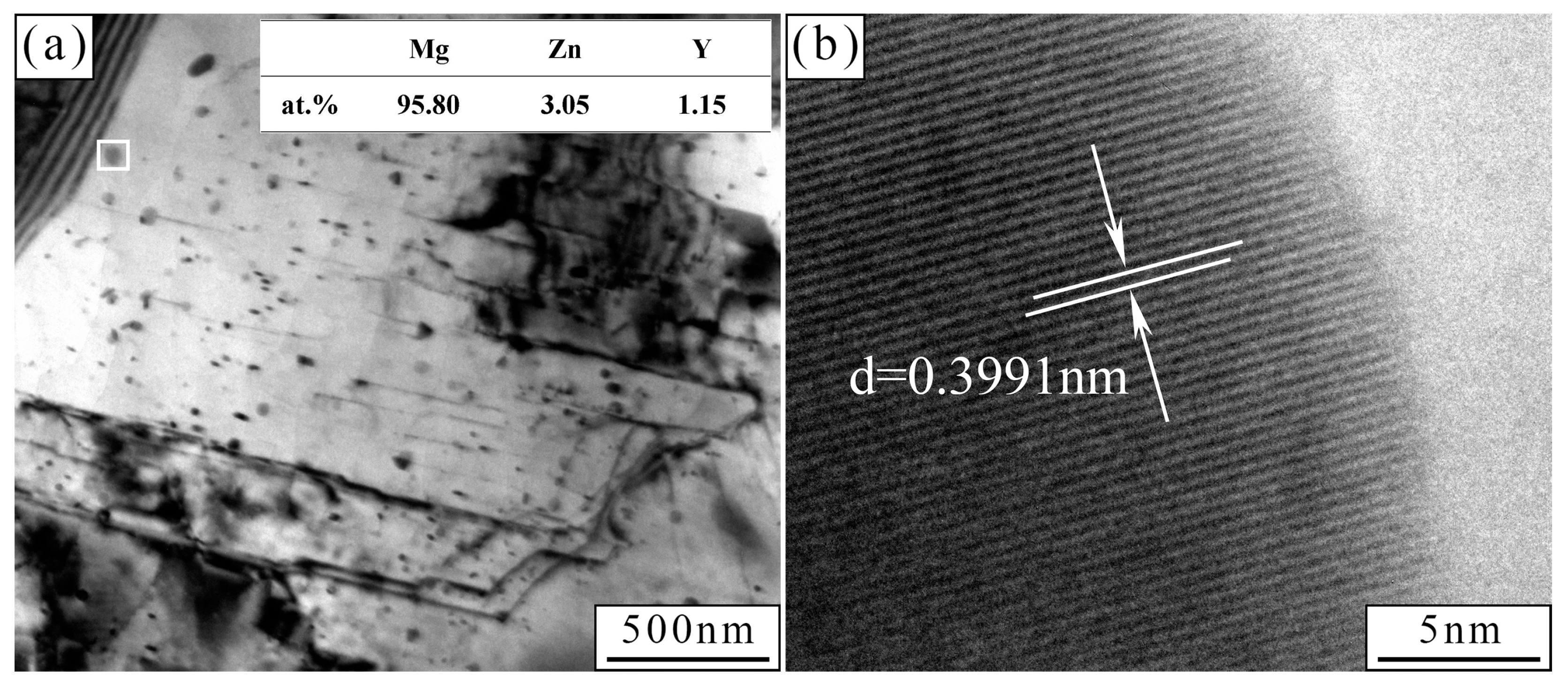

| Zn | Y | Sr | Fe | Si | Mn | Al | Mg |
|---|---|---|---|---|---|---|---|
| 1.4724 | 1.1923 | 0.1028 | 0.0036 | 0.0027 | 0.0018 | 0.0009 | Bal. |
| Alloys | Ecorr (V) | Jcorr (μA/cm2) | Ept (V) |
|---|---|---|---|
| As-cast | −1.625 ± 0.007 | 45.548 ± 3.191 | −1.510 ± 0.009 |
| As-extruded | −1.584 ± 0.012 | 17.492 ± 1.570 | −1.549 ± 0.011 |
| Samples | Rs (Ωcm2) | CPEf (snΩ−1cm−2) | Rf (Ωcm2) | n1 | CPEdl (snΩ−1cm−2) | Rct (Ωcm2) | n2 | RL (Ωcm2) | L (Hcm2) |
|---|---|---|---|---|---|---|---|---|---|
| As-cast | 7.331 | 1.87 × 10−5 | 85.07 | 0.72 | 1.37 × 10−5 | 5993 | 0.86 | 9067 | 755,160 |
| As-extruded | 13.990 | 2.58 × 10−5 | 306.6 | 0.65 | 5.00 × 10−6 | 9218 | 0.94 | 26,266 | 915,480 |
| Positions | C | O | Mg | P | Ca | Na | Cl |
|---|---|---|---|---|---|---|---|
| I | 8.91 | 69.00 | 21.81 | 0.09 | - | 0.19 | - |
| II | 6.71 | 69.52 | 13.87 | 5.26 | 4.25 | - | 0.39 |
| III | 6.90 | 67.58 | 7.36 | 8.56 | 9.19 | 0.41 | - |
| IV | 7.54 | 67.43 | 24.39 | 0.48 | 0.16 | - | - |
| V | 6.15 | 61.24 | 15.13 | 8.00 | 8.80 | 0.53 | 0.15 |
| VI | 7.29 | 65.99 | 7.03 | 9.00 | 10.23 | 0.46 | - |
| VII | 5.85 | 60.63 | 15.76 | 7.97 | 9.27 | 0.52 | - |
| VIII | 8.62 | 67.74 | 22.57 | 0.49 | 0.58 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, T.; Yang, L.; Xu, C.; Xu, C.; Zhang, Q.; Zhang, J.; Song, Z. Effect of Extrusion on Mechanical Property, Corrosion Behavior, and In Vitro Biocompatibility of the As-Cast Mg-Zn-Y-Sr Alloy. Materials 2024, 17, 1297. https://doi.org/10.3390/ma17061297
Huang T, Yang L, Xu C, Xu C, Zhang Q, Zhang J, Song Z. Effect of Extrusion on Mechanical Property, Corrosion Behavior, and In Vitro Biocompatibility of the As-Cast Mg-Zn-Y-Sr Alloy. Materials. 2024; 17(6):1297. https://doi.org/10.3390/ma17061297
Chicago/Turabian StyleHuang, Tao, Lijing Yang, Chunxiang Xu, Cheng Xu, Qingke Zhang, Jinshan Zhang, and Zhenlun Song. 2024. "Effect of Extrusion on Mechanical Property, Corrosion Behavior, and In Vitro Biocompatibility of the As-Cast Mg-Zn-Y-Sr Alloy" Materials 17, no. 6: 1297. https://doi.org/10.3390/ma17061297





