Impact of Metal Salt Oxidants and Preparation Technology on Efficacy of Bacterial Cellulose/Polypyrrole Flexible Conductive Fiber Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of BC/PPy Conductive Fiber Membrane
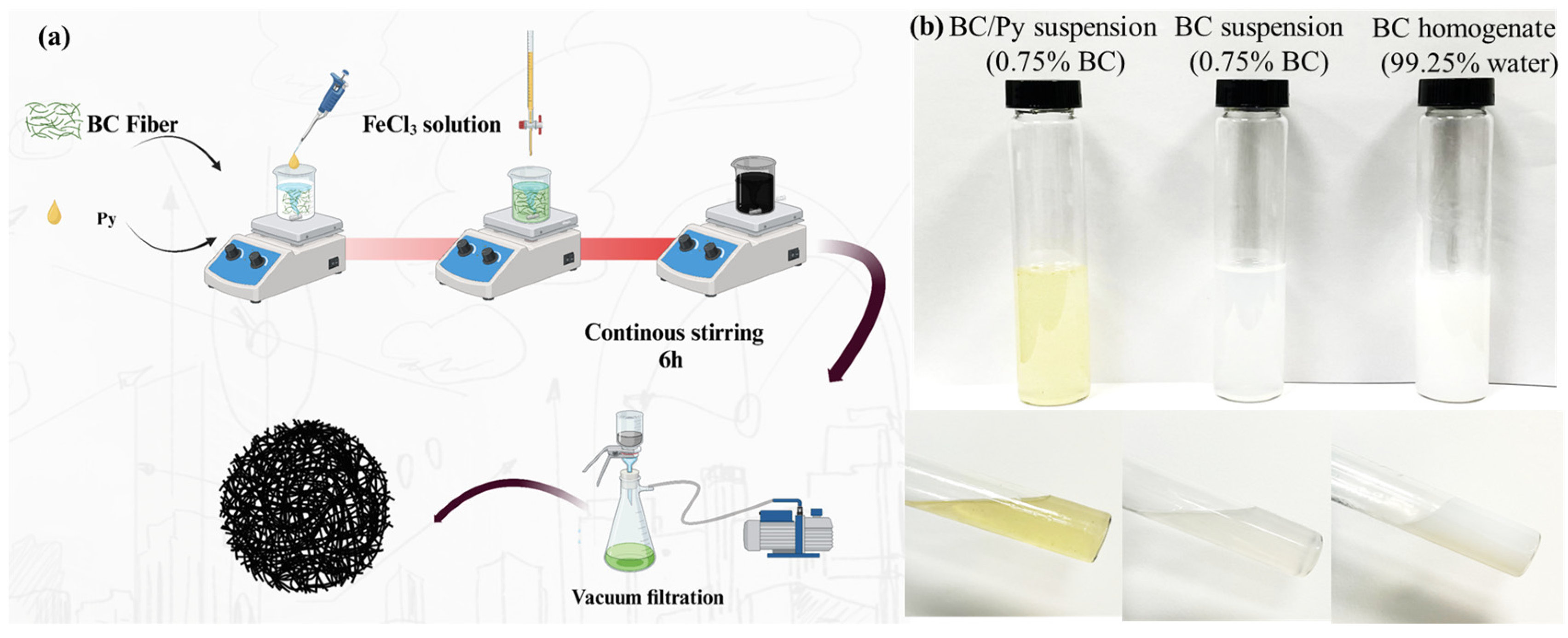
2.2.1. BC/PPy Conductive Fiber Membrane Prepared by In Situ Polymerization
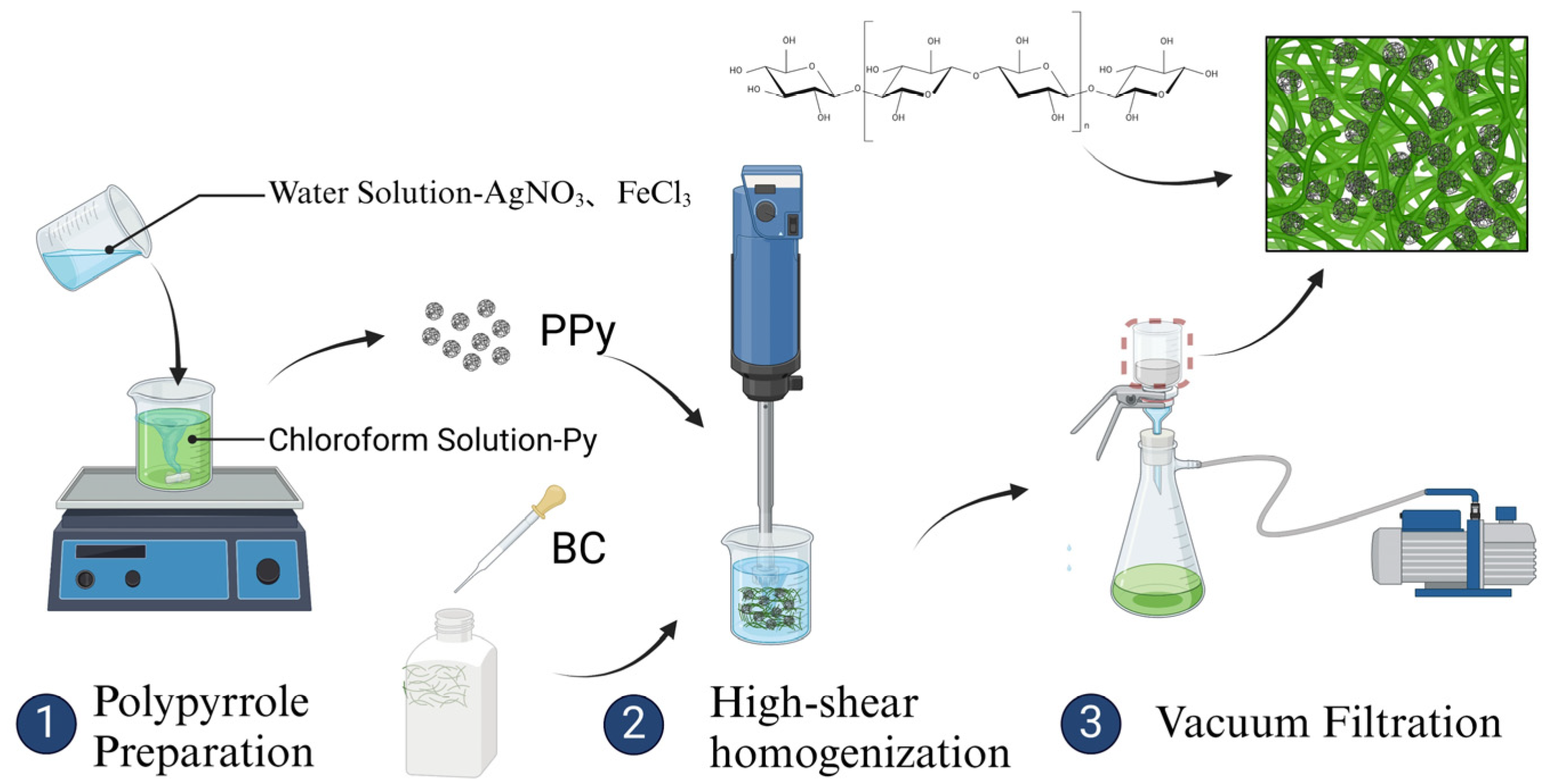
2.2.2. BC/PPy Fiber Membrane Prepared by Co-Blended Method
2.3. Characterization
2.3.1. FTIR Analysis
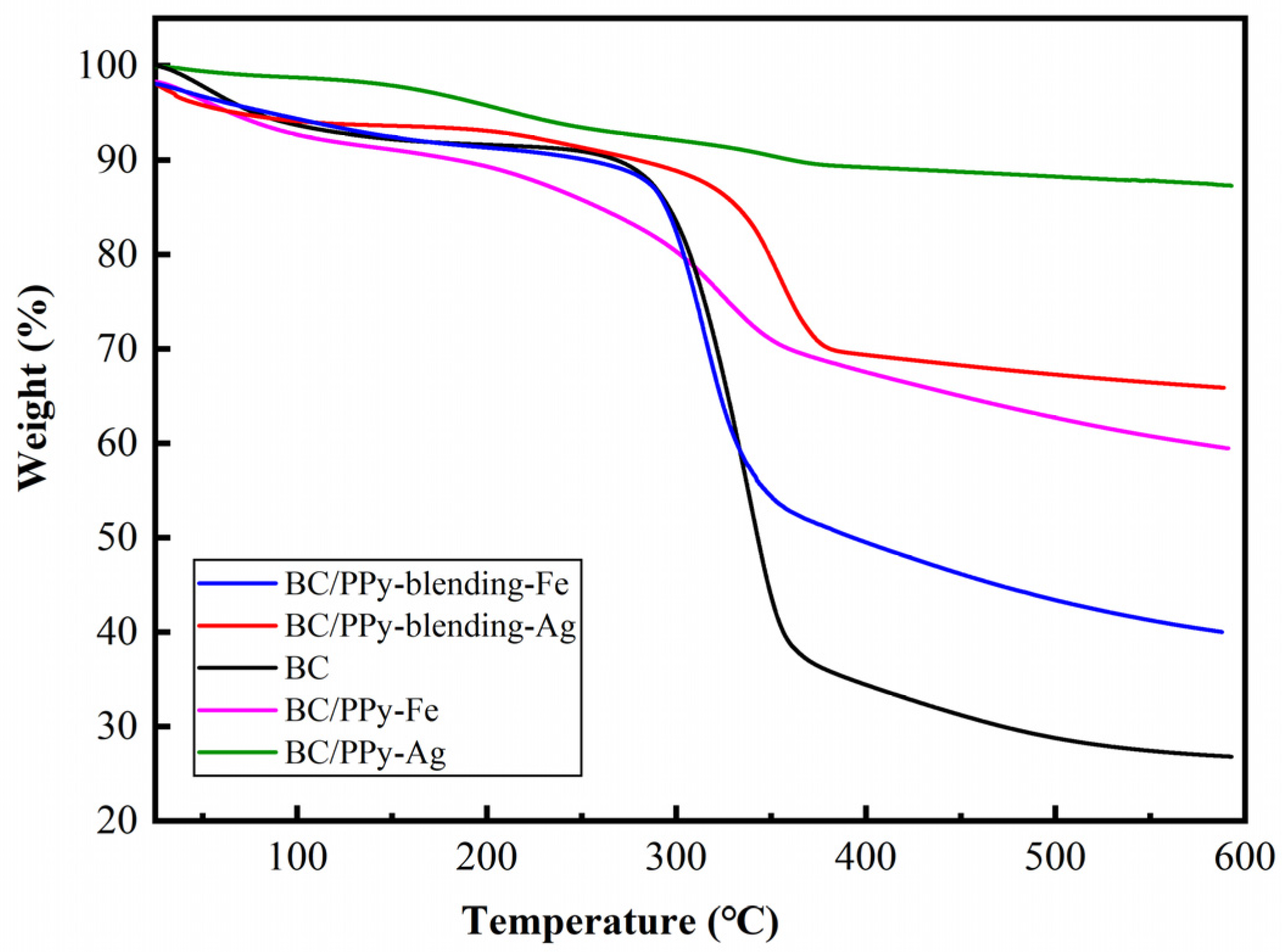
2.3.2. Thermogravimetric Analysis
2.3.3. SEM Analysis
2.3.4. XPS Analysis
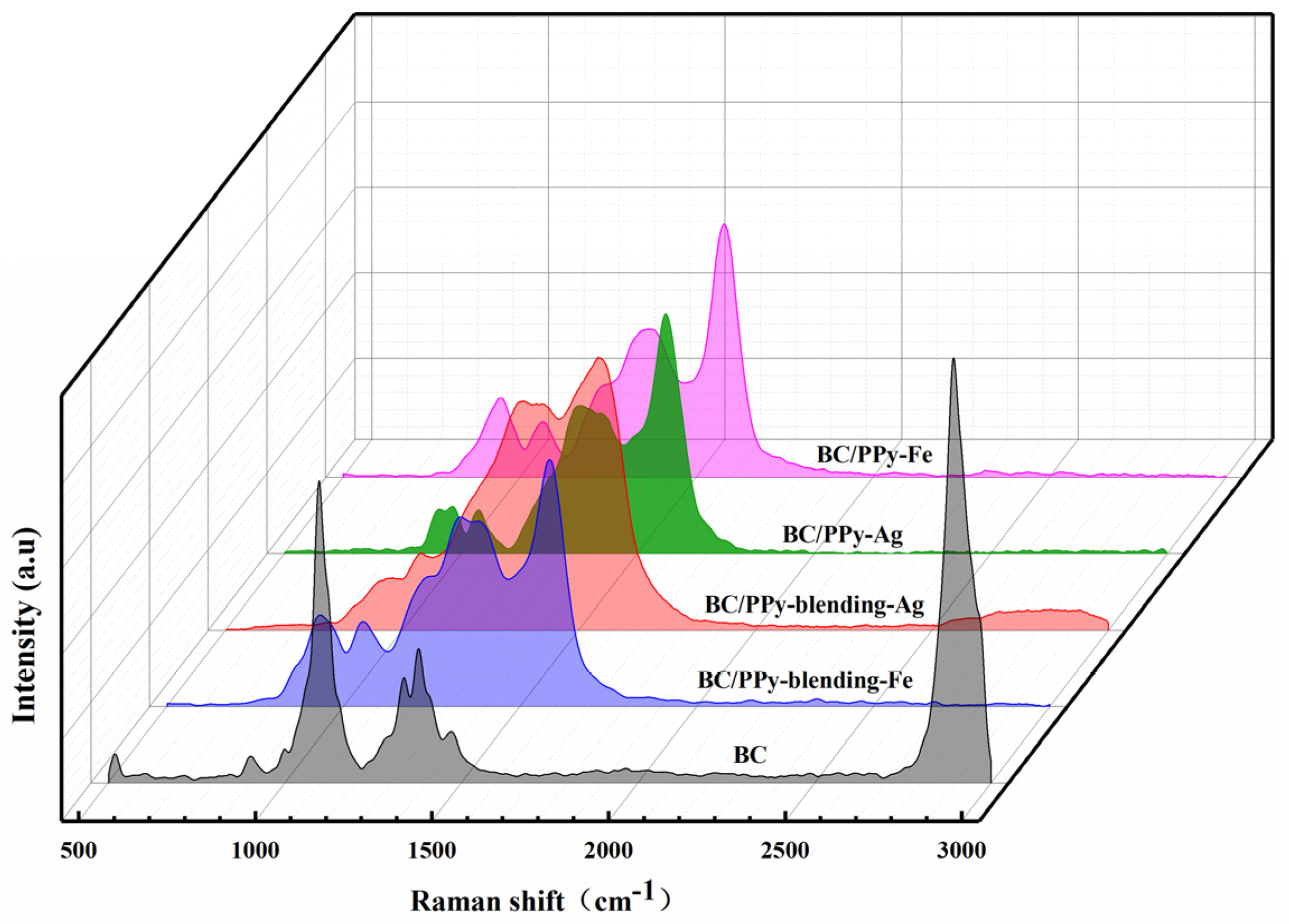
2.3.5. Raman Analysis
2.3.6. Resistance Testing
2.3.7. Tensile Strength Testing
3. Results and Discussion
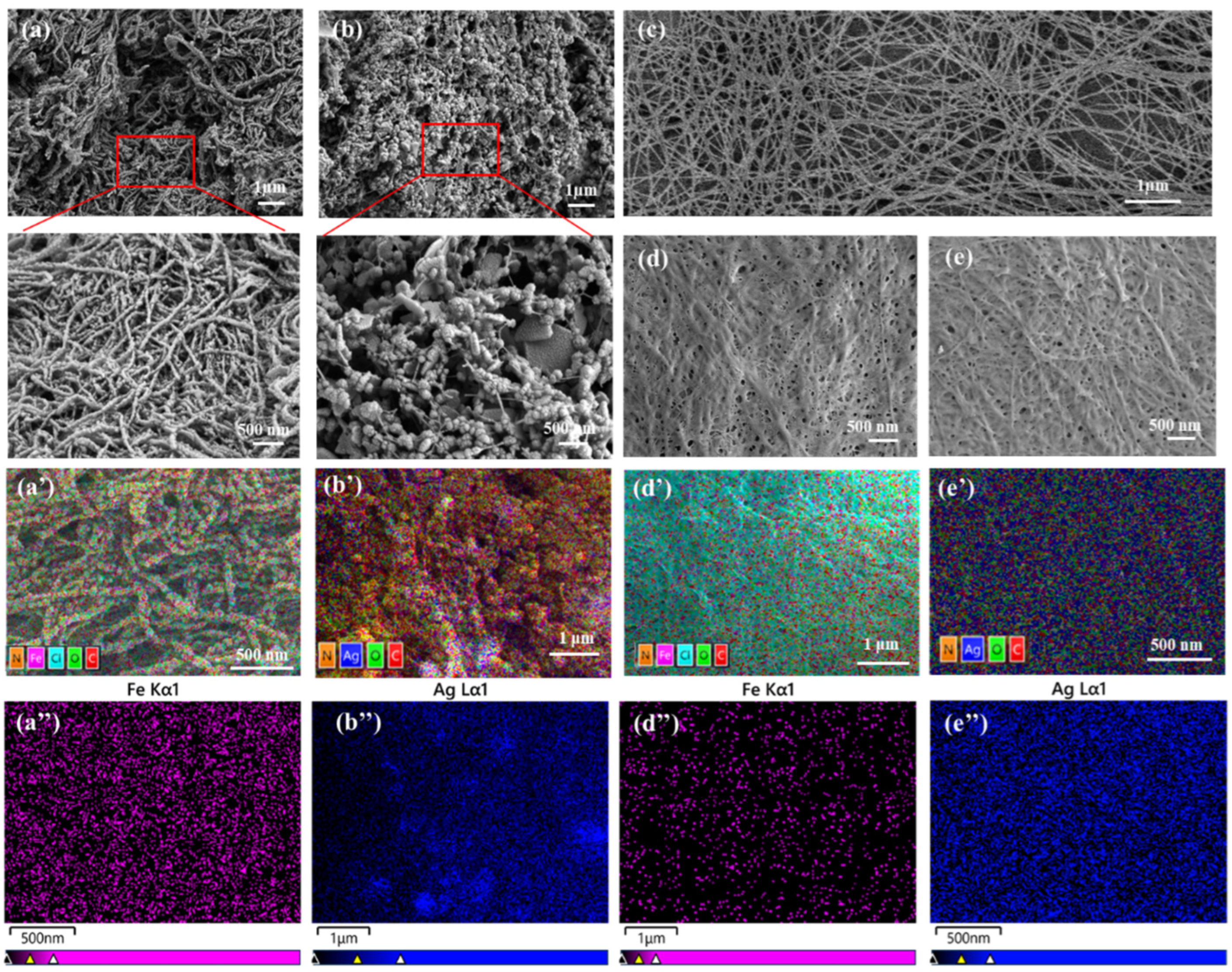
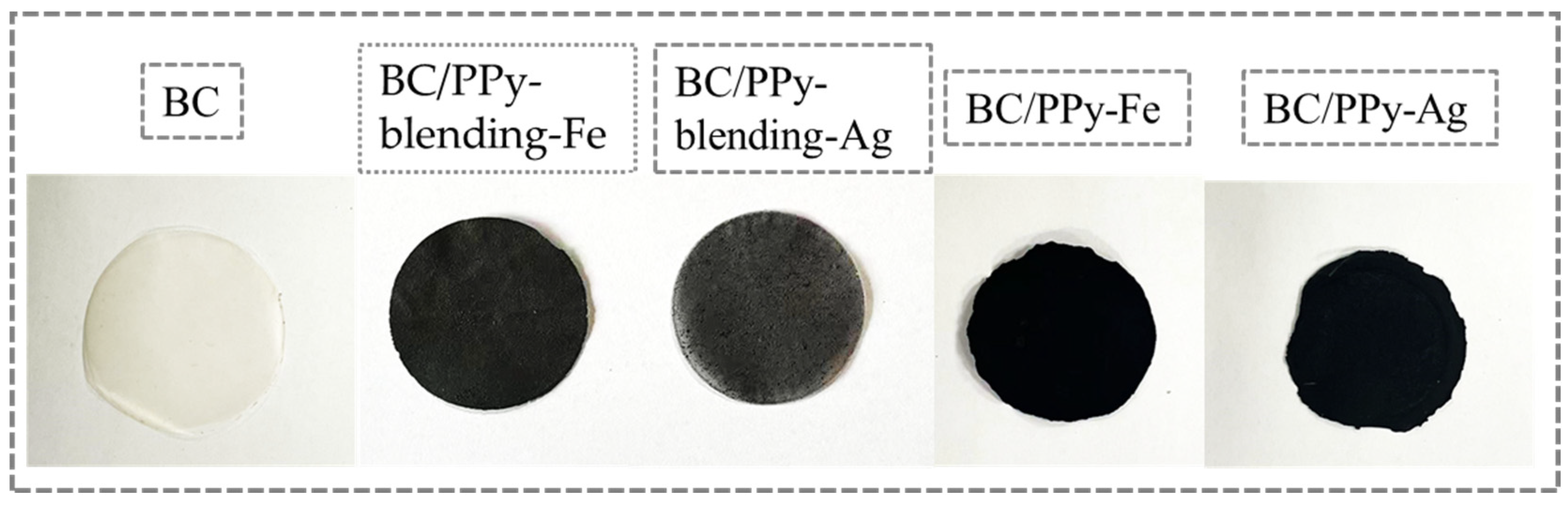
3.1. Micromorphology of BC/PPy Fiber Membranes
3.2. Resistance of BC/PPy Conductive Fiber Membrane
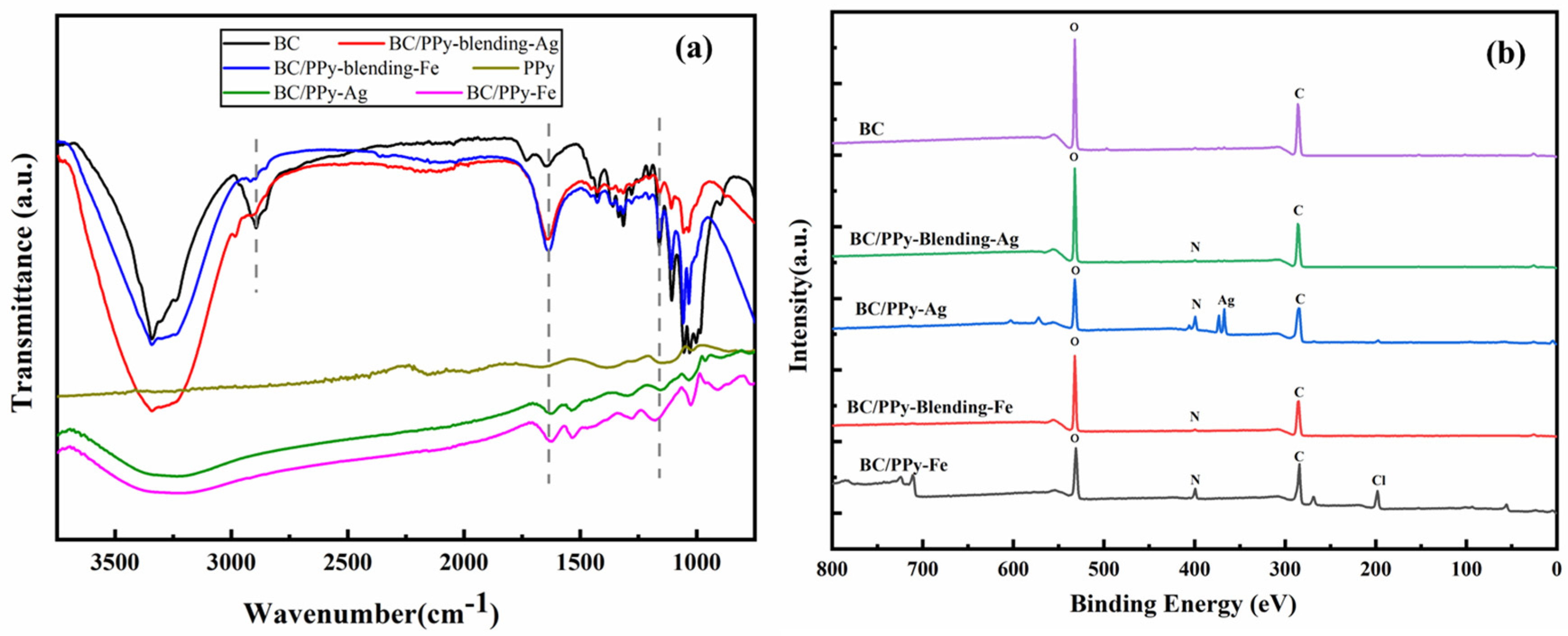
3.3. Chemical Structure of BC/PPy Conductive Fiber Membrane
3.4. Thermal Properties of BC/PPy Conductive Fiber Membrane
3.5. Mechanical Properties of BC/PPy Conductive Fiber Membrane
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhou, Z.; Zhang, J.; Tang, J.; Wu, P.; Wang, K.; Zhao, Y. Properties of graphene-thermoplastic polyurethane flexible conductive film. Coatings 2020, 10, 400. [Google Scholar] [CrossRef]
- Li, M.X.; Wu, D.Y.; Tang, R.Y.; Zhou, S.Y.; Liang, W.H.; Liu, J.; Li, L. Liquid metal integrated PU/CNT fibrous membrane for human health monitoring. Front. Bioeng. Biotechnol. 2023, 11, 1169411. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xiao, H.; Chen, X. MOFs/PVA Hybrid membranes with enhanced mechanical and ion-conductive properties. E-Polym. 2021, 21, 160–165. [Google Scholar] [CrossRef]
- Balser, S.; Bernd, J.; Fritsche, L.; Terfort, A. Preparation and characterization of highly conductive and biorepulsive polypyrrole/polyglycerol surface films. ACS Appl. Polym. Mater. 2022, 4, 8344–8356. [Google Scholar] [CrossRef]
- Shibata, Y.; Suizu, R.; Awaga, K.; Hirotani, J.; Omachi, H. Fabrication of MXene transparent conductive films via transfer process. Appl. Phys. Express 2023, 16, 037001. [Google Scholar] [CrossRef]
- Maleski, K.; Ren, C.E.; Zhao, M.-Q.; Anasori, B.; Gogotsi, Y. Size-Dependent physical and electrochemical properties of two-dimensional MXene flakes. ACS Appl. Mater. Interfaces 2018, 10, 24491–24498. [Google Scholar] [CrossRef]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.-A.; Maiti, T.K.; et al. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Hao, L.; Yu, D. Progress of conductive polypyrrole nanocomposites. Synth. Met. 2022, 290, 117138. [Google Scholar] [CrossRef]
- Ye, J.; Guo, L.; Feng, Y.; Sun, F.; Zhang, T.; Yang, Z.; Shen, G.; Zhang, Z. Fabrication of bacterial cellulose-based ATO-PPy nanocomposites as flexible conductive materials. J. Electron. Mater. 2020, 49, 6686–6694. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, S.P.; Kleinberg, M.N.; Gupta, A.; Arnusch, C.J. Laser-induced graphene–PVA composites as robust electrically conductive water treatment membranes. ACS Appl. Mater. Interfaces 2019, 11, 10914–10921. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Y.; Nie, M.; Wang, Q.; Liu, Y. Highly stretchable and conductive carbon fiber/polyurethane conductive films featuring interlocking interfaces. ACS Appl. Mater. Interfaces 2021, 13, 38656–38665. [Google Scholar] [CrossRef] [PubMed]
- Jinhong, T.; Qun, Y.; Gegnhao, H.; Hongjuan, Z.; Liujun, P.; Jiping, W. Experimental study on the temperature-sensitive behavior of poly-n-isopropylacrylamide/graphene oxide composites and the flexible conductive cotton fabrics. Polym. Test. 2022, 110, 107563. [Google Scholar]
- Rani, P.; Ahamed, B.; Deshmukh, K. Electromagnetic interference shielding properties of graphene quantum-dots Reinforced Poly(Vinyl Alcohol)/Polypyrrole Blend Nanocomposites. J. Appl. Polym. Sci. 2020, 137, 49392. [Google Scholar] [CrossRef]
- Saafan, S.A.; El-Nimr, M.K.; El-Ghazzawy, E.H. Study of dielectric properties of polypyrrole prepared using two different oxidizing agents. J. Appl. Polym. Sci. 2006, 99, 3370–3379. [Google Scholar] [CrossRef]
- Yussuf, A.; Al-Saleh, M.; Al-Enezi, S.; Abraham, G. Synthesis and characterization of conductive polypyrrole: The influence of the oxidants and monomer on the electrical, thermal, and morphological properties. Int. J. Polym. Sci. 2018, 2018, 4191747. [Google Scholar] [CrossRef]
- Shuo, P.; Qi, X.; Lingling, F.; Chengzhuo, W.; Haifeng, B.; Weilin, X.; Jie, X. Flexible polypyrrole/cobalt sulfide/bacterial cellulose composite membranes for supercapacitor application. Synth. Met. 2016, 222, 285–292. [Google Scholar]
- Lina, M.; Rong, L.; Haijun, N.; Fang, W.; Li, L.; Yudong, H. Freestanding conductive film based on polypyrrole/bacterial cellulose/graphene paper for flexible supercapacitor: Large areal mass exhibits excellent areal capacitance. Electrochim. Electrochim. Acta 2016, 222, 429–437. [Google Scholar]
- Jianbin, Y.; Linxuan, G.; Shanshan, Z.; Yingjie, F.; Tingting, Z.; Zongcan, Y.; Qishan, Y.; Guopeng, S.; Zhan, Z. Synthesis of bacterial cellulose based SnO2-PPy nanocomposites as potential flexible, highly conductive material. Mater. Lett. Mater. Lett. 2019, 253, 372–376. [Google Scholar]
- Sasso, C.; Zeno, E.; Petit-Conil, M.; Chaussy, D.; Belgacem, M.N.; Tapin-Lingua, S.; Beneventi, D. Highly conducting polypyrrole/cellulose nanocomposite films with enhanced mechanical properties: Highly conducting polypyrrole/cellulose nanocomposite films with enhanced macromol. Mater. Eng. 2010, 295, 934–941. [Google Scholar]
- Li, F.; Li, H.; Jiang, H.; Zhang, K.; Chang, K.; Jia, S.; Jiang, W.; Shang, Y.; Lu, W.; Deng, S.; et al. Polypyrrole nanoparticles fabricated via triton x-100 micelles template approach and their acetone gas sensing property. Appl. Surf. Sci. 2013, 280, 212–218. [Google Scholar] [CrossRef]
- Lay, M.; González, I.; Tarrés, J.A.; Pellicer, N.; Bun, K.N.; Vilaseca, F. High electrical and electrochemical properties in bacterial cellulose/polypyrrole membranes. Eur. Polym. J. 2017, 91, 1–9. [Google Scholar] [CrossRef]
- Šetka, M.; Calavia, R.; Vojkůvka, L.; Llobet, E.; Drbohlavová, J.; Vallejos, S. Raman and XPS studies of ammonia sensitive polypyrrole nanorods and nanoparticles. Sci. Rep. 2019, 9, 8465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Polypyrrole/nylon membrane composite film for ultra-flexible all-solid supercapacitor. J. Mater. 2020, 6, 339–347. [Google Scholar] [CrossRef]
- Zeyu, X.; En, Z.; Ziwei, X.; Haibo, S.; Yingchun, L.; Jianming, W. Photo-Initiated in situ synthesis of polypyrrole Fe-Coated porous silicon microspheres for High-performance Lithium-ion battery anodes. Chem. Eng. J. 2023, 459, 141543. [Google Scholar]
- Muller, D. Structure and properties of polypyrrole/bacterial cellulose nanocomposites. Carbohydr. Polym. 2013, 94, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.F. One pot synthesis of polypyrrole silver nanocomposite on cotton fabrics for multifunctional property. Carbohydr. Polym. 2012, 90, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Singu, B.S.; Yoon, K.R. Highly exfoliated GO-PPy-Ag ternary nanocomposite for electrochemical supercapacitor. Electrochim. Acta 2018, 268, 304–315. [Google Scholar] [CrossRef]
- Neoh, K.G.; Lau, K.K.S.; Wong, V.V.T.; Kang, E.T.; Tan, K.L. Structure and degradation behavior of polypyrrole doped with sulfonate anions of different sizes subjected to undoping-redoping cycles. Chem. Mater. 1996, 8, 167–172. [Google Scholar] [CrossRef]
- Mao, J.; Liu, C.; Cheng, C.; Zhang, W.; Liao, X.; Wang, J.; Li, L.; Yang, X.; He, Y.; Ma, Z. A porous and interconnected polypyrrole film with high conductivity and ion accessibility as electrode for flexible all-solid-state supercapacitors. Chem. Electro. Chem. 2019, 6, 5479–5485. [Google Scholar] [CrossRef]
- Wang, C.; Ding, Y.; Yuan, Y.; Cao, A.; He, X.; Peng, Q.; Li, Y. Multifunctional, highly flexible, free-standing 3D polypyrrole foam. Small 2016, 12, 4070–4076. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Song, X.; Liu, J.; Zhao, L.; Zhang, P.; Gao, L. Engineering the volumetric effect of polypyrrole for auto-deformable supercapacitor. Chem. Eng. J. 2019, 374, 59–67. [Google Scholar] [CrossRef]
- Rambo, C.R.; Recouvreux, D.O.S.; Carminatti, C.A.; Pitlovanciv, A.K.; Antônio, R.V.; Porto, L.M. Cellulose biosynthesis by the beta-proteobacterium, Chromobacterium violaceum. Curr. Microbiol. 2008, 57, 469–476. [Google Scholar]
- Shao, W.; Liu, H.; Liu, X.; Sun, H.; Wang, S.; Zhang, R. pH-responsive release behavior and anti-bacterial activity of bacterial cellulose-silver nanocomposites. Int. J. Biol. Macromol. 2015, 76, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.F.; Feitosa, J.P.A.; da Gama, F.M.P.; Morais, J.P.S.; Andrade, F.K.; De Souza Filho, M.D.S.M.; Rosa, M.D.F. Bacterial cellulose nanocrystals produced under different hydrolysis conditions: Properties and morphological features. Carbohydr. Polym. 2017, 155, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Shuo, P.; Lingling, F.; Chengzhuo, W.; Xiaohong, L.; Hongwei, Z.; Weilin, X.; Jie, X. Flexible polypyrrole/copper sulfide/bacterial cellulose nanofibrous composite membranes as supercapacitor electrodes. Carbohydr. Polym. 2017, 157, 344–352. [Google Scholar]

| Samples | Resistance (Ω) |
|---|---|
| BC/PPy-Fe | 13 |
| BC/PPy-Ag | 130 |
| BC/PPy-blending-Fe | 5000 |
| BC/PPy-blending-Ag | >22,000 |
| BC | >22,000 |
| BC-SnO2-PPy [18] | 35.7 |
| Samples | Tensile Strength (Mpa) | Elongation at Break (%) |
|---|---|---|
| BC/PPy-Fe | 6.94 ± 1.0 | 4.66 ± 0.4 |
| BC/PPy-Ag | 5.78 ± 0.8 | 3.12 ± 0.3 |
| BC/PPy-blending-Fe | 16.7 ± 1.5 | 12.1 ± 0.5 |
| BC/PPy-blending-Ag | 16.1 ± 1.4 | 11.7 ± 0.6 |
| BC | 15.4 ± 1.3 | 14.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, S.; Yang, Q.; Qiu, H.; Zhu, J.; Zhou, W.; Su, J.; Zhang, N.; Xu, L.; Pan, H.; Zhang, H.; et al. Impact of Metal Salt Oxidants and Preparation Technology on Efficacy of Bacterial Cellulose/Polypyrrole Flexible Conductive Fiber Membranes. Materials 2024, 17, 1281. https://doi.org/10.3390/ma17061281
Tao S, Yang Q, Qiu H, Zhu J, Zhou W, Su J, Zhang N, Xu L, Pan H, Zhang H, et al. Impact of Metal Salt Oxidants and Preparation Technology on Efficacy of Bacterial Cellulose/Polypyrrole Flexible Conductive Fiber Membranes. Materials. 2024; 17(6):1281. https://doi.org/10.3390/ma17061281
Chicago/Turabian StyleTao, Sixuan, Qun Yang, Huili Qiu, Jie Zhu, Weimian Zhou, Juan Su, Ning Zhang, Lihui Xu, Hong Pan, Hongjuan Zhang, and et al. 2024. "Impact of Metal Salt Oxidants and Preparation Technology on Efficacy of Bacterial Cellulose/Polypyrrole Flexible Conductive Fiber Membranes" Materials 17, no. 6: 1281. https://doi.org/10.3390/ma17061281





