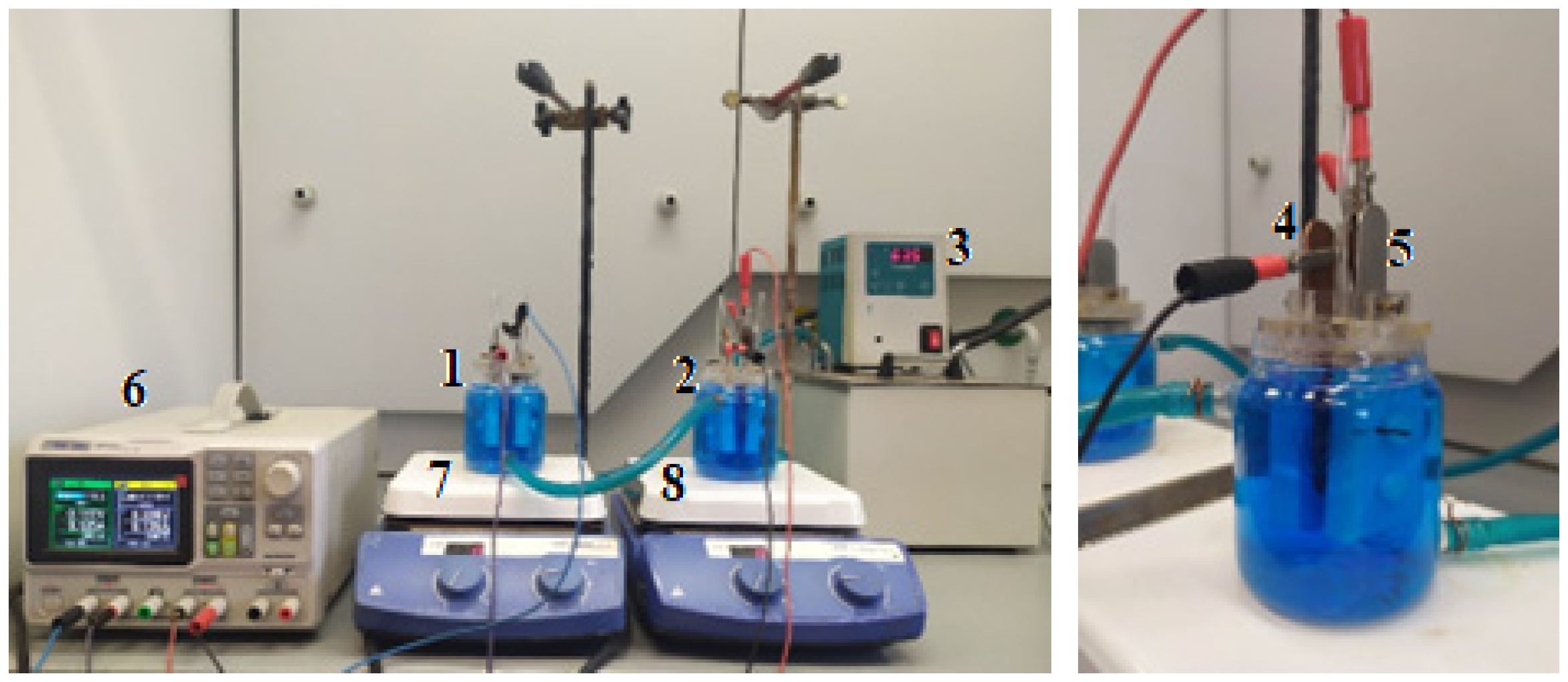
Figure 1.
A station for testing the processes of copper electrorefining on a small laboratory scale: (1) and (2) glass electrolyzers, (3) ultra-thermostat, (4) anode, (5) cathode, (6) power supply, (7) and (8) magnetic stirrers.
Figure 1.
A station for testing the processes of copper electrorefining on a small laboratory scale: (1) and (2) glass electrolyzers, (3) ultra-thermostat, (4) anode, (5) cathode, (6) power supply, (7) and (8) magnetic stirrers.
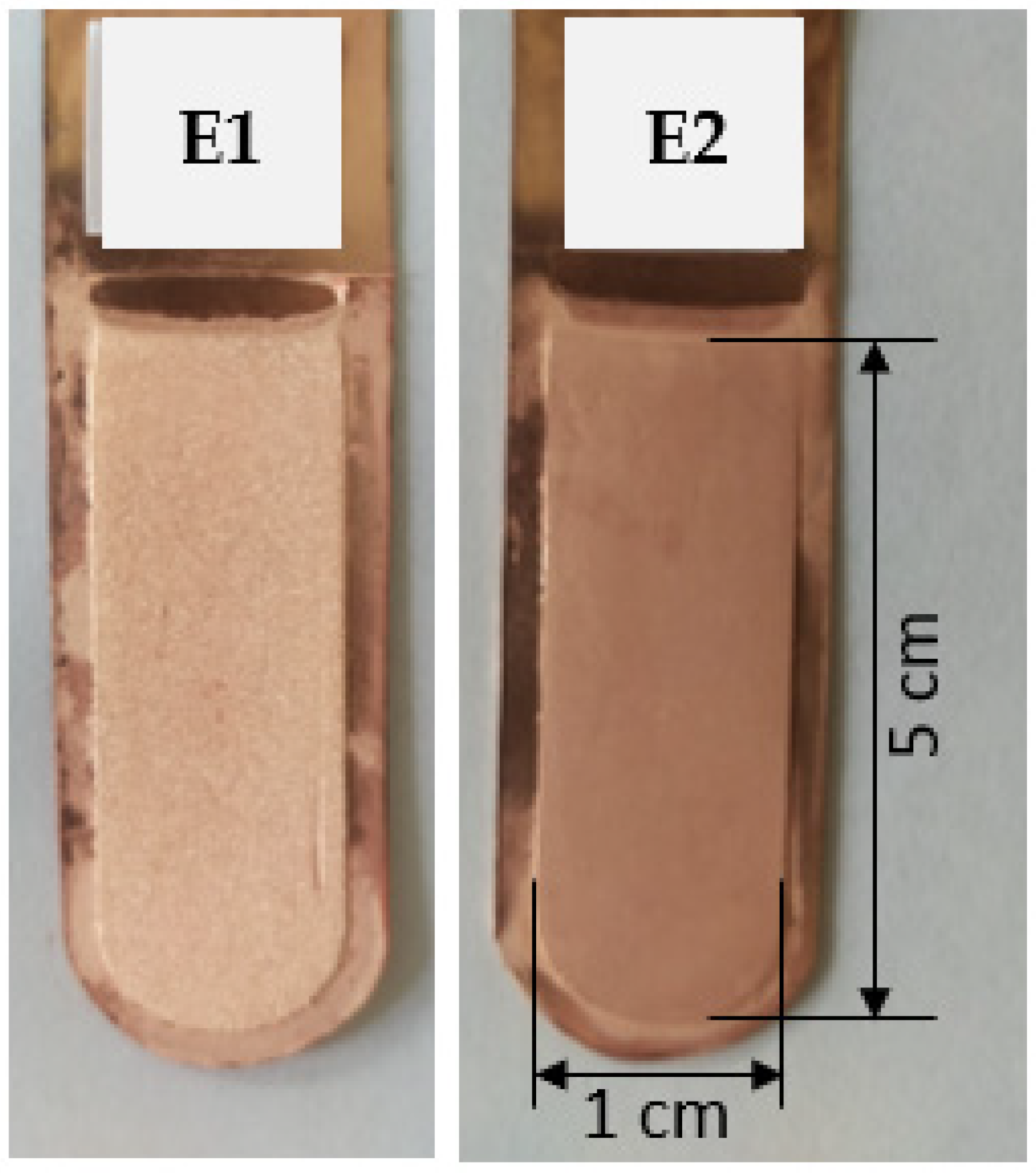
Figure 2.
Appearance of the obtained cathode Cu deposits: (E1) without organic additives and (E2) with the addition of bone glue and thiourea.
Figure 2.
Appearance of the obtained cathode Cu deposits: (E1) without organic additives and (E2) with the addition of bone glue and thiourea.
Figure 3.
Appearance of the obtained cathode Cu deposits with the addition of safranin at concentrations of 10 mg/dm3 (E3), 25 mg/dm3 (E4), and 50 mg/dm3 (E5).
Figure 3.
Appearance of the obtained cathode Cu deposits with the addition of safranin at concentrations of 10 mg/dm3 (E3), 25 mg/dm3 (E4), and 50 mg/dm3 (E5).
Figure 4.
Appearance of the obtained cathode Cu deposits with the addition of polyhexamethyleneguanidine at concentrations of 0.05 mg/dm3 (E6), 0.25 mg/dm3 (E7), and 0.50 mg/dm3 (E8).
Figure 4.
Appearance of the obtained cathode Cu deposits with the addition of polyhexamethyleneguanidine at concentrations of 0.05 mg/dm3 (E6), 0.25 mg/dm3 (E7), and 0.50 mg/dm3 (E8).
Figure 5.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine at concentrations of 0.005 mg/dm3 (E16), 0.01 mg/dm3 (E9), 0.05 mg/dm3 (E10), 0.50 mg/dm3 (E12), 5.0 mg/dm3 (E13), 50.0 mg/dm3 (E14), and 250.0 mg/dm3 (E15).
Figure 5.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine at concentrations of 0.005 mg/dm3 (E16), 0.01 mg/dm3 (E9), 0.05 mg/dm3 (E10), 0.50 mg/dm3 (E12), 5.0 mg/dm3 (E13), 50.0 mg/dm3 (E14), and 250.0 mg/dm3 (E15).
Figure 6.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and bone glue (BG) at concentrations of 0.005 mg/dm3 IL + 5.0 mg/dm3 BG (E19), 0.05 mg/dm3 IL + 5.0 mg/dm3 BG (E31), 0.15 mg/dm3 IL + 5.0 mg/dm3 BG (E46), 0.5 mg/dm3 IL + 5.0 mg/dm3 BG (E32), and 5.0 mg/dm3 IL + 5.0 mg/dm3 BG (E34).
Figure 6.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and bone glue (BG) at concentrations of 0.005 mg/dm3 IL + 5.0 mg/dm3 BG (E19), 0.05 mg/dm3 IL + 5.0 mg/dm3 BG (E31), 0.15 mg/dm3 IL + 5.0 mg/dm3 BG (E46), 0.5 mg/dm3 IL + 5.0 mg/dm3 BG (E32), and 5.0 mg/dm3 IL + 5.0 mg/dm3 BG (E34).
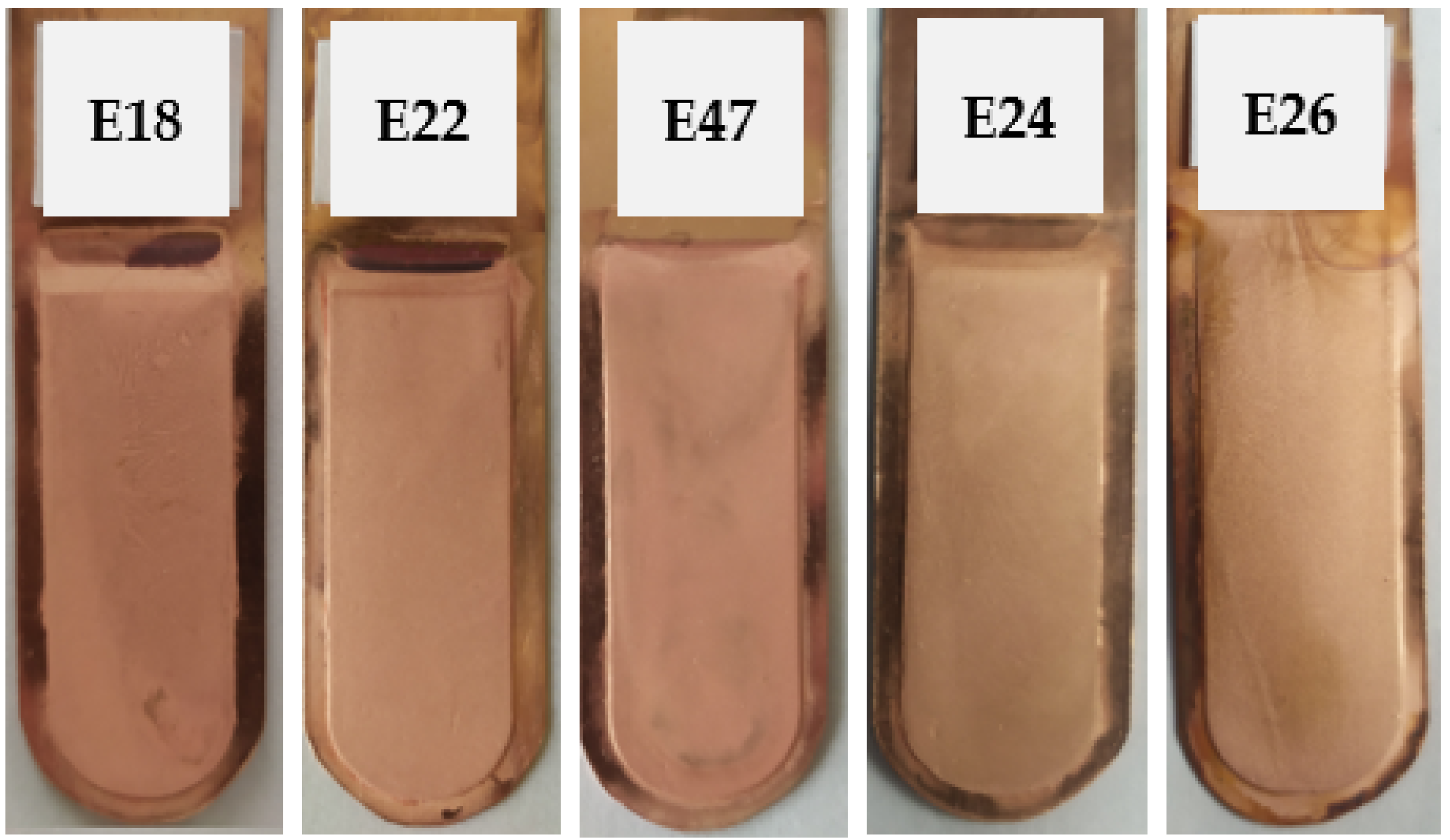
Figure 7.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and thiourea (T) at concentrations of 0.005 mg/dm3 IL + 5.0 mg/dm3 T (E18), 0.05 mg/dm3 IL + 5.0 mg/dm3 T (E22), 0.15 mg/dm3 IL + 5.0 mg/dm3 T (E47), 0.5 mg/dm3 IL + 5.0 mg/dm3 T (E24), and 5.0 mg/dm3 IL + 5.0 mg/dm3 T (E26).
Figure 7.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and thiourea (T) at concentrations of 0.005 mg/dm3 IL + 5.0 mg/dm3 T (E18), 0.05 mg/dm3 IL + 5.0 mg/dm3 T (E22), 0.15 mg/dm3 IL + 5.0 mg/dm3 T (E47), 0.5 mg/dm3 IL + 5.0 mg/dm3 T (E24), and 5.0 mg/dm3 IL + 5.0 mg/dm3 T (E26).
Figure 8.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and safranin (S) at concentrations of 0.005 mg/dm3 IL + 50 mg/dm3 S (E44), 0.05 mg/dm3 IL + 50 mg/dm3 S (E45), 0.15 mg/dm3 IL + 50 mg/dm3 S (E60), and 0.5 mg/dm3 IL + 50 mg/dm3 S (E61).
Figure 8.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and safranin (S) at concentrations of 0.005 mg/dm3 IL + 50 mg/dm3 S (E44), 0.05 mg/dm3 IL + 50 mg/dm3 S (E45), 0.15 mg/dm3 IL + 50 mg/dm3 S (E60), and 0.5 mg/dm3 IL + 50 mg/dm3 S (E61).
Figure 9.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and polyhexamethyleneguanidin (P) at concentrations of 0.005 mg/dm3 IL + 0.5 mg/dm3 P (E63), 0.05 mg/dm3 IL + 0.5 mg/dm3 P (E64), 0.15 mg/dm3 IL + 0.5 mg/dm3 P (E66), 0.5 mg/dm3 IL + 0.5 mg/dm3 P (E65), and 5.0 mg/dm3 IL + 0.5 mg/dm3 P (E67).
Figure 9.
Appearance of the obtained cathode Cu deposits with the addition of ionic liquid based on polyhexamethylenebiguanidine (IL) and polyhexamethyleneguanidin (P) at concentrations of 0.005 mg/dm3 IL + 0.5 mg/dm3 P (E63), 0.05 mg/dm3 IL + 0.5 mg/dm3 P (E64), 0.15 mg/dm3 IL + 0.5 mg/dm3 P (E66), 0.5 mg/dm3 IL + 0.5 mg/dm3 P (E65), and 5.0 mg/dm3 IL + 0.5 mg/dm3 P (E67).
Table 1.
Evaluation of the copper surface appearance.
Table 1.
Evaluation of the copper surface appearance.
| Number of Points | Surface Appearance |
|---|
| 0 | Smooth deposit |
| 1 | Rough sediment, single surface irregularities |
| 2 | Distinct surface irregularities of copper, growths on the surface |
| 3 | Dendritic sediment, growths loosely associated with the cathode surface falling off the cathode or not covered with copper sediment |
Table 2.
Evaluation of the copper crystalline structure.
Table 2.
Evaluation of the copper crystalline structure.
| Number of Points | Crystalline Structure |
|---|
| 0 | Very fine, even crystal, matte surface |
| 1 | Small crystal, noticeable often on a fragment of the surface |
| 2 | Distinct, medium crystal, shiny surface |
| 3 | Coarse crystalline precipitate |
Table 3.
The initial concentrations of additives introduced into the electrolyte in the process of copper electrorefining—small laboratory scale.
Table 3.
The initial concentrations of additives introduced into the electrolyte in the process of copper electrorefining—small laboratory scale.
| Trial | Initial Concentration in Electrolyte, mg/dm3 |
|---|
| IL | P | S | BG | T |
|---|
| E1 | - | - | - | - | - |
| E2 | - | - | - | 5 | 5 |
| E3 | - | - | 10 | - | - |
| E4 | - | - | 25 | - | - |
| E5 | - | - | 50 | - | - |
| E6 | - | 0.05 | - | - | - |
| E7 | - | 0.25 | - | - | - |
| E8 | - | 0.50 | - | - | - |
| E16 | 0.005 | - | - | - | - |
| E9 | 0.01 | - | - | - | - |
| E10 | 0.05 | - | - | - | - |
| E12 | 0.50 | - | - | - | - |
| E13 | 5 | - | - | - | - |
| E14 | 50 | - | - | - | - |
| E15 | 250 | - | - | - | - |
| E19 | 0.005 | - | - | 5 | - |
| E31 | 0.05 | - | - | 5 | - |
| E46 | 0.15 | - | - | 5 | - |
| E32 | 0.50 | - | - | 5 | - |
| E34 | 5 | - | - | 5 | - |
| E18 | 0.005 | - | - | - | 5 |
| E22 | 0.05 | - | - | - | 5 |
| E47 | 0.15 | - | - | - | 5 |
| E24 | 0.50 | - | - | - | 5 |
| E26 | 5 | - | - | - | 5 |
| E44 | 0.005 | - | 50 | - | - |
| E45 | 0.05 | - | 50 | - | - |
| E60 | 0.15 | - | 50 | - | - |
| E61 | 0.50 | - | 50 | - | - |
| E63 | 0.005 | 0.50 | - | - | - |
| E64 | 0.05 | 0.50 | - | - | - |
| E66 | 0.15 | 0.50 | - | - | - |
| E65 | 0.50 | 0.50 | - | - | - |
| E67 | 5 | 0.50 | - | - | - |
Table 4.
Average profile roughness parameters of the E1 and E2 copper deposits.
Table 4.
Average profile roughness parameters of the E1 and E2 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E1 | 5.26 | 29.30 | 33.62 | 4.78 | 28.03 | 31.92 |
| E2 | 0.73 | 5.40 | 8.37 | 0.81 | 5.73 | 7.91 |
Table 5.
The indicators for the E1 the E2 electrorefining processes with an evaluation of the Cu deposit quality.
Table 5.
The indicators for the E1 the E2 electrorefining processes with an evaluation of the Cu deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E1 | 100.0 | 0.180 | 147.4 | 1 | 2 | 3 |
| E2 | 97.4 | 0.126 | 109.1 | 0 | 0 | 0 |
Table 6.
Average profile roughness parameters of the E3, E4, and E5 copper deposits.
Table 6.
Average profile roughness parameters of the E3, E4, and E5 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E3 | 2.92 | 17.00 | 19.18 | 4.54 | 25.15 | 33.37 |
| E4 | 4.59 | 27.59 | 41.53 | 2.83 | 19.15 | 25.27 |
| E5 | 1.61 | 10.97 | 14.29 | 1.64 | 11.35 | 15.54 |
Table 7.
The indicators of the E3, E4, and E5 electrorefining processes with the evaluation of the Cu deposit quality.
Table 7.
The indicators of the E3, E4, and E5 electrorefining processes with the evaluation of the Cu deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E3 | 99.9 | 0.128 | 107.0 | 2 | 1 | 3 |
| E4 | 98.4 | 0.118 | 101.1 | 2 | 1 | 3 |
| E5 | 98.2 | 0.101 | 86.7 | 1 | 0 | 1 |
Table 8.
Average profile roughness parameters of the E6, E7, and E8 copper deposits.
Table 8.
Average profile roughness parameters of the E6, E7, and E8 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E6 | 2.76 | 17.31 | 18.78 | 2.68 | 16.32 | 20.07 |
| E7 | - | - | - | 8.56 | 44.42 | 51.05 |
| E8 | - | - | - | - | - | - |
Table 9.
The indicators of the E6, E7, and E8 electrorefining processes with the evaluation of the Cu deposit quality.
Table 9.
The indicators of the E6, E7, and E8 electrorefining processes with the evaluation of the Cu deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E6 | 97.5 | 0.122 | 105.5 | 1 | 2 | 3 |
| E7 | 99.2 | 0.156 | 132.6 | 1 | 2 | 3 |
| E8 | 97.1 | 0.186 | 161.6 | 3 | 1 | 4 |
Table 10.
Average profile roughness parameters of the E16, E9, E10, E12, E13, E14, and E15 copper deposits.
Table 10.
Average profile roughness parameters of the E16, E9, E10, E12, E13, E14, and E15 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E16 | 9.34 | 48.74 | 59.04 | 10.26 | 51.72 | 59.67 |
| E9 | 4.12 | 31.31 | 51.96 | 2.57 | 15.34 | 20.24 |
| E10 | 2.75 | 15.93 | 19.44 | 2.96 | 18.29 | 22.42 |
| E12 | 6.73 | 41.87 | 51.18 | 5.65 | 33.48 | 39.69 |
| E13 | 10.75 | 53.74 | 62.30 | 8.95 | 44.85 | 59.86 |
| E14 | 6.54 | 35.67 | 46.56 | 7.61 | 39.80 | 49.77 |
| E15 | 8.96 | 56.60 | 64.75 | 7.68 | 42.34 | 52.15 |
Table 11.
The indicators of the E16, E9, E10, E12, E13, E14, and E15 electrorefining processes with the evaluation of the Cu deposit quality.
Table 11.
The indicators of the E16, E9, E10, E12, E13, E14, and E15 electrorefining processes with the evaluation of the Cu deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E16 | 97.5 | 0.138 | 119.3 | 0 | 2 | 2 |
| E9 | 99.2 | 0.157 | 133.5 | 1 | 0 | 1 |
| E10 | 98.4 | 0.165 | 141.4 | 1 | 0 | 1 |
| E12 | 97.7 | 0.142 | 122.6 | 0 | 1 | 1 |
| E13 | 99.1 | 0.153 | 130.2 | 2 | 2 | 4 |
| E14 | 96.9 | 0.171 | 148.8 | 2 | 2 | 4 |
| E15 | 94.1 | 0.238 | 213.3 | 0 | 1 | 1 |
Table 12.
Average profile roughness parameters of the E19, E31, E46, E32, and E34 copper deposits.
Table 12.
Average profile roughness parameters of the E19, E31, E46, E32, and E34 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E19 | 8.06 | 47.26 | 56.99 | 7.11 | 40.68 | 54.27 |
| E31 | 2.68 | 16.50 | 19.38 | 2.60 | 16.62 | 18.64 |
| E46 | 3.75 | 22.61 | 24.89 | 3.55 | 21.09 | 24.06 |
| E32 | 3.02 | 17.91 | 20.52 | 2.97 | 17.67 | 20.63 |
| E34 | 3.81 | 22.46 | 25.91 | 3.93 | 22.95 | 26.61 |
Table 13.
The indicators of the E19, E31, E46, E32, and E34 electrorefining processes with the evaluation of the Cu deposit quality.
Table 13.
The indicators of the E19, E31, E46, E32, and E34 electrorefining processes with the evaluation of the Cu deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E19 | 99.6 | 0.158 | 133.8 | 0 | 2 | 2 |
| E31 | 98.3 | 0.173 | 148.6 | 0 | 0 | 0 |
| E46 | 98.5 | 0.179 | 153.3 | 0 | 1 | 1 |
| E32 | 98.2 | 0.163 | 140.0 | 0 | 0 | 0 |
| E34 | 99.9 | 0.199 | 167.5 | 0 | 1 | 1 |
Table 14.
Average profile roughness parameters of the E18, E22, E47, E24, and E26 copper deposits.
Table 14.
Average profile roughness parameters of the E18, E22, E47, E24, and E26 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E18 | 1.26 | 8.35 | 10.87 | 1.18 | 8.02 | 9.80 |
| E22 | 1.12 | 7.05 | 7.86 | 1.27 | 8.11 | 10.60 |
| E47 | 1.02 | 7.11 | 8.54 | 1.05 | 6.62 | 8.00 |
| E24 | 0.91 | 5.87 | 7.23 | 1.00 | 6.99 | 8.96 |
| E26 | 1.70 | 11.12 | 46.96 | 1.63 | 10.73 | 13.79 |
Table 15.
The indicators of the E18, E22, E47, E24, and E26 electrorefining processes with the evaluation of the cathode deposit quality.
Table 15.
The indicators of the E18, E22, E47, E24, and E26 electrorefining processes with the evaluation of the cathode deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E18 | 97.1 | 0.113 | 98.0 | 0 | 0 | 0 |
| E22 | 96.9 | 0.116 | 101.0 | 0 | 0 | 0 |
| E47 | 96.1 | 0.113 | 99.2 | 0 | 0 | 0 |
| E24 | 97.4 | 0.118 | 102.2 | 0 | 0 | 0 |
| E26 | 97.7 | 0.174 | 150.3 | 0 | 0 | 0 |
Table 16.
Average profile roughness parameters of the E44, E45, E60, and E61 copper deposits.
Table 16.
Average profile roughness parameters of the E44, E45, E60, and E61 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E44 | 2.16 | 13.00 | 15.41 | 2.34 | 15.09 | 17.73 |
| E45 | 4.86 | 27.13 | 28.56 | 4.86 | 27.69 | 31.32 |
| E60 | 3.64 | 21.10 | 24.68 | 3.64 | 21.60 | 28.32 |
| E61 | 3.94 | 26.50 | 30.15 | 4.06 | 26.13 | 32.39 |
Table 17.
The indicators of the E44, E45, E60, and E61 processes with the evaluation of the Cu deposit quality.
Table 17.
The indicators of the E44, E45, E60, and E61 processes with the evaluation of the Cu deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E44 | 97.9 | 0.108 | 93.1 | 0 | 1 | 1 |
| E45 | 96.7 | 0.116 | 101.2 | 0 | 1 | 1 |
| E60 | 100.0 | 0.149 | 125.6 | 0 | 1 | 1 |
| E61 | 98.5 | 0.201 | 172.1 | 0 | 1 | 1 |
Table 18.
Average profile roughness parameters of the E63, E64, E66, E65, and E67 copper deposits.
Table 18.
Average profile roughness parameters of the E63, E64, E66, E65, and E67 copper deposits.
| Trial | Average Profile Roughness Parameters, µm |
|---|
| Horizontal | Vertical |
|---|
| RA | RZ | Rm | RA | RZ | Rm |
|---|
| E63 | - | - | - | - | - | - |
| E64 | - | - | - | - | - | - |
| E66 | 4.82 | 30.33 | 44.59 | 4.73 | 26.67 | 30.65 |
| E65 | 5.51 | 36.41 | 45.88 | 5.55 | 32.26 | 40.92 |
| E67 | 4.90 | 26.47 | 31.16 | 4.53 | 26.48 | 30.81 |
Table 19.
The indicators of the E63, E64, E66, E65, and E67 electrorefining processes with the evaluation of the cathode deposit quality.
Table 19.
The indicators of the E63, E64, E66, E65, and E67 electrorefining processes with the evaluation of the cathode deposit quality.
| Trial | η, % | Vcell, V | SEC, kWh/tCu | Evaluation of Cathode Deposit Quality |
|---|
Surface
Appearance | Crystalline Structure | Total Points |
|---|
| E63 | 98.5 | 0.230 | 197.0 | 3 | 3 | 6 |
| E64 | 99.1 | 0.215 | 183.1 | 3 | 3 | 6 |
| E66 | 99.2 | 0.147 | 124.9 | 1 | 2 | 3 |
| E65 | 98.1 | 0.159 | 137.0 | 1 | 2 | 3 |
| E67 | 98.5 | 0.297 | 254.3 | 1 | 2 | 3 |