Improving Mechanical and Corrosion Properties of 6061 Al Alloys via Differential Speed Rolling and Plasma Electrolytic Oxidation
Abstract
:1. Introduction
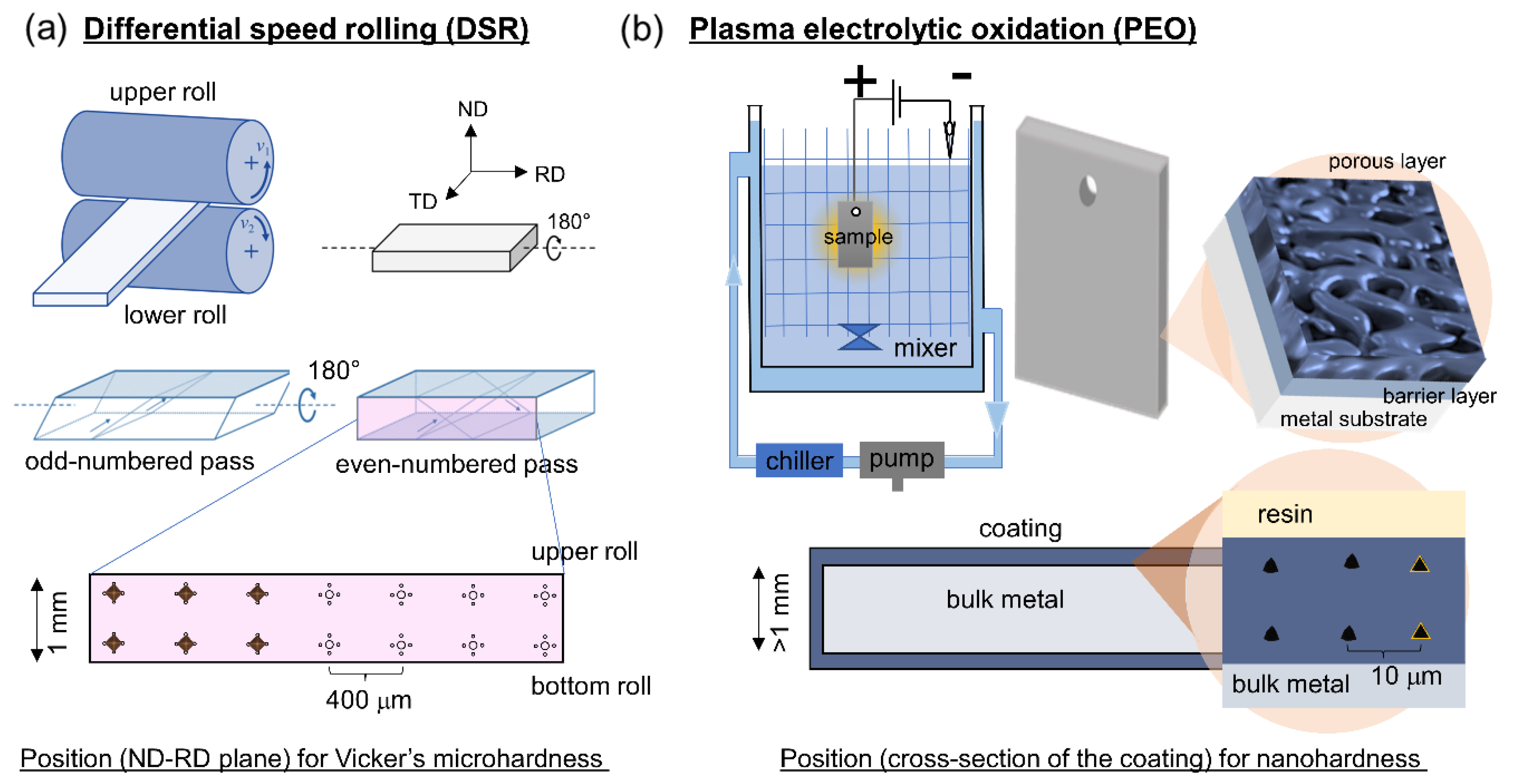
2. Experimental Procedure
2.1. Differential Speed Rolling
2.2. Plasma Electrolytic Oxidation
3. Results and Discussion
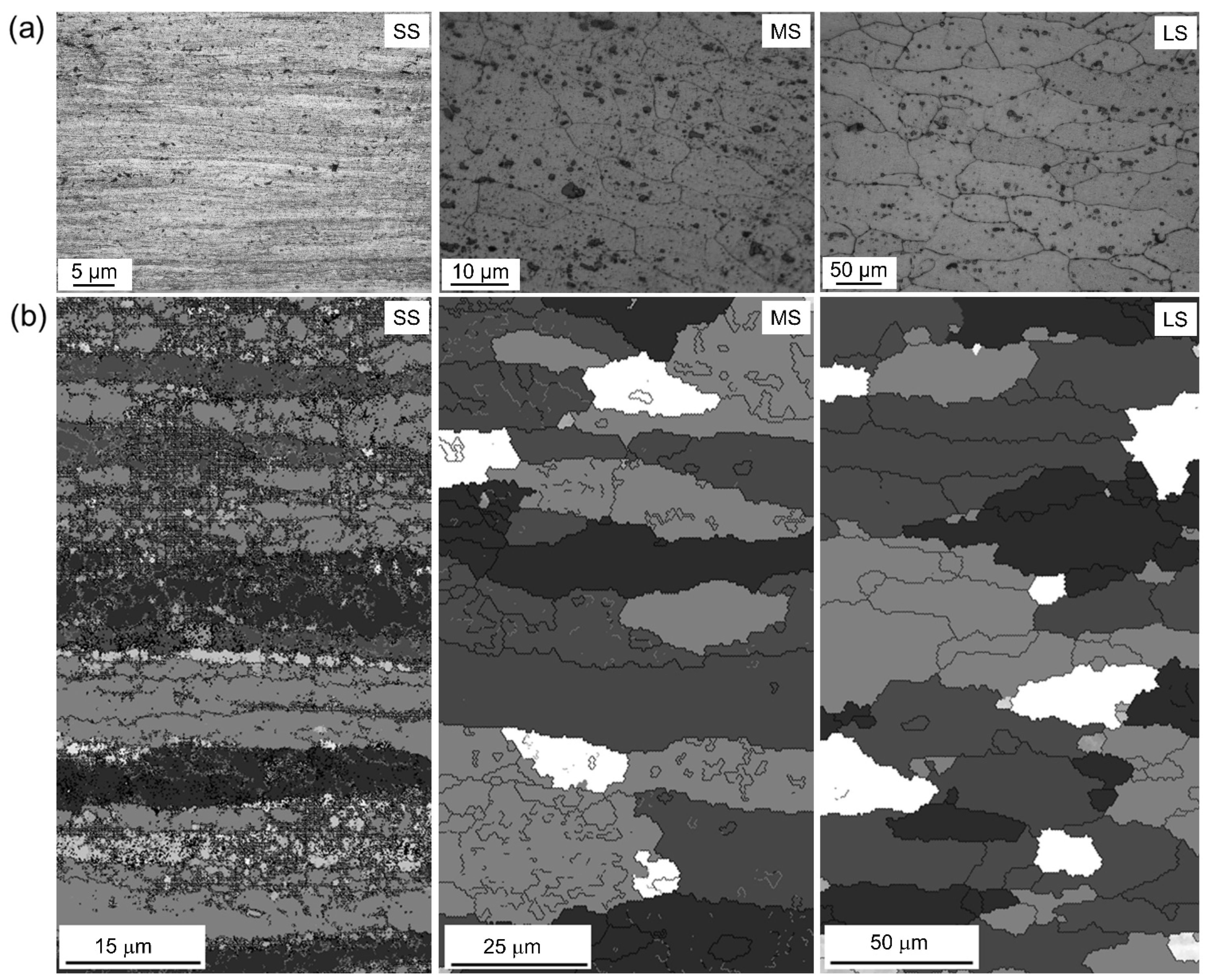
3.1. Deformation of Al Sheet Alloy via DSR
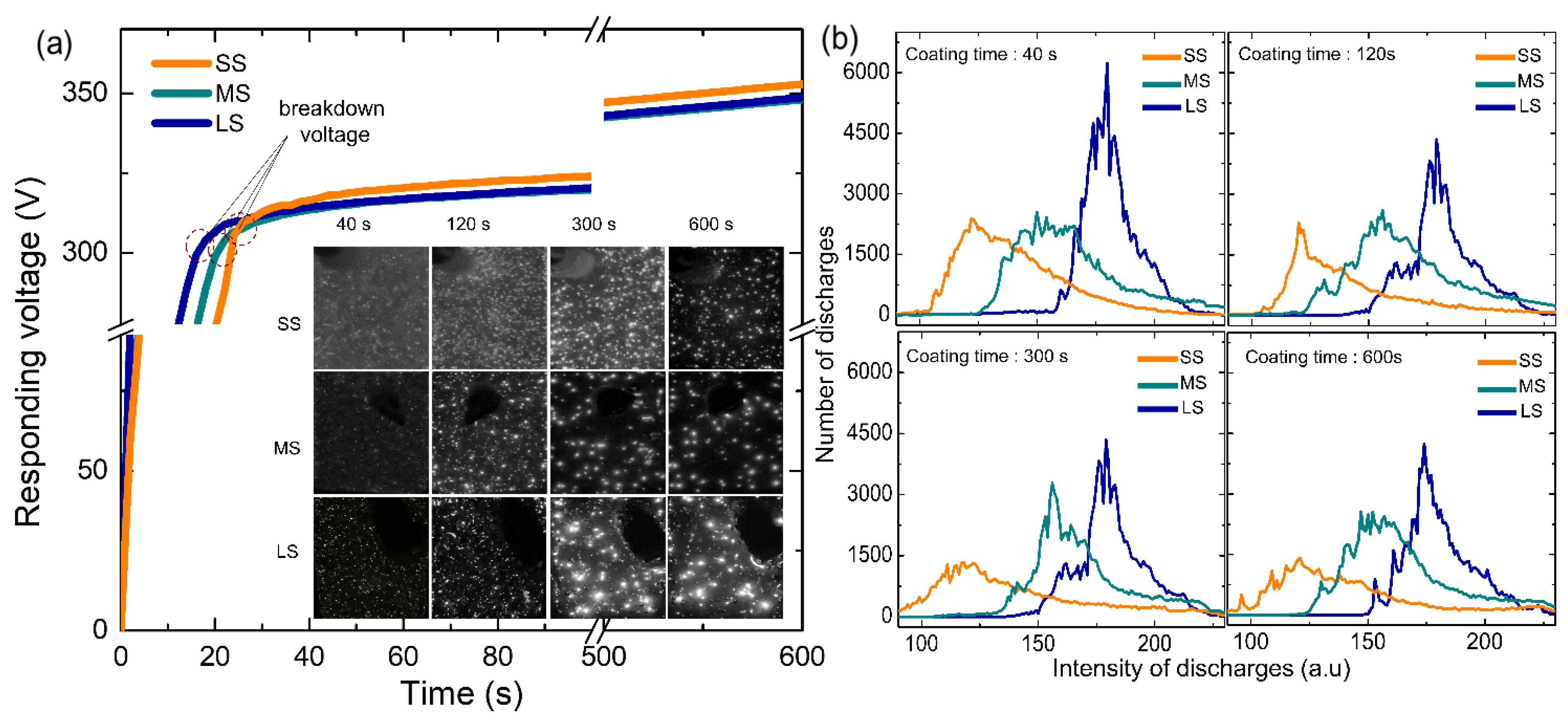
3.2. Voltage Response and Plasma Characteristics
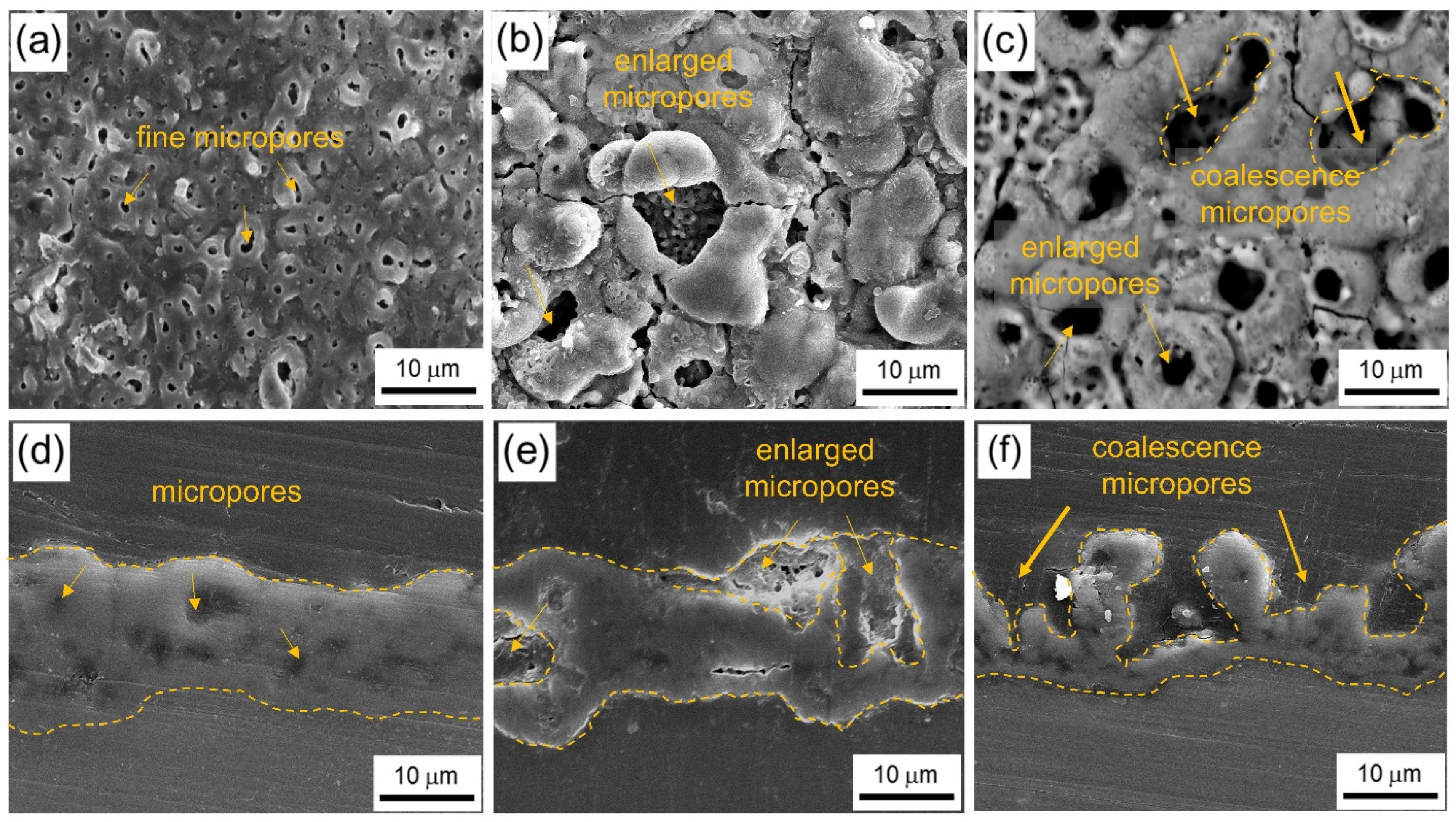
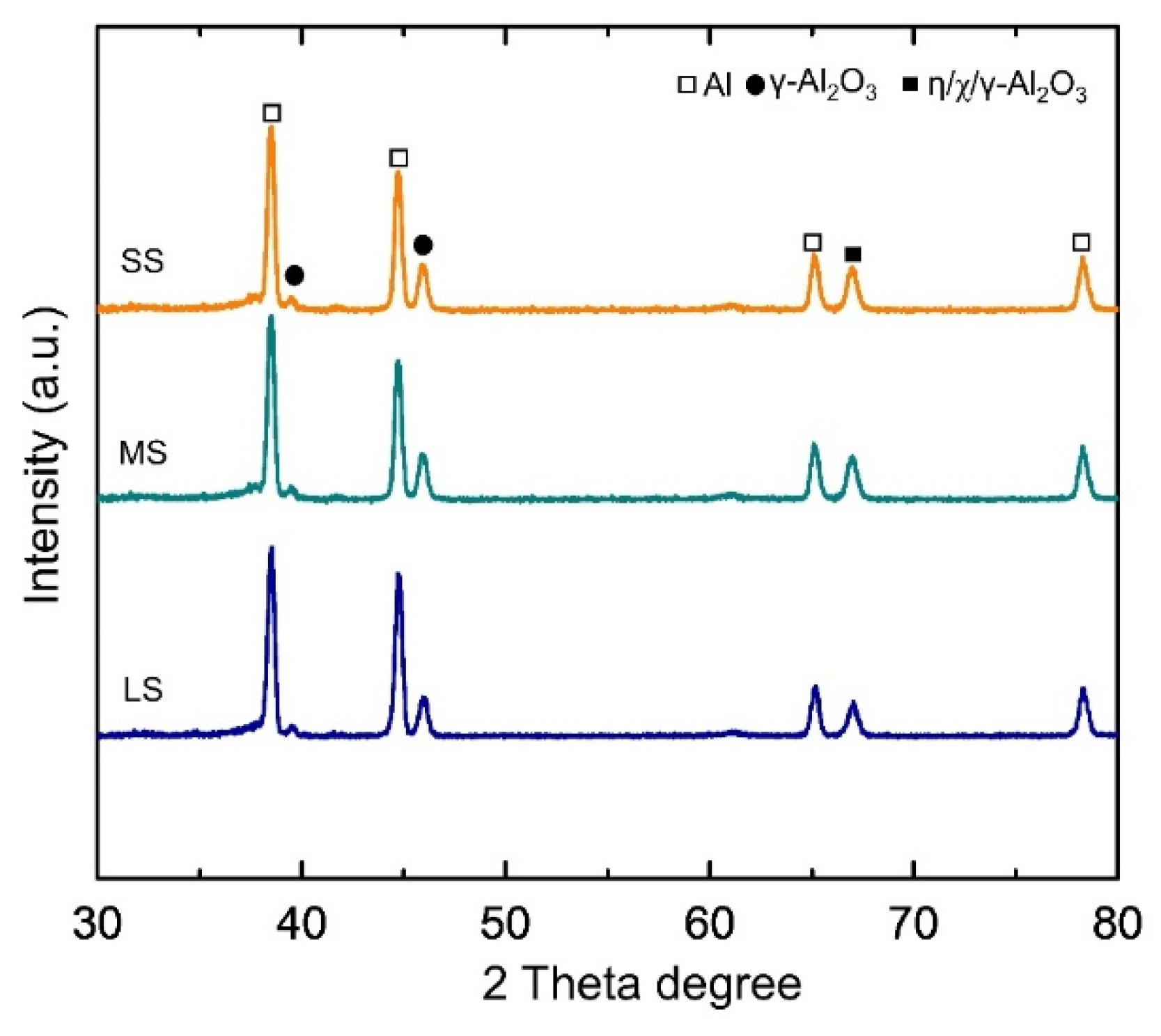
3.3. Morphologies and Composition of the Coating
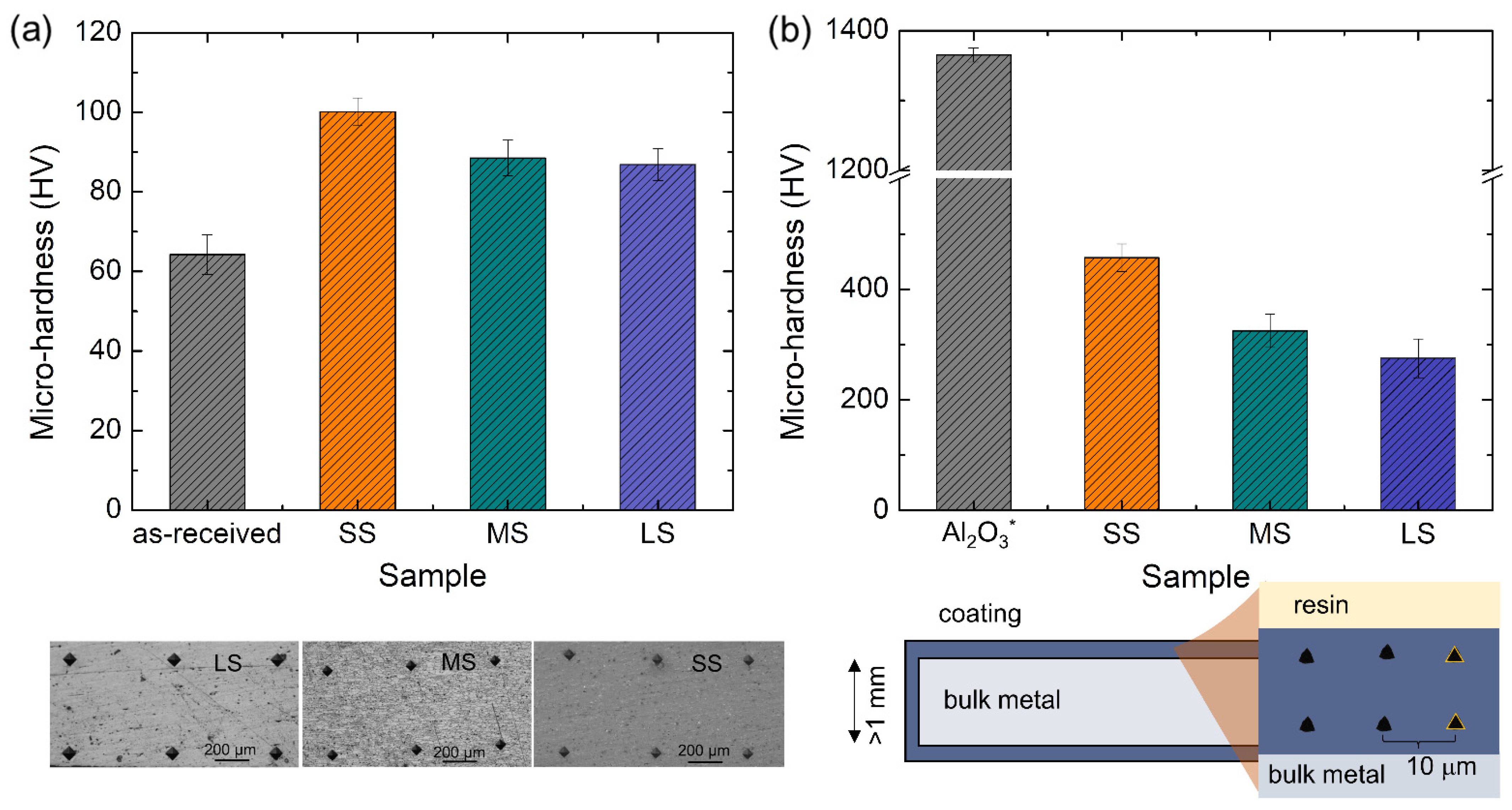
3.4. Microhardness of the Coating
3.5. The Corrosion-Resistant Properties of the Coating
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma Electrolysis for Surface Engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent Progress in Surface Modification of Metals Coated by Plasma Electrolytic Oxidation: Principle, Structure, and Performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Amegadzie, M.Y.; Bishop, D.P. Effect of Asymmetric Rolling on the Microstructure and Mechanical Properties of Wrought 6061 Aluminum. Mater. Today Commun. 2020, 25, 101283. [Google Scholar] [CrossRef]
- Hamad, K.; Ko, Y.G. Continuous Differential Speed Rolling for Grain Refinement of Metals: Processing, Microstructure, and Properties. Crit. Rev. Solid State Mater. Sci. 2019, 44, 470–525. [Google Scholar] [CrossRef]
- Vincze, G.; Simões, F.; Butuc, M. Asymmetrical Rolling of Aluminum Alloys and Steels: A Review. Metals 2020, 10, 1126. [Google Scholar] [CrossRef]
- Bahmani, A.; Kim, W.J. Effect of Grain Refinement and Dispersion of Particles and Reinforcements on Mechanical Properties of Metals and Metal Matrix Composites through High-Ratio Differential Speed Rolling. Materials 2020, 13, 4159. [Google Scholar] [CrossRef]
- Son, I.J.; Nakano, H.; Oue, S.; Kobayashi, S.; Fukushima, H.; Horita, Z. Effect of Annealing on the Pitting Corrosion Resistance of Anodized Aluminum-Magnesium Alloy Processed by Severe Plastic Deformation. Mater. Trans. 2008, 49, 2656–2663. [Google Scholar] [CrossRef]
- Osório, W.R.; Freire, C.M.; Garcia, A. The Role of Macrostructural Morphology and Grain Size on the Corrosion Resistance of Zn and Al Castings. Mater. Sci. Eng. A 2005, 402, 22–32. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Ma, L.; Cheng, X.; Li, X. Effect of Grain Ultra-Refinement on Microstructure, Tensile Property, and Corrosion Behavior of Low Alloy Steel. Mater. Charact. 2021, 179, 111385. [Google Scholar] [CrossRef]
- Reshadi, F.; Faraji, G.; Baniassadi, M.; Tajeddini, M. Surface Modification of Severe Plastically Deformed Ultrafine Grained Pure Titanium by Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2017, 316, 113–121. [Google Scholar] [CrossRef]
- Chung, M.K.; Choi, Y.S.; Kim, J.G.; Kim, Y.M.; Lee, J.C. Effect of the Number of ECAP Pass Time on the Electrochemical Properties of 1050 Al Alloys. Mater. Sci. Eng. A 2004, 366, 282–291. [Google Scholar] [CrossRef]
- Fatimah, S.; Hazmatulhaq, F.; Sheng, Y.; Suhartono, T.; Oh, J.M.; Nashrah, N.; Kang, J.H.; Ko, Y.G. Effect of Ultrasonic Frequency on Structure and Corrosion Properties of Coating Formed on Magnesium Alloy via Plasma Electrolytic Oxidation. Materials 2023, 16, 5424. [Google Scholar] [CrossRef]
- Ko, Y.G.; Masood Chaudry, U.; Hamad, K. Microstructure and Mechanical Properties of AA6061 Alloy Deformed by Differential Speed Rolling. Mater. Lett. 2020, 259, 126870. [Google Scholar] [CrossRef]
- Ikonopisov, S. Theory of Electrical Breakdown during Formation of Barrier Anodic Films. Electrochim. Acta 1977, 22, 1077–1082. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A. Characterisation of Oxide Films Produced by Plasma Electrolytic Oxidation of a Ti-6Al-4V Alloy. Surf. Coat. Technol. 2000, 130, 195–206. [Google Scholar] [CrossRef]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma Electrolytic Oxidation Coatings with Particle Additions—A Review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Zygmuntowicz, J.; Miazga, A.; Konopka, K.; Kaszuwara, W. Metal Particles Size Influence on Graded Structure in Composite Al2O3-Ni. Mater. Tehnol. 2016, 50, 537–541. [Google Scholar] [CrossRef]
- Paneto, F.J.; Pereira, J.L.; Lima, J.O.; Jesus, E.J.; Silva, L.A.; Sousa Lima, E.; Cabral, R.F.; Santos, C. Effect of Porosity on Hardness of Al2O3-Y3Al5O12 Ceramic Composite. Int. J. Refract. Met. Hard Mater. 2015, 48, 365–368. [Google Scholar] [CrossRef]
- Ruppi, S.; Larsson, A.; Flink, A. Nanoindentation Hardness, Texture and Microstructure of α-Al2O3 and κ-Al2O3 Coatings. Thin Solid Films 2008, 516, 5959–5966. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, H.; Liu, S.; Qi, Y.; Liang, J. Wear and Corrosion Resistance of Plasma Electrolytic Oxidation Coatings on 6061 Al Alloy in Electrolytes with Aluminate and Phosphate. Materials 2021, 14, 4037. [Google Scholar] [CrossRef] [PubMed]
- Musil, J.; Blažek, J.; Zeman, P.; Prokšová, Š.; Šašek, M.; Čerstvý, R. Thermal Stability of Alumina Thin Films Containing γ-Al2O3 Phase Prepared by Reactive Magnetron Sputtering. Appl. Surf. Sci. 2010, 257, 1058–1062. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, S.J.; Ahn, J.W.; Kim, D.H.; Kim, W.J. Ultrafine Grained Titanium Sheets with High Strength and High Corrosion Resistance. Mater. Sci. Eng. A 2011, 528, 8479–8485. [Google Scholar] [CrossRef]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.A.; Stolyarov, V.V.; Valiev, R.Z.; Liao, X.Z.; Zhao, Y.H.; Jiang, Y.B.; Xu, H.F.; Lowe, T.C.; et al. Corrosion Resistance of Ultra Fine-Grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the Relationship between Grain Size and Corrosion Rate of Metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Lejček, P. Grain Boundaries: Description, Structure and Thermodynamics. In Grain Boundary Segregation in Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 5–24. [Google Scholar]
- Kwapuliński, P.; Rasek, J.; Gierak, Z. Scattering of Conductivity Electrons on Grain Boundaries in Metals. Phys. Status Solidi 1988, 107, 299–304. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, M.; Ren, W.; Saetang, V.; Lu, J.; Wu, Y.; Xu, K.; Liu, Y.; Zhang, Z. Laser-Induced Localized and Maskless Electrodeposition of Micro-Copper Structure on Silicon Surface: Simulation and Experimental Study. Opt. Laser Technol. 2024, 170, 110315. [Google Scholar] [CrossRef]
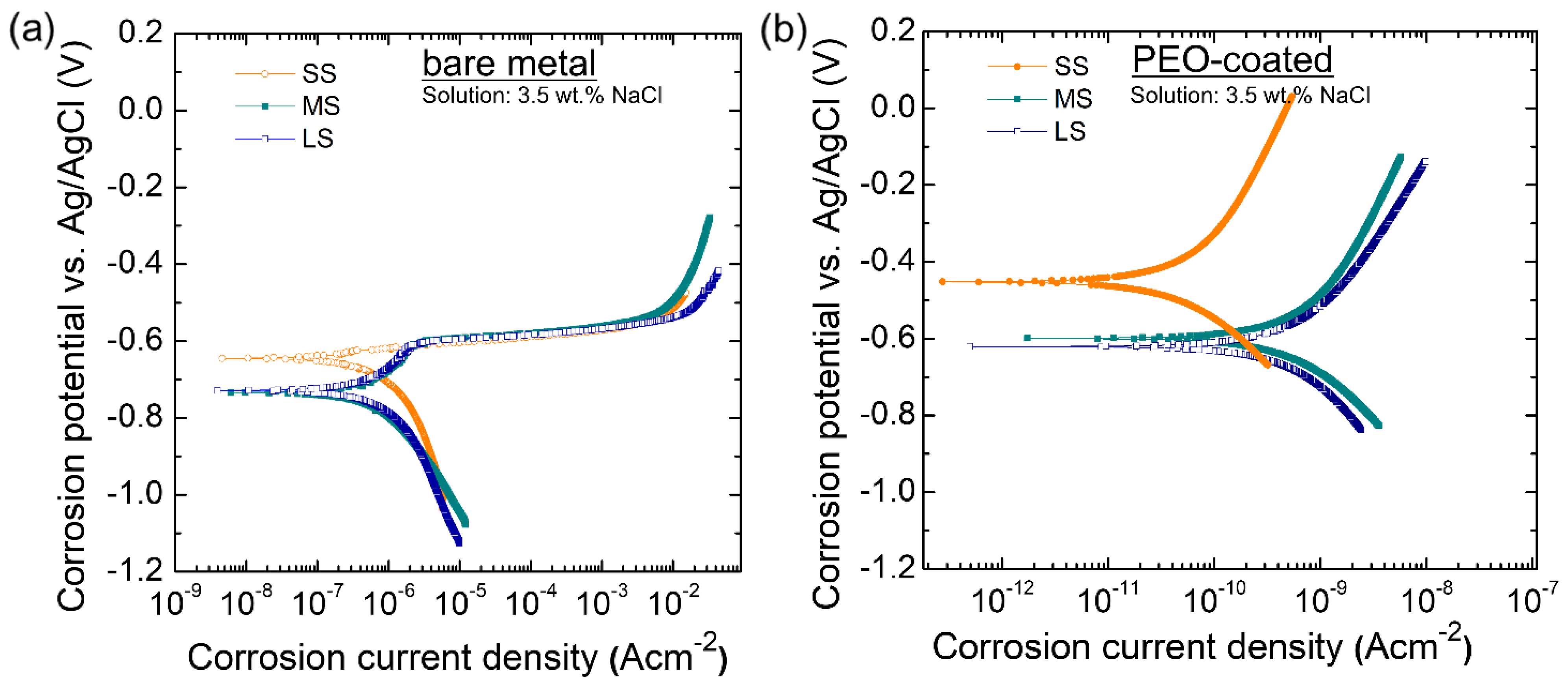
| Sample | icorr (A·cm2) | Ecorr (V) | βa (V/decade) | βc (V/decade) | Rp |
|---|---|---|---|---|---|
| SS (bare) | 1.07 × 10−6 | −0.645 | 0.271 | −0.510 | 7.18 × 104 |
| MS (bare) | 1.34 × 10−6 | −0.734 | 0.298 | −0.320 | 5.90 × 104 |
| LS (bare) | 3.47 × 10−6 | −0.729 | 0.985 | −0.904 | 5.00 × 104 |
| SS (coated) | 7.58 × 10−11 | −0.453 | 0.589 | −0.346 | 1.25 × 109 |
| MS (coated) | 9.74 × 10−10 | −0.580 | 0.680 | −0.771 | 1.24 × 108 |
| LS (coated) | 1.05 × 10−9 | −0.628 | 0.676 | −0.410 | 1.06 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatimah, S.; Bahanan, W.; Widiantara, I.P.; Go, J.H.; Kang, J.-H.; Ko, Y.G. Improving Mechanical and Corrosion Properties of 6061 Al Alloys via Differential Speed Rolling and Plasma Electrolytic Oxidation. Materials 2024, 17, 1252. https://doi.org/10.3390/ma17061252
Fatimah S, Bahanan W, Widiantara IP, Go JH, Kang J-H, Ko YG. Improving Mechanical and Corrosion Properties of 6061 Al Alloys via Differential Speed Rolling and Plasma Electrolytic Oxidation. Materials. 2024; 17(6):1252. https://doi.org/10.3390/ma17061252
Chicago/Turabian StyleFatimah, Siti, Warda Bahanan, I Putu Widiantara, Jae Hoon Go, Jee-Hyun Kang, and Young Gun Ko. 2024. "Improving Mechanical and Corrosion Properties of 6061 Al Alloys via Differential Speed Rolling and Plasma Electrolytic Oxidation" Materials 17, no. 6: 1252. https://doi.org/10.3390/ma17061252





