A Novel Method of Synthesizing Polymeric Aluminum Ferric Sulfate Flocculant and Preparing Red Mud-Based Ceramsite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Red Mud Pre-Treatment Experiment
2.2.2. Preparation of Polymeric Aluminum Ferric Sulfate
2.2.3. The Synthesized Flocculant Performance Verification
2.2.4. Preparation of Red Mud-Based Ceramsite
2.2.5. Phosphate Adsorption Capacities of ARMC
2.2.6. Fe and Al Analyses and Adsorbents Characterization
2.2.7. Statistical Method
3. Results and Analysis
3.1. Effect of the Different Sintering Temperature on Red Mud Leaching of Al and Fe
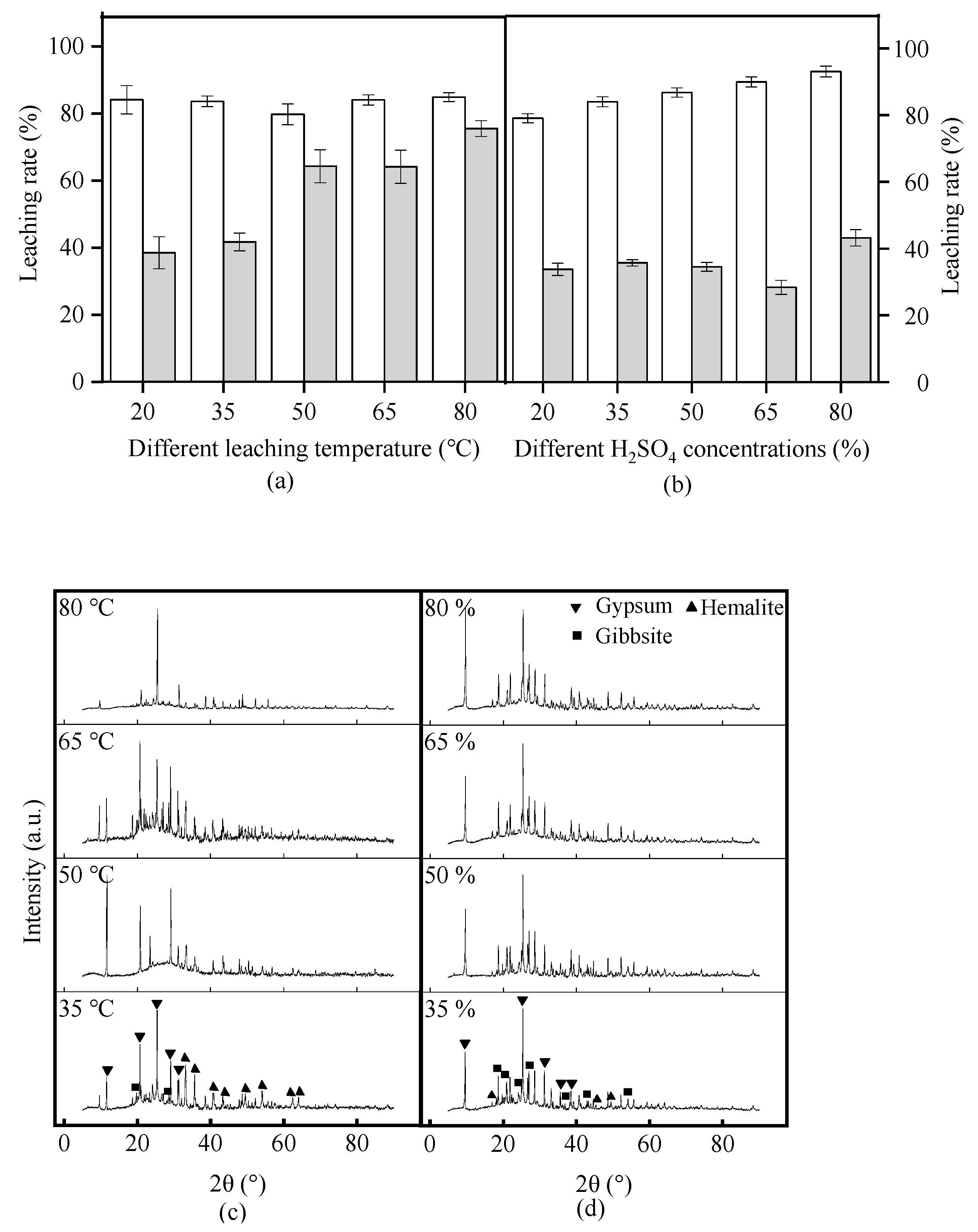
3.2. Effect of Reaction Temperature on Red Mud Leaching of Al and Fe
3.3. Effect of the Initial H2SO4 Concentration on Al and Fe Leaching from Red Mud
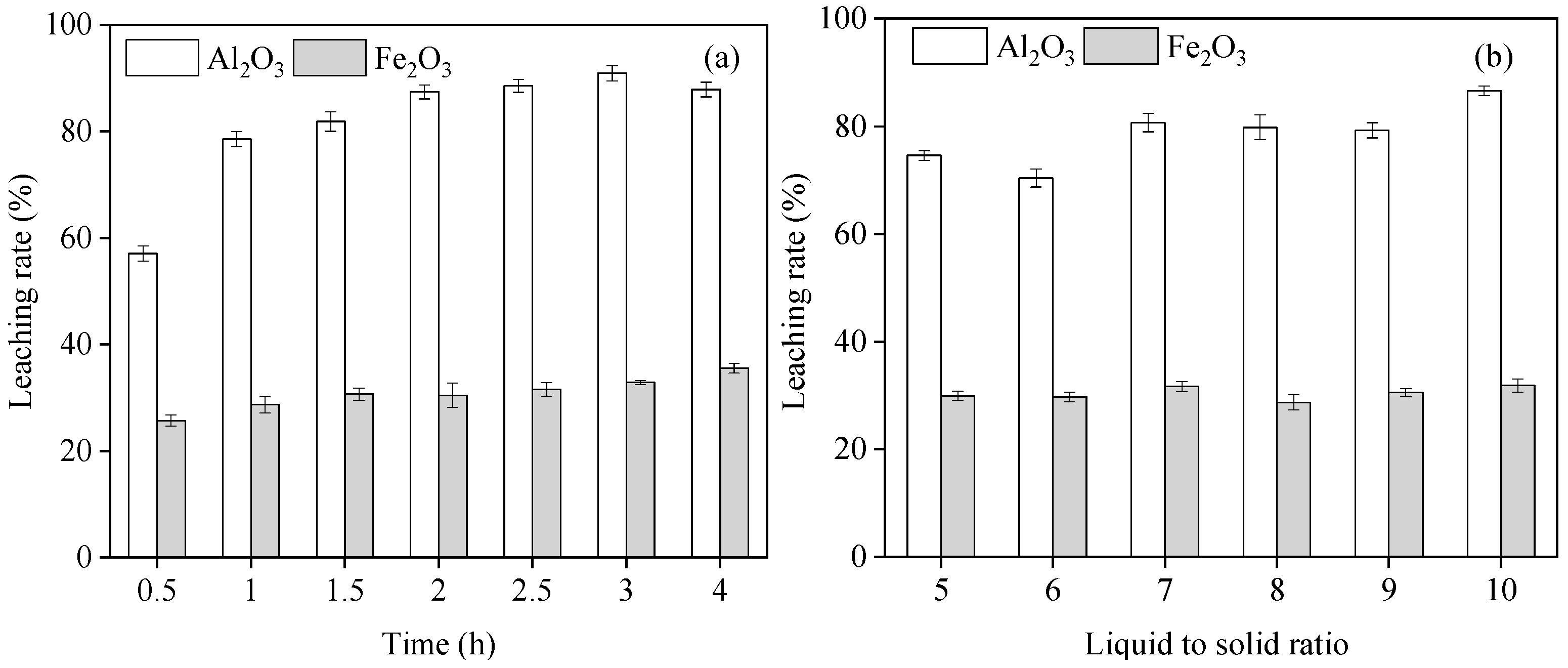
3.4. Effect of the Reaction Time on Al and Fe Leaching from Red Mud
3.5. Effect of Liquid-to-Solid Ratio on Al and Fe Leaching from Red Mud
3.6. Characteristics of the Synthesized Flocculant
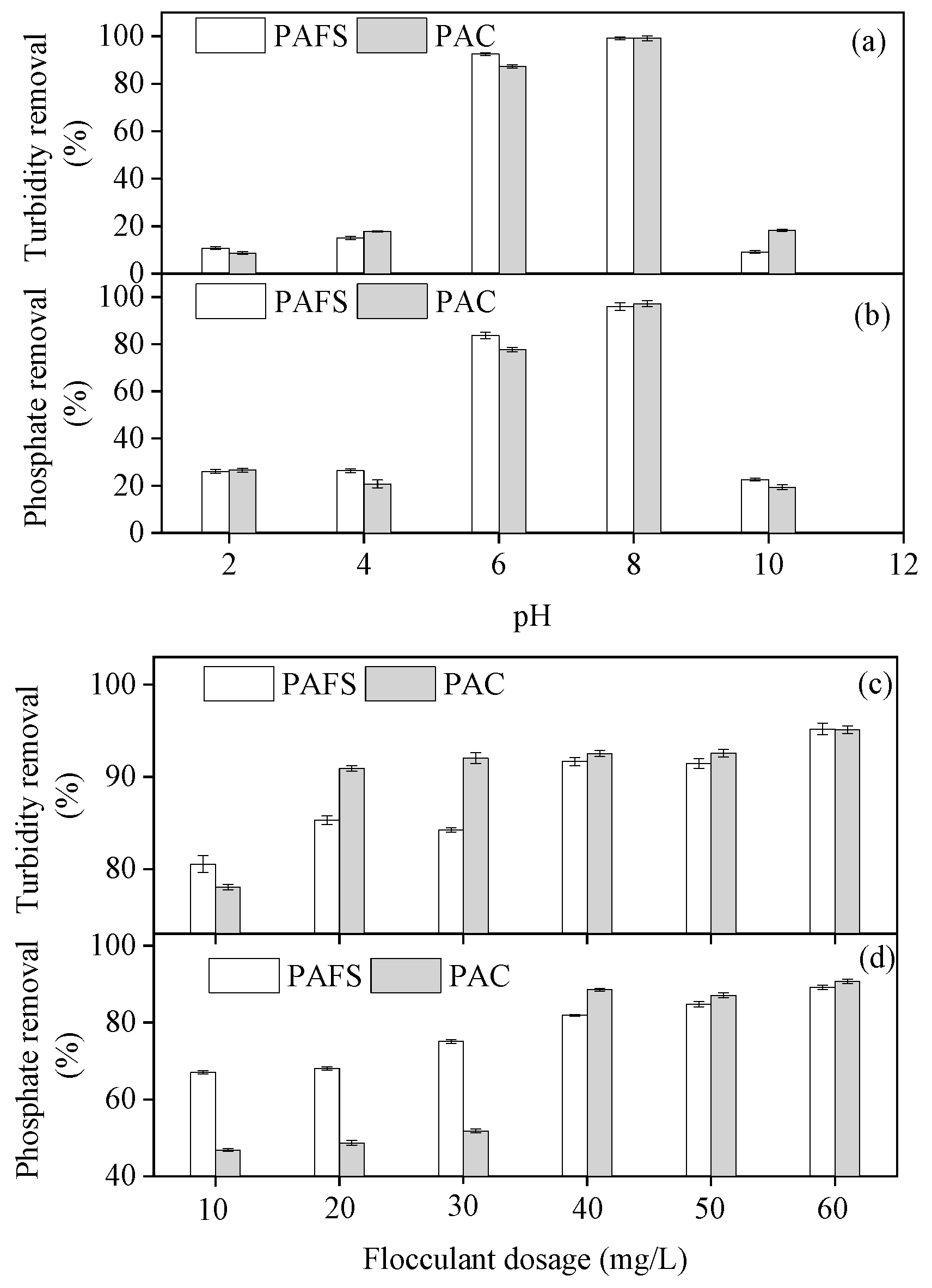
3.7. Turbidity and Phosphate Removal by the Synthesized Flocculant
3.7.1. Effect of pH on Turbidity and Phosphate Removal
3.7.2. Effect of the Synthesized Flocculant Dosage on Turbidity and Phosphate Removal
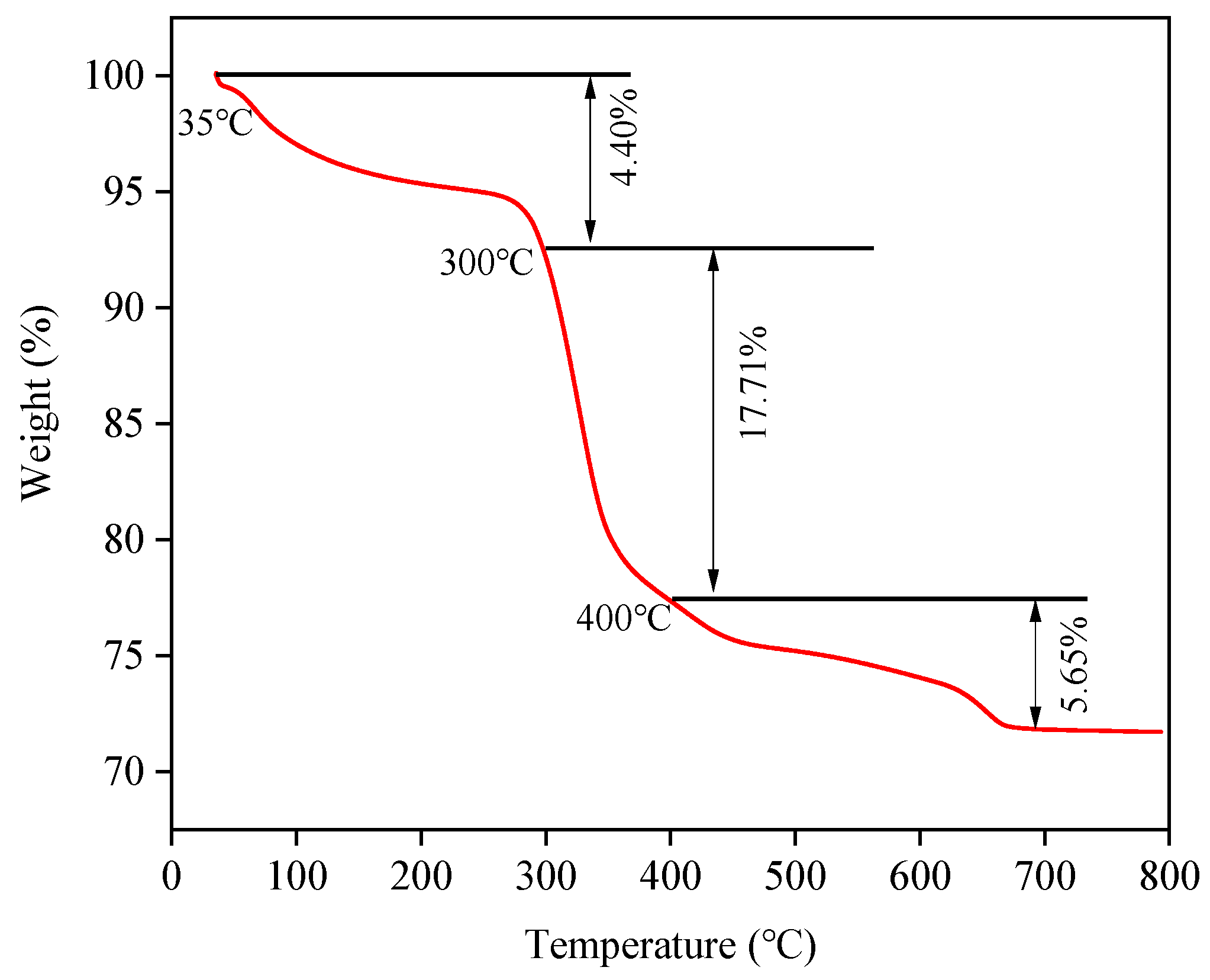
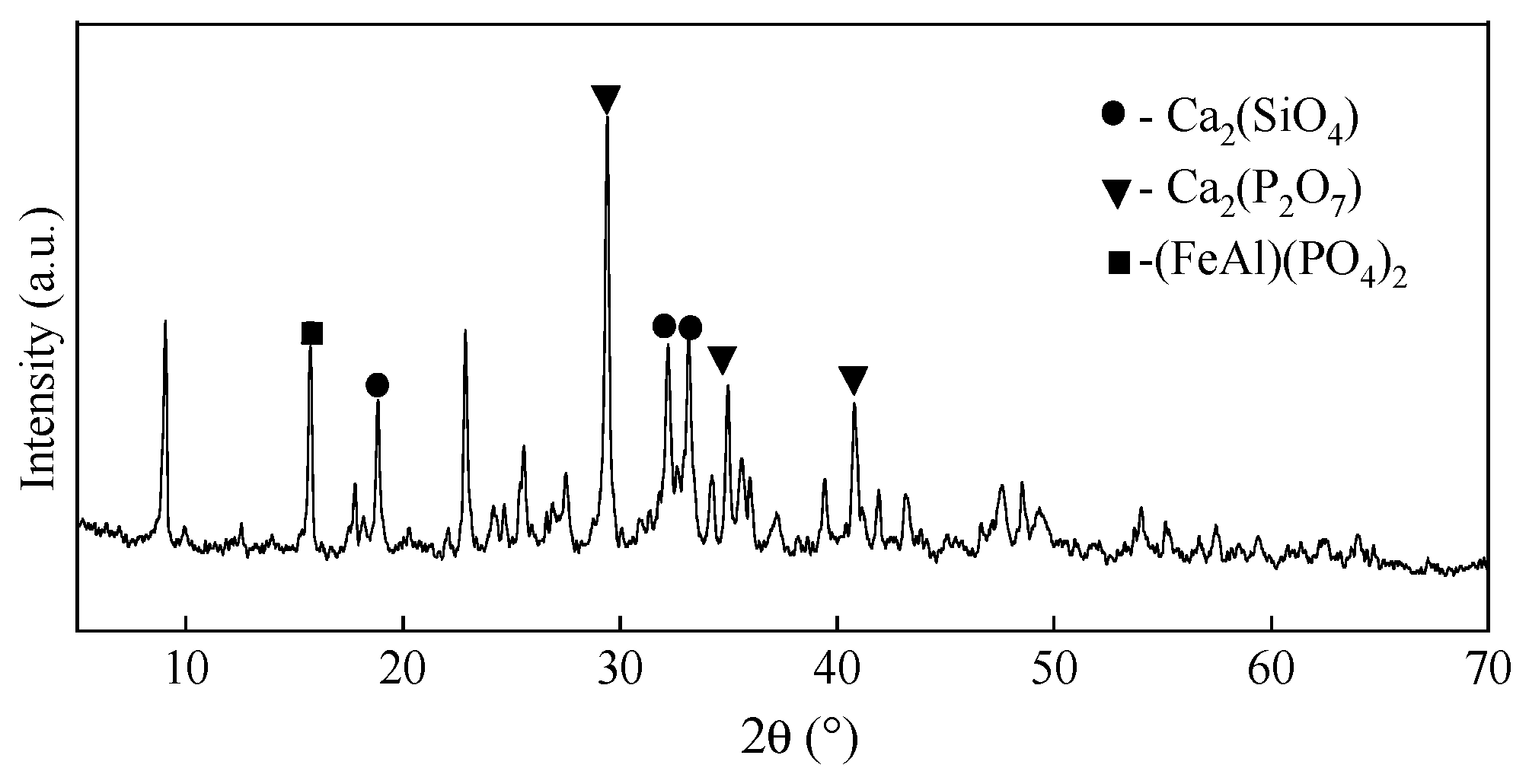
3.8. Properties of the Red Mud-Based Ceramsite
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qaidi, S.M.; Tayeh, B.A.; Isleem, H.F.; de Azevedo, A.R.; Ahmed, H.U.; Emad, W. Sustainable utilization of red mud waste (bauxite residue) and slag for the production of geopolymer composites: A review. Case Stud. Constr. Mater. 2022, 16, e00994. [Google Scholar] [CrossRef]
- Prasad, R.; Pai, A.R.; Oyadiji, S.O.; Thomas, S.; Parashar, S.K.S. Utilization of hazardous red mud in silicone rubber/MWCNT nanocomposites for high performance electromagnetic interference shielding. J. Clean. Prod. 2022, 377, 134290. [Google Scholar] [CrossRef]
- Ozden, B.; Brennan, C.; Landsberger, S. Investigation of bauxite residue (red mud) in terms of its environmental risk. J. Radioanal. Nucl. Chem. 2019, 319, 339–346. [Google Scholar] [CrossRef]
- Agrawal, V.; Paulose, R.; Arya, R.; Rajak, G.; Giri, A.; Bijanu, A.; Sanghi, S.K.; Mishra, D.; Prasanth, N.; Khare, A.K.; et al. Green conversion of hazardous red mud into diagnostic X-ray shielding tiles. J. Hazard. Mater. 2022, 424, 127507. [Google Scholar] [CrossRef]
- Rai, S.; Nimje, M.T.; Chaddha, M.J.; Modak, S.; Rao, K.R.; Agnihotri, A. Recovery of iron from bauxite residue using advanced separation techniques. Miner. Eng. 2019, 134, 222–231. [Google Scholar] [CrossRef]
- Dai, X.; Nhung, N.T.H.; Hamza, M.F.; Guo, Y.; Chen, L.; He, C.; Ning, S.; Wei, Y.; Dodbiba, G.; Fujita, T. Selective adsorption and recovery of scandium from red mud leachate by using phosphoric acid pre-treated pitaya peel biochar. Sep. Purif. Technol. 2022, 292, 121043. [Google Scholar] [CrossRef]
- Gao, J.; Qu, X.; Lan, X.; Li, Y.; Guo, Z. A green method for solidification and recovery of soluble sodium in red mud via super-gravity. Process Saf. Environ. Prot. 2022, 161, 384–391. [Google Scholar] [CrossRef]
- Hena, S.; bt Abdullah, N.F.; Keong, L.C.; Mohamed Najar, P.A.; Gutierrez, L.; Croué, J.P. Zero residual heavy metals in aqueous media using composite coagulant converted from bauxite residue. Int. J. Environ. Sci. Technol. 2023, 20, 5453–5470. [Google Scholar] [CrossRef]
- Anawar, H.; Strezov, V.; Hossain, M. Comparison of different nanoprocesses and industrial waste-based adsorbents such as red mud, steel slag, and fly ashes for treating wastewater nanomaterial contaminants. In Emerging and Nanomaterial Contaminants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2019; pp. 107–136. [Google Scholar] [CrossRef]
- Vidya, A.; Poojitha, A.S.; Reddy, N.G.; Samal, K. Utilization of Red Mud (Bauxite Residue) in Environmental and Geotechnical Engineering Applications—An Overview. In Transportation and Environmental Geotechnics; Springer: Singapore, 2021; pp. 243–251. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Zhang, Y.; Liu, J.; Xiao, X.; Meng, K.; Chu, B.; Wang, C.; Chu, P.K. Multiple flocculant prepared with dealkalized red mud and fly ash: Properties and characterization. J. Water Process Eng. 2020, 34, 101173. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Liu, D.; Hou, X.; Zou, J.; Ma, X.; Shang, F.; Wang, Z. Aluminum and iron leaching from power plant coal fly ash for preparation of polymeric aluminum ferric chloride. Environ. Technol. 2019, 40, 1568–1575. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Q.; Tian, C.; Zhan, W.; Chen, S.; He, Z. High-Efficiency Preparation of Fe-Al Based Flocculants from Red Mud by Microwave Selective Carbothermic Reduction and Magnetic Separation. Environ. Prog. Sustain. Energy 2020, 39, e13466. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, L.; Liu, C. Red mud-based polyaluminium ferric chloride flocculant: Preparation, characterisation, and flocculation performance. Environ. Technol. Innov. 2022, 27, 102509. [Google Scholar] [CrossRef]
- Ke, Y.; Liang, S.; Hou, H.; Hu, Y.; Li, X.; Chen, Y.; Li, X.; Cao, L.; Yuan, S.; Xiao, K.; et al. A zero-waste strategy to synthesize geopolymer from iron-recovered Bayer red mud combined with fly ash: Roles of Fe, Al and Si. Constr. Build. Mater. 2022, 322, 126176. [Google Scholar] [CrossRef]
- Zhang, D.R.; Chen, H.R.; Zhao, X.-J.; Xia, J.L.; Nie, Z.-y.; Zhang, R.Y.; Shu, W.S.; Pakostova, E. Fe(II) bio-oxidation mediates red mud transformations to form Fe(III)/Al (hydr)oxide adsorbent for efficient As(V) removal under acidic conditions. Chem. Eng. J. 2022, 439, 135753. [Google Scholar] [CrossRef]
- Khanna, R.; Konyukhov, Y.; Zinoveev, D.; Jayasankar, K.; Burmistrov, I.; Kravchenko, M.; Mukherjee, P.S. Red mud as a secondary resource of low-grade iron: A global perspective. Sustainability 2022, 14, 1258. [Google Scholar] [CrossRef]
- Deihimi, N.; Irannajad, M.; Rezai, B. Characterization studies of red mud modification processes as adsorbent for enhancing ferricyanide removal. J. Environ. Manag. 2018, 206, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Joghataei, A.; Mostafapour, F.K.; Bazrafshan, E. Ciprofloxacin antibiotics removal from effluent using heat-acid activated red mud. J. Pharm. Res. Int. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Shabnam, N.; Ahn, Y.; Maksachev, A.; Lee, J.H.; Huang, C.-P.; Kim, H. Application of red-mud based ceramic media for phosphate uptake from water and evaluation of their effects on growth of Iris latifolia seedling. Sci. Total Environ. 2019, 688, 724–731. [Google Scholar] [CrossRef]
- Didanovic, S.; Vrhovsek, D. Significance of substrate selection in the efficiency of wastewater treatment in constructed wetlands (cws). J. Water Resour. Prot. 2023, 15, 424–441. [Google Scholar] [CrossRef]
- Özcan, M.; Birol, B.; Dülger Kutlu, Ö.; Kaya, F. Photocatalytic properties of ZnO nanoparticle coating on porous ceramic substrates with varying porosities produced from fly ash and red mud. Int. J. Appl. Ceram. Technol. 2023. earlyview. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Campostrini, R.; Maurina, S.; Carturan, G.; Monagheddu, M.; Budroni, G.; Cocco, G. Bauxite ‘red mud’ in the ceramic industry. Part 1: Thermal behaviour. J. Eur. Ceram. Soc. 2000, 20, 235–244. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of China. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002. [Google Scholar]
- GB/T 17431.2-2010; Lightweight Aggregates and Its Test Methods-Part 2: Test Methods for Lightweight Aggregates. China Building Materials Federation: Beijing, China, 2011.
- Yashima, S.; Kanda, Y.; Sano, S. Relationships between particle size and fracture energy or impact velocity required to fracture as estimated from single particle crushing. Powder Technol. 1987, 51, 277–282. [Google Scholar] [CrossRef]
- Hou, D.; Wu, D.; Wang, X.; Gao, S.; Yu, R.; Li, M.; Wang, P.; Wang, Y. Sustainable use of red mud in ultra-high performance concrete (UHPC): Design and performance evaluation. Cem. Concr. Compos. 2021, 115, 103862. [Google Scholar] [CrossRef]
- Kurfman, L.A.; Odbadrakh, T.T.; Shields, G.C. Calculating Reliable Gibbs Free Energies for Formation of Gas-Phase Clusters that Are Critical for Atmospheric Chemistry: (H2SO4)3. J. Phys. Chem. A 2021, 125, 3169–3176. [Google Scholar] [CrossRef]
- Moraes, C.V.; Demétrio da Silva, V.C.; Castegnaro, M.V.; Morais, J.; Schrekker, H.S.; Amico, S.C. Lightweight composites through imidazolium ionic liquid enhanced aramid–epoxy resin interactions. ACS Appl. Polym. Mater. 2020, 2, 1754–1763. [Google Scholar] [CrossRef]
- Tolkou, A.; Zouboulis, A. Synthesis and characterization of a novel composite pre-polymerized coagulant for water and wastewater treatment. Int. J. Environ. Eng. 2015, 2, 154–157. [Google Scholar]
- Oladipo, A.A.; Mustafa, F.S.; Ezugwu, O.N.; Gazi, M. Efficient removal of antibiotic in single and binary mixture of nickel by electrocoagulation process: Hydrogen generation and cost analysis. Chemosphere 2022, 300, 134532. [Google Scholar] [CrossRef] [PubMed]
- Agbovi, H.; Wilson, D. Design of amphoteric chitosan flocculants for phosphate and turbidity removal in wastewater. Carbohydr. Polym. 2018, 189, 360–370. [Google Scholar] [CrossRef]
- Ferasat, Z.; Panahi, R.; Mokhtarani, B. Natural polymer matrix as safe flocculant to remove turbidity from kaolin suspension: Performance and governing mechanism. J. Environ. Manag. 2020, 255, 109939. [Google Scholar] [CrossRef]
- Kunjie, L.; Jun, W.; Jianfeng, M.; Da, G.; Yao, B.; Yaoling, L.; Xiaorun, C. Effect of municipal sludge on the performance of coal gasification slag-based ceramsite. Ceram. Silikáty 2021, 65, 176–186. [Google Scholar] [CrossRef]
- Tancredi, N.; Medero, N.; Mller, F.; Píriz, J.; Plada, C.; Cordero, T. Phenol adsorption onto powdered and granular activated carbon, prepared from Eucalyptus wood. In Proceedings of the Joint International Conference on Information Sciences, Hainan, China, 22–24 March 2004; Volume 279, pp. 357–363. [Google Scholar] [CrossRef]
- Samal, S. Utilization of Red Mud as a Source for Metal Ions-A Review. Materials 2021, 14, 2211. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, A.A.; Bazhin, V.Y.; Kuskova, Y.V.; Abdelrahim, A.; Ahmed, Y.M.Z. Study the recycling of red mud in iron ore sintering process. J. Ecol. Eng. 2021, 22, 191–201. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Investigation of mechanical and thermal activation on metal extraction from red mud. Sustain. Mater. Technol. 2021, 27, e00246. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, C. Analysis on Physical and Mechanical Properties of Red Mud Materials and Stockpile Stability after Dilatation. Adv. Mater. Sci. Eng. 2018, 2018, 8784232. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.-Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Unuabonah, E.I.; Amuda, O.S.; Kolawole, O.M. Polysaccharides as a Green and Sustainable Resources for Water and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Pan, L.T.; Wang, W.L.; Yu, B.; Shu, Y.B. The preparation and characterization of a new high polymeric flocculant-polymeric aluminum ferric sulfate chloride. Adv. Mater. Res. 2012, 356–360, 445–450. [Google Scholar] [CrossRef]
- Taneez, M.; Hurel, C. A review on the potential uses of red mud as amendment for pollution control in environmental media. Environ. Sci. Pollut. Res. 2019, 26, 22106–22125. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. Preparation of high-strength ceramsite from red mud, fly ash, and bentonite. Ceram. Int. 2021, 47, 18218–18229. [Google Scholar] [CrossRef]
- Ma, L.; Li, G.Z. The Preparation of Red Mud Lightweight Ceramsite. Adv. Mater. Res. 2013, 648, 96–99. [Google Scholar] [CrossRef]






| Components | Na2O | MgO | Al2O3 | SiO2 | CaO | TiO2 | Fe2O3 | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| Mass percentage | 10.77 | 1.12 | 23.11 | 20.25 | 11.50 | 4.01 | 26.96 | 0.65 | 1.63 |
| Water Quality Index | COD (mg/L) | TP (mg/L) | TN (mg/L) | NH3-N (mg/L) | pH |
|---|---|---|---|---|---|
| Concentration | 240.45 | 2.98 | 40.78 | 55.32 | 7.32 |
| Parameters | ARMC-70%-600 °C | ARMC-60%-600 °C | ARMC-50%-600 °C | ARMC-40%-600 °C |
|---|---|---|---|---|
| BET surface area (m2/g) | 40.62 | 56.16 | 25.24 | 4.79 |
| Pore volume (cm3/g) | 0.149 | 0.167 | 0.128 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhen, Z.; He, C.; Wang, Y.; Ma, H. A Novel Method of Synthesizing Polymeric Aluminum Ferric Sulfate Flocculant and Preparing Red Mud-Based Ceramsite. Materials 2024, 17, 1239. https://doi.org/10.3390/ma17061239
Zhen Z, He C, Wang Y, Ma H. A Novel Method of Synthesizing Polymeric Aluminum Ferric Sulfate Flocculant and Preparing Red Mud-Based Ceramsite. Materials. 2024; 17(6):1239. https://doi.org/10.3390/ma17061239
Chicago/Turabian StyleZhen, Zhilei, Chenxi He, Yanrong Wang, and Haotian Ma. 2024. "A Novel Method of Synthesizing Polymeric Aluminum Ferric Sulfate Flocculant and Preparing Red Mud-Based Ceramsite" Materials 17, no. 6: 1239. https://doi.org/10.3390/ma17061239






