A Study of Hyaluronic Acid’s Theoretical Reactivity and of Magnetic Nanoparticles Capped with Hyaluronic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Simulation
2.2. Reagents
2.3. Preparation of HA-MNP Aqueous Suspensions
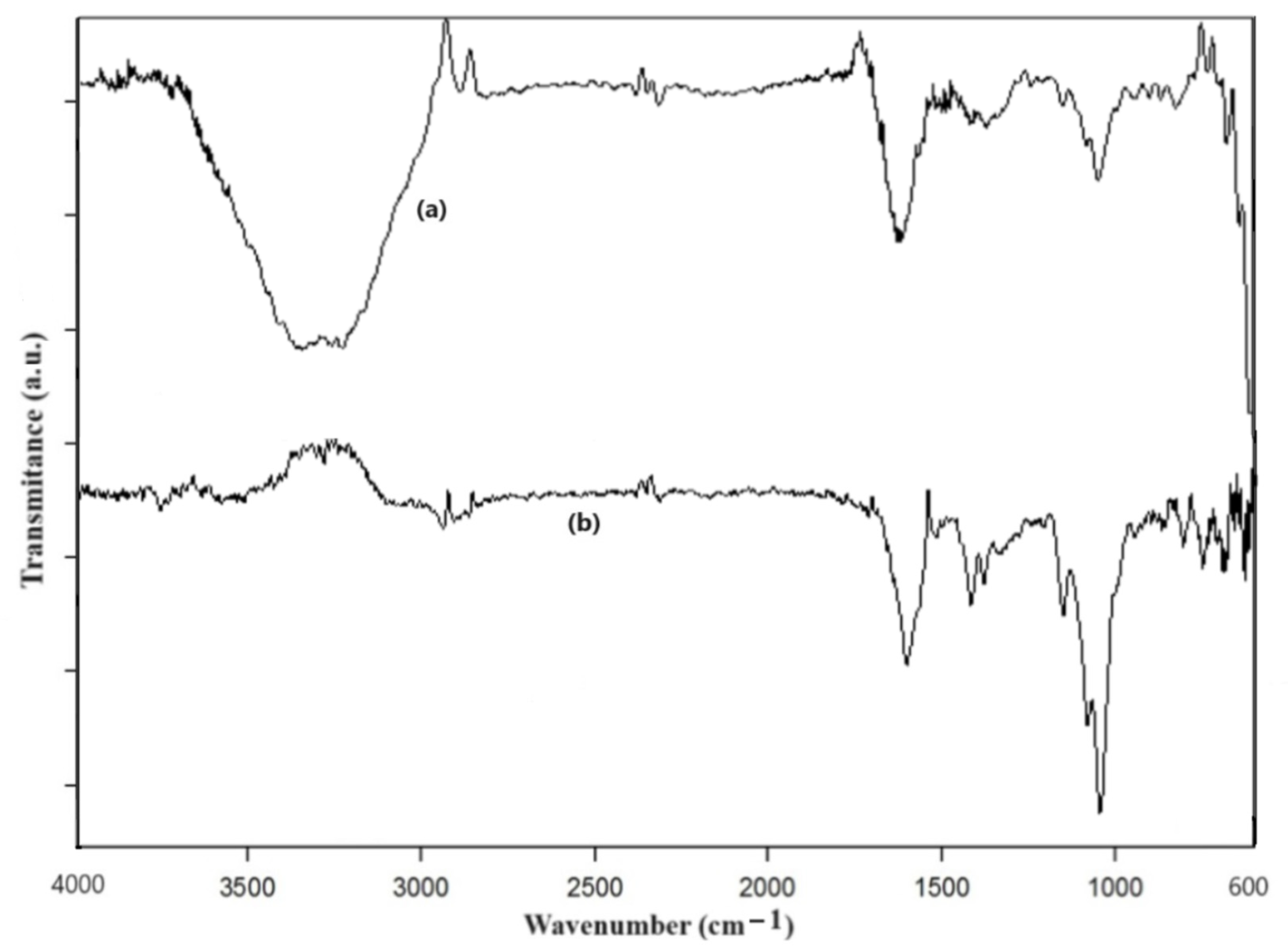
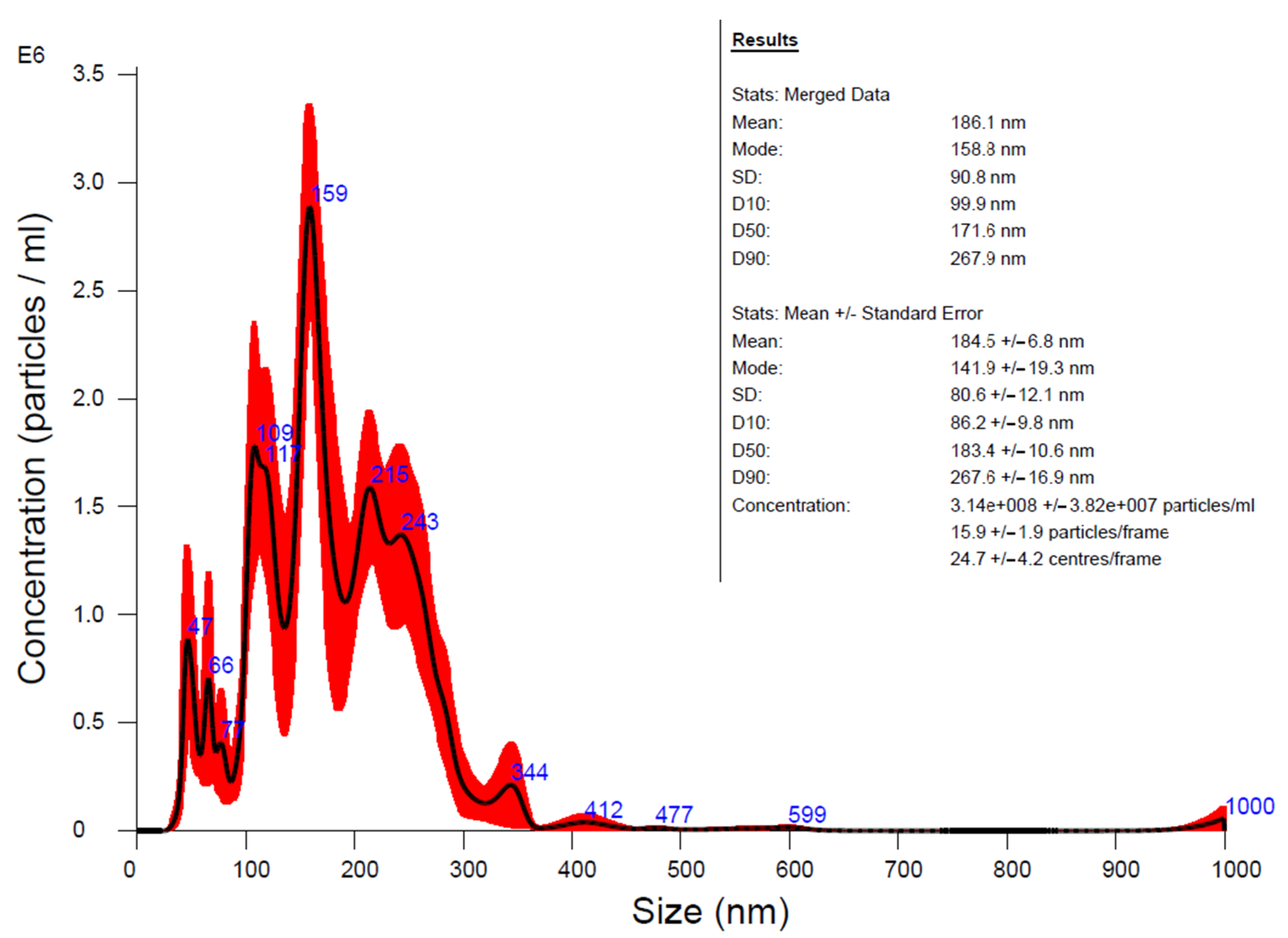
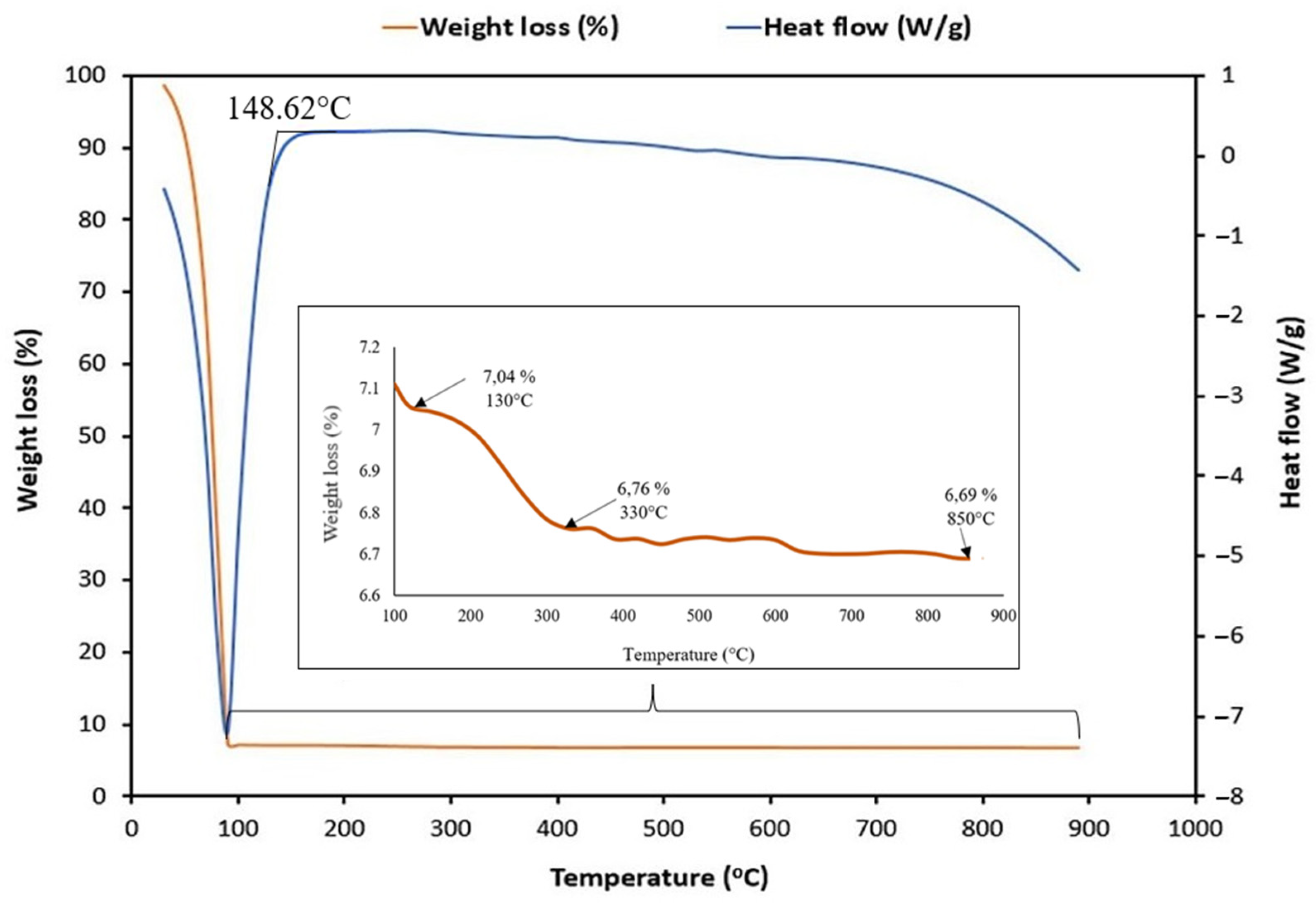
2.4. Characterization of HA-MNPs
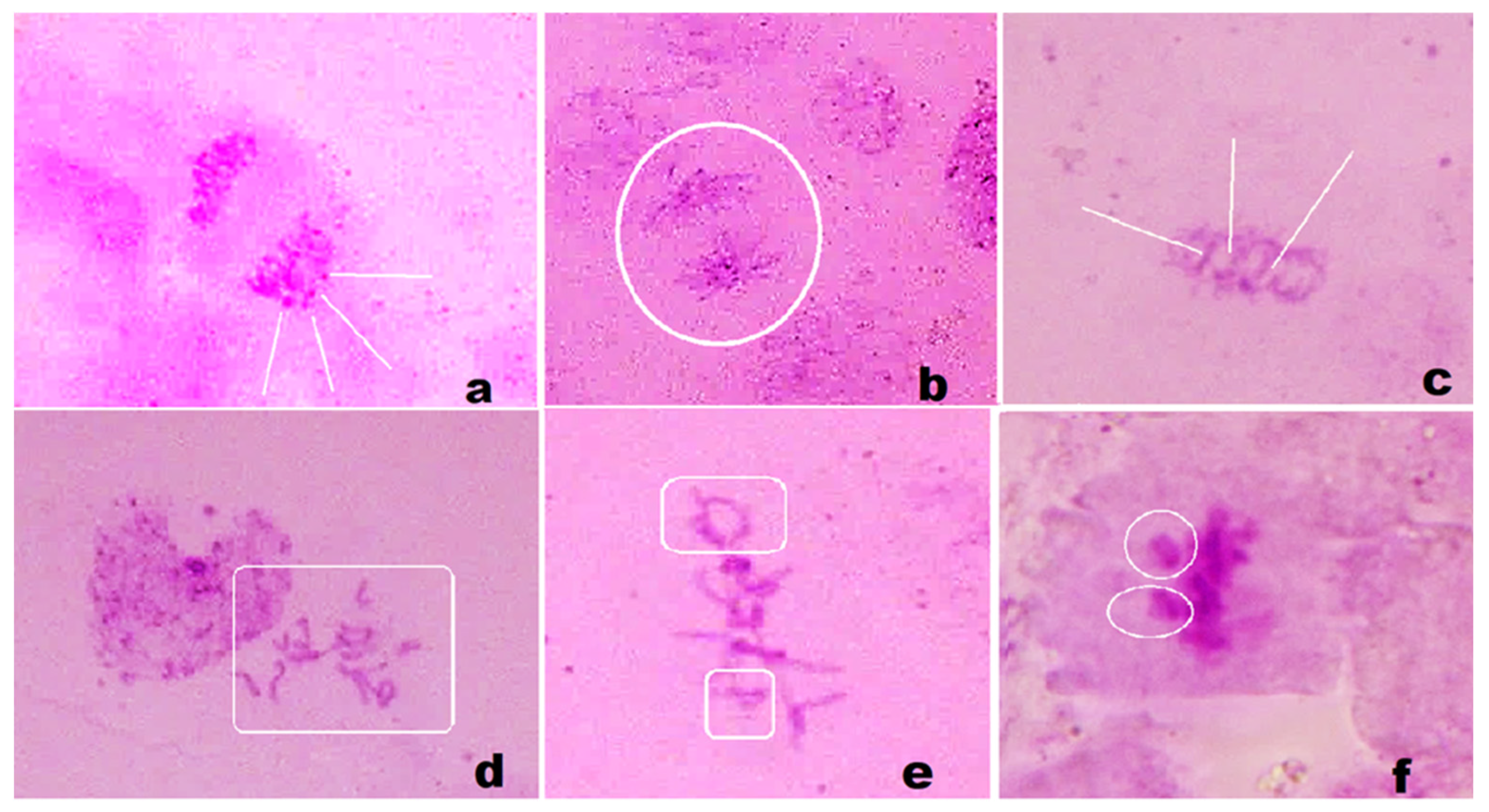
2.5. Nanotoxicity Test
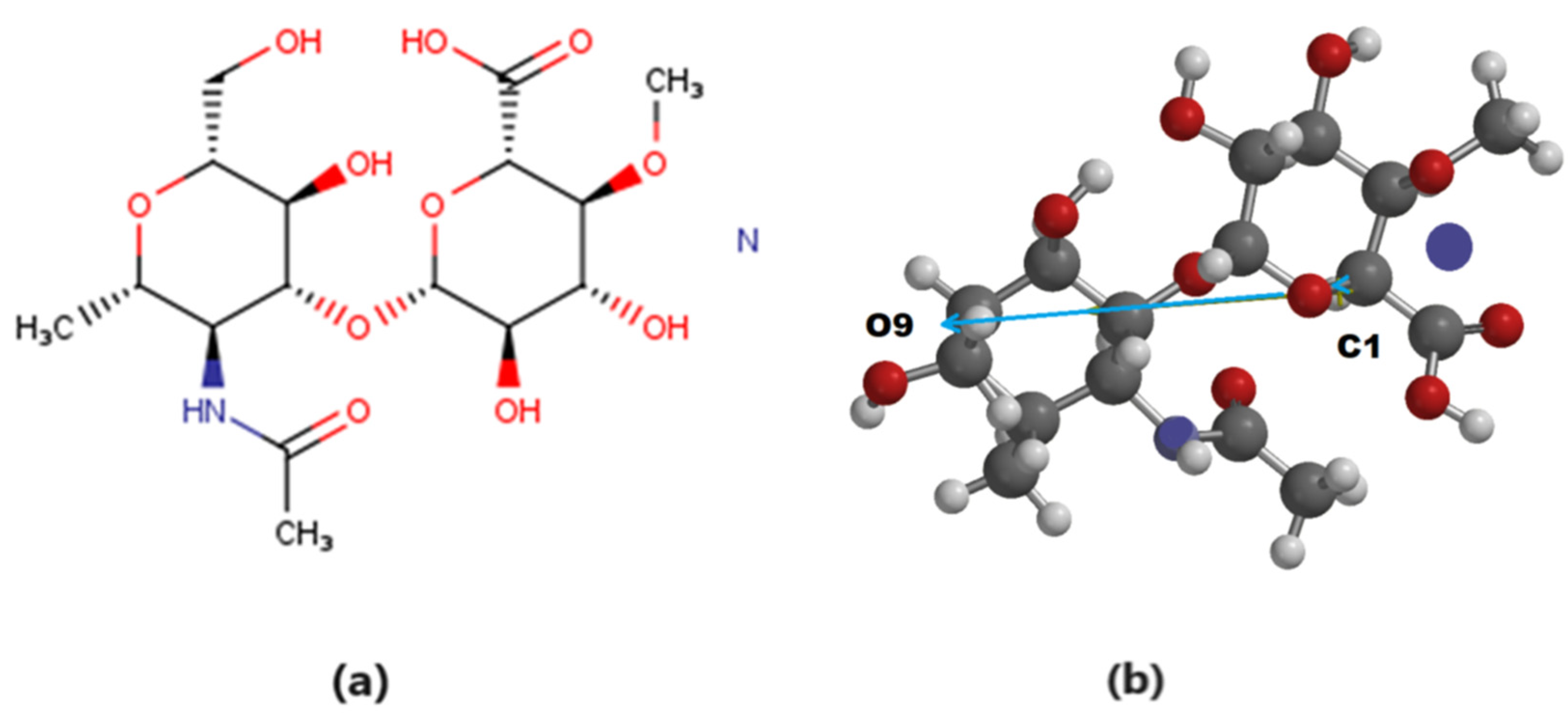
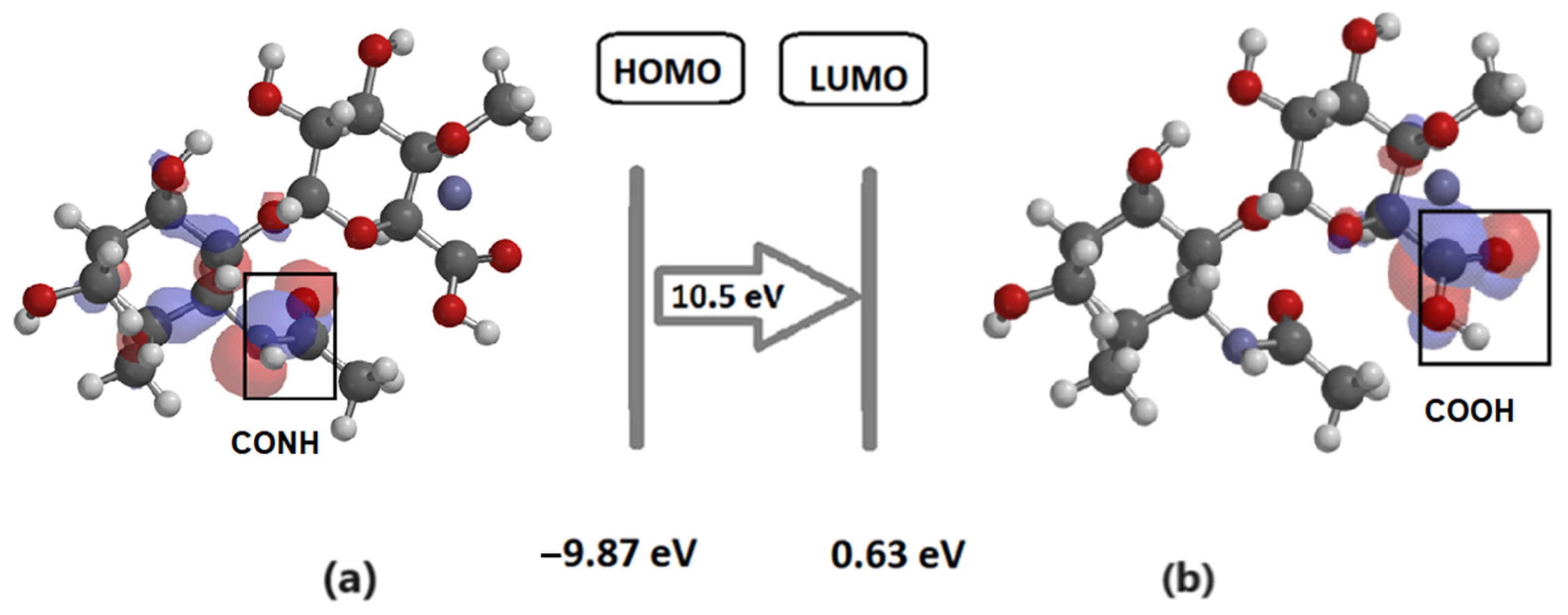
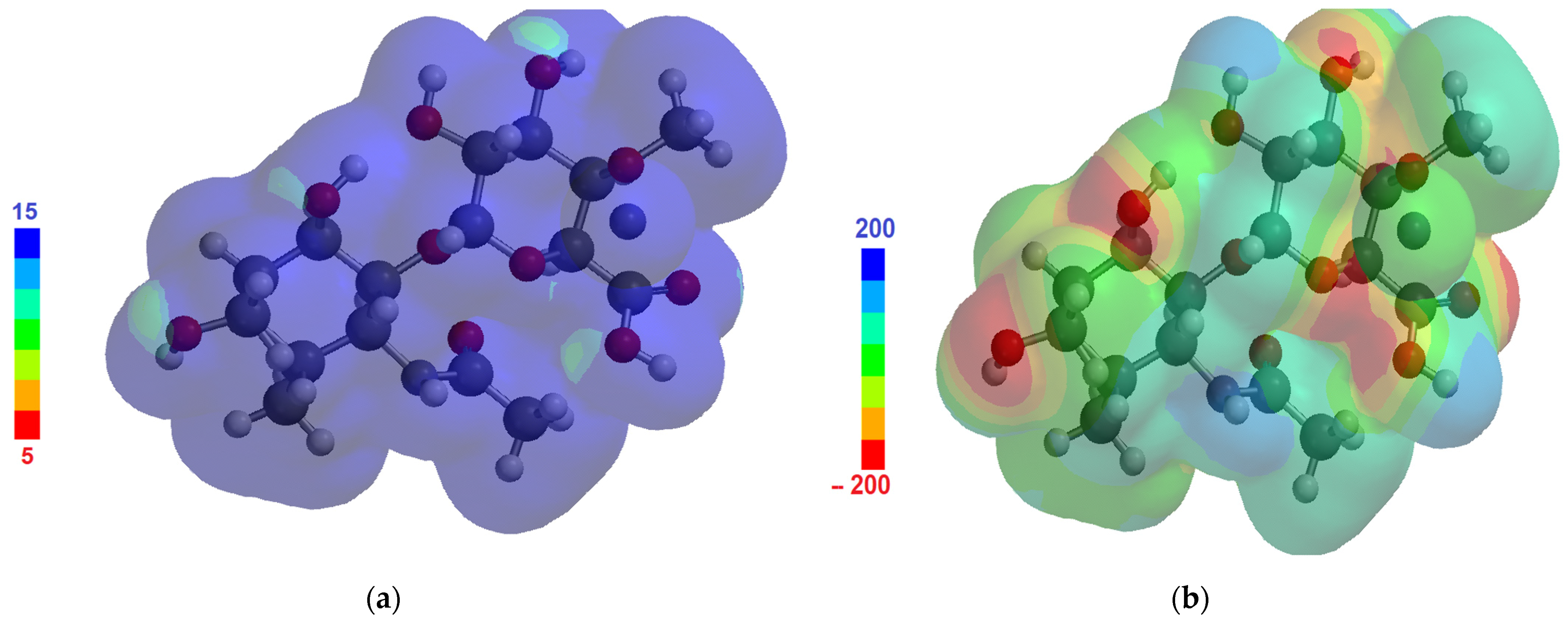
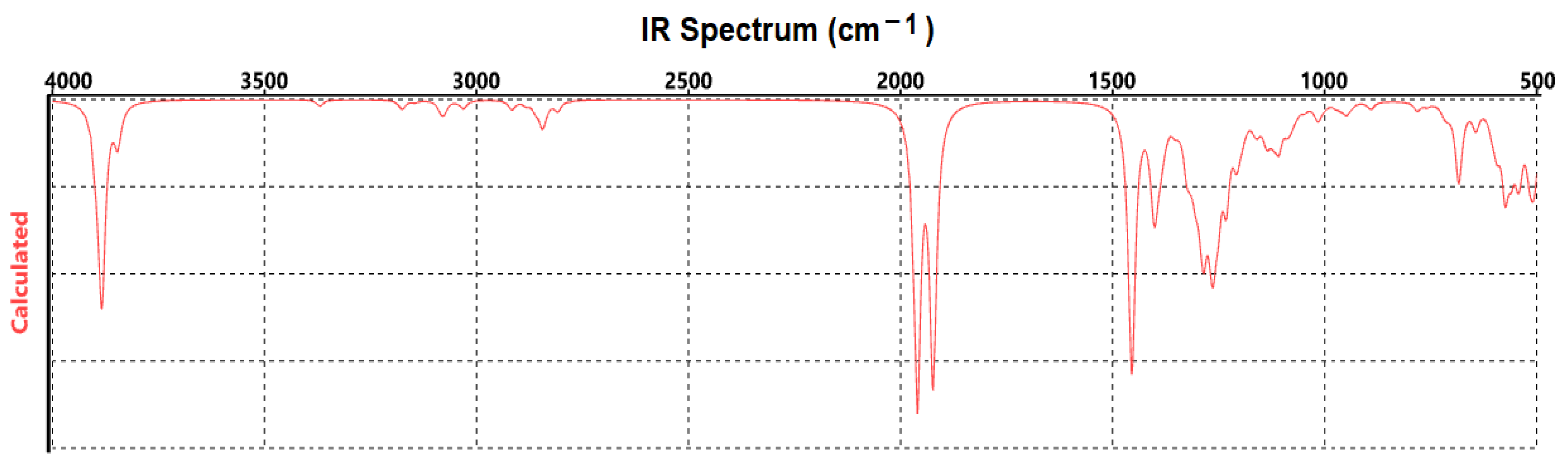
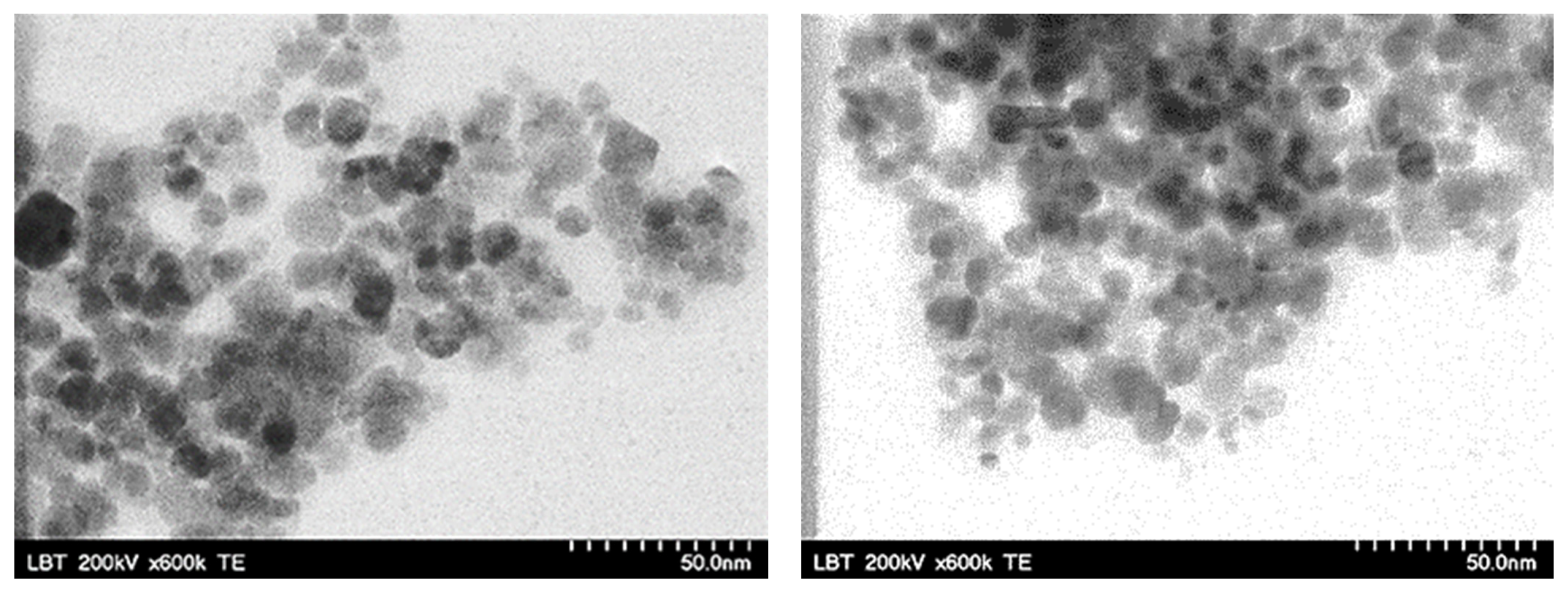
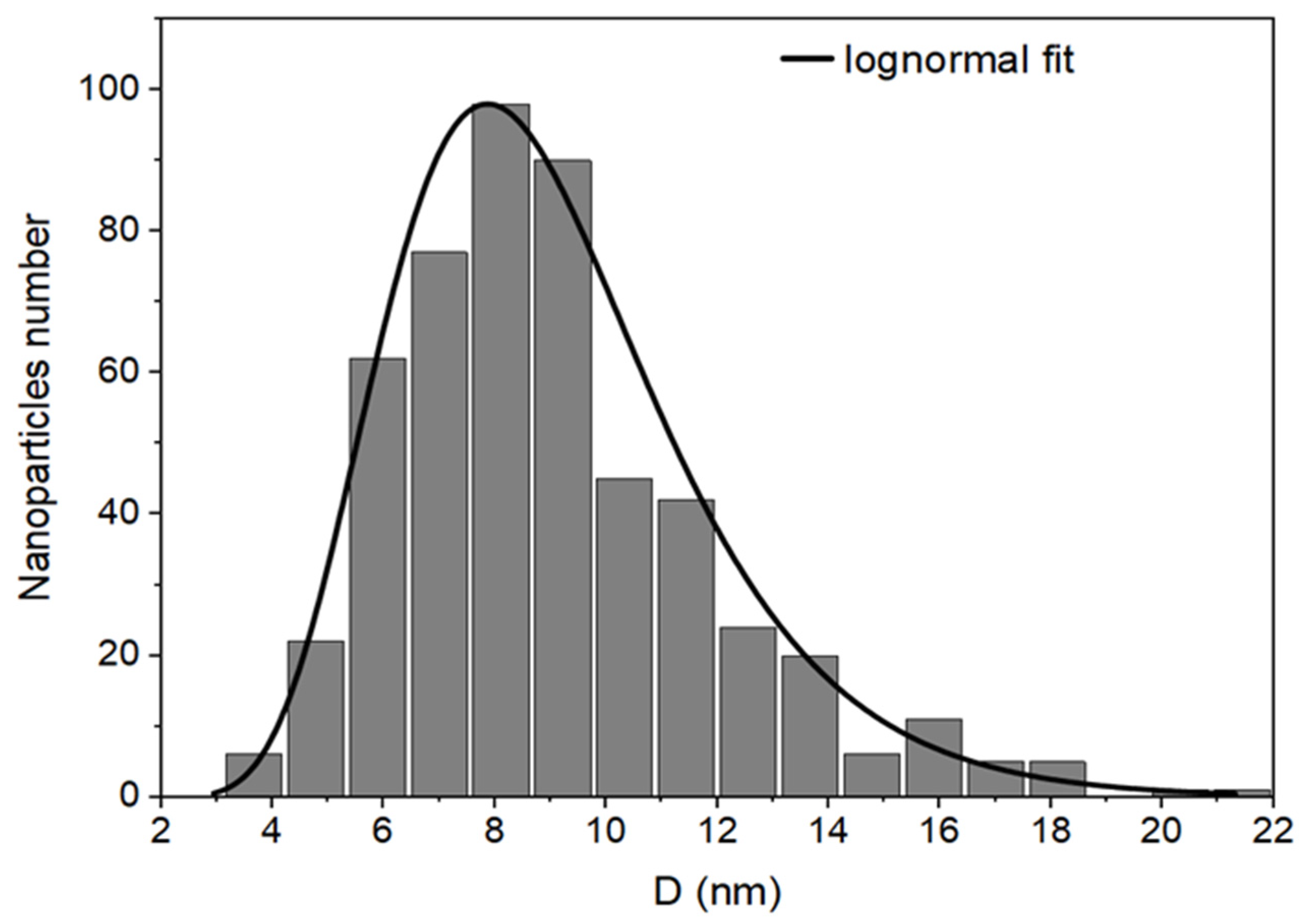
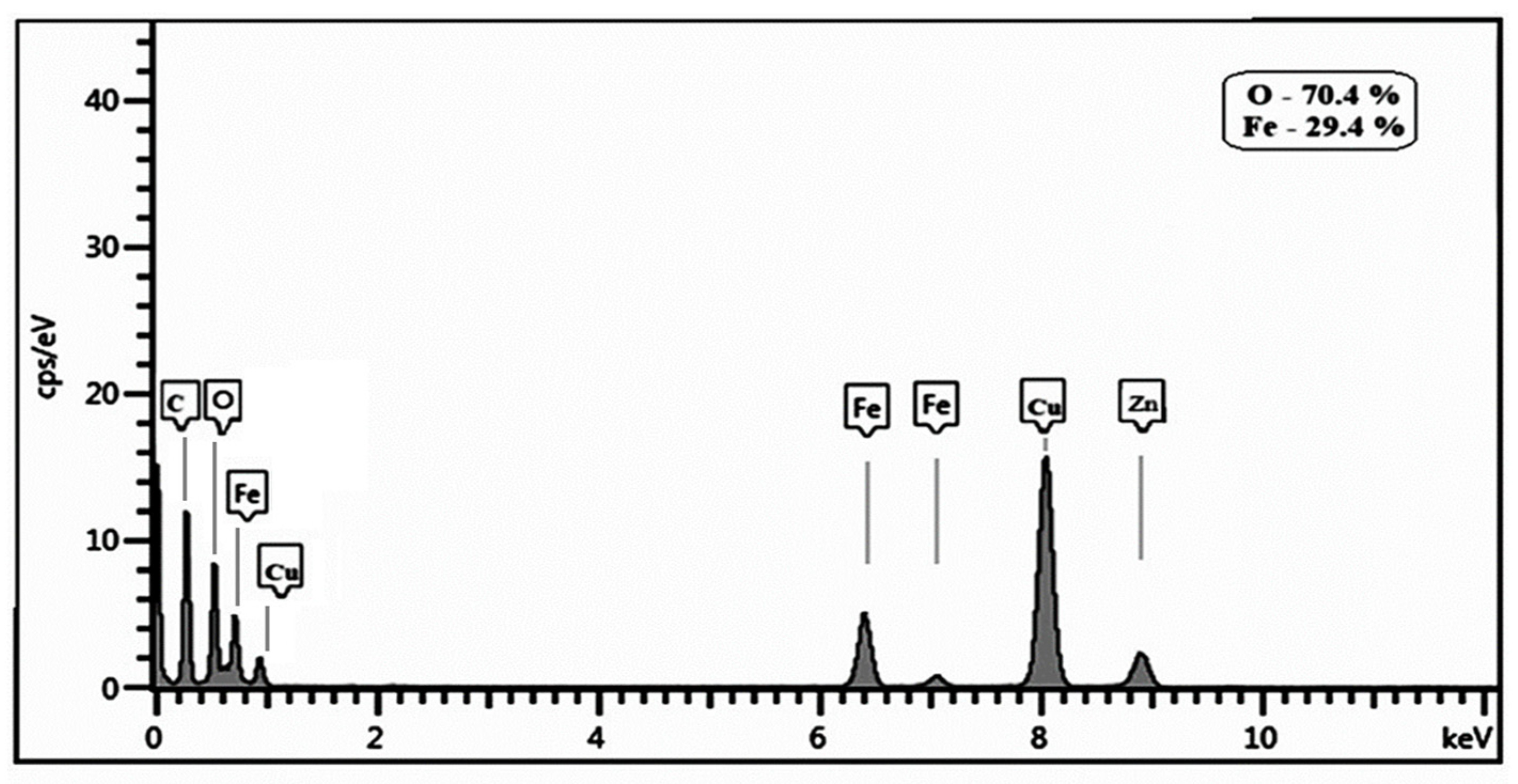
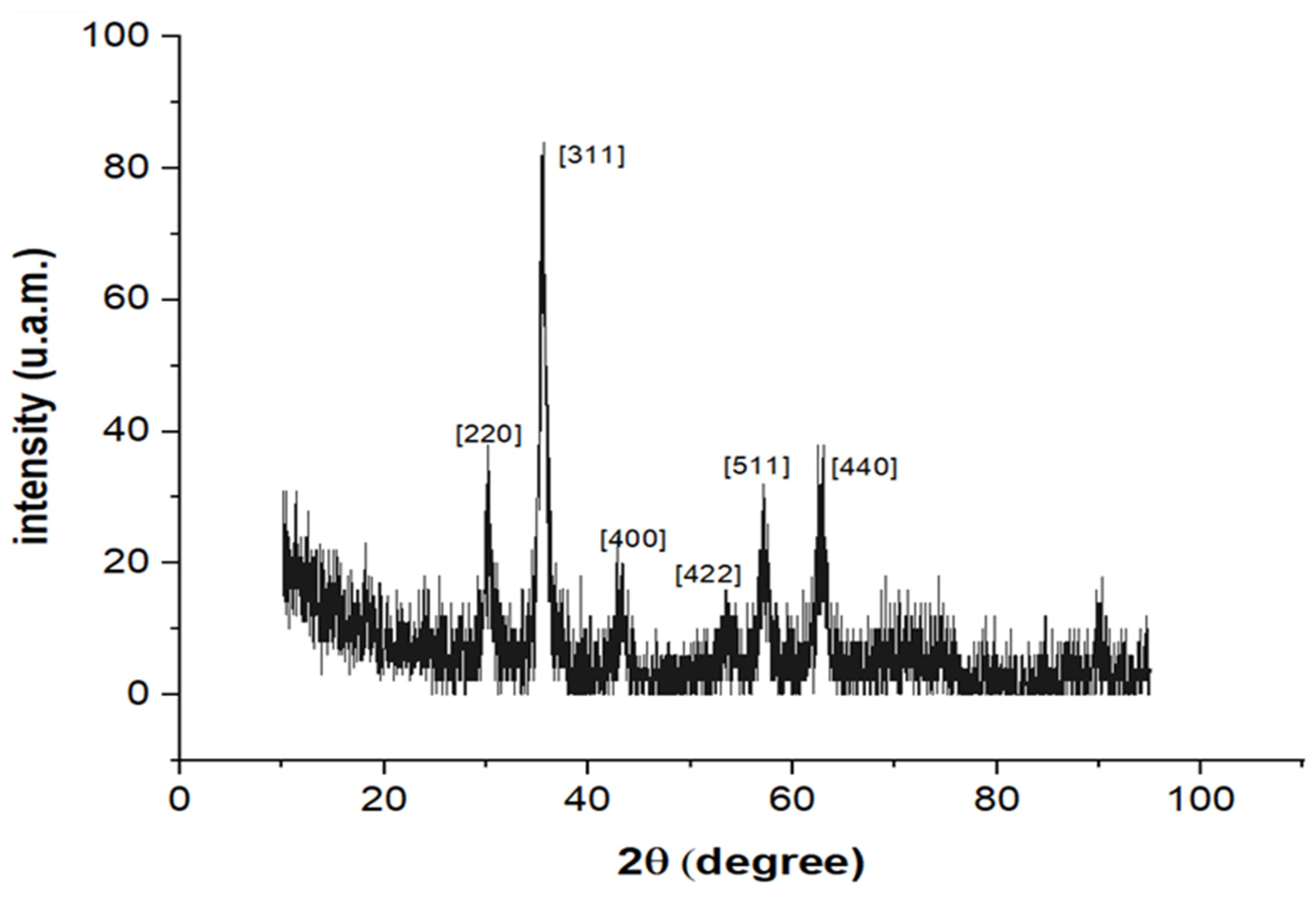
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yildiz, I. Applications of magnetic nanoparticles in biomedical separation and purification. Nanotechnol. Rev. 2016, 5, 331–340. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D.; Yuan, S.; Chen, Y.; Fletcher, E.E.; Shi, H.; Han, B. Selective binding, magnetic separation and purification of histidine-tagged protein using biopolymer magnetic core-shell nanoparticles. Protein Expr. Purif. 2018, 144, 5–11. [Google Scholar] [CrossRef]
- He, M.; Wei, Y.; Wang, R.; Wang, C.; Zhang, B.; Han, L. Boronate affinity magnetic nanoparticles with hyperbranched polymer brushes for the adsorption of cis-diol biomolecules. Mikrochim. Acta 2019, 186, 683. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo Torres, E.; Leal, M.P.; García Martín, M.L. Magnetic nanoparticles as MRI contrast agents. Top. Curr. Chem. 2020, 378, 49–91. [Google Scholar]
- Zhong, J.; Schilling, M.; Ludwig, F. Magnetic nanoparticle temperature imaging with a scanning magnetic particle spectrometer. Meas. Sci. Technol. 2018, 29, 115903. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. Int. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Yu, X.; Ding, S.; Yang, R.; Wu, C.; Zhang, W. Research progress on magnetic nanoparticles for magnetic induction hyperthermia of malignant tumor. Ceram. Int. 2021, 47, 5909–5917. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Commun. 2015, 468, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhou, X.; Liu, J.; Huang, Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020, 188, 106943. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Ahmad, M.Z.; Malankowska, M.; Coronas, J. A new relevant membrane application: CO2 direct air capture (DAC). J. Chem. Eng. 2022, 446, 137047. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 16, 3–7. [Google Scholar] [CrossRef]
- Le Floc’h, J.; Lu, H.D.; Lim, T.L.; D’emor’e, C.; Prud’homme, R.K.; Hynynen, K.; Foster, F.S. Transcranial photoacoustic detection of blood-brain barrier disruption following focused ultrasound-mediated nanoparticle delivery. Mol. Imaging Biol. 2020, 22, 324–334. [Google Scholar] [CrossRef]
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Sachdeva, V.; Monga, A.; Vashisht, R.; Singh, D.; Singh, A.; Bedi, N. Iron Oxide Nanoparticles: The precise strategy for targeted delivery of genes, oligonucleotides and peptides in cancer therapy. J. Drug Deliv. Sci. Technol. 2022, 74, 103585. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef]
- Martins, P.M.; Lima, A.C.; Ribeiro, S.; Lanceros-Mendez, S.; Martins, P. Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. ACS Appl. Bio Mater. 2021, 4, 5839–5870. [Google Scholar] [CrossRef] [PubMed]
- Berry, C. Possible exploitation of magnetic nanoparticle-cell interaction for biomedical applications. J. Mater. Chem. 2005, 15, 543–547. [Google Scholar] [CrossRef]
- Talluri, S.; Malla, R.R. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) for Diagnosis and Treatment of Breast, Ovarian and Cervical Cancers. Curr. Drug Metab. 2020, 20, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Marecos, E.; Bogdanov, A.; Weissleder, R. Tumoral distribution of long circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology 2000, 214, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic nanoparticles: Design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, M.R.; Kashefi, M.; Shams, S.F.; Jaafari, M.R. Perspective of Fe3O4 Nanoparticles Role in Biomedical Applications. Biochem. Res. Int. 2016, 2016, 7840161. [Google Scholar]
- Choi, K.Y.; Saravanakumar, G.; Park, J.H.; Park, K. Hyaluronic acid-based nanocarriers for intracellular targeting: Interfacial interactions with proteins in cancer. Colloids Surf. B 2012, 99, 82–94. [Google Scholar] [CrossRef]
- Platt, V.M.; Szoka, F.C. Anticancer Therapeutics: Targeting Macromolecules and Nanocarriers to Hyaluronan or CD44, a Hyaluronan Receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Suneetha, M.; Ajeya, K.V.; Ji, S.M. Hyaluronic Acid Modified Metal Nanoparticles and Their Derived Substituents for Cancer Therapy: A Review. Pharmaceutics 2023, 15, 1713. [Google Scholar] [CrossRef]
- Rodriguez-Marquez, C.D.; Arteaga-Marin, S.; Rivas-Sánchez, A.; Autrique-Hernández, R.; Castro-Muñoz, R. A review on current strategies for extraction and purification of hyaluronic acid. Int. J. Mol. Sci. 2022, 23, 6038. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Montemayor, M.I.; Fraguas, J.; Murado, M.A. Hyaluronic acid production by Streptococcus zooepidemicus in marine by-products media from mussel processing wastewaters and tuna peptone viscera. Microb. Cell Factories 2010, 9, 46. [Google Scholar] [CrossRef]
- Amado, I.R.; Vázquez, J.A.; Pastrana, L.; Teixeira, J.A. Cheese whey: A cost-effective alternative for hyaluronic acid Production by Streptococcus zooepidemicus. Food Chem. 2016, 198, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cerminati, S.; Leroux, M.; Anselmi, P.; Peirú, S.; Alonso, J.C.; Priem, B.; Menzella, H.G. Low Cost and Sustainable Hyaluronic Acid Production in a Manufacturing Platform Based on Bacillus Subtilis 3NA Strain. Appl. Microbiol. Biotechnol. 2021, 8, 3075–3086. [Google Scholar] [CrossRef]
- Rajendran, V.; Puvendran, K.; Guru, B.R.; Jayaraman, G. Design of aqueous two-phase systems for purification of hyaluronic acid produced by metabolically engineered Lactococcus Lactis. J. Sep. Sci. 2016, 39, 655–662. [Google Scholar] [CrossRef]
- Woo, J.E.; Seong, H.J.; Lee, S.Y.; Jang, Y.S. Metabolic engineering of Escherichia Coli for the production of hyaluronic acid from glucose and galactose. Front. Bioeng. Biotechnol. 2019, 7, 351. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; do Rosário Bronze, M. Hyaluronic acid and Chondroitin sulfate from marine and terrestrial sources: Extraction and purification methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef] [PubMed]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.L.; Vázquez, J.A.; González, M.P. Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod. Process. 2012, 90, 491–498. [Google Scholar] [CrossRef]
- Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. [Google Scholar] [CrossRef]
- El-Dakdouki, M.H.; El-Boubbou, K.; Zhu, D.C.; Huang, X. A simple method for the synthesis of hyaluronic acid coated magnetic nanoparticles for highly efficient cell labelling and in vivo imaging. RSC Adv. 2011, 1, 1449–1452. [Google Scholar] [CrossRef]
- Khodayari, H.; Heydarinasab, A.; Moniri, E.; Miralinaghi, M. Synthesis and characterization of magnetic nanoparticles-grafted-hyaluronic acid/β-cyclodextrin as a novel pH-sensitive nanocarrier for targeted delivery of doxorubicin. Inorg. Chem. Commun. 2023, 148, 110366. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Lou, S.; Chang, Z.; Wen, B.; Zhang, T. Hyaluronic Acid–Stabilized Fe3O4 Nanoparticles for Promoting In Vivo Magnetic Resonance Imaging of Tumors. Front. Pharmacol. 2022, 13, 918819. [Google Scholar] [CrossRef]
- Pramanik, N.; Ranganathan, S.; Rao, S.; Suneet, K.; Jain, S.; Rangarajan, A.; Jhunjhunwala, S. A composite of hyaluronic acid-modified graphene oxide and iron oxide nanoparticles for targeted drug delivery and magnetothermal therapy. ACS Omega 2019, 4, 9284–9293. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Sun, W.; Luo, Y.; Cai, H.; Pan, Y.; Shen, M.; Xia, J.; Shi, X. Hyaluronic acid-modified hydrothermally synthesized iron oxide nanoparticles for targeted tumor MR imaging. Biomaterials 2014, 35, 3666–3677. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Q. Hyaluronic acid-coated magnetic nanoparticles-based selective collection and detection of leukemia cells with quartz crystal microbalance. Sens. Actuators B Chem. 2016, 223, 9–14. [Google Scholar] [CrossRef]
- Lee, C.R.; Kim, G.G.; Park, S.B.; Kim, S.W. Synthesis of Hyaluronic Acid-Conjugated Fe3O4@CeO2 Composite Nanoparticles for a Target-Oriented Multifunctional Drug Delivery System. Micromachines 2021, 12, 1018. [Google Scholar] [CrossRef]
- Spartan’18 Software, Wavefunction, Inc.: Irvine, CA, USA, 2018.
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Goodarzi, A.; Sahoo, Y.; Swihart, M.T.; Prasad, P.N. Aqueous Ferrofluid of Citric Acid Coated Magnetite Particles. Mat. Res. Soc. Symp. Proc. 2004, 789, N6.6.1–N6.6.6. [Google Scholar]
- Petcharoen, K.; Sirivat, A.J.M.S. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Kandpal, N.D.; Sah, N.; Loshali, R.; Joshi, R.; Prasad, J. Co-precipitation method of synthesis and characterization of iron oxide nanoparticles. J. Sci. Ind. Res. 2014, 73, 87–90. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1974; pp. 505–565. [Google Scholar]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall: New York, NY, USA, 2001; pp. 88–106. [Google Scholar]
- Kákay, A.; Gutowski, M.W.; Takacs, L.; Franco, V.; Varga, L.K. Langevin granulometry of the particle size distribution. J. Phys. A Math. Gen. 2004, 37, 6027–6041. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Ferrohydrodynamics; Cambridge University Press: New York, NY, USA, 1985; pp. 55–60. [Google Scholar]
- Gamborg, O.L.; Wetter, L.R. Plant Tissue Culture Methods; National Research Council of Canada, Prairie Regional Laboratory: Saskatoon, SK, Canada, 1975. [Google Scholar]
- Singh, R.J. Plant Cytogenetics, 3rd ed.; CRC Press: New York, NY, USA, 2016. [Google Scholar]
- Truta, E.; Vochita, G.; Zamfirache, M.M.; Olteanu, Z.; Rosu, C.M. Copper-induced genotoxic effects in root meristems of Triticum aestivum L. cv. Beti. Carpath. J. Earth Environ. Sci. 2013, 8, 83–92. [Google Scholar]
- Key Organics. Available online: https://www.keyorganics.net/hyaluronicacid-9004-61-9-c16h27n2o11.html (accessed on 26 January 2024).
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.D.L.C.; Vasconcelos, A.F.D.; Celligoi, M.A.P.C. Improvement production of hyaluronic acid by Streptococcus zooepidemicus in sugarcane molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef]
- Arora, A.K.; Sharma, M.; Kumari, R.; Jaswal, V.S.; Kumar, P. Synthesis, characterization, and magnetic studies of-nanoparticles. J. Nanotechnol. 2014, 2014, 474909. [Google Scholar] [CrossRef]
- Hushiarian, R.; Yusof, N.A.; Abdullah, A.H.; Ahmad, S.A.A.; Dutse, S.W. A Novel DNA Nanosensor Based on CdSe/ZnS Quantum Dots and Synthesized Fe3O4 Magnetic Nanoparticles. Molecules 2014, 19, 4355–4368. [Google Scholar] [CrossRef]
- Baki, A.; Remmo, A.; Löwa, N.; Wiekhorst, F.; Bleul, R. Albumin-Coated Single-Core Iron Oxide Nanoparticles for Enhanced Molecular Magnetic Imaging (MRI/MPI). Int. J. Mol. Sci. 2021, 22, 6235. [Google Scholar] [CrossRef]
- Gorski, C.A.; Scherer, M.M. Determination of Nanoparticulate Magnetite Stoichiometry by Mossbauer Spectroscopy, Acidic Dissolution, and Powder X-ray Diffraction: A Critical Review. Am. Mineral. 2010, 95, 1017–1026. [Google Scholar] [CrossRef]
- Petkov, V.; Cozzoli, P.D.; Buonsanti, R.; Cingolani, R.; Ren, Y. Size, Shape, and Internal Atomic Ordering of Nanocrystals by Atomic Pair Distribution Functions: A Comparative Study of γ-Fe2O3 Nanosized Spheres and Tetrapods. J. Am. Chem. Soc. 2009, 131, 14264–14266. [Google Scholar] [CrossRef]
- Cuenca, J.A.; Bugler, K.; Taylor, S.; Morgan, D.; Williams, P.; Bauer, J.; Porch, A. Study of the magnetite to maghemite transition using microwave permittivity and permeability measurements. J. Phys. Condens. Matter. 2016, 28, 106002. [Google Scholar] [CrossRef]
- Răcuciu, M.; Oancea, S. Characterization of the Oxidation of Magnetite Nanoparticles during Their Synthesis by Different Structural Analysis Techniques. Anal. Lett. 2021, 54, 173–183. [Google Scholar] [CrossRef]
- Blaney, L. Magnetite (Fe3O4): Properties, Synthesis, and Applications. Lehigh Preserv. Lehigh Univ. 2007, 15, 33–81. [Google Scholar]
- WebSpectra—Problems in NMR and IR Spectroscopy. Available online: https://webspectra.chem.ucla.edu/irtable.html (accessed on 28 October 2023).
- Jia, W.; Li, M.; Kang, L.; Gu, G.; Guo, Z.; Chen, Z. Fabrication and comprehensive characterization of biomimetic extracellular matrix electrospun scaffold for vascular tissue engineering applications. J. Mater. Sci. 2019, 54, 10871–10883. [Google Scholar] [CrossRef]
- Laffleur, F.; Hörmann, N.; Gust, R.; Ganner, A. Synthesis, characterization and evaluation of hyaluronic acid-based polymers for nasal delivery. Int. J. Pharm. 2023, 631, 122496. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf. B 2019, 177, 41–49. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides. Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2003; pp. 253–296. [Google Scholar]
- Sklute, E.C.; Kashyap, S.; Dyar, M.D.; Holden, J.F.; Tague, T.; Wang, P.; Jaret, S.J. Spectral and morphological characteristics of synthetic nanophase iron (oxyhydr)oxides. Phys. Chem. Miner. 2018, 45, 1–26. [Google Scholar] [CrossRef]
- Antoniac, I.; Cernea, A.; Petcu, C.; Laptoiu, D.; Tabaras, D.; Tecu, C.; Antoniac, A.; Gradinaru, S. Synthesis and Characterization of Coated Iron Oxide Nanoparticles Produced for Drug Delivery in Viscoelastic Solution. Rev. Chim. 2020, 71, 145–154. [Google Scholar] [CrossRef]
- Soleymani, M.; Velashjerdi, M.; Shaterabadi, Z.; Barati, A. One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydr. Polym. 2020, 237, 116130. [Google Scholar] [CrossRef]
- Mondek, J.; Kalina, M.; Simulescu, V.; Pekar, M. Thermal degradation of high molar mass hyaluronan in solution and in powder; comparison with BSA. Polym. Degrad. Stabil. 2015, 120, 107–113. [Google Scholar] [CrossRef]
- Ahire, J.J.; Robertson, D.; Neveling, D.P.; van Reenen, A.J.; Dicks, L.M.T. Hyaluronic acid-coated poly(D, L-lactide) (PDLLA) nanofibers prepared by electrospinning and coating. RSC Adv. 2016, 6, 34791–34796. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef]
- Atrei, A.; Innocenti, C.; Lamponi, S.; Paesano, S.; Leone, G.; Reale, A.; Paolino, M.; Cappelli, A. Covalent hyaluronic-based coating of magnetite nanoparticles: Preparation, physicochemical and biological characterization. Mater. Sci. Eng. C 2020, 107, 110271. [Google Scholar] [CrossRef]
- Hasan, K.; Shehadi, I.A.; Al-Bab, N.D.; Elgamouz, A. Magnetic Chitosan-Supported Silver Nanoparticles: A Heterogeneous Catalyst for the Reduction of 4-Nitrophenol. Catalysts 2019, 9, 839. [Google Scholar] [CrossRef]
- BYJU’S. Available online: https://byjus.com/chemistry/dipole-moment/ (accessed on 30 January 2024).
- Pitt Quantum Repository. Available online: https://pqr.pitt.edu/mol/KRKNYBCHXYNGOX-UHFFFAOYSA-N (accessed on 22 February 2024).
- Oh, J.J.; Drouin, B.J.; Cohen, E.A. The torsion–rotation spectrum of perchloric acid, HClO4. J. Mol. Spectrosc. 2005, 234, 10–24. [Google Scholar] [CrossRef]
- Pardeshi, S.; Dhodapkar, R.; Kumar, A. Quantum Chemical Density Functional Theory Studies on the Molecular Structure and Vibrational Spectra of Gallic Acid Imprinted Polymers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 562–573. [Google Scholar] [CrossRef]
- Les, A.; Popescu, L.; Creanga, D.; Dorohoi, D.O.; Sacarescu, L. Study of gallic acid antioxidant molecule in interaction with solvents, aiming its utilization as a stabilizer of magnetic nanoparticles in suspensions. Mol. Cryst. Liq. 2023, 762, 1–12. [Google Scholar] [CrossRef]
- Răcuciu, M.; Barbu-Tudoran, L.; Oancea, S.; Drăghici, O.; Morosanu, C.; Grigoras, M.; Brînză, F.; Creangă, D.E. Aspartic Acid Stabilized Iron Oxide Nanoparticles for Biomedical Applications. Nanomaterials 2022, 12, 1151. [Google Scholar] [CrossRef]
- Osman, O.; Zanini, L.F.; Frénéa-Robin, M.; Dumas-Bouchiat, F.; Dempsey, N.M.; Reyne, G.; Buret, F.; Haddour, N. Monitoring the endocytosis of magnetic nanoparticles by cells using permanent micro-flux sources. Biomed. Microdevices 2012, 14, 947–954. [Google Scholar] [CrossRef]
- Herrera, A.P.; Rodríguez, M.; Torres-Lugo, M.; Rinaldi, C. Multifunctional magnetite nanoparticles coated with fluorescent thermo-responsive polymeric shells. J. Mater. Chem. 2008, 18, 855–858. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Zelepukin, I.V.; Ivanov, I.N.; Melikov, R.O.; Pechnikova, N.A.; Dzhalilova, D.S.; Mirkasymov, A.B.; Bragina, V.A.; Nikitin, M.P.; Deyev, S.M.; et al. Influence of magnetic nanoparticle biotransformation on contrasting efficiency and iron metabolism. J. Nanobiotechnol. 2022, 20, 535. [Google Scholar] [CrossRef]
- Fang, Z.; Li, X.; Xu, Z.; Du, F.; Wang, W.; Shi, R.; Gao, D. Hyaluronic acid-modified mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles for targeted drug delivery. Int. J. Nanomed. 2019, 14, 5785–5797. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fahling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Singh, B.C.S. Toxicity Analysis of Hybrid Nanodiamond/Fe3O4 Nanoparticles on Allium cepa L. J. Toxicol. 2022, 2022, 5903409. [Google Scholar]
- Pandey, A.K.; Shahi, A.K.; Srivastava, N.; Kumar, G.; Gopal, R. Synthesis and cytogenetic effect of magnetic nanoparticles. Adv. Mater. Lett. 2015, 6, 954–960. [Google Scholar] [CrossRef]
- Akaneme, F.I.; Amaefule, C.C. Evaluation of the cytotoxicity and genotoxicity of aqueous leaf extracts of Azadirachta indica A. Juss using the Allium test. J. Med. Plant Res. 2012, 6, 3898–3907. [Google Scholar] [CrossRef]
- Sreeranjini, S.; Siril, E.A. Evaluation of anti-genotoxicity of the leaf extracts of Morinda citrifolia Linn. Plant Soil Environ. 2011, 57, 222–227. [Google Scholar] [CrossRef]
- Vochita, G.; Creangă, D.; Focanici-Ciurlica, E.L. Magnetic nanoparticle genetic impact on root tip cells of sunflower seedlings. Water Air Soil Pollut. 2012, 223, 2541–2549. [Google Scholar] [CrossRef]
- Pavel, A.; Trifan, M.; Băra, I.I.; Creangă, D.; Cotae, C. Accumulation dynamics and somecytogenetical tests at Chelidonium majus and Papaver somniferum callus under the magnetic liquid effect. J. Magn. Magn. Mater. 1999, 201, 443–445. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răcuciu, M.; Oancea, S.; Barbu-Tudoran, L.; Drăghici, O.; Agavriloaei, A.; Creangă, D. A Study of Hyaluronic Acid’s Theoretical Reactivity and of Magnetic Nanoparticles Capped with Hyaluronic Acid. Materials 2024, 17, 1229. https://doi.org/10.3390/ma17061229
Răcuciu M, Oancea S, Barbu-Tudoran L, Drăghici O, Agavriloaei A, Creangă D. A Study of Hyaluronic Acid’s Theoretical Reactivity and of Magnetic Nanoparticles Capped with Hyaluronic Acid. Materials. 2024; 17(6):1229. https://doi.org/10.3390/ma17061229
Chicago/Turabian StyleRăcuciu, Mihaela, Simona Oancea, Lucian Barbu-Tudoran, Olga Drăghici, Anda Agavriloaei, and Dorina Creangă. 2024. "A Study of Hyaluronic Acid’s Theoretical Reactivity and of Magnetic Nanoparticles Capped with Hyaluronic Acid" Materials 17, no. 6: 1229. https://doi.org/10.3390/ma17061229







