One-Step Synthesis of TiO2/SiO2-np Nanocomposite Photocatalytic Multilayer Films: Effect of Incorporation Time Sequences of SiO2 Nanoparticles during the TiO2 Film Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. TiO2/SiO2-np Nanocomposite Thin Film Synthesis
2.2. Substrate Preparation
2.3. TiO2 Film Synthesis
2.4. SiO2 Nanoparticle Incorporation
2.5. TiO2/SiO2-np Nanocomposite Architecture
2.6. Characterization of the Nanocomposite Films
2.7. Photocatalytic Tests
3. Results and Discussion
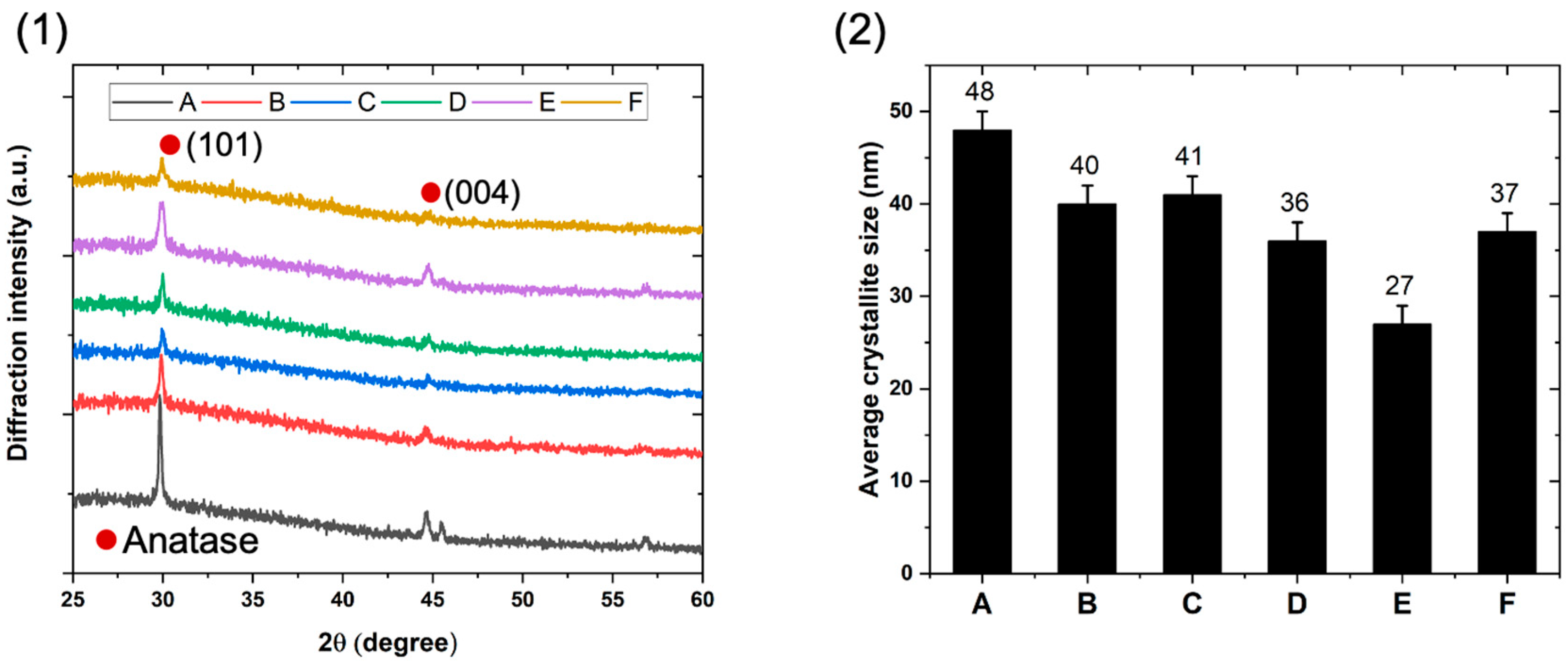
3.1. Structural Properties of the TiO2/SiO2-np Nanocomposite Films
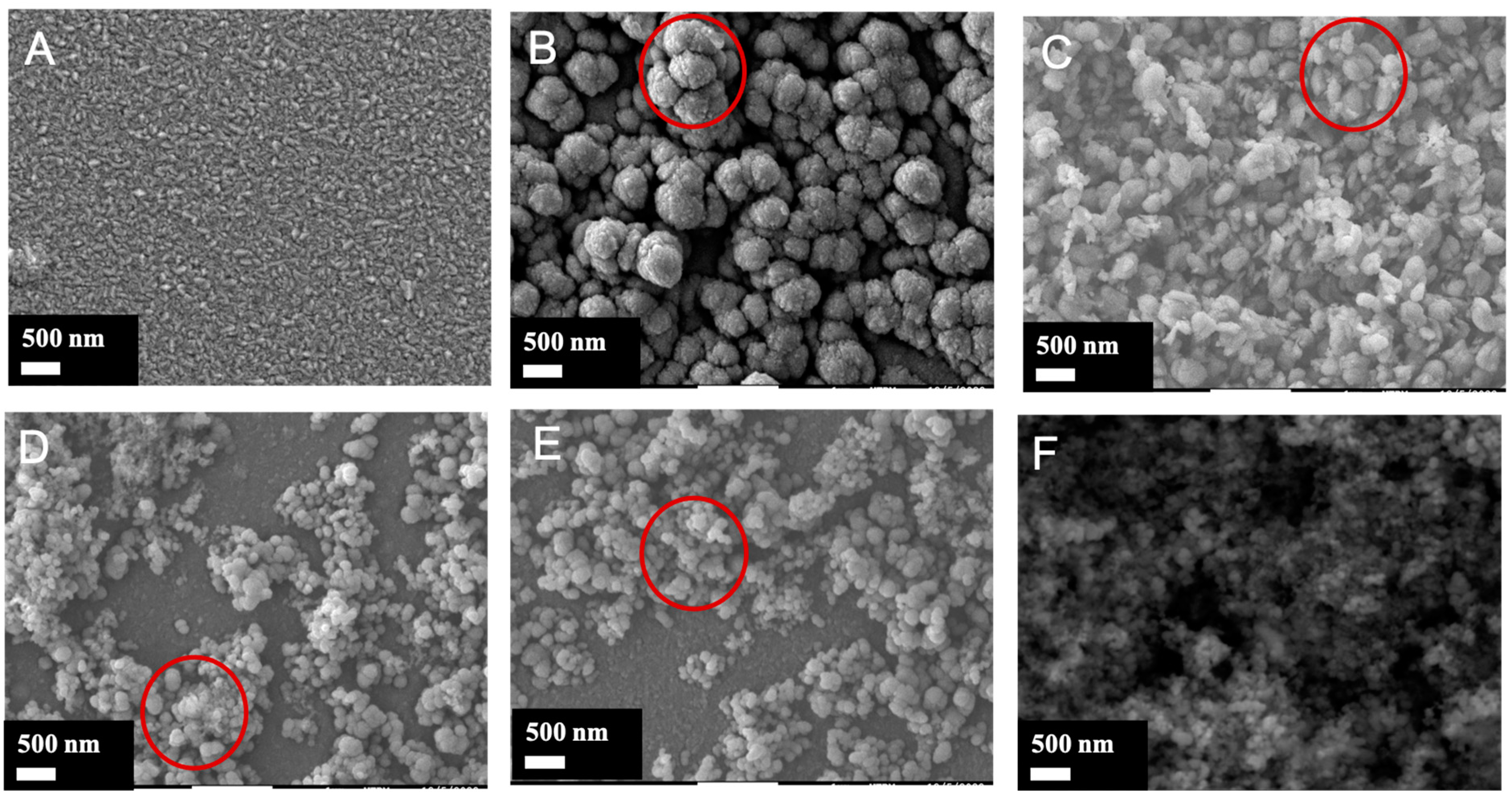
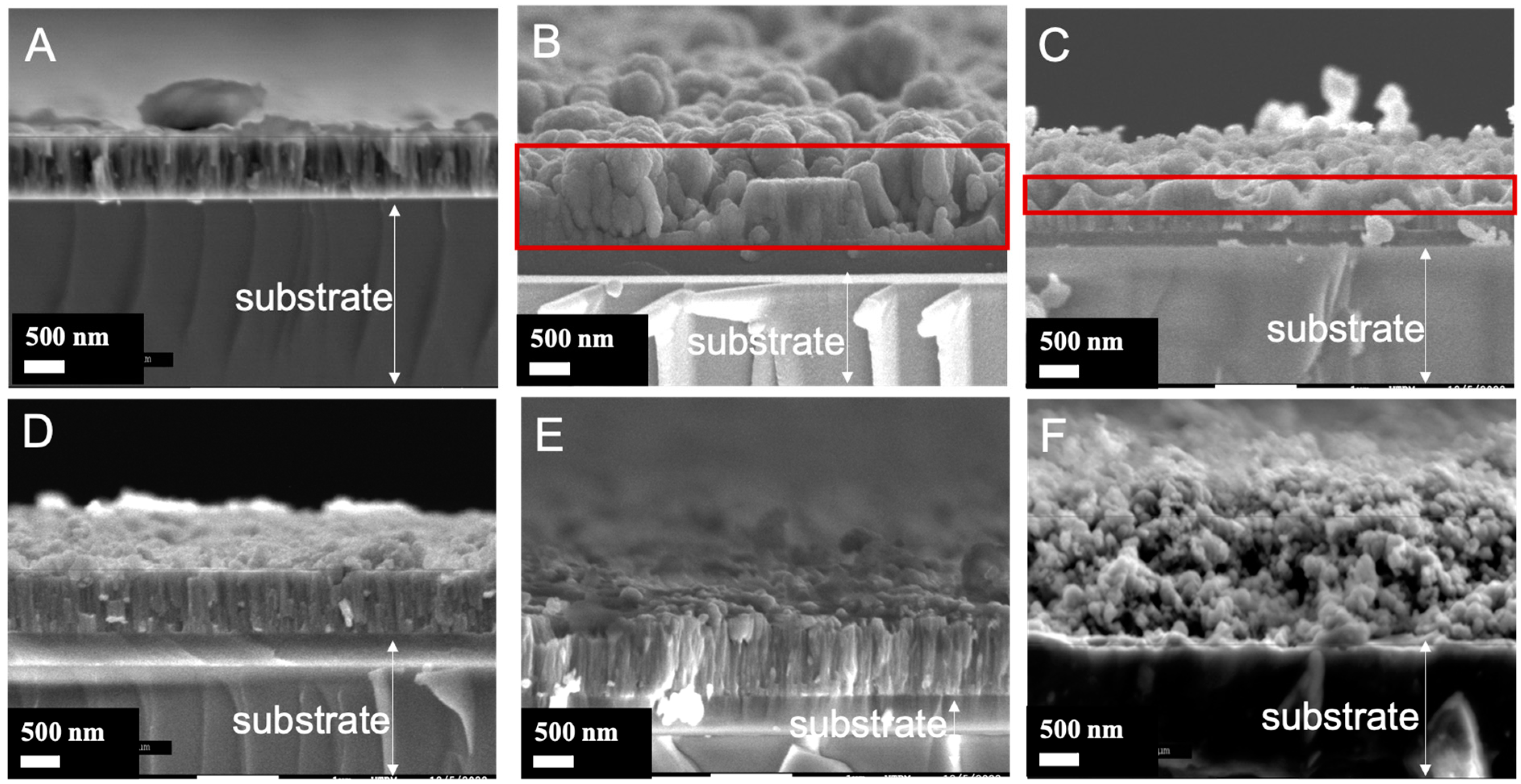
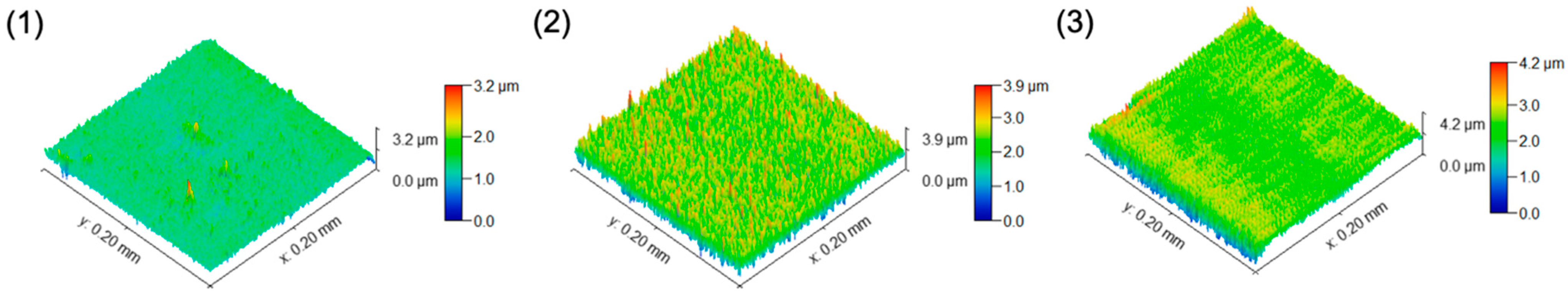
3.2. Morphological Features of the TiO2/SiO2-np Nanocomposite Films
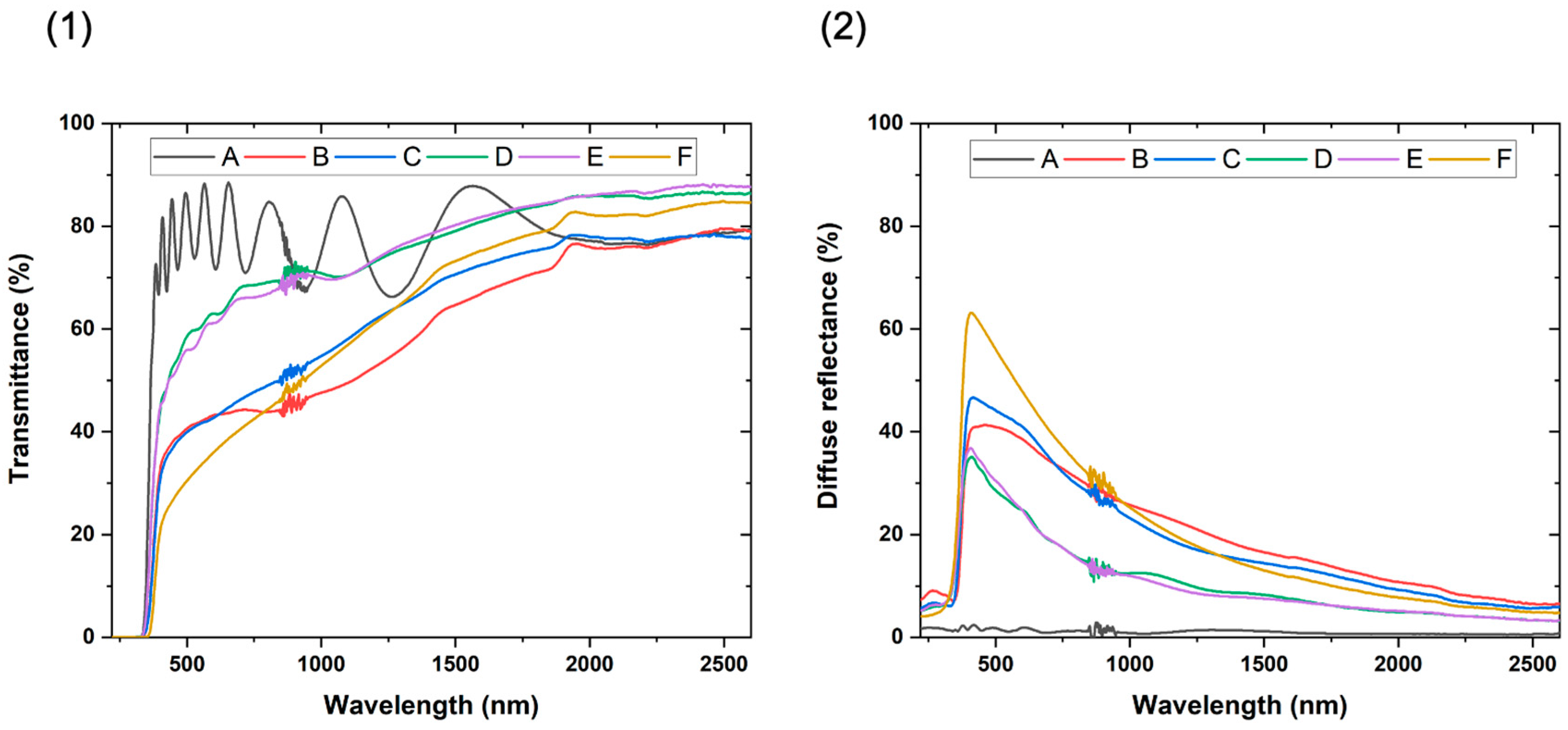
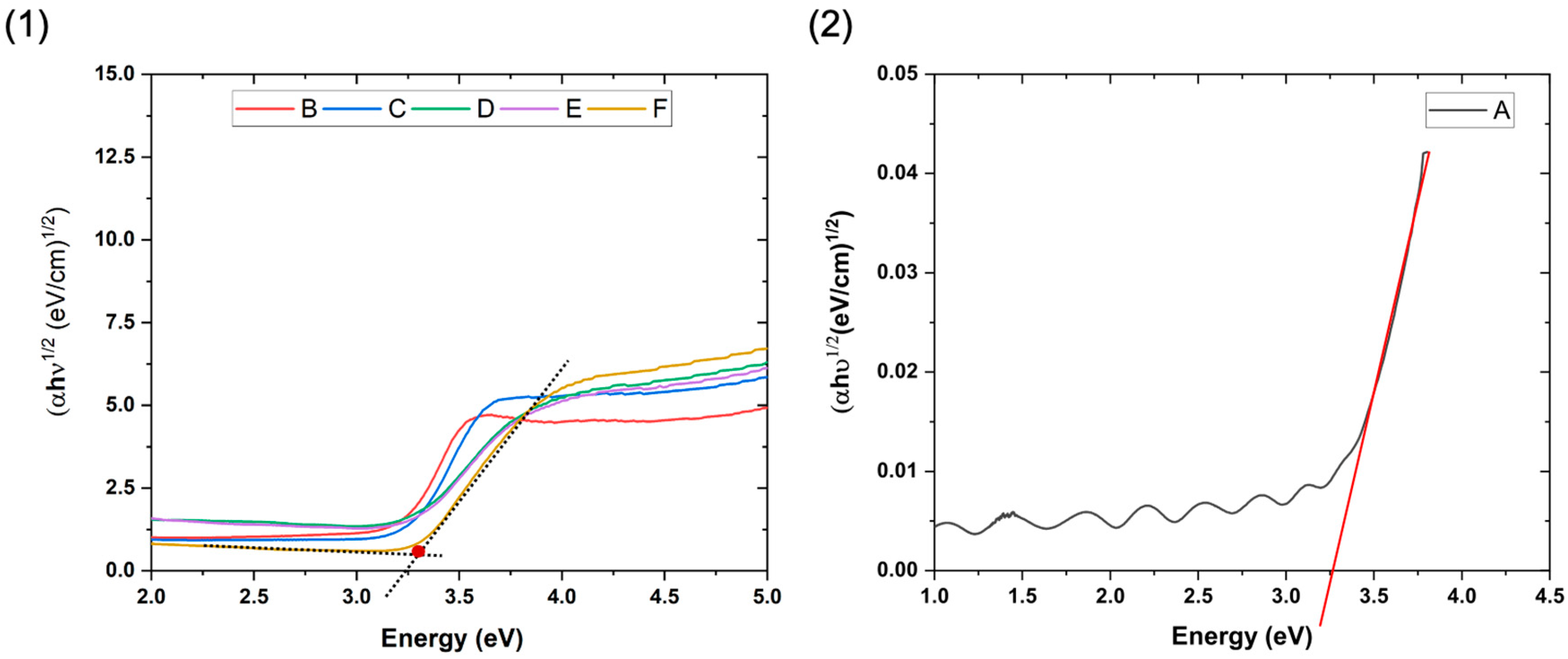
3.3. Optical Properties of the TiO2/SiO2-np Nanocomposite Films
3.4. Surface Feature Estimations
3.5. Bandgap Calculations
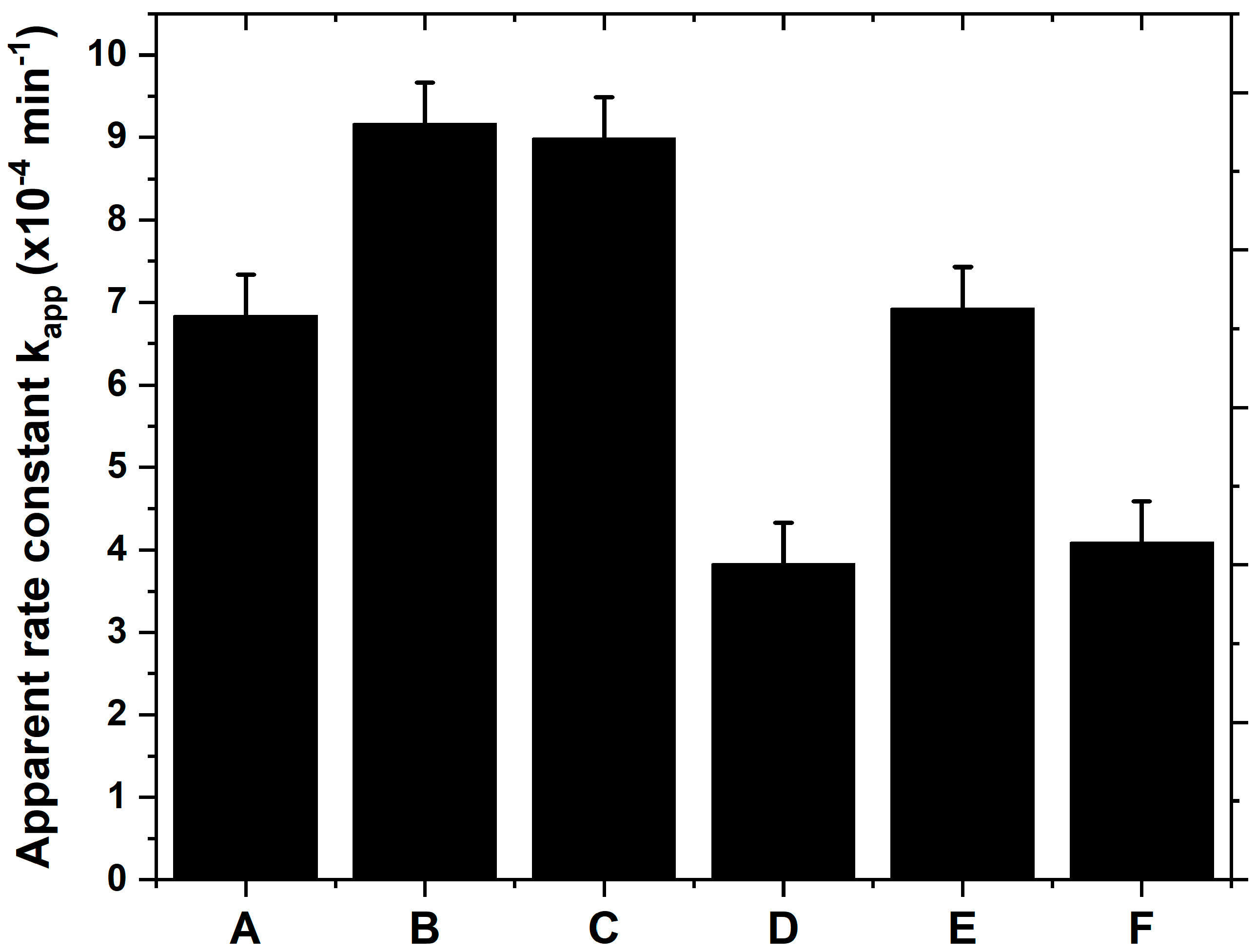
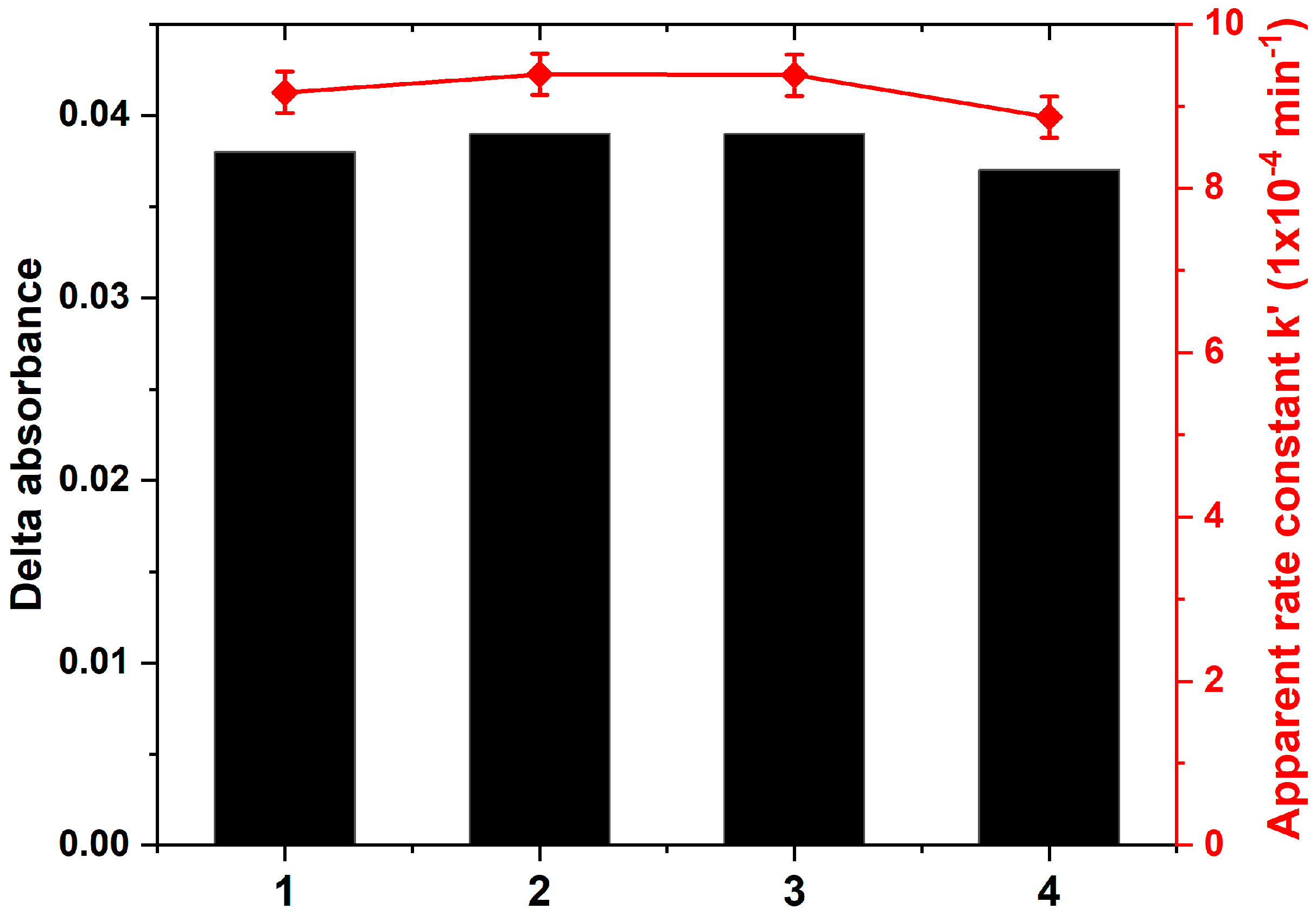
3.6. Photocatalytic Performance and Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ameta, R.; Ameta, S.C. Photocatalysis: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kisch, H. Semiconductor Photocatalysis: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. JJAP 2005, 44, 8269. [Google Scholar] [CrossRef]
- Lopez, L.; Daoud, W.A.; Dutta, D.; Panther, B.C.; Turney, T.W. Effect of substrate on surface morphology and photocatalysis of large-scale TiO2 films. Appl. Surf. Sci. 2013, 265, 162–168. [Google Scholar] [CrossRef]
- Antoniou, M.G.; Nicolaou, P.A.; Shoemaker, J.A.; Armah, A.; Dionysiou, D.D. Impact of the morphological properties of thin TiO2 photocatalytic films on the detoxification of water contaminated with the cyanotoxin, microcystin-LR. Appl. Catal. B Environ. 2009, 91, 165–173. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, S.; Tian, B.; Zhang, J.; Anpo, M. Preparation of TiO2–SiO2 film with high photocatalytic activity on PET substrate. Mater. Lett. 2006, 60, 396–399. [Google Scholar] [CrossRef]
- Guan, C.; Yue, X.; Fan, J.; Xiang, Q. MXene quantum dots of Ti3C2: Properties, synthesis, and energy-related applications. Chin. J. Catal. 2022, 43, 2484. [Google Scholar] [CrossRef]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of the functional group modifications on the photocatalytic performance of g-C3N4. Small 2023, 19, 2300109. [Google Scholar] [CrossRef]
- Devi, K.P.; Goswami, P.; Chaturvedi, H. Fabrication of nanocrystalline TiO2 thin films using Sol-Gel spin coating technology and investigation of its structural, morphology and optical characteristics. Appl. Surf. Sci. 2022, 591, 153226. [Google Scholar] [CrossRef]
- Lopez, L.; Daoud, W.A.; Dutta, D. Preparation of large scale photocatalytic TiO2 films by the sol–gel process. Surf. Coat. Technol. 2010, 205, 251–257. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Zhang, T.; Yin, W.; Cao, G.Y. Relationship between photocatalytic activity and structure of TiO2 thin film. CJCP 2007, 20, 95–98. [Google Scholar] [CrossRef]
- Blanco, E.; González-Leal, J.M.; Solar, M.R.-D. Photocatalytic TiO2 sol–gel thin films: Optical and morphological characterization. Sol. Energy 2015, 122, 11–23. [Google Scholar] [CrossRef]
- Raut, N.C.; Mathews, T.; Sundari, S.; Sairam, T.N.; Dash, S.; Tyagi, A.K. Structural and morphological characterization of TiO2 thin films synthesized by spray pyrolysis technique. J. Nanosci. Nanotechnol. 2009, 9, 5298–5302. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.; Olguin, D.; Conde-Gallardo, A.; Jiménez-Sandoval, S. Structural and morphological properties of TiO2thin films prepared by spray pyrolysis. Rev. Mex. Fís. 2004, 50, 382–387. [Google Scholar]
- Kim, S.H.; Choi, Y.L.; Song, Y.S.; Lee, D.Y.; Lee, S.J. Influence of sputtering parameters on microstructure and morphology of TiO2 thin films. Mater. Lett. 2002, 57, 343–348. [Google Scholar] [CrossRef]
- Zhang, C.; Wanyu DI, N.G.; Hualin, W.; Weiping, C.; Dongying, J.U. Influences of working pressure on properties for TiO2 films deposited by DC pulse magnetron sputtering. J. Environ. Sci. 2009, 21, 741–744. [Google Scholar] [CrossRef]
- Zeman, P.; Takabayashi, S. Effect of total and oxygen partial pressures on structure of photocatalytic TiO2 films sputtered on unheated substrate. Surf. Coat. Technol. 2002, 153, 93. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Souier, T.; Tala-Ighil, R.; Toubane, M.; Cai, W. Al doping effect on the morphological, structural and photocatalytic properties of TiO2 thin layers. Thin Solid Films 2016, 616, 655–661. [Google Scholar] [CrossRef]
- Andronic, L.; Enesca, A.; Vladuta, C.; Duta, A. Photocatalytic activity of cadmium doped TiO2 films for photocatalytic degradation of dyes. Chem. Eng. J. 2009, 152, 64–71. [Google Scholar] [CrossRef]
- Bensouici, F.; Bououdina, M.; Dakhel, A.A.; Tala-Ighil, R.; Tounane, M.; Iratni, A.; Cai, W. Optical, structural and photocatalysis properties of Cu-doped TiO2 thin films. Appl. Surf. Sci. 2017, 395, 110–116. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, Y.; Wang, J.; Luo, D.; Fan, L.; Wu, J. Controllable fabrication of Bi2O3/TiO2 heterojunction with excellent visible-light responsive photocatalytic performance. Appl. Surf. Sci 2017, 423, 119–130. [Google Scholar] [CrossRef]
- Kim, S.; Chang, H.K.; Kim, K.B.; Kim, H.J.; Lee, H.N.; Park, T.J.; Park, Y.M. Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition. Catalysts 2021, 11, 1144. [Google Scholar] [CrossRef]
- Kaishu, G. Relationship Between Photocatalytic Activity, Hydrophilicity and Self-Cleaning Effect of TiO2/SiO2 Films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, E.; Zhang, R.; Liu, Z. The effect of SiO2 on TiO2-SiO2 composite film for self-cleaning application. Surf. Interfaces 2019, 16, 194–198. [Google Scholar] [CrossRef]
- Huang, T.; Huang, W.; Zhou, C.; Situ, Y.; Huang, H. Superhydrophilicity of TiO2/SiO2 thin films: Synergistic effect of SiO2 and phase-separation-induced porous structure. Surf. Coat. Technol. 2012, 213, 126–132. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of growth defects in thin films prepared by PVD techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Müller-Pfeiffer, S.; Anklam, H.-J. Computer simulation of hillock growth. Vacuum 1991, 42, 113–116. [Google Scholar] [CrossRef]
- Lai, S.; Sublemontier, O.; Aubry, E.; Rousseau, Y.; Billard, A.; Briois, P. Aerosol-based functional nanocomposite coating process for large surface areas. Sci. Rep. 2023, 13, 4709. [Google Scholar] [CrossRef]
- Sublemontier, O.; Kintz, H.; Leconte, Y.U.S. Method for the Synthesis of a Nanostructured Composite Material and a Device for Implementing Said. Method. Patent No. 9925557, 27 March 2018. [Google Scholar]
- Sublemontier, O.; Rousseau, Y. Process for Controlling, under Void, a Jet of Particles with an Aerodynamic Lens and Associated Aerodynamic Lens. U.S. Patent No. 11076476, 27 July 2021. [Google Scholar]
- Huang, C.; Bai, H.; Huang, Y.; Liu, S.; Yen, S.; Tseng, Y. Synthesis of neutral SiO2/TiO2 Hydrosol and Its Application as Antireflective Self-Cleaning Thin Film. Int. J. Photoenergy 2012, 2012, 620764. [Google Scholar] [CrossRef]
- Güler, E.; Uğur, G.; Uğur, Ş.; Güler, M. A theoretical study for the band gap energies of the most common silica polymorphs. Chin. J. Phys. 2020, 65, 472–480. [Google Scholar] [CrossRef]
- Liu, P.; Ziemann, P.J.; Kittelson, D.B.; McMurry, P.H. Generating particle beams of controlled dimensions and divergence: I. Theory of particle motion in aerodynamic lenses and nozzle expansions. Aerosol Sci. Technol. 1995, 22, 293–313. [Google Scholar] [CrossRef]
- Liu, P.; Ziemann, P.J.; Kittelson, D.B.; McMurry, P.H. Generating particle beams of controlled dimensions and divergence: II. Experimental evaluation of particle motion in aerodynamic lenses and nozzle expansions. Aerosol Sci. Technol. 1995, 22, 314–324. [Google Scholar] [CrossRef]
- Aubry, E.; Ghazzal, M.N.; Demange, V.; Chaoui, N.; Robert, D.; Billard, A. Poisoning prevention of TiO2 photocatalyst coatings sputtered on soda-lime glass by intercalation of SiNx diffusion barriers. Surf. Coat. Technol. 2007, 201, 7706–7712. [Google Scholar] [CrossRef]
- Ghazzal, M.N.; Aubry, E.; Chaoui, N.; Robert, D. Effect of SiNx diffusion barrier thickness on the structural properties and photocatalytic activity of TiO2 films obtained by sol–gel dip coating and reactive magnetron sputtering. Beilstein J. Nanotechnol. 2015, 6, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Aubry, E.; Lambert, J.; Demange, V.; Billard, A. Effect of Na diffusion from glass substrate on the microstructural and photocatalytic properties of post-annealed TiO2 films synthesised by reactive sputtering. Surf. Coat. Technol. 2012, 206, 4999–5005. [Google Scholar] [CrossRef]
- Hébert, M.; Roger, D.; Hersch, P. Fundamentals of optics and radiometry for color reproduction. In Handbook of Digital Imaging; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.P.; Li, W.; Ni, C.; Shah, S.I.; Tseng, Y.H. Size dependency of nanocrystalline TiO2 on its optical property and photocatalytic reactivity exemplified by 2-chlorophenol. Appl. Catal. B Environ. 2006, 68, 1–11. [Google Scholar] [CrossRef]
- Imam, S.; Muhammad, A.I.; Babamale, H.F.; Zango, Z.U. Removal of orange G dye from aqueous solution by adsorption: A short review. JETT 2021, 9, 318–327. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, A.F. Langmuir–Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Anders, A. A structure zone diagram including plasma-based deposition and ion etching. Thin Solid Films 2010, 518, 4087–4090. [Google Scholar] [CrossRef]
- Stolz, C.J.; Genin, F.Y.; Pistor, T.V. Electric-field enhancement by nodular defects in multilayer coatings irradiated at normal and 45 incidence. In Laser-Induced Damage in Optical Materials, Proceedings of the Boulder Damage Symposium 35th Annual Symposium on Optical Materials for High Power Lasers, Boulder, CO, USA, 21–24 September 2003; SPIE: Cergy, France, 2004; Volume 5273, pp. 41–49. [Google Scholar] [CrossRef]
- Manifacier, J.C.; Gasiot, J.; Fillard, J.P. A simple method for the determination of the optical constants n, k and the thickness of a weakly absorbing thin film. J. Phys. E Sci. Instr. 1976, 9, 1002. [Google Scholar] [CrossRef]
- Galy, T.; Marszewski, M.; King, S.; Yan, Y.; Tolbert, S.H.; Pilon, L. Comparing methods for measuring thickness, refractive index, and porosity of mesoporous thin films. Microporous Mesoporous Mater. 2020, 291, 109677. [Google Scholar] [CrossRef]
- Peelen, J.G.J. Light transmission of sintered alumina. Philips Tech. Rev. 1976, 36, 47–52. [Google Scholar]
- Hulst, H.C.; van de Hulst, H.C. Light Scattering by Small Particles; Courier Corporation: North Chelmsford, MA, USA, 1981. [Google Scholar]
- Hříbalová, S.; Pabst, W. The van de Hulst approximation for light scattering and its use for transmittance predictions in transparent ceramics. J. Eur. Ceram. 2020, 40, 2141–2150. [Google Scholar] [CrossRef]
- Hříbalová, S.; Pabst, W. Modeling light scattering by spherical pores for calculating the transmittance of transparent ceramics–All you need to know. J. Eur. Ceram. 2021, 41, 2169–2192. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–Vis spectra. J. Phys. Chem. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Mahanty, S.; Roy, S.; Heintz, O.; Bourgeois, S.; Chaumont, D. Investigation on sol–gel synthesized Ag-doped TiO2 cermet thin films. Thin Solid Films 2005, 474, 245–249. [Google Scholar] [CrossRef]
- Wang, Z.; Helmersson, U.; Käll, P.O. Optical properties of anatase TiO2 thin films prepared by aqueous sol–gel process at low temperature. Thin Solid Films 2002, 405, 50–54. [Google Scholar] [CrossRef]
- Ziani, N.; Aubry, E.; Martin, N.; Hirsinger, L.; Billard, A.; Briois, P.; Belkaid, M.S.; Yazdi MA, P. Influence of substrate temperature on delafossite CuFeO2 films synthesized by reactive magnetron sputtering. J. Alloys Compd. 2021, 876, 160169. [Google Scholar] [CrossRef]
- Mishra, V.; Warshi, M.K.; Sati, A.; Kumar, A.; Mishra, V.; Kumar, R.; Sagdeo, P.R. Investigation of temperature-dependent optical properties of TiO2 using diffuse reflectance spectroscopy. SN Appl. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef]
- Madhavan, J.; Grieser, F.; Ashokkumar, M. Degradation of orange-G by advanced oxidation processes. Ultrasonics Sonochem. 2010, 17, 338–343. [Google Scholar] [CrossRef]
- Aubry, E.; Miska, P.; Gigleux, L.; Mézin, A.; Demange, V.; Billard, A. Microstructural and photocatalytic properties distribution of TiO2 coatings reactively sputtered as a function of the substrate position relatively to the Ti target. Surf. Coat. Technol. 2008, 202, 4980–4985. [Google Scholar] [CrossRef]
- Aubry, E.; Demange, V.; Billard, A. Effect of the internal stress relaxation during the post-annealing on the photo-induced properties of TiO2 coatings reactively sputtered. Surf. Coat. Technol. 2008, 202, 6120–6126. [Google Scholar] [CrossRef]
- Wang, X.; Fu, C.; Zhang, C.; Qiu, Z.; Wang, B. A comprehensive review of wetting transition mechanism on the surfaces of microstructures from theory and testing methods. Materials 2022, 15, 4747. [Google Scholar] [CrossRef] [PubMed]





| Power per Target (W) | Frequency (kHz) | “Off” Time (µs) | Air Flow Rate (sccm) | O2 Flow Rate (sccm) | Total Pressure (Pa) | Deposition Rate (nm/min) | Substrate Temperature (°C) |
|---|---|---|---|---|---|---|---|
| 1000 | 50 | 4.0 | 120 | 40 | 0.5 | 11.3 | 300 |
| Sample | Micro-Strain (σ) (×10−2) (nm) |
Dislocation Density (δ) (×10−3) (lines/nm2) |
|---|---|---|
| A | 20 ± 1 | 4 ± 1 |
| B | 23 ± 1 | 6 ± 1 |
| C | 23 ± 1 | 6 ± 1 |
| D | 26 ± 1 | 8 ± 1 |
| E | 35 ± 1 | 14 ± 1 |
| F | 25 ± 1 | 7 ± 1 |
| Samples | Bandgap Energy (eV) | Urbach Energy (meV) |
|---|---|---|
| A | 3.26 ± 0.02 | 137 ± 5 |
| B | 3.21 ± 0.02 | 141 ± 5 |
| C | 3.25 ± 0.02 | 140 ± 5 |
| D | 3.27 ± 0.02 | 214 ± 5 |
| E | 3.27 ± 0.02 | 214 ± 5 |
| F | 3.30 ± 0.02 | 118 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, S.; Aubry, E.; Sublemontier, O.; Briois, P. One-Step Synthesis of TiO2/SiO2-np Nanocomposite Photocatalytic Multilayer Films: Effect of Incorporation Time Sequences of SiO2 Nanoparticles during the TiO2 Film Growth. Materials 2024, 17, 1227. https://doi.org/10.3390/ma17061227
Lai S, Aubry E, Sublemontier O, Briois P. One-Step Synthesis of TiO2/SiO2-np Nanocomposite Photocatalytic Multilayer Films: Effect of Incorporation Time Sequences of SiO2 Nanoparticles during the TiO2 Film Growth. Materials. 2024; 17(6):1227. https://doi.org/10.3390/ma17061227
Chicago/Turabian StyleLai, Shutong, Eric Aubry, Olivier Sublemontier, and Pascal Briois. 2024. "One-Step Synthesis of TiO2/SiO2-np Nanocomposite Photocatalytic Multilayer Films: Effect of Incorporation Time Sequences of SiO2 Nanoparticles during the TiO2 Film Growth" Materials 17, no. 6: 1227. https://doi.org/10.3390/ma17061227






