Synthesis of Orthorhombic Tin Dioxide Nanowires in Track Templates
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
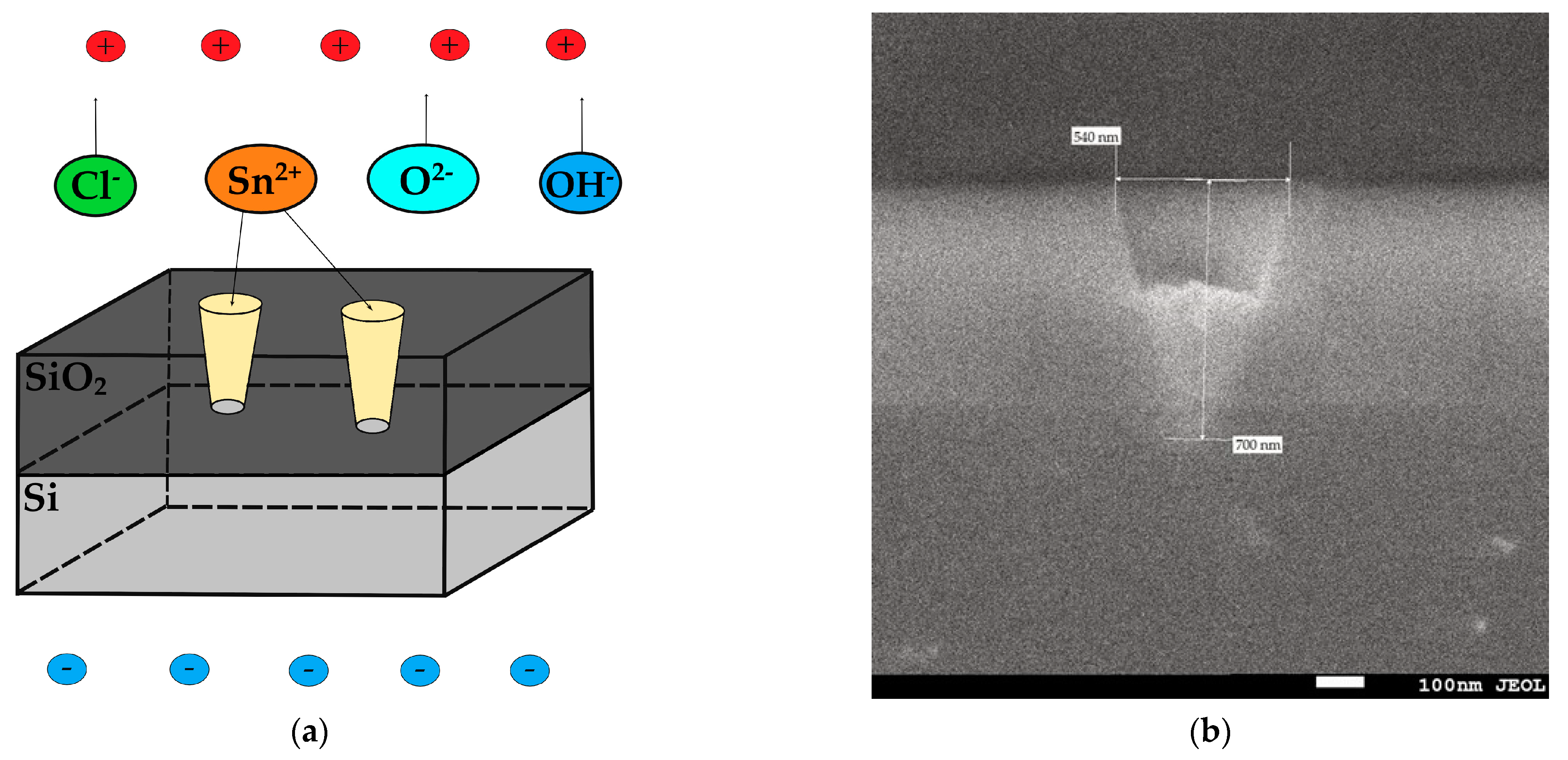
3.1. SEM and XRD Analysis of Deposited Samples
3.2. The Photoluminescence (PL) End Electrical Properties of Orthorhombic SnO2-NWs/SiO2/Si
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenz, M.; Ramachandra Rao, M.S.; Venkatesan, T.; Fortunato, E.; Barquinha, P.; Branquinho, R. Topical Review: The oxide electronic materials and oxide interfaces roadmap. J. Phys. D Appl. Phys. 2016, 49, 433001. [Google Scholar] [CrossRef]
- Varghese, B.; Hoong, T.C.; Yanwu, Z.; Reddy, M.V.; Chowdari, B.V.R.; Wee, A.T.S.; Vincent, T.B.C.; Lim, C.T.; Sow, C.H. Co3O4 nanostructures with different morphologies and their field-emission properties. Adv. Funct. Mater. 2007, 17, 1932–1939. [Google Scholar] [CrossRef]
- Fang, X.S.; Yan, J.; Hu, L.F.; Liu, H.; Lee, P.S. Thin SnO2 nanowires with uniform diameter as excellent field emitters: A stability of more than 2400 minutes. Adv. Funct. Mater. 2012, 22, 1613–1622. [Google Scholar] [CrossRef]
- Bie, Y.Q.; Liao, Z.M.; Zhang, H.Z.; Li, G.R.; Ye, Y.; Zhou, Y.B.; Xu, J.; Qin, Z.X.; Dai, L.; Yu, D.P. Self-powered, ultrafast, visible-blind UV detection and optical logical operation based on ZnO/GaN nanoscale p-n junctions. Adv. Funct. Mater. 2011, 23, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Rigutti, L.; Tchernycheva, M.; Bugallo, A.D.; Jacopin, G.; Julien, F.H.; Zagonel, L.F.; March, K.; Stephan, O.; Kociak, M.; Songmuang, R. Ultraviolet photodetector based on GaN/AlN quantum discs in a single nanowire. Nano Lett. 2010, 10, 2939–2943. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.S.; Wang, C.Y.; Xiu, F.X.; Lang, M.R.; Chu, L.W.; Tsai, C.J.; Chueh, Y.L.; Chen, L.J.; Wang, K.L. Oxide-confined formation of germanium nanowire heterostructures for high-performance transistors. Am. Chem. Soc. Nano 2011, 5, 6008–6015. [Google Scholar] [CrossRef] [PubMed]
- Kulmala, T.S.; Colli, A.; Fasoli, A.; Lombardo, A.; Haque, S.; Ferrari, A.C. Self-Aligned coupled nanowire. Am. Chem. Soc. Nano 2011, 5, 6910–6915. [Google Scholar] [CrossRef]
- Vaseashta, A.; Dimova-Malinovska, D. Nanostructured and nanoscale devices, sensors and detectors. Sci. Technol. Adv. Mater. 2005, 6, 312–318. [Google Scholar] [CrossRef]
- Chou, J.C.; Wang, Y.F. Preparation and study on the drift and hysteresis properties of the tin oxide gate ISFET by the sol-gel method. Sens. Actuators B Chem. 2002, 86, 58–62. [Google Scholar] [CrossRef]
- Lee, J.S.; Sim, S.K.; Min, B.; Cho, K.; Kim, S.W.; Kim, S. Structural and optoelectronic properties of SnO2 nanowires synthesized from ball-milled SnO2 powders. J. Cryst. Growth 2004, 267, 145–149. [Google Scholar] [CrossRef]
- Ying, Z.; Wan, Q.; Song, Z.T.; Feng, S.L. Controlled synthesis of branched SnO2 nanowhiskers. Mater. Lett. 2005, 59, 1670–1672. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Lu, H. Hierarchical structure SnO2 supported Pt nanoparticles as enhanced electrocatalyst for methanol oxidation. Electrochim. Acta 2012, 76, 475–479. [Google Scholar] [CrossRef]
- Heiss, M.; Fontana, Y.; Gustafsson, A.; Wust, G.; Magen, C.; O’Regan, D.; Luo, J.; Ketterer, B.; Conesa-Boj, S.; Kuhlmann, A.; et al. Self-assembled quantum dots in a nanowire system for quantum photonics. Nat. Mater. 2013, 12, 439–444. [Google Scholar] [CrossRef]
- Kozlovskii, A.L.; Kadyrzhanov, K.K.; Zdorovets, M.V. Structural and Conductive Characteristics of Fe/Co Nanotubes. Russ. J. Electrochem. 2018, 54, 178–185. [Google Scholar] [CrossRef]
- Kozlovskiy, A.; Zhanbotin, A.; Zdorovets, M.; Manakova, I.; Ozernoy, A.; Kadyrzhanov, K.; Rusakov, V. Study of Ni/Fe nanotube properties. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 365, 663–667. [Google Scholar] [CrossRef]
- Mashentseva, A.; Borgekov, D.; Kislitsin, S.; Sdorovets, M.; Migunova, A. Comparative catalytic activity of PET track-etched membranes with embedded silver and gold nanotubes. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 365, 70–74. [Google Scholar] [CrossRef]
- Demyanov, S.; Kaniukov, E.; Petrov, A.; Sivakov, V. Positive magnetoresistive effect in Si/SiO2 (Cu/Ni) nanostructures. Sens. Actuators A Phys. 2014, 216, 64–68. [Google Scholar] [CrossRef]
- Sivakov, V.; Kaniukov, E.Y.; Petrov, A.; Korolik, O.; Mazmanik, A.; Bochmann, A.; Teichert, S.; Hidi, I.J.; Schleusener, A.; Gialla, D.; et al. Novel silver nanostructures formation in porous Si/SiO2 matrix. J. Cryst. Growth 2014, 400, 21–26. [Google Scholar] [CrossRef]
- Fertig, N.; Blick, R.H.; Behrends, J.C. Whole cell patch clamp recording performed on a planar glass chip. Biophys. J. 2002, 82, 3056–3062. [Google Scholar] [CrossRef]
- Hoppe, K.; Fahrner, W.R.; Fink, D.; Dhamodoran, S.; Petrov, A.; Chandra, A.; Saad, A.; Faupel, F.; Chakravadhanula, V.S.K.; Zaporotchenko, V. An ion track based approach to nano-and micro-electronics. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 1642–1646. [Google Scholar] [CrossRef]
- Kaniukov, E.; Bundyukova, V.; Kutuzau, M.; Yakimchuk, A. Preculiarities of Formation and Characterization of SiO2/Si Ion-Track Template. In Fundamental and Applied Nano-Electromagnetics II: THz Circuits, Materials, Devices; Springer: Dordrecht, The Netherlands, 2019; pp. 41–57. [Google Scholar] [CrossRef]
- Dallanora, A.; Marcondes, T.L.; Bermudez, G.G.; Fichtner, P.F.P.; Trautman, C.; Toulemonde, M.; Papaleo, R.M. Nanoporous SiO2/Si thin layers produced by ion track etching: Dependence on the ion energy and criterion for etchability. J. Appl. Phys. 2008, 104, 024307. [Google Scholar] [CrossRef]
- Giniyatova, S.; Dauletbekova, A.; Baimukhanov, Z.; Vlasukova, L.; Akilbekov, A. Structure, electrical properties and lum. of ZnO NCs deposited in SiO2/Si track templates. Radiat. Meas. 2019, 125, 52–56. [Google Scholar] [CrossRef]
- Balakhayeva, R.; Akilbekov, A.; Baimukhanov, Z.; Giniyatova, S.; Zdorovets, M.; Gorin, Y.; Popov, A.I.; Dauletbekova, A. Structure properties of CdTe nanocrystals created in SiO2/Si ion track templates. Surf. Coat. Technol. 2020, 401, 126269. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Akylbekova, A.; Sarsekhan, G.; Usseinov, A.; Baimukhanov, Z.; Kozlovskiy, A.; Vlasukova, L.A.; Komarov, F.F.; Popov, A.I.; Akilbekov, A.T. Ion-track template synthesis and characterization of ZnSeO3 Nanocrystals. Crystals 2022, 12, 817. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Dauletbekova, A.K.; Alzhanova, A.Y.; Akilbekov, A.T.; Mashentseva, A.A.; Zdorovets, M.V.; Balabekov, K.N. Synthesis of Si/SiO2/ZnO nanoporous materials using chemical and electrochemical deposition techniques. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1767, p. 020005. [Google Scholar] [CrossRef]
- Laun, J.; Bredow, T. BSSE-corrected consistent Gaussian basis sets of triple-zeta valence with polarization quality of the fifth period for solid-state calculations. J. Comput. Chem. 2022, 12, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Vilela Oliveira, D.; Peintinger, M.F.; Laun, J.; Bredow, T. BSSE-correction scheme for consistent gaussian basis sets of double-and triple-zeta valence with polarization quality for solid-state calculations. J. Comput. Chem. 2019, 27, 2364–2376. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a Functional of the Electron Density. Phys. Rev. B 1998, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. II. Overlap populations, bond orders, and covalent bond energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Slassi, A.; Hammi, M.; Oumekloul, Z.; Nid-bahami, A.; Arejdal, M.; Ziat, Y.; El Rhazouani, O. Effect of halogens doping on transparent conducting properties of SnO2 rutile: An ab initio investigation. Opt. Quantum Electron. 2018, 50, 8. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Veerapandy, V.; Fjellvag, V.; Vajeeston, P. First-principles exploration into the physical and chemical properties of certain newly identified SnO2 polymorphs. ACS Omega 2022, 12, 10382–10393. [Google Scholar] [CrossRef]
- Arlinghaus, F.J. Energy bands in stannic oxide (SnO2). J. Phys. Chem. Solids 1974, 8, 931–935. [Google Scholar] [CrossRef]
- Barbarat, P.; Matar, S.F. First-principles investigations of the electronic, optical and chemical bonding properties of SnO2. Comput. Mater. Sci. 1998, 1–4, 368–372. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Baumann, T.F.; Sterne, P.A.; Wang, Y.M.; van Buuren, T.; Hamza, A.V.; Terminello, L.J.; Willey, T.M. Surface electronic states in three-dimensional SnO2 nanostructures. Phys. Rev. B 2005, 3, 035404. [Google Scholar] [CrossRef]
- Nagasawa, M.; Shionoya, S. Temperature dependence of the fundamental optical absorption edge in stannic oxide. J. Phys. Soc. Jpn. 1971, 4, 1118–1123. [Google Scholar] [CrossRef]
- Themlin, J.M.; Sporken, R.; Darville, K.; Caudano, R.; Gilles, J.M.; Johnson, R.L. Resonant-photoemission study of SnO2: Cationic origin of the defect band-gap states. Phys. Rev. B 1990, 18, 11914. [Google Scholar] [CrossRef] [PubMed]
- Maki-Jaskari, M.A.; Rantala, T.T. Band structure and optical parameters of the SnO2 (110) surface. Phys. Rev. B 2001, 7, 075407. [Google Scholar] [CrossRef]
- Suito, K.; Kawai, N.; Masuda, Y. High pressure synthesis of orthorhombic SnO2. Mater. Res. Bull. 1975, 10, 677–680. [Google Scholar] [CrossRef]
- Shieh, S.R.; Kubo, A.; Duffy, T.S.; Prakapenka, V.B.; Shen, G. High-pressure phases in SnO2 to 117 GPa. Phys. Rev. B 2006, 73, 014105. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, J.K.L.; Shek, C.H. Facile strategy and mechanism for orthorhombic SnO2 thin films. Appl. Phys. Lett. 2006, 89, 231902. [Google Scholar] [CrossRef]
- Kaplan, L.; Ben-Shalom, A.; Boxman, R.L.; Goldsmith, S.; Rosenberg, U.; Nathan, M. Annealing and Sb-doping of Sn—O films produced by filtered vacuum arc deposition: Structure and electro-optical properties. Thin Solid Film. 1994, 253, 1–8. [Google Scholar] [CrossRef]
- Lamelas, F.J.; Reid, S.A. Thin-film synthesis of the orthorhombic phase of SnO2. Phys. Rev. B 1999, 60, 9347. [Google Scholar] [CrossRef]
- Bae, J.Y.; Park, J.; Kim, H.Y.; Kim, H.S.; Park, J.S. Facile route to the controlled synthesis of tetragonal and orthorhombic SnO2 films by mist chemical vapor deposition. ACS Appl. Mater. Interfaces 2015, 7, 12074–12079. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E. RHEED-Untersuchungen einer grenzschichtstruktur von SnO2 auf quarz. Acta Crystallogr. Sect. B Struct. Sci. 1984, 40, 359–363. [Google Scholar] [CrossRef]
- Sangaletti, L.; Depero, L.E.; Dieguez, A.; Marca, G.; Morante, J.R.; Romano-Rodriguez, A.; Sberveglieri, G. Microstructure and morphology of tin dioxide multilayer thin film gas sensors. Sens. Actuators B Chem. 1997, 44, 268–274. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Faglia, G.; Groppelli, S.; Nelli, P.; Taroni, A. A novel PVD technique for the preparation of SnO2 thin films as C2H5OH sensors. Sens. Actuators B Chem. 1992, 7, 721–726. [Google Scholar] [CrossRef]
- Masuda, Y. Recent advances in SnO2 nanostructure based gas sensors. Sens. Actuators B Chem. 2022, 364, 131876. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 gas sensors based on SnO2 nanowires. Sens. Actuators B Chem. 2021, 345, 130334. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Vivod, D.; Kim, S.; Mirzaei, A.; Zahn, D.; Park, C.; Kim, S.S.; Halik, M. Chemical-recognition-driven selectivity of SnO2-nanowire-based gas sensors. Nano Today 2021, 40, 101265. [Google Scholar] [CrossRef]
- Sharma, A.; Khosla, A.; Arya, S. Synthesis of SnO2 nanowires as a reusable and flexible electrode for electrochemical detection of riboflavin. Microchem. J. 2020, 156, 104858. [Google Scholar] [CrossRef]
- Dai, Z.R.; Gole, J.L.; Stout, J.D.; Wang, Z.L. Tin oxide nanowires, nanoribbons, and nanotubes. J. Phys. Chem. B 2002, 106, 1274–1279. [Google Scholar] [CrossRef]
- Dai, Z.R.; Pan, Z.W.; Wang, Z.L. Novel nanostructures of functional oxides synthesized by thermal evaporation. Adv. Funct. Mater. 2003, 13, 9–24. [Google Scholar] [CrossRef]
- Ihn, S.G.; Song, J.I.; Kim, T.W.; Leem, D.S.; Lee, T.; Lee, S.G.; Koh, E.K.; Song, K. Morphology-and orientation-controlled gallium arsenide nanowires on silicon substrates. Nano Lett. 2007, 7, 39–44. [Google Scholar] [CrossRef]
- Arbiol, J.; Kalache, B.; Roca i Cabarrocas, P.; Morante, J.R.; Fontcuberta i Morral, A. Influence of Cu as a catalyst on the properties of silicon nanowires synthesized by the vapour–solid–solid mechanism. Nanotechnology 2007, 18, 305606. [Google Scholar] [CrossRef]
- Dauletbekova, A.; Vlasukova, L.; Baimukhanov, Z.; Akilbekov, A.; Kozlovskiy, A.; Giniyatova, S.; Seitbayev, A.; Usseinov, A.; Akylbekova, A. Synthesis of ZnO Nanocrystals in SiO2/Si Track Template: Effect of Electrodeposition Parameters on Structure. Phys. Status Solidi B 2019, 256, 1800408. [Google Scholar] [CrossRef]
- Arbiol, J.; Comini, E.; Faglia, G.; Sberveglieri, G.; Morante, J.R. Orthorhombic Pbcn SnO2 nanowires for gas sensing applications. J. Cryst. Growth 2008, 310, 253–260. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.F.; Lu, M.K.; Zhou, G.J.; Xu, D.; Yuan, D.R. Photoluminescence properties of SnO2 nanoparticles synthesized by sol−gel method. J. Phys. Chem. B 2004, 108, 8119–8123. [Google Scholar] [CrossRef]
- Chowdhury, P.S.; Saha, S.; Patra, A. Influence of nanoenvironment on luminescence of Eu3+ activated SnO2 nanocrystals. Solid State Commun. 2004, 131, 785–788. [Google Scholar] [CrossRef]
- Faglia, G.; Baratto, C.; Sberveglieri, G.; Zha, M.; Zappettini, A. Adsorption effects of NO2 at ppm level on visible photoluminescence response of SnO2 nanobelts. Appl. Phys. Lett. 2005, 86, 011923. [Google Scholar] [CrossRef]
- Maestre, D.; Cremades, A.; Piqueras, J. Growth and luminescence properties of micro-and nanotubes in sintered tin oxide. J. Appl. Phys. 2005, 97, 044316. [Google Scholar] [CrossRef]
- Gu, F.; Wang, S.F.; Song, C.F.; Lu, M.K.; Qi, Y.X.; Zhou, G.J.; Xu, D.; Yuan, D.R. Synthesis and luminescence properties of SnO2 nanoparticles. Chem. Phys. Lett. 2003, 372, 451–454. [Google Scholar] [CrossRef]
- Munnix, S.; Schmeits, M. Electronic structure of tin dioxide surfaces. Phys. Rev. B 1983, 27, 7624. [Google Scholar] [CrossRef]
- Chiodini, N.; Paleari, A.; DiMartino, D.; Spinolo, G. SnO2 nanocrystals in SiO2: A wide-band-gap quantum-dot system. Appl. Phys. Lett. 2002, 81, 1702–1704. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q.; Xu, C. Single-narrow-band red upconversion fluorescence of ZnO nanocrystals codoped with Er and Yb and its achieving mechanism. J. Appl. Phys. 2008, 104, 064701. [Google Scholar] [CrossRef]
- Godinho, K.G.; Walsh, A.; Watson, G.W. Energetic and electronic structure analysis of intrinsic defects in SnO2. J. Phys. Chem. C 2009, 113, 439–448. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhang, M.S.; Yin, Z.; Chen, Q. Photoluminescence in anatase titanium dioxide nanocrystals. Appl. Phys. B 2000, 70, 261–265. [Google Scholar] [CrossRef]
- Bhatnagar, M.; Kaushik, V.; Kaushal, A.; Singh, M.; Mehta, B. Structural and photoluminescence properties of tin oxide and tin oxide: C core–shell and alloy nanoparticles synthesised using gas phase technique. AIP Adv. 2016, 6, 095321. [Google Scholar] [CrossRef]
- Rani, S.; Roy, S.; Karar, N.; Bhatnagar, M. Structure, microstructure and photoluminescence properties of Fe doped SnO2 thin films. Solid State Commun. 2007, 141, 214–218. [Google Scholar] [CrossRef]
- Her, Y.C.; Wu, J.Y.; Lin, Y.R.; Tsai, S.Y. Low-temperature growth and blue luminescence of SnO2 nanoblades. Appl. Phys. Lett. 2006, 89, 043115. [Google Scholar] [CrossRef]
- Hu, J.Q.; Bando, Y.; Golberg, D. Self-catalyst growth and optical properties of novel SnO2 fishbone-like nanoribbons. Chem. Phys. Lett. 2003, 372, 758–762. [Google Scholar] [CrossRef]
- Cai, D.; Su, Y.; Chen, Y.; Jiang, J.; He, Z.; Chen, L. Synthesis and photoluminescence properties of novel SnO2 asterisk-like nanostructures. Mater. Lett. 2005, 59, 1984–1988. [Google Scholar] [CrossRef]
- Sinha, S.K.; Bhattacharya, R.; Ray, S.K.; Manna, I. Influence of deposition temperature on structure and morphology of nanostructured SnO2 films synthesized by pulsed laser deposition. Mater. Lett. 2011, 65, 146–149. [Google Scholar] [CrossRef]
- Duan, J.; Gong, J.; Huang, H.; Zhao, X.; Cheng, G.; Yu, Z.; Yang, S. Multiform structures of SnO2 nanobelts. Nanotechnology 2007, 18, 055607. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, S.; Zuo, Y.; Zhang, B.; Xi, L. Influence of oxygen flow rate on the morphology and magnetism of SnO2 nanostructures. J. Phys. Chem. C 2010, 114, 7541–7547. [Google Scholar] [CrossRef]
- Hu, J.Q.; Bando, Y.; Liu, Q.L.; Golberg, D. Laser-ablation growth and optical properties of wide and long single-crystal SnO2 ribbons. Adv. Funct. Mater. 2003, 13, 493–496. [Google Scholar] [CrossRef]
- He, J.H.; Wu, T.H.; Hsin, C.L.; Li, K.M.; Chen, L.J.; Chueh, Y.L.; Chou, L.J.; Wang, Z.L. Beaklike SnO2 nanorods with strong photoluminescent and field-emission properties. Small 2006, 2, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Touskova, J.; Tousek, J.; Klier, E.; Kuzel, R. Preparation and basic electrical properties of CdTe thick films. Phys. Status Solidi 1979, 56, 315–322. [Google Scholar] [CrossRef]
- Davis, E.A. States in the gap and defects in amorphous semiconductors. Amorph. Semicond. 2005, 36, 41–72. [Google Scholar] [CrossRef]
- Belyaev, A.P.; Rubets, V.P.; Nuzhdin, M.Y. Electrical properties of cadmium telluride films synthesized in a thermal field with a temperature gradient. Semiconductors 2003, 37, 646–648. [Google Scholar] [CrossRef]
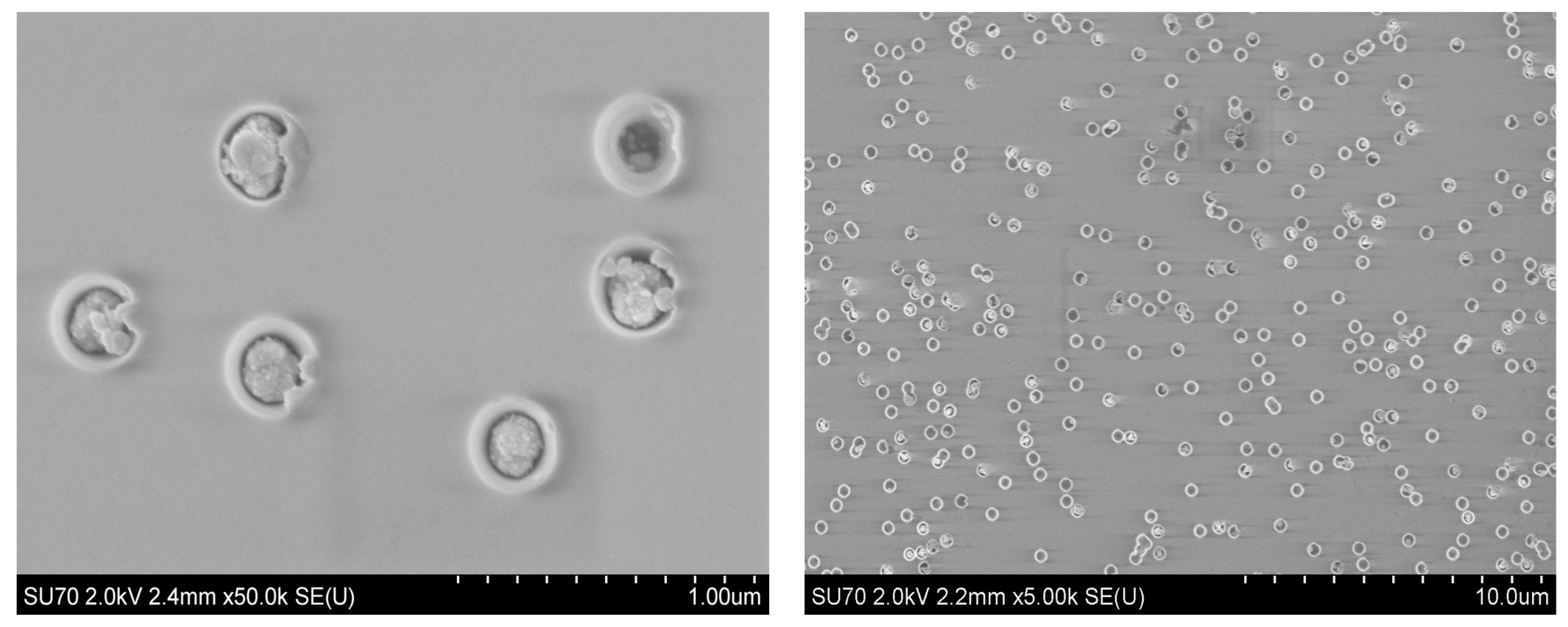
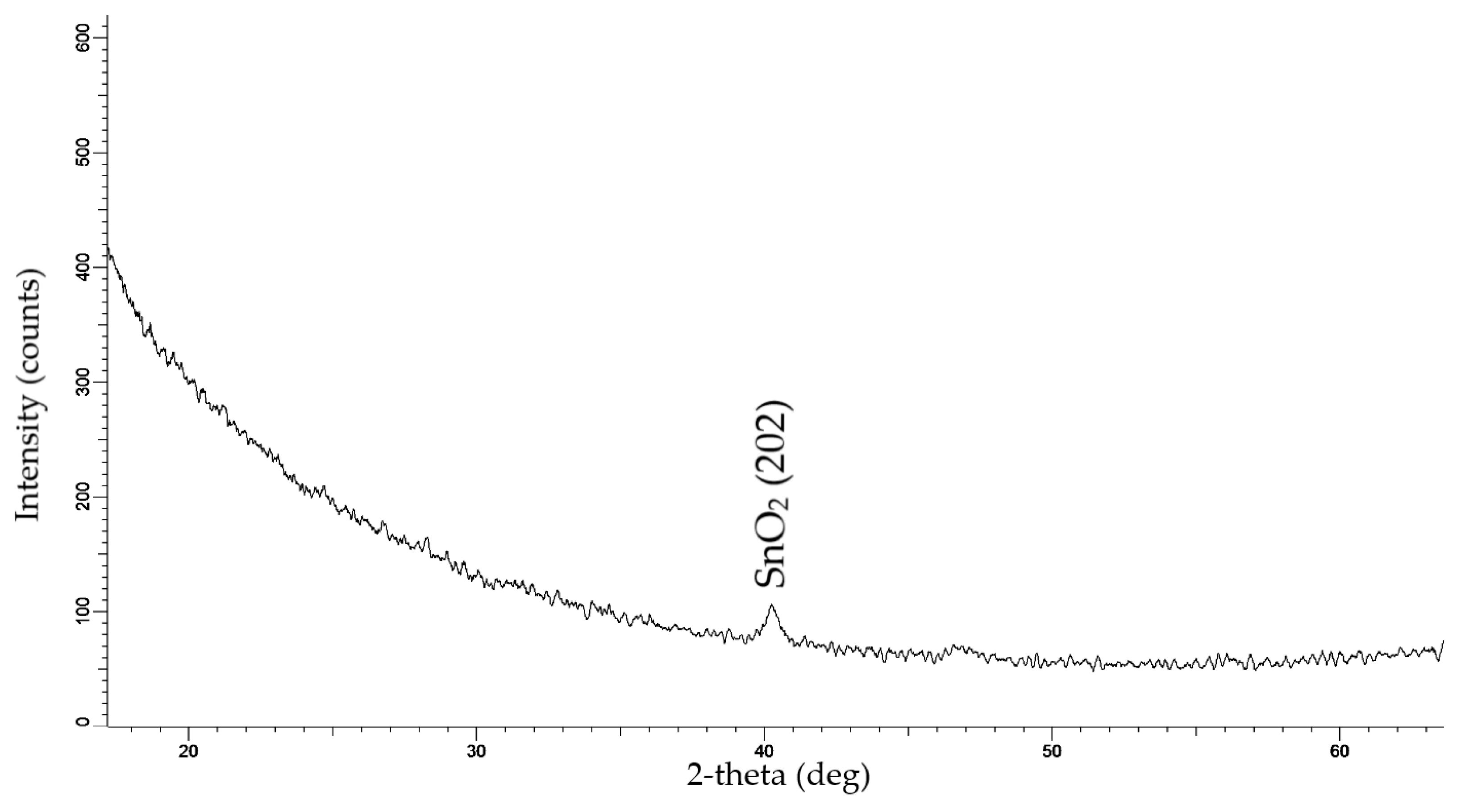

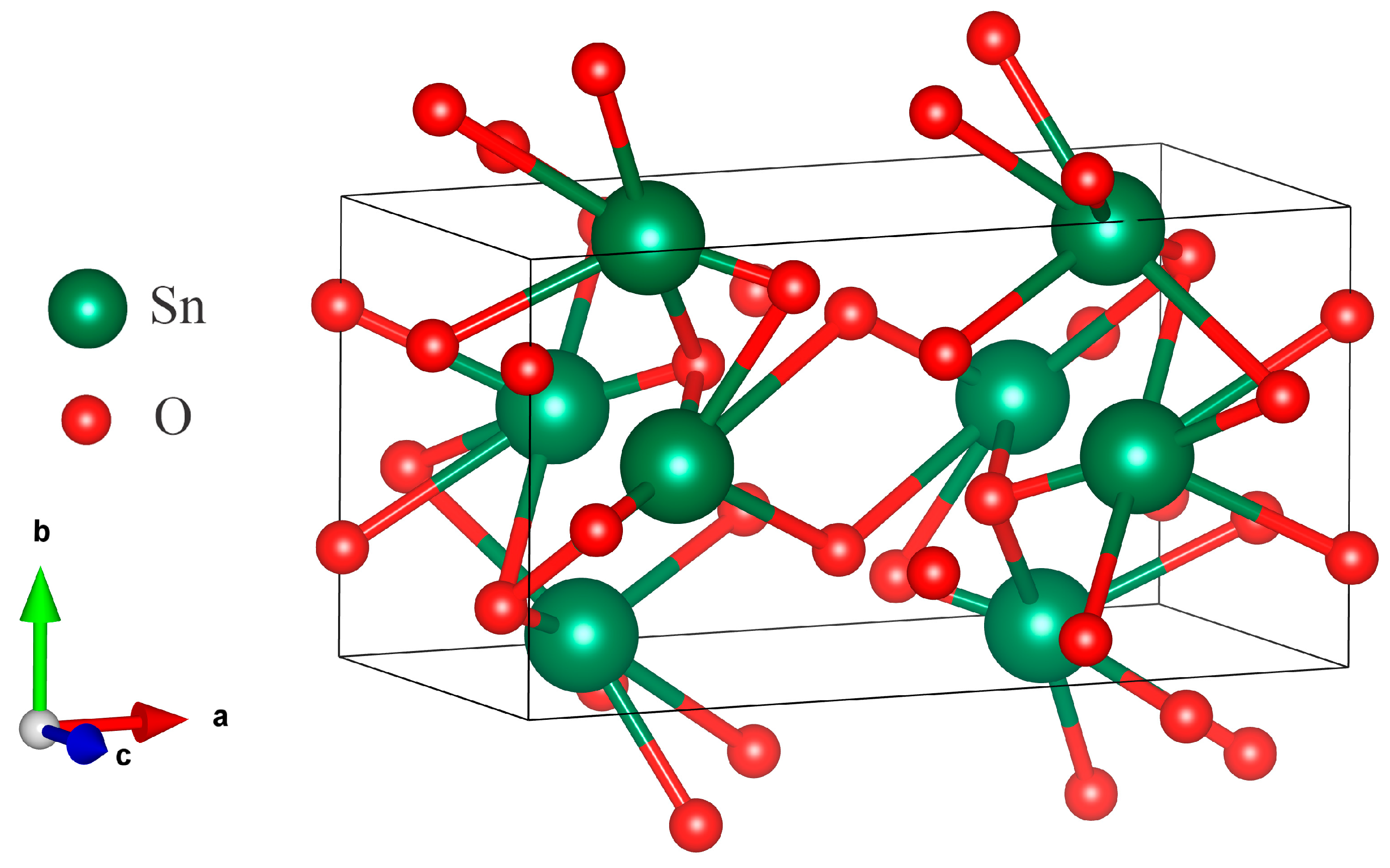
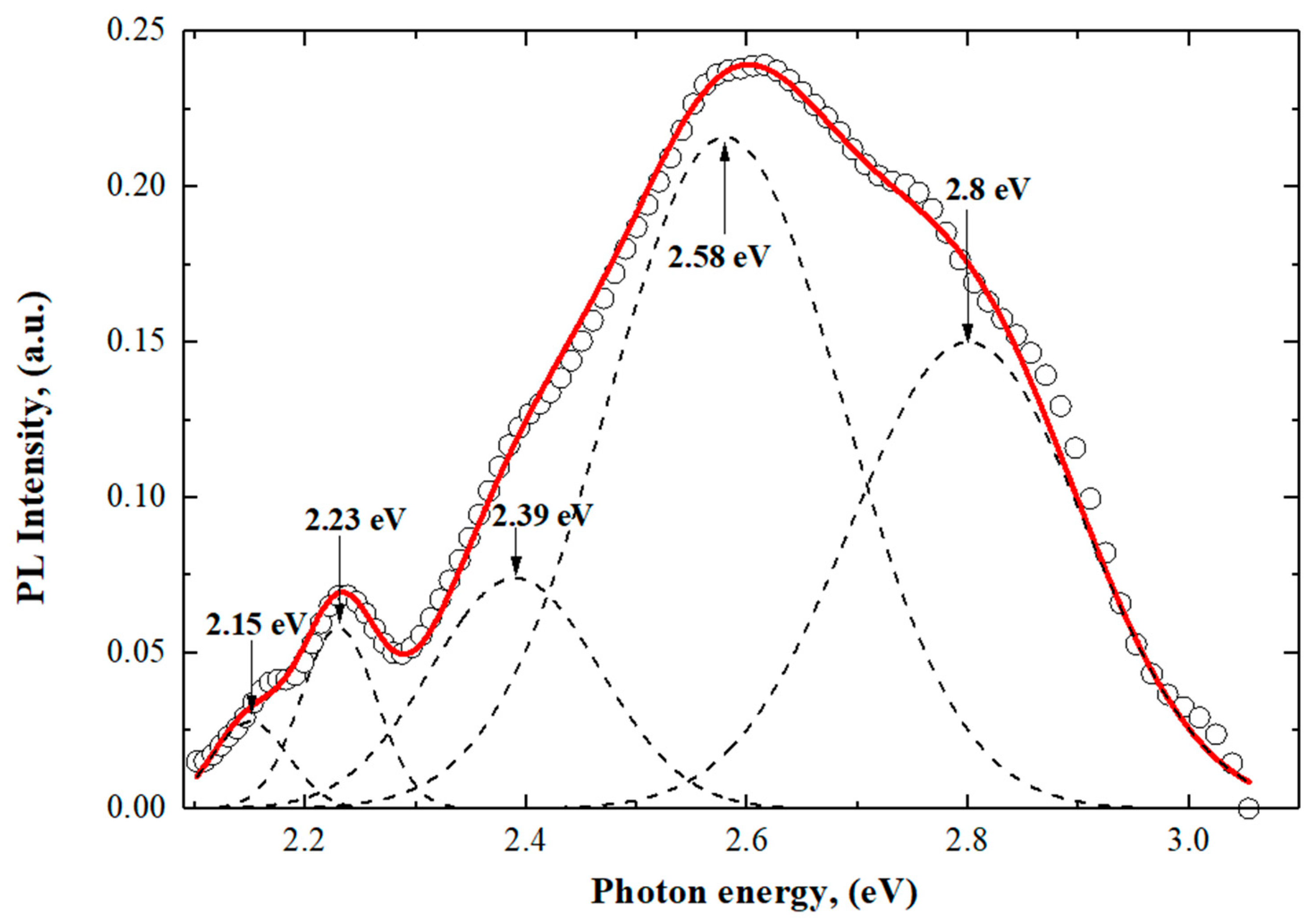

| Phase Name | Structure Type | Space Group | (hkl) | 2θ | d, Å | L, nm | FWHM | Cell Parameters, Å | Volume, Å3 | Density, g/cm3 | Degree of Crystallinity, % | Phase Content, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SnO2 | Orthorhombic | Pbca (61) | 202 | 40.219 | 2.24046 | 19.39 | 0.485 | a = 9.97195 (10.05) b = 5.11601 (5.10) c = 5.03283 (5.18) | 256.76 (266.26) | 7.819 (7.57) | 41.8 | 100 |
| H, nm | T, K | m | hk, Å | Barrier Height E, eV |
|---|---|---|---|---|
| 700 | 300 | 1044 | 6.7 | 3.82 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baimukhanov, Z.; Dauletbekova, A.; Junisbekova, D.; Kalytka, V.; Akilbekov, A.; Akylbekova, A.; Baubekova, G.; Aralbayeva, G.; Bazarbek, A.-D.; Usseinov, A.; et al. Synthesis of Orthorhombic Tin Dioxide Nanowires in Track Templates. Materials 2024, 17, 1226. https://doi.org/10.3390/ma17061226
Baimukhanov Z, Dauletbekova A, Junisbekova D, Kalytka V, Akilbekov A, Akylbekova A, Baubekova G, Aralbayeva G, Bazarbek A-D, Usseinov A, et al. Synthesis of Orthorhombic Tin Dioxide Nanowires in Track Templates. Materials. 2024; 17(6):1226. https://doi.org/10.3390/ma17061226
Chicago/Turabian StyleBaimukhanov, Zein, Alma Dauletbekova, Diana Junisbekova, Valeriy Kalytka, Abdirash Akilbekov, Aiman Akylbekova, Guldar Baubekova, Gulnara Aralbayeva, Assyl-Dastan Bazarbek, Abay Usseinov, and et al. 2024. "Synthesis of Orthorhombic Tin Dioxide Nanowires in Track Templates" Materials 17, no. 6: 1226. https://doi.org/10.3390/ma17061226







