Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review
Abstract
1. Introduction
2. Soft Tissue Substitutes
2.1. Dermal Matrix
2.2. Human Amniotic Membrane
2.3. Porcine-Derived Collagen Matrix
2.4. Polymeric Matrices
3. Materials and Methods
- Population (P): adults (≥18 years) presenting reduced keratinized tissue around teeth and implants,
- Intervention (I): root coverage procedures, soft tissue augmentations,
- Comparisons (C): soft tissue substitutes vs. no treatment or connective tissue graft/free gingival graft,
- Outcomes (O): keratinized tissue width, soft tissue thickness, root/implant coverage with more than 6 months of follow-up,
- Study design (S): systematic reviews, randomized clinical trials
3.1. Inclusion Criteria
3.2. Exclusion Criteria
3.3. Outcomes
3.4. Strategy Search
3.5. Selection of Studies, Data Extraction and Synthesis
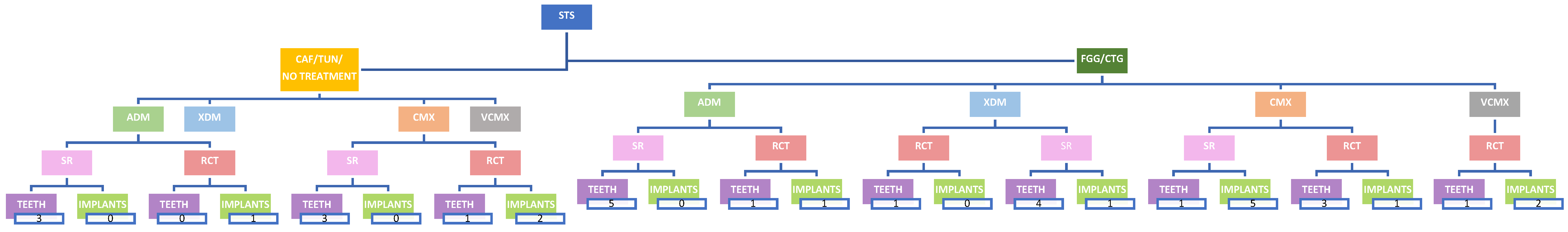
4. Results
| Study Type | Authors | Year | Surgical Procedure | Test Group | Control Group | No. of Patients/ No. of Teeth or Implants | Follow-Up (Months) | CRC | mRC | KTW | STT | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT [23] | Jepsen et al. [23] | 2017 | CAF for single recessions | CAF + CMX | CAF | 18/36 | 36 | T: 61.1% C: 38.9% | T: 91.7 ± 12.05% C: 82.77 ± 17.03% | T: from 2.14 ± 1.21 mm to 4.06 ± 1.55 mm C: from 2.22 ± 1.39 mm to 3.25 ± 0.81 mm | T: from 0.93 ± 0.27 mm to 1.52 ± 0.41 mm C: from 0.96 ± 0.34 mm to 1.11 ± 0.41 | In CAF + CMX group, mRC, CRC, KTW, and STT showed better outcomes than CAF alone |
| SR [4] | Chambrone et al. | 2018 | 1. CAF for multiple recessions 2. CAF for single recession | 1. CAF + ADM2. CAF + ADMG | 1. CAF 2. CAF | 48 studies in total, 2 studies evaluated | >6 | from 0% to 91.6% for ADMG from 7.7% to 81.8% for CAF | from 50% to 96% for ADMG from 55.9% to 95.4% for CAF | NA | NA | ADMG appears as the soft tissue substitute that may provide the most similar outcomes to those achieved by SCTG |
| SR [21] | Moraschini et al. | 2020 | 1. CAF + CMX 2. CAF + CMX 3. CAF + CMX 4. CAF + ADM | 1. CAF 2. CAF 3. CAF 4. CAF | 27 studies in total, 2 studies evaluated | 12 | NA | C: 81.4% ± 23.4 T: 93.2% ± 10 C: 75% ± 26.2 T: 76.2% ± 28 C: 75% ± 30 T: 87% ± 19 C: 74.9% ± 28.0 T: 94.8% | C: 0.7 ± 1.04 mm T: 1.07 ± 0.87 mm C: 0.64 ± 1.16 mm T: 1.05 ± 1.19 mm C: 1.1 ± 1.3 mm T: 0.6 ± 1.7 mm C: 0.60 ± 0.36 mm T: 1.21 ± 0.23 mm | NA | Biomaterials increase the effectiveness of RC in comparison with CAF alone. ADM demonstrated the best results |
| Study Type | Authors | Year | Surgical Procedure | Test Group | Control Group | No. of Patients/ No. of Teeth or Implants | Follow-Up (Months) | CRC | mRC | KTW | STT | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT [18] | Frizzera et al. | 2018 | STA (Immediate implant placement and provisionalization) | STA + CMX | No soft tissue augmentation | 16/16 | 12 | T: NA C: NA | T: NA C: NA | T: NA T: NA C: NA | T: from 0.98 to 2.1 mm C: from 1 to 2.11 mm | CMX reduced MPR, provided better contour of the alveolar ridge, and increased STT |
| RCT [19] | Zuiderveld et al. | 2018 | STA (in conjunction with implant placement) | STA + CMX | No soft tissue augmentation | 40/40 | 12 | T: NA C: NA | T: loss of 0.17 ± 1.3 mm C: loss of 0.48 ± 1.5 mm | T: NA C: NA | T: NA C: NA | CMX does not result in a more favorable esthetic outcome than when no soft tissue graft was applied |
| RCT [20] | Lee et al. | 2023 | STA in conjunction with implant placement | STA + ADM | No soft tissue augmentation | 31/31 | 12 | ADM maintained buccal soft tissue contours 3–5 mm below the initial soft tissue margin | T: NA C: NA | Changes were not significantly different between the groups | T: from 1.34 ± 0.25mm to 2.57 ± 0.30 mm C: from 1.18 ± 0.31 mm to 1.18 ± 0.31 mm | STA enhanced STT and maintained soft tissue contours but did not prevent peri-implant mucosal recession |
| Study Type | Authors | Year | Surgical Procedure | Test Group | Control Group | No. of Patients/ No. of Teeth or Implants | Follow-Up (Months) | CRC | mRC | KTW | STT | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT [24] | Aroca et al. | 2013 | TUN for multiple recessions | MCAT + CMX | MCAT + CTG | 22/156 | 12 | T: 42% C: 85% | T: 71 ± 21% C: 90 ± 18% | T: from 2.1 ± 0.9 mm to 2.4 ± 0.7 mm C: from 2.0 ± 0.7 mm to 2.7 ± 0.8 mm | T: from 0.8 ± 0.2 mm to 1.0 ± 0.3 mm C: from 0.8 ± 0.3 mm to 1.3 ± 0.4 mm | CMX reduce surgical time and patient morbidity but give lower CRC when used in conjunction with MCAT |
| SR [4] | Chambrone et al. | 2018 | 1. CAF for single recession 2. CAF for multiple recessions 3. CAF for single recession | 1. CAF + ADMG 2. CAF + CMX 3. CAF + ADMG | 1. CAF + CTG 2. CAF + CTG 3. CAF + CTG | 48 studies in total, 3 studies evaluated | >6 | from 0% to 91.6% for ADMG from 18.1% to 95.6% for SCTG | from 50% to 96% for ADMG from 64.7% to 99.3% for SCTG | NA | NA | There was insufficient evidence of a difference in GR reduction and KTW gain between ADMG + CAF and SCTG + CAF |
| SR [16] | de Carvalho Formiga et al. | 2020 | CAF | 1. ADM 2. ADM 3. XDM 4. CMX | 1. CTG 2. CTG 3. CTG 4. CTG | 14 studies in total, 4 studies evaluated (conducted after 2010) | >6 | No statistically significant differences | The CTG increased the MRC (+7.6 percentage points) | On 2 mm recessions, CTG showed superiority above other biomaterials, but on 3 mm recessions, it seemed to have the same results | NA | CTG, acellular dermal matrix allograft and xenogenic collagen matrix provided similar results for root coverage |
| RCT [25] | McGuire et al. | 2021 | VP | VP + CMX | VP + FGG | 23/ | 6-8 y | T: NA C: NA | T: −0.07 ± 1.26 C: −0.17 ± 0.78 | T: −0.09 ± 1.30 C: 0.20 ± 0.72 | T: NA C: NA | Recession levels were maintained equivalently by both therapies |
| RCT [26] | Tonetti et al. | 2021 | CAF for multiple recessions | CAF + CMX | CAF + CTG | 125/307 | 36 | T: 3% C: 3% | T: NA C: NA | C: from 2.8 ± 1.3 mm to 0.5 ± 1.0 mm T: from 2.6 ± 1.2 mm to 0.0 ± 1.2 mm | T: NA C: NA | CMX reported shorter time to recovery, lower morbidity, and a more natural appearance of tissue texture and contour |
| RCT [27] | Elmahdi et al. | 2022 | TUN for multiple recessions | MCAT + ADM | MCAT + CTG | 12/69 | 9 | T: from 2.87 ± 0.31mm to 0.76 ± 0.65 mm C: from 2.76 ± 0.89mm to 0.53 ± 0.48 mm | T: NA C: NA | T: from 3.03 ± 0.72mm to 3.12 ± 0.69 mm C: from 2.65 ± 0.92 mm to 3.82 ± 1.3 mm | T: from 1.10 ± 0.20 mm to 1.65 ± 0.39 mm C: from 1.33 ± 0.54 mm to 2.26 ± 0.63 mm | The use of ADM may represent a valid alternative to SCTG when used in conjunction with MCAT |
| RCT [28] | Molnár et al. | 2022 | TUN for multiple recessions | MCAT + XDM | MCAT + CTG | 22/114 | 9 years | T: 1% C: 1% | T: 23.07 ± 44.5% C: 39.7 ± 35.17% | T: from 2.00 ± 0.9 mm to 2.97 ± 0.95 mm C: from 2.03 ± 0.65 mm to 3.28 ± 1.14 mm | T: from 0.83 ± 0.26 mm to 1.49 ± 0.32 mm C: from 0.86 ± 0.29 mm to 1.57 ± 0.35 mm | MCAT in conjunction with either CM or CTG for MAGR is likely to show a relapse over a period of 9 years |
| RCT [29] | McGuire et al. | 2022 | CAF for single recessions | CAF + VCMX | CAF + CTG | SPLIT MOUTH 30/60 | 12 | T: 63.2% C: 70.7% | T: NA C: NA | T: from 2.5 ± 1.25 mm to 3.3 ± 1.3 mm C: from 2.3 ± 0.88 mm to 3.6 ± 1.31 mm | T: from 158.37 ± 72.89 to 72.35 ± 38.40 mm2 C: from 189.40 ± 73.87 to 39.23 ± 30.92 mm2 | VCMX + CAF root coverage was inferior to CTG + CAF but produced less morbidity |
| SR [17] | Halim et al. | 2023 | CAF | 1. XDM 2. XDM 3. XDM 4. XDM 5. ADM | 1. CTG 2. CTG 3. CTG 4. CTG 5. CTG | 5 studies in total, 5 studies evaluated | >6 | T: 70.3 C: 83.3 T: 24.3 ± 8.2 C: 48.7± 6.8 T: 70.7 C: 87.7 T: 51.9 C: 46.8 T: NA C: NA | T: 91.79 ± 10.1 C: 89.19 ± 16.3 T: 80.6 ± 23.7 C: 68.8± 23.4 T: NA C: NA T: 87.6 ± 15.1 C: 85.25 ± 14.9 T: NA C: NA | T: 0.85 ± 0.25 C: 0.81 ± 0.23 T: 0.8 ± 0.3 C: 0.8 ± 0.2 T: NA C: NA T: 0.69 ± 0.26 C: 0.61 ± 0.2 T: NA C: NA | T: 2.42 ± 1.29 C: 2.43 ± 1.12 T: 2.2 ± 1.3 C: 2.1± 1.6 T: 3.7 ± 1.10 C: 3.40 ± 1.2 T: 2.43 ± 1.4 C: 2.44 ± 1.3 T: 2.05 ± 0.78 C:1.90 ± 0.54 | CTG is considered superior for gingival recession therapy. If it is contraindicated, the AADM and XDM might be considered as alternatives |
| Study Type | Authors | Year | Surgical Procedure | Test Group | Control Group | No. of Patients/ No. of Teeth or Implants | Follow-Up (Months) | CRC | mRC | KTW | STT | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT [18] | Frizzera et al. | 2018 | STA (Immediate implant placement and provisionalization) | STA + CMX | STA + CTG | 16/16 | 12 | T: NA C: NA | T: NA C: NA | T: NA C: NA | T: from 0.98 to 3.04 mm C: from 1 to 2.11 mm | CTG avoided marginal peri-implant recession and provided greater thickness of the soft tissue at the implant facial aspect |
| RCT [30] | Thoma et al. | 2020 | STA before implant placement | STA + VCMX | STA + CTG | 20/20 | 3 years | T: NA C: NA | T: NA C: NA | T: NA C: NA | T: from 3.0 to 3.5 mm C: from 3.0 to 3.3 mm | Both VCMX and CTGdemonstrated negligible differences, stable buccal tissue contour, esthetics, and STT slightly increased |
| SR [22] | Moraschini et al. | 2022 | CAF | 1. CAF + CMX 2. CAF + CMX 3. CAF + CMX 4. CAF + CMX 5. CAF + CMX | 1. CAF + CTG 2. CAF + CTG 3. CAF + CTG 4. CAF + CTG 5. CAF + CTG | 11 studies in total, 5 studies evaluated | >6 | T: NA C: NA | T: NA C: NA | 1. XCM: 2.1 ± 1.2 mm CTG: 3.2 ± 0.8 mm 2. T: NA C: NA 3. XCM:1.7 ± 1.3 mm CTG:4.0 ± 1.1 mm 4. T: NA C: NA 5. XCM:6.51 ± 1.98 mm FGG: 7.76 ± 1.99 mm | 1. XCM: 2.8 ± 0.7 mm CTG:3.1 ± 1.3 mm 2. XCM: 2.5 ± 1.3 mm CTG: 3.28 ± 1.7 mm 3. T: NA C: NA 4. XCM: 1.66 ± 0.01 mm CTG: 2.86 ± 0.01 mm 5. T: NA C: NA | CTG demonstrated best treatment ranking of probability results than CMX |
| RCT [20] | Lee et al. | 2023 | STA in conjunction with implant placement | STA + ADM | STA + CTG | 30/30 | 12 | ADM showed soft tissue margin 3–5 mm below the initial level | T: NA C: NA | Changes between the groups were not significantly different | T: from 1.34 ± 0.25 mm to 2.57 ± 0.3 mm C: from 1.24 ± 0.25 mm to 2.38 ± 0.32 mm | STA enhanced soft tissue thickness and maintained soft tissue contours but did not prevent peri-implant mucosal recession |
| RCT [31] | Thoma et al. | 2023 | STA after implant placement | STA + VCMX | STA + CTG | 20/20 | 5 years | T: NA C: NA | T: NA C: NA | T: NA C: NA | T: from 3.0 to 3.0 mm C: from 3.0 to 3.3 mm | Both groups resulted in stable peri-implant tissues, favorable esthetics, and clinically negligible contour changes |
5. Discussion
- 1.
- Allogeneic dermal matrix (ADM)
- 2.
- Xenogeneic acellular dermal matrix (XDM)
- 3.
- Bilayered collagen matrix (CMX)
- 4.
- Volume-stable collagen matrix (VCMX)
- 1.
- Allogeneic dermal matrix (ADM)
- 2.
- Xenogeneic acellular dermal matrix (XDM)
- 3.
- Bilayered collagen matrix (CMX)
- 4.
- Volume-stable collagen matrix (VCMX)
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Björn, H. Coverage of denuded root surfaces with a lateral sliding flap. Use of free gingival grafts. Odontol. Revy. 1971, 22, 37–44. [Google Scholar]
- Sullivan, H.C.; Atkins, J.H. Free autogenous gingival grafts. I. Principles of successful grafting. Periodontics 1968, 6, 121–129. [Google Scholar]
- Miller, P.D., Jr. Root coverage using the free soft tissue autograft following citric acid application. III. A successful and predictable procedure in areas of deep-wide recession. Int. J. Periodontics Restor. Dent. 1985, 5, 14–37. [Google Scholar]
- Chambrone, L.; Salinas Ortega, M.A.; Sukekava, F.; Rotundo, R.; Kalemaj, Z.; Buti, J.; Pini Prato, G.P. Root coverage procedures for treating localised and multiple recession-type defects. Cochrane Database Syst. Rev. 2018, 10, CD007161. [Google Scholar] [CrossRef]
- Stefanini, M.; Barootchi, S.; Sangiorgi, M.; Pispero, A.; Grusovin, M.G.; Mancini, L.; Zucchelli, G.; Tavelli, L. Do soft tissue augmentation techniques provide stable and favorable peri-implant conditions in the medium and long term? A systematic review. Clin. Oral. Implants Res. 2023, 34 (Suppl. S26), 28–42. [Google Scholar] [CrossRef]
- Montero, E.; Molina, A.; Matesanz, P.; Monje, A.; Sanz-Sánchez, I.; Herrera, D. Efficacy of soft tissue substitutes, in comparison with autogenous grafts, in surgical procedures aiming to increase the peri-implant keratinized mucosa: A systematic review. Clin. Oral. Implants Res. 2022, 33, 32–46. [Google Scholar] [CrossRef]
- Barber, F.A.; Aziz-Jacobo, J. Biomechanical Testing of Commercially Available Soft-Tissue Augmentation Materials. Arthroscopy. J. Arthrosc. Relat. Surg. 2009, 25, 1233–1239. [Google Scholar] [CrossRef]
- Rotundo, R.; Pini-Prato, G. Use of a new collagen matrix (mucograft) for the treatment of multiple gingival recessions: Case reports. Int. J. Periodontics Restor. Dent. 2012, 32, 413–419. [Google Scholar]
- Griffin, T.J.; Cheung, W.S.; Zavras, A.I.; Damoulis, P.D. Postoperative complications following gingival augmentation pro-cedures. J. Periodontol. 2006, 77, 2070–2079. [Google Scholar] [CrossRef]
- Buff, L.R.; Burklin, T.; Eickholz, P.; Monting, J.S.; Ratka-Kruger, P. Does harvesting connective tissue grafts from the palate cause persistent sensory dysfunction? A pilot study. Quintessence Int. 2009, 40, 479–489. [Google Scholar]
- Tavelli, L.; Barootchi, S.; Cairo, F.; Rasperini, G.; Shedden, K.; Wang, H.L. The Effect of Time on Root Coverage Outcomes: A Network Meta-analysis. J. Dent. Res. 2019, 98, 1195–1203. [Google Scholar] [CrossRef]
- Lu, W.; Qi, G.; Ding, Z.; Li, X.; Qi, W.; He, F. Clinical efficacy of acellular dermal matrix for plastic periodontal and implant surgery: A systematic review. Int. J. Oral Maxillofac. Surg. 2020, 49, 1057–1066. [Google Scholar] [CrossRef]
- Kesting, M.R.; Wolff, K.D.; Nobis, C.P.; Rohleder, N.H. Amniotic membrane in oral and maxillofacial surgery. Oral Maxillofac. Surg. 2014, 18, 153–164. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Maia, L.C.; Antonio, A.G. Systematic reviews in dental research. a guideline. J. Clin. Pediatr. Dent. 2012, 37, 117–124. [Google Scholar] [CrossRef]
- De Carvalho Formiga, M.; Nagasawa, M.A.; Moraschini, V.; Ata-Ali, J.; Sculean, A.; Shibli, J.A. Clinical efficacy of xenogeneic and allogeneic 3D matrix in the management of gingival recession: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 2229–2245. [Google Scholar] [CrossRef]
- Halim, F.C.; Sulijaya, B. Allogenic Acellular Dermal Matrix and Xenogeneic Dermal Matrix as Connective Tissue Graft Substitutes for Long-Term Stability Gingival Recession Therapy: A Systematic Review and Meta-Analysis. Eur. J. Dent. 2023. [Google Scholar] [CrossRef]
- Frizzera, F.; de Freitas, R.M.; Muñoz-Chávez, O.F.; Cabral, G.; Shibli, J.A.; Marcantonio, E., Jr. Impact of Soft Tissue Grafts to Reduce Peri-implant Alterations after Immediate Implant Placement and Provisionalization in Compromised Sockets. Int. J. Periodontics Restor. Dent. 2019, 39, 381–389. [Google Scholar] [CrossRef]
- Zuiderveld, E.G.; Meijer, H.J.A.; Vissink, A.; Raghoebar, G.M. The influence of different soft-tissue grafting procedures at single implant placement on esthetics: A randomized controlled trial. J. Periodontol. 2018, 89, 903–914. [Google Scholar] [CrossRef]
- Lee, C.; Tran, D.; Tsukiboshi, Y.; Min, S.; Kim, S.K.; Ayilavarapu, S.; Weltman, R. Clinical efficacy of soft-tissue augmentation on tissue preservation at immediate implant sites: A randomized controlled trial. J. Clin. Periodontol. 2023, 50, 1010–1020. [Google Scholar] [CrossRef]
- Moraschini, V.; Calasans-Maia, M.D.; Dias, A.T.; de Carvalho Formiga, M.; Sartoretto, S.C.; Sculean, A.; Shibli, J.A. Effectiveness of connective tissue graft substitutes for the treatment of gingival recessions compared with coronally advanced flap: A network meta-analysis. Clin. Oral Investig. 2020, 24, 3395–3406. [Google Scholar] [CrossRef]
- Moraschini, V.; Kischinhevsky, I.C.C.; Sartoretto, S.C.; Shibli, J.A.; Dias, A.T.; Sacco, R.; Yates, J.; Calasans-Maia, M.D. Is there any biomaterial substitute for peri-implant soft tissue phenotype modification? A network meta-analysis of the appraisal literature. Int. J. Oral Maxillofac. Surg. 2022, 51, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.; Stefanini, M.; Sanz, M.; Zucchelli, G.; Jepsen, S. Long-Term Stability of Root Coverage by Coronally Advanced Flap Procedures. J. Periodontol. 2017, 88, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Aroca, S.; Molnár, B.; Windisch, P.; Gera, I.; Salvi, G.E.; Nikolidakis, D.; Sculean, A. Treatment of multiple adjacent Miller class I and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. J. Clin. Periodontol. 2013, 40, 713–720. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Scheyer, E.T.; Lipton, D.I.; Gunsolley, J.C. Randomized, controlled, clinical trial to evaluate a xenogeneic collagen matrix as an alternative to free gingival grafting for oral soft tissue augmentation: A 6- to 8-year follow-up. J Periodontol. 2021, 92, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Cortellini, P.; Bonaccini, D.; Deng, K.; Cairo, F.; Allegri, M.; Conforti, G.; Graziani, F.; Guerrero, A.; Halben, J.; et al. Autologous connective tissue graft or xenogenic collagen matrix with coronally advanced flaps for coverage of multiple adjacent gingival recession. 36-month follow-up of a randomized multicentre trial. J. Clin. Periodontol. 2021, 48, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Elmahdi, F.; Reda, A.; Hosny, M. Evaluation of Subepithelial Connective Tissue Graft Versus Acellular Dermal Matrix with Modified Coronally Advanced Tunnel Technique in Treatment of Multiple Gingival Recessions: A Randomized, Parallel-Design Clinical Trial. Int. J. Periodontics Restor. Dent. 2022, 42, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Molnár, B.; Aroca, S.; Dobos, A.; Orbán, K.; Szabó, J.; Windisch, P.; Stähli, A.; Sculean, A. Treatment of multiple adjacent RT 1 gingival recessions with the modified coronally advanced tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: 9-year results of a split-mouth randomized clinical trial. Clin. Oral Investig. 2022, 26, 7135–7142. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; Janakievski, J.; Scheyer, E.T.; Velásquez, D.; Gunsolley, J.C.; Heard, R.H.; Morelli, T. Efficacy of a harvest graft substitute for recession coverage and soft tissue volume augmentation: A randomized controlled trial. J. Periodontol. 2022, 93, 333–342. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gasser, T.J.W.; Jung, R.E.; Hämmerle, C.H.F. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J. Clin. Periodontol. 2020, 47, 630–639. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gasser, T.J.W.; Hämmerle, C.H.F.; Strauss, F.J.; Jung, R.E. Soft tissue augmentation with a volume-stable collagen matrix or an autogenous connective tissue graft at implant sites: Five-year results of a randomized controlled trial post implant loading. J. Periodontol. 2023, 94, 230–243. [Google Scholar] [CrossRef]
- Woodyard, J.G.; Greenwell, H.; Hill, M.; Drisko, C.; Iasella, J.M.; Scheetz, J. The clinical effect of acellular dermal matrix on gingival thickness and root coverage compared to coronally positioned flap alone. J. Periodontol. 2004, 75, 44–56. [Google Scholar] [CrossRef]
- de Queiroz Côrtes, A.; Sallum, A.W.; Casati, M.Z.; Nociti, F.H., Jr.; Sallum, E.A. A two-year prospective study of coronally positioned flap with or without acellular dermal matrix graft. J. Clin. Periodontol. 2006, 33, 683–689. [Google Scholar] [CrossRef]
- Ahmedbeyli, C.; Ipçi, Ş.D.; Cakar, G.; Kuru, B.E.; Yılmaz, S. Clinical evaluation of coronally advanced flap with or without acellular dermal matrix graft on complete defect coverage for the treatment of multiple gingival recessions with thin tissue biotype. J. Clin. Periodontol. 2014, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Khijmatgar, S.; Arbildo-Vega, H.; Das, A.C.; Kumar, M.; Das, M.; Mancini, L.; Del Fabbro, M. Stability of biomaterials used in adjunct to coronally advanced flap: A systematic review and network meta-analysis. Clin. Exp. Dent. Res. 2022, 8, 421–438. [Google Scholar] [CrossRef]
- Santamaria, M.P.; Rossato, A.; Miguel, M.M.V.; Fonseca, M.B.; Bautista, C.R.G.; de Marco, A.C.; Mathias-Santamaria, I.F.; Ferreira Ferraz, L.F. Comparison of two types of xenogeneic matrices to treat single gingival recessions: A randomized clinical trial. J. Periodontol. 2022, 93, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Meza-Mauricio, J.; Cortez-Gianezzi, J.; Duarte, P.M.; Tavelli, L.; Rasperini, G.; De Faveri, M. Comparison between a xenogeneic dermal matrix and connective tissue graft for the treatment of multiple adjacent gingival recessions: A randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 6919–6929. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Bugnas, S.; Laurent, J.; Naman, E.; Charbit, M.; Borie, G. Treatment of multiple gingival recessions with xenogeneic acellular dermal matrix compared to connective tissue graft: A randomized split-mouth clinical trial. J. Periodontal Implants Sci. 2021, 51, 77. [Google Scholar] [CrossRef] [PubMed]
- Gürlek, Ö.; Gümüş, P.; Nizam, N.; Buduneli, N. Coronally advanced flap with connective tissue graft or xenogeneic acellular dermal matrix in the treatment of multiple gingival recessions: A split-mouth randomized clinical trial. J. Esthet. Restor. Dent. 2020, 32, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Rakasevic, D.L.; Milinkovic, I.Z.; Jankovic, S.M.; Soldatovic, I.A.; Aleksic, Z.M.; Nikolic-Jakoba, N.S. The use of collagen porcine dermal matrix and connective tissue graft with modified coronally advanced tunnel technique in the treatment of multiple adjacent type I gingival recessions: A randomized, controlled clinical trial. J. Esthet. Restor. Dent. 2020, 32, 681–690. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotundo, R.; Pancrazi, G.L.; Grassi, A.; Ceresoli, L.; Di Domenico, G.L.; Bonafede, V. Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review. Materials 2024, 17, 1221. https://doi.org/10.3390/ma17051221
Rotundo R, Pancrazi GL, Grassi A, Ceresoli L, Di Domenico GL, Bonafede V. Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review. Materials. 2024; 17(5):1221. https://doi.org/10.3390/ma17051221
Chicago/Turabian StyleRotundo, Roberto, Gian Luca Pancrazi, Alessia Grassi, Lara Ceresoli, Giovanna Laura Di Domenico, and Vanessa Bonafede. 2024. "Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review" Materials 17, no. 5: 1221. https://doi.org/10.3390/ma17051221
APA StyleRotundo, R., Pancrazi, G. L., Grassi, A., Ceresoli, L., Di Domenico, G. L., & Bonafede, V. (2024). Soft Tissue Substitutes in Periodontal and Peri-Implant Soft Tissue Augmentation: A Systematic Review. Materials, 17(5), 1221. https://doi.org/10.3390/ma17051221






