Amino-Modified Graphene Oxide from Kish Graphite for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings
Abstract
1. Introduction
2. Experimental Section
2.1. Raw Materials
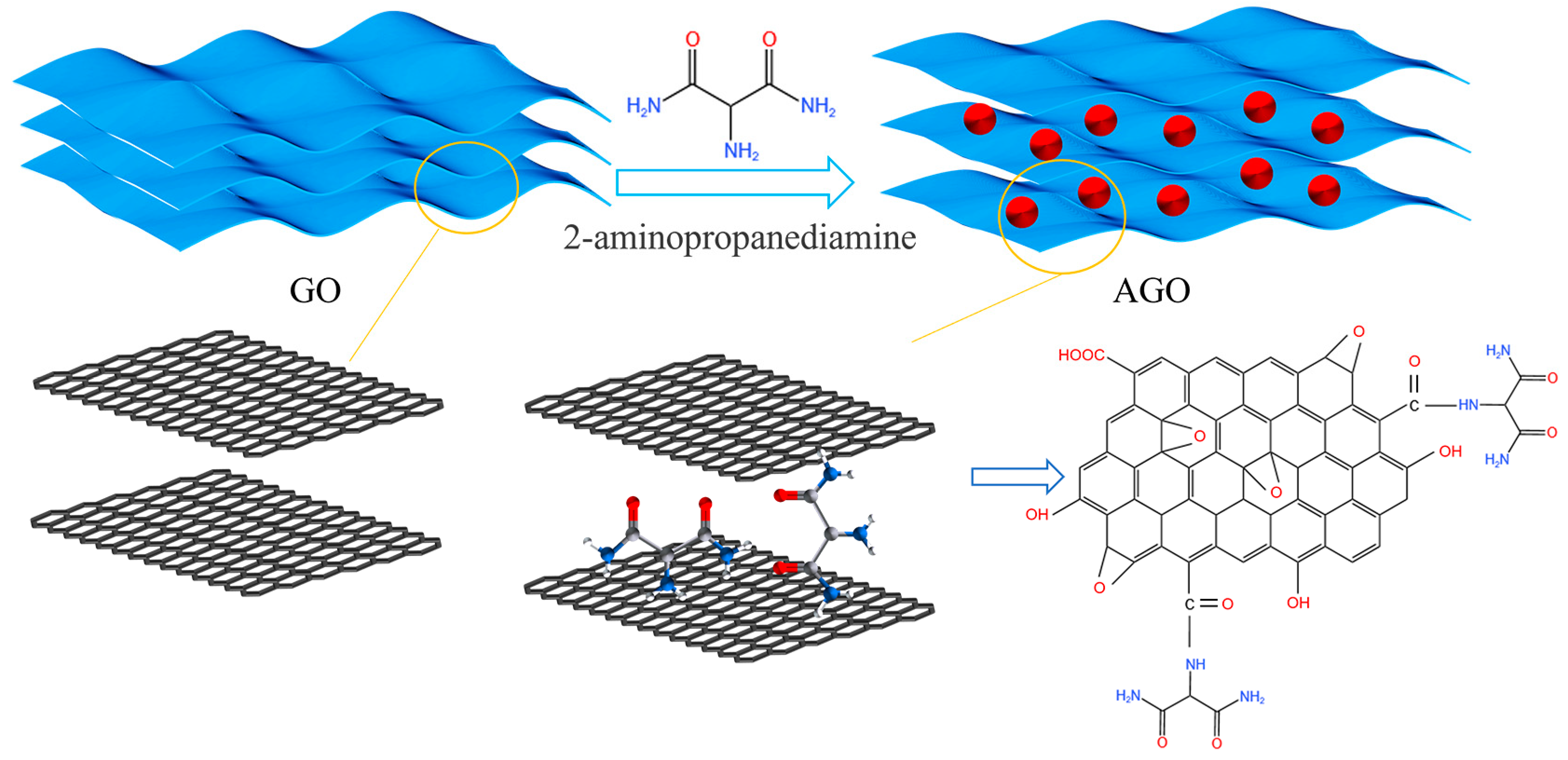
2.2. Synthesis of GO and AGO
2.3. Preparation of GO/WEP and AGO/WEP Composite Coatings
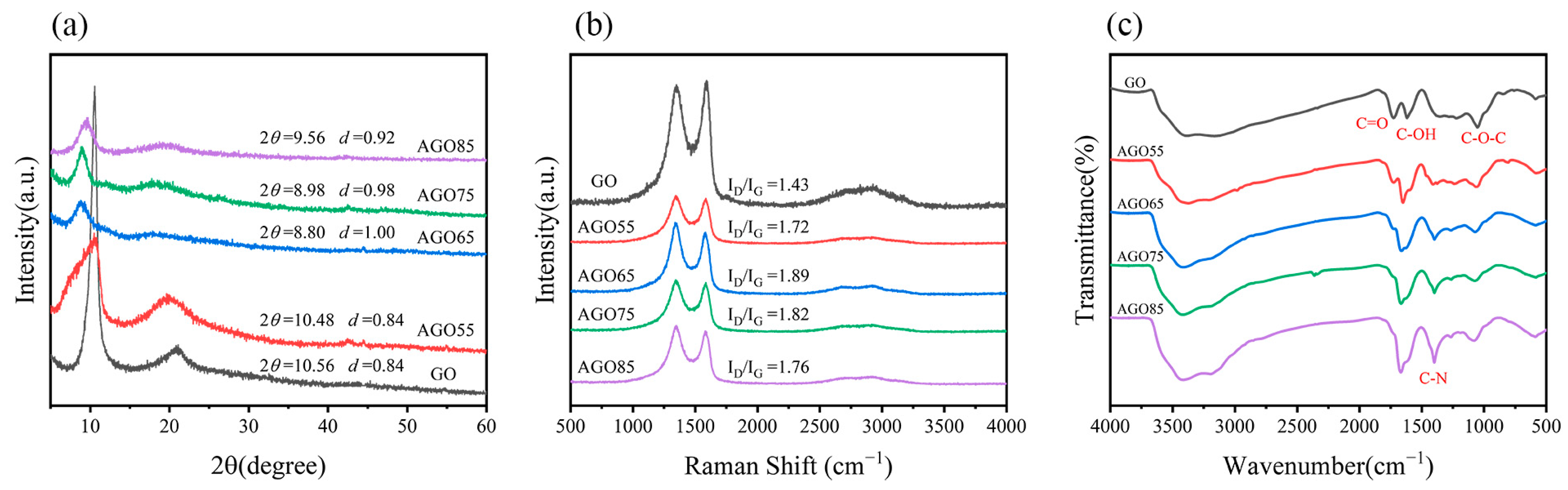
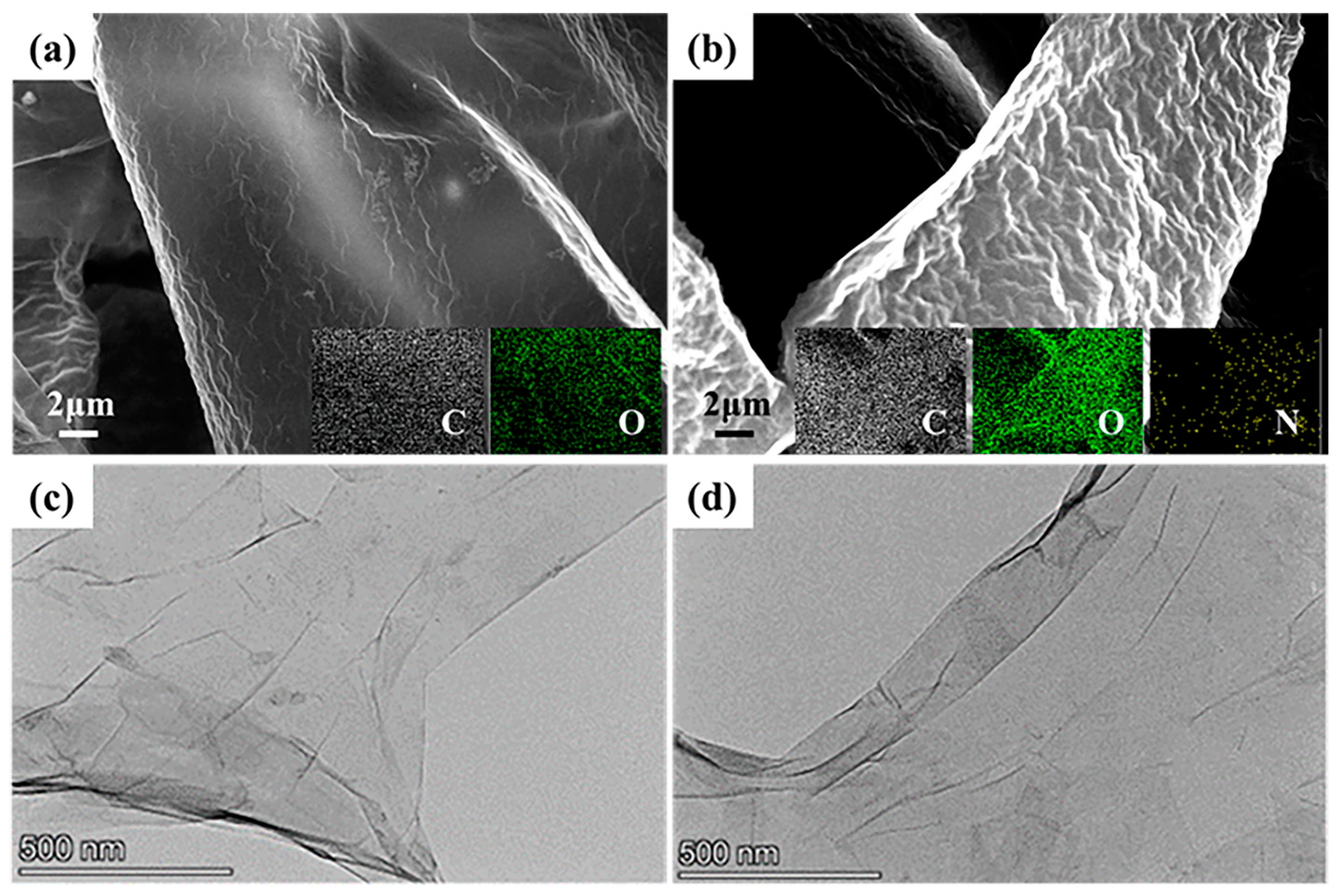
2.4. Characterizations
2.5. Electrochemical and Salt Spray Tests
3. Results and Discussion
3.1. Characterization of AGO and AGO/WEP Composite Coatings
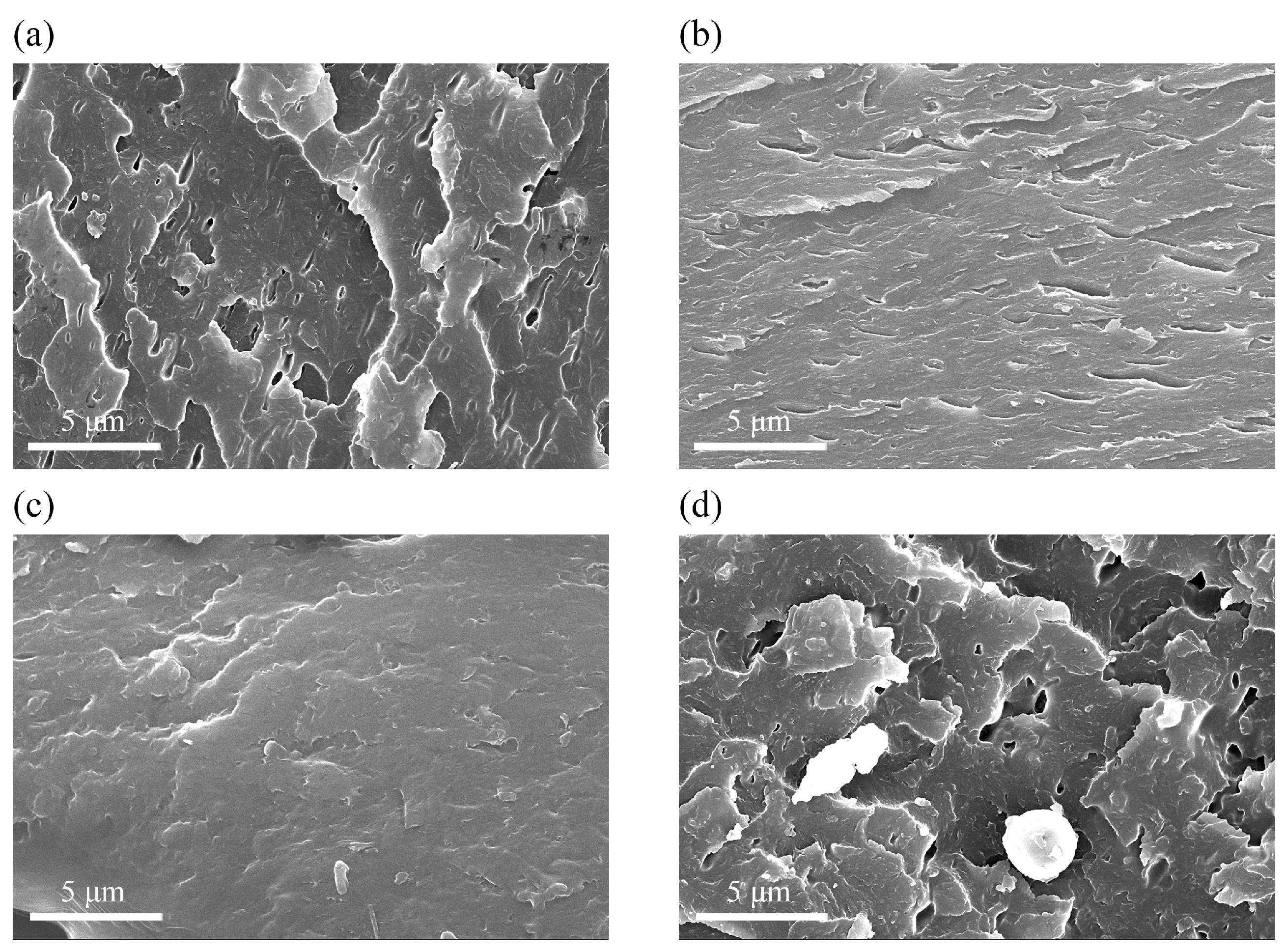
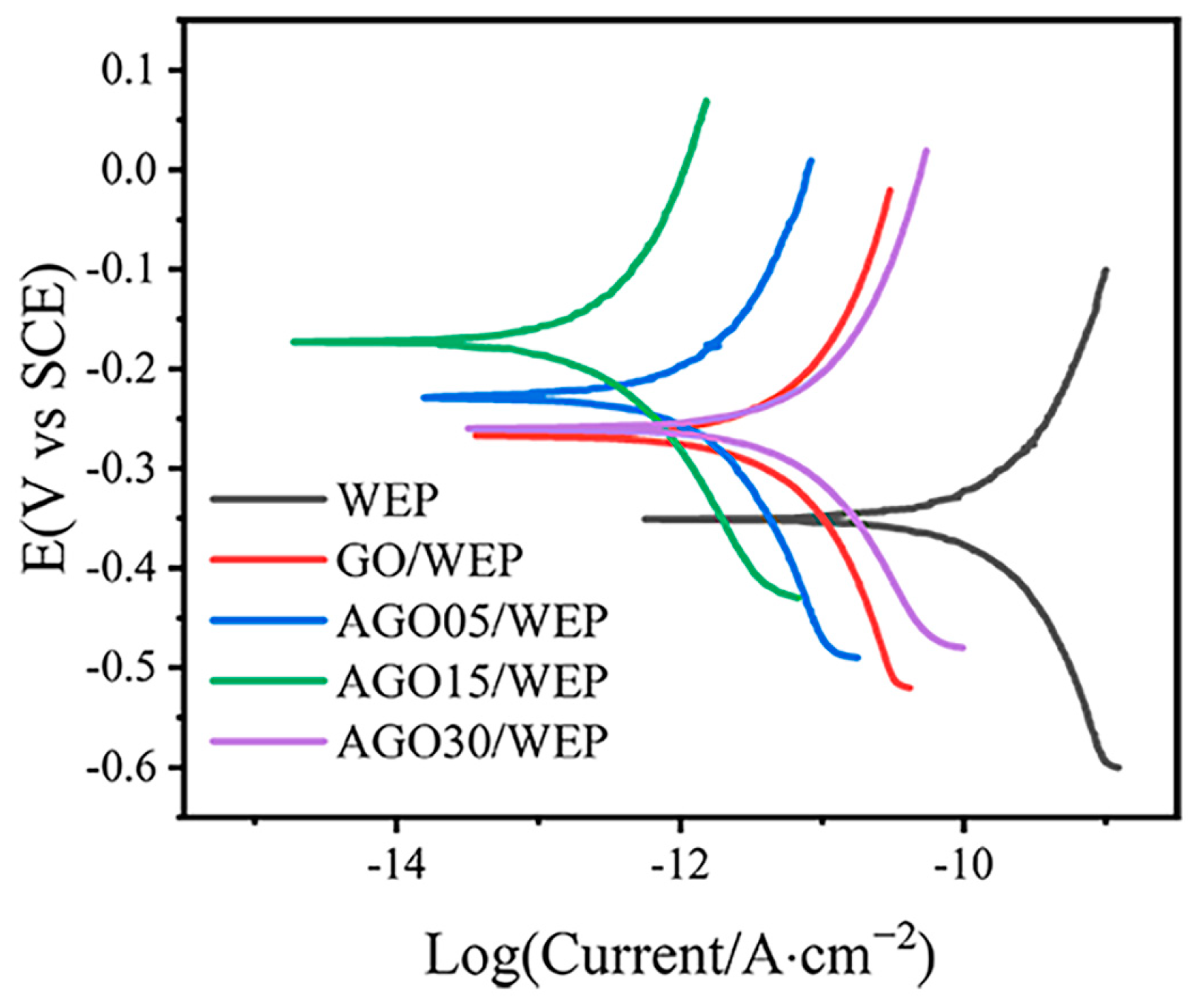
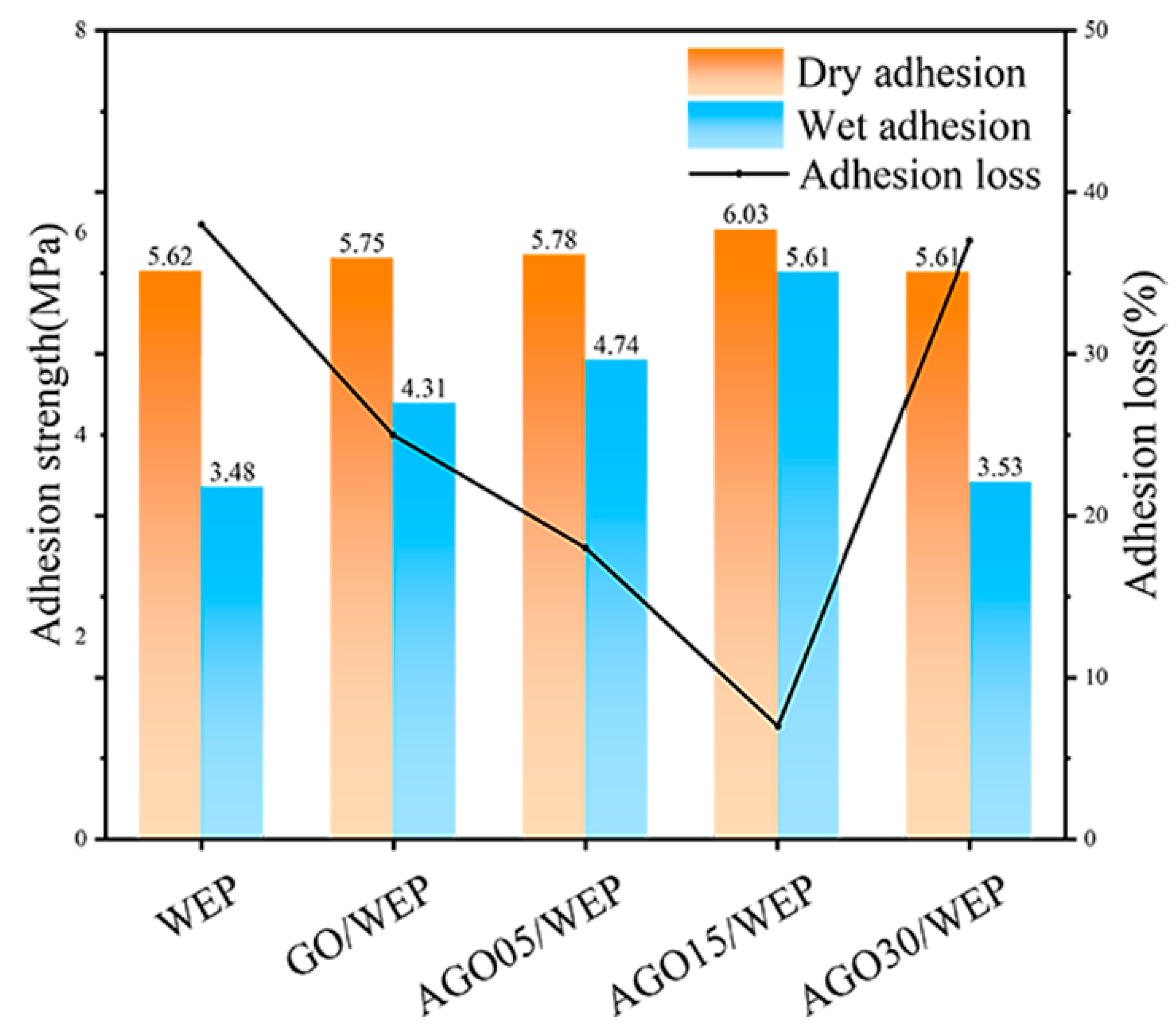
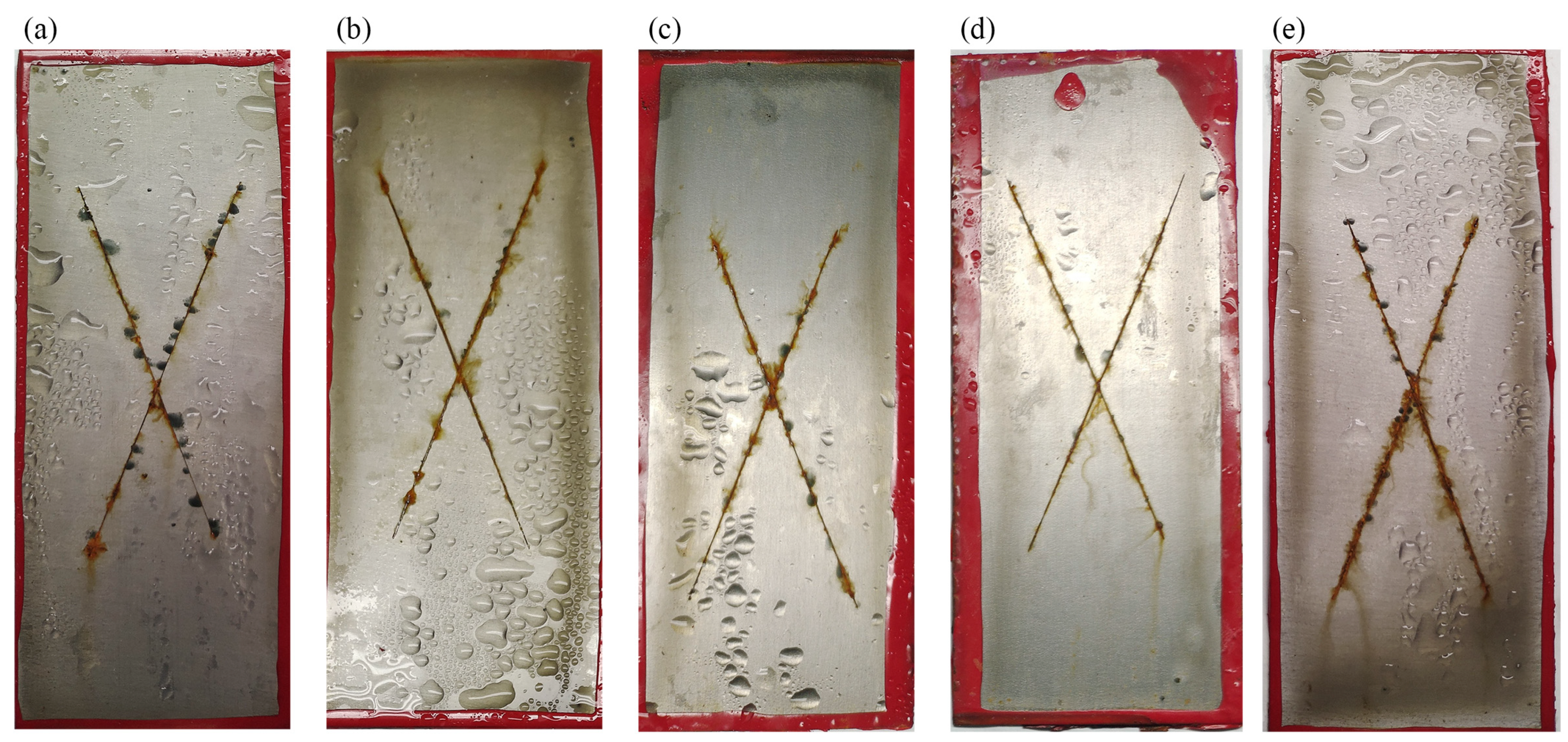
3.2. Anti-Corrosive Performance of AGO/WEP Composite Coatings
3.3. Corrosion Protective Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A. Current Trends on Mechanical, Corrosion Resistance, and Antibacterial Properties of Metallic Materials. Materials 2022, 15, 3822. [Google Scholar] [CrossRef]
- Wang, R.; Yi, H. Mechanical-induced functionalization of graphene with sodium carboxymethyl cellulose toward enhancing anticorrosion performance of waterborne epoxy coatings. J. Coat. Technol. Res. 2023, 20, 2069–2080. [Google Scholar] [CrossRef]
- Li, J.; Tao, Z.; Cui, J.; Shen, S.; Qiu, H. Facile Fabrication of Dual Functional Graphene Oxide Microcapsules Carrying Corrosion Inhibitor and Encapsulating Self-Healing Agent. Polymers 2022, 14, 4067. [Google Scholar] [CrossRef]
- Wegmann, A. Chemical resistance of waterborne epoxy/amine coatings. Prog. Org. Coat. 1997, 32, 231–239. [Google Scholar] [CrossRef]
- Huang, H.; Sheng, X.; Tian, Y.; Zhang, L.; Chen, Y.; Zhang, X. Two-Dimensional Nanomaterials for Anticorrosive Polymeric Coatings: A Review. Ind. Eng. Chem. Res. 2020, 59, 15424–15446. [Google Scholar] [CrossRef]
- Zhao, Y.; He, Y.; Yan, S.; Li, C.; Li, H.; Chen, W.; Yan, J.; Wu, G.; Yuan, X. Eco-friendly design of α-zirconium phosphate modified by phytic acid for reinforcing the corrosion resistance of waterborne epoxy coating. Colloids Surf. Physicochem. Eng. Asp. 2023, 656, 130472. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Lin, Y.; Wu, Y.; Chen, J. Self-assembled graphene oxide-graphene hybrids for enhancing the corrosion resistance of waterborne epoxy coating. Appl. Surf. Sci. 2019, 488, 801–812. [Google Scholar] [CrossRef]
- Irfan, M.; Iqbal, S.; Ahmad, S. Waterborne reduced graphene oxide dispersed sebacic acid modified soy epoxy nanocomposite: A green and sustainable approach for high performance mechanically robust anticorrosive coatings. Prog. Org. Coat. 2022, 170, 106984. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, P.; Shen, L.; Chu, L.; Wu, J.; Ding, Y.; Zhong, B.; Zhang, X.; Bao, N. Modified graphene oxide/waterborne epoxy composite coating with enhanced corrosion resistance. Prog. Org. Coat. 2022, 172, 107100. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Li, S.; Chen, Y.; Zhang, L.; Sheng, X. An eco-friendly and pH response cerium-based melamine phytate (PM) nanosheets with active and passive anticorrosion ability in water-borne epoxy coating. Appl. Surf. Sci. 2022, 597, 153726. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, W.; Wu, Y.; Dong, J.; Zhou, K.; Lu, G.; Pu, J. Anti-corrosion behaviors of epoxy composite coatings enhanced via graphene oxide with different aspect ratios. Prog. Org. Coat. 2019, 127, 70–79. [Google Scholar] [CrossRef]
- Nan, D.; Li, X.; Li, D.; Liu, Q.; Wang, B.; Gao, X.; Ma, T.; He, N.; Xu, Y.; Dong, J. Preparation and Anticorrosive Performance of Waterborne Epoxy Resin Composite Coating with Amino-Modified Graphene Oxide. Polymers 2022, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, S.; Xing, B.; Ma, M.; Shi, C.; Cheng, G.; Meng, W.; Zhang, C. High efficiency purification of natural flake graphite by flotation combined with alkali-melting acid leaching: Application in energy storage. J. Mater. Res. Technol. 2022, 21, 4212–4223. [Google Scholar] [CrossRef]
- Singh, S.B.; Haskin, N.; Dastgheib, S.A. Coal-based graphene oxide-like materials: A comprehensive review. Carbon 2023, 215, 118447. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Amiri, A.; Fazaeli, Y.; Zare, H.; Eslami-Kalantari, M.; Feizi, S.; Shahedi, Z.; Afrasyabi, M. Radiolabeled florescent-magnetic graphene oxide nanosheets: Probing the biodistribution of a potential PET-MRI hybrid imaging agent for detection of fibrosarcoma tumor. Ann. Nucl. Med. 2024, 14, 15. [Google Scholar] [CrossRef]
- Wu, W.; Ge, H. Preparation of amino-silane and thiosemicarbazide modified graphene oxide composite for high uptake adsorption of Hg(II) and Pb(II). J. Dispers. Sci. Technol. 2024, 1–12. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Keshipour, S.; Ahour, F. 2-Aminopyridine-Reduced Graphene Oxide Modified with Platinum Nanoparticles as a Photocatalyst of Water Splitting. Iran. J. Sci. 2023, 48, 77–86. [Google Scholar] [CrossRef]
- Liu, S.; Loper, C.R., Jr. Kish, a source of crystalline graphite. Carbon 1991, 29, 1119–1124. [Google Scholar] [CrossRef]
- Kazmi, K.R.; Anwar, M.S.; Bhatti, M.A.; Mehmood, A. Recovery of flake graphite from Kish. Pak. J. Sci. Ind. Res. 2007, 50, 284–287. [Google Scholar]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- An, J.-C.; Kim, H.J.; Hong, I. Preparation of Kish graphite-based graphene nanoplatelets by GIC (graphite intercalation compound) via process. J. Ind. Eng. Chem. 2015, 26, 55–60. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Ma, L.; Wei, L.; Cao, L.; Shen, W.; Kang, F.; Huang, Z.-H. Combining Multiple Methods for Recycling of Kish Graphite from Steelmaking Slags and Oil Sorption Performance of Kish-Based Expanded Graphite. ACS Omega 2021, 6, 9868–9875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bao, X.; Zhang, D.; Sun, Z.; Lu, X.; Hong, R. Application of purified kish flake graphite as a potential cathode material for high-performance aluminum ion batteries. J. Alloys Compd. 2023, 954, 170197. [Google Scholar] [CrossRef]
- Guo, H.; Chao, B.; Zhao, Z.; Nan, D. Preparation of aniline trimer modified graphene oxide new composite coating and study on anticorrosion performance. Mater. Res. Express 2020, 7, 125601. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% NaCl Solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef]
- ASTM B117; Standard Practice for Operating Salt Spray (Fog) Apparatus. ASTM–American Society for Testing and Materials: West Conshohocken, PA, USA, 2009.
- He, X.-X.; Zhao, J.-H.; Lai, W.-H.; Li, R.; Yang, Z.; Xu, C.-m.; Dai, Y.; Gao, Y.; Liu, X.-H.; Li, L.; et al. Soft-Carbon-Coated, Free-Standing, Low-Defect, Hard-Carbon Anode to Achieve a 94% Initial Coulombic Efficiency for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 44358–44368. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Sainsbury, T.; Liaghat, G.H.; Vahid, S. Improving the fracture toughness and the strength of epoxy using nanomaterials—A review of the current status. Nanoscale 2015, 7, 10294–10329. [Google Scholar] [CrossRef]
- Conradi, M.; Kocijan, A.; Kek-Merl, D.; Zorko, M.; Verpoest, I. Mechanical and anticorrosion properties of nanosilica-filled epoxy-resin composite coatings. Appl. Surf. Sci. 2014, 292, 432–437. [Google Scholar] [CrossRef]
- Hinderliter, B.R.; Croll, S.G.; Tallman, D.E.; Su, Q.; Bierwagen, G.P. Interpretation of EIS data from accelerated exposure of coated metals based on modeling of coating physical properties. Electrochim. Acta 2006, 51, 4505–4515. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Cui, J.; Qiu, H.; Yang, G.; Zheng, S.; Yang, J. Dispersion and parallel assembly of sulfonated graphene in waterborne epoxy anticorrosion coatings. J. Mater. Chem. A 2019, 7, 17937–17946. [Google Scholar] [CrossRef]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]


| GO | Modifier | Preparation Method | Application |
|---|---|---|---|
| Angxing Technology Co., Ltd. (Changzhou, China) | Polydopamine | 24 h at room temperature | Anti-corrosion coatings [16] |
| Modified hummers | Cysteine | 2 h at room temperature | Aiagnostic agent in nuclear medicine [17] |
| Modified hummers | (3-aminopropyl) triethoxysilane (APTES) | 24 h at 30 °C | Adsorbent for removal of Hg2+ and Pb2+ from wastewaters [18] |
| Modified hummers | 2-Aminopyridine | 24 h at 90 °C with carboxylic acid activators (DCC/DMAP) | Photocatalyst of Water Splitting [19] |
| Element | Fe | Mn | Si | P | S | C |
|---|---|---|---|---|---|---|
| wt.% | Balance | 0.47 | 0.28 | 0.033 | 0.047 | 0.17 |
| Proximate Analysis | XRF Analysis (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FC (wt.%) | PS (μm) | SiO2 | Fe2O3 | CaO | Al2O3 | Cr2O3 | ZnO | TiO2 | MnO | Others | Total |
| 98.47 | 50–75 | 56.97 | 19.89 | 4.88 | 3.52 | 3.07 | 2.90 | 2.81 | 2.26 | 3.70 | 100 |
| Sample | icorr (A·cm−2) | vcorr (mm·Year−1) | Rp (Ohm·cm−2) |
|---|---|---|---|
| WEP | 1.53 × 10−10 | 1.79 × 10−6 | 2.94 × 109 |
| GO/WEP | 5.29 × 10−12 | 6.17 × 10−8 | 8.35 × 1010 |
| AGO05/WEP | 1.39 × 10−12 | 1.64 × 10−8 | 8.60 × 1011 |
| AGO15/WEP | 3.11 × 10−13 | 3.63 × 10−9 | 1.29 × 1012 |
| AGO30/WEP | 7.32 × 10−12 | 8.56 × 10−8 | 1.47 × 1011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, S.; Wan, S.; Hou, S.; Yuan, B.; Luan, C.; Nan, D.; Huang, G.; Xu, D.; Huang, Z.-H. Amino-Modified Graphene Oxide from Kish Graphite for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings. Materials 2024, 17, 1220. https://doi.org/10.3390/ma17051220
Hao S, Wan S, Hou S, Yuan B, Luan C, Nan D, Huang G, Xu D, Huang Z-H. Amino-Modified Graphene Oxide from Kish Graphite for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings. Materials. 2024; 17(5):1220. https://doi.org/10.3390/ma17051220
Chicago/Turabian StyleHao, Shengle, Siming Wan, Shiyu Hou, Bowen Yuan, Chenhui Luan, Ding Nan, Gen Huang, Deping Xu, and Zheng-Hong Huang. 2024. "Amino-Modified Graphene Oxide from Kish Graphite for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings" Materials 17, no. 5: 1220. https://doi.org/10.3390/ma17051220
APA StyleHao, S., Wan, S., Hou, S., Yuan, B., Luan, C., Nan, D., Huang, G., Xu, D., & Huang, Z.-H. (2024). Amino-Modified Graphene Oxide from Kish Graphite for Enhancing Corrosion Resistance of Waterborne Epoxy Coatings. Materials, 17(5), 1220. https://doi.org/10.3390/ma17051220







