Crevice Corrosion Behavior of 201 Stainless Steel in NaCl Solutions with Different pH Values by In Situ Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Solution Preparation
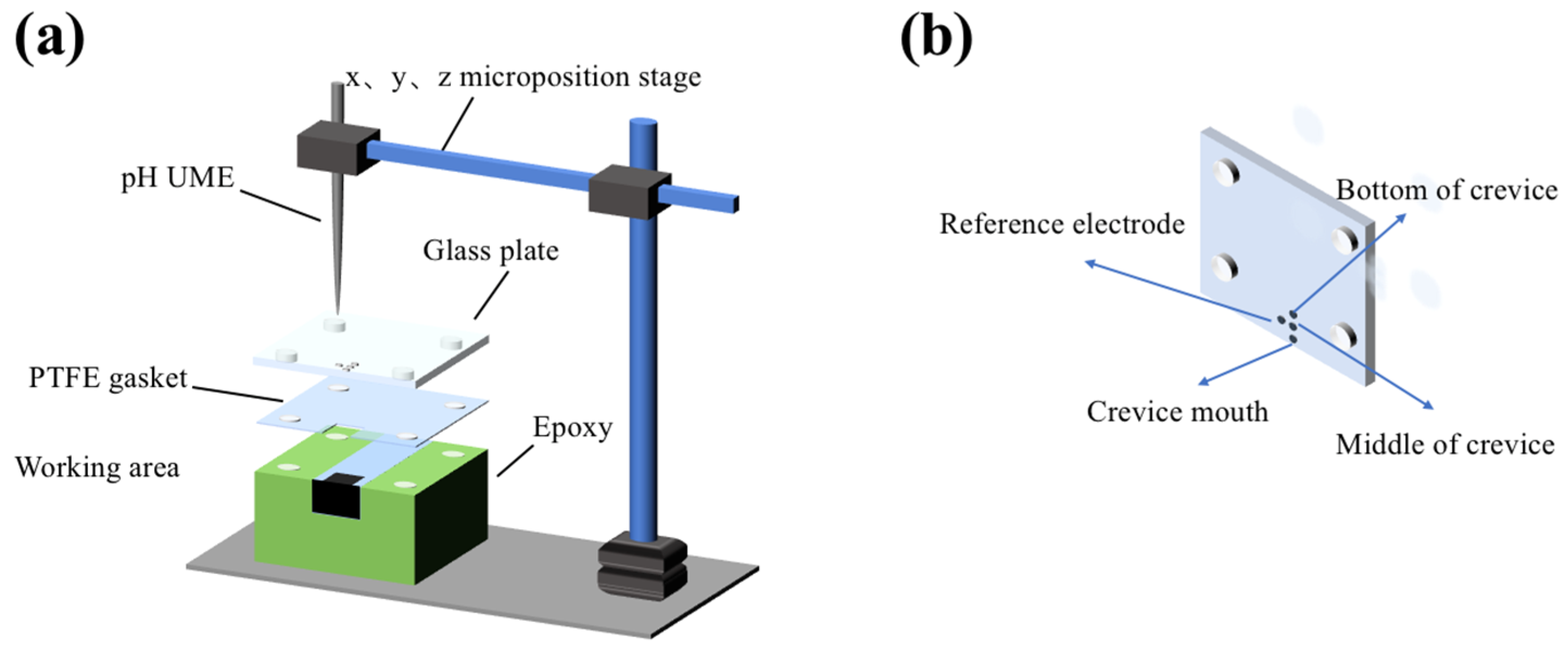
2.2. CC Test
2.3. Corrosion Morphology Observation
3. Results
3.1. Effect of Bulk Solution pH Value on OCP
3.2. Effect of Bulk Solution pH Value on Corrosion Potentials
3.3. Effect of Bulk Solution pH Value on the Radial pH Distribution Variation Inside
3.4. In Situ CC Morphologies Observation
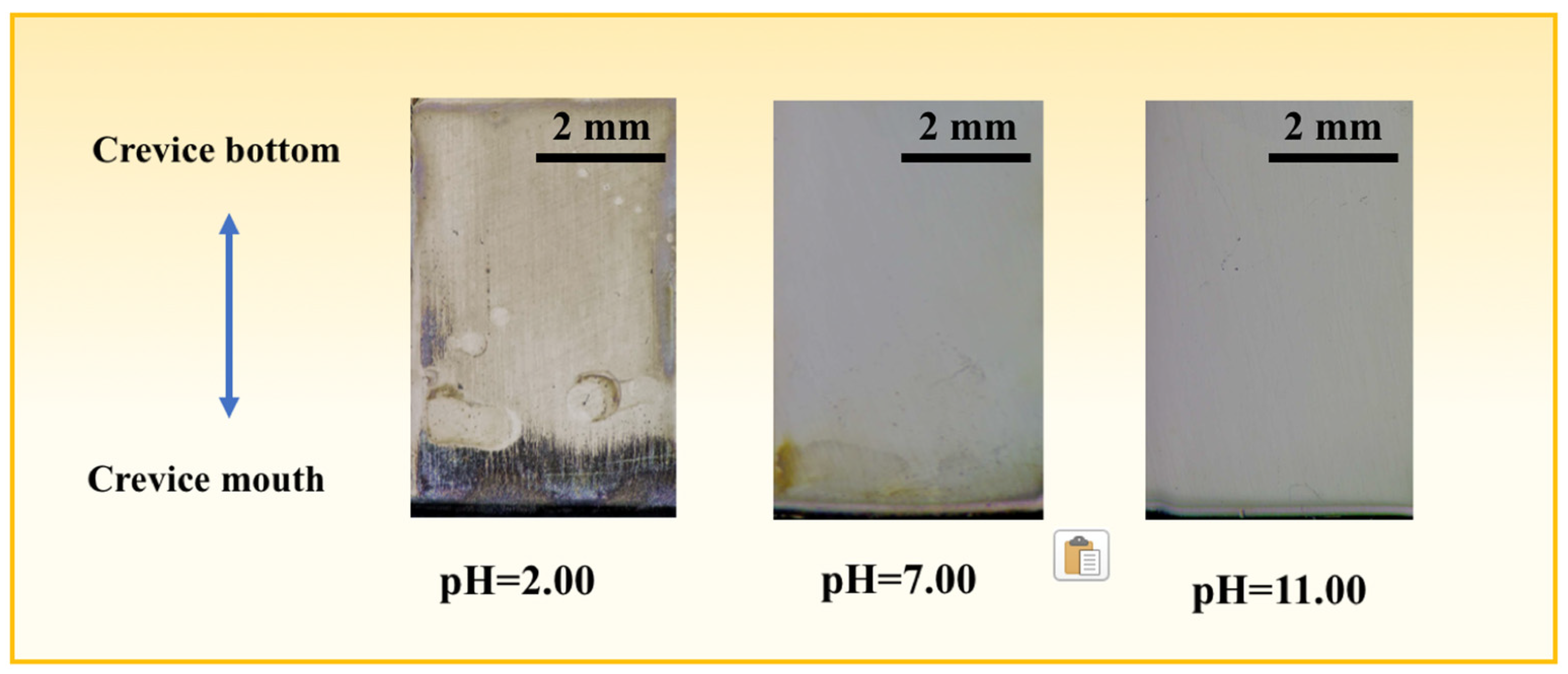
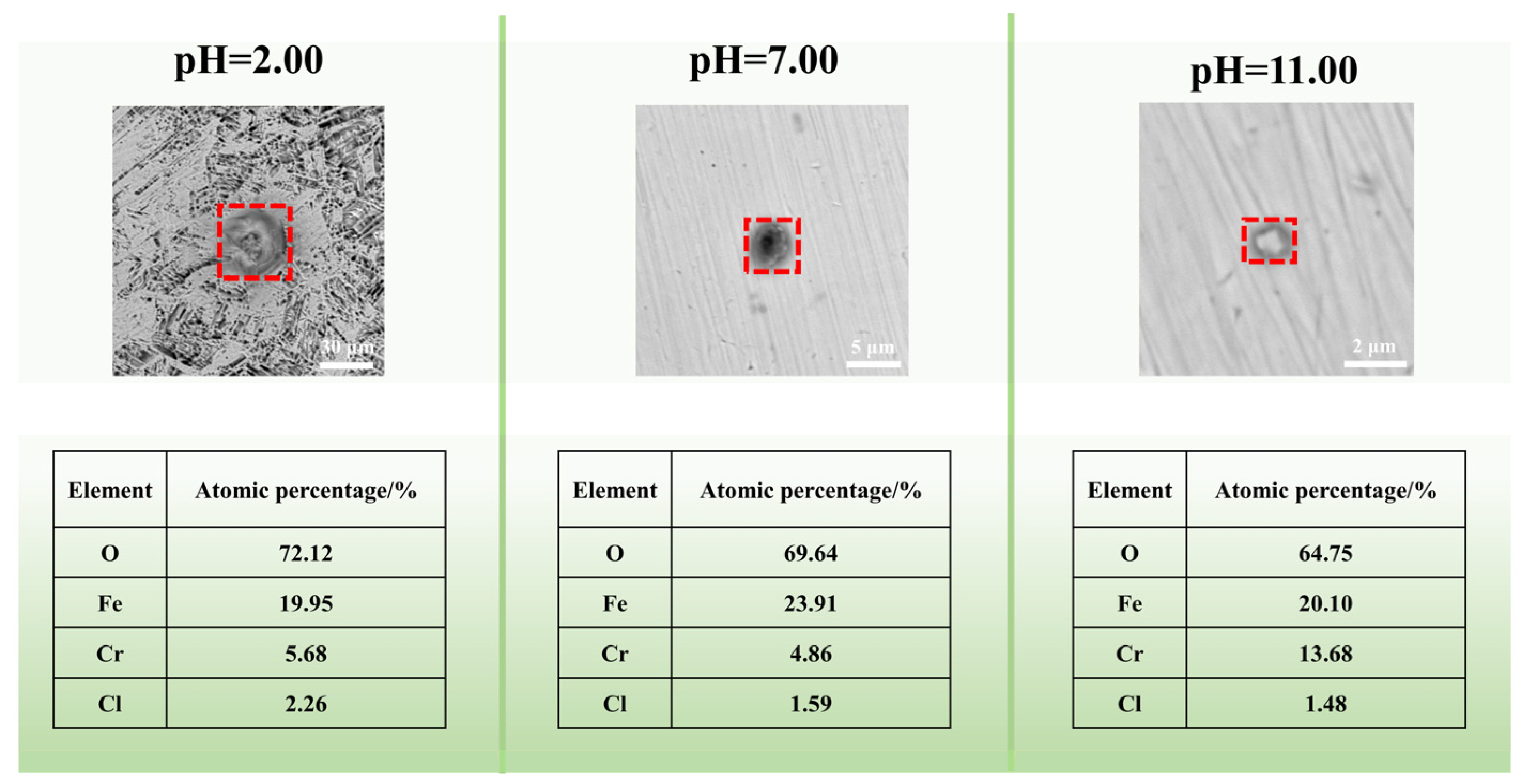
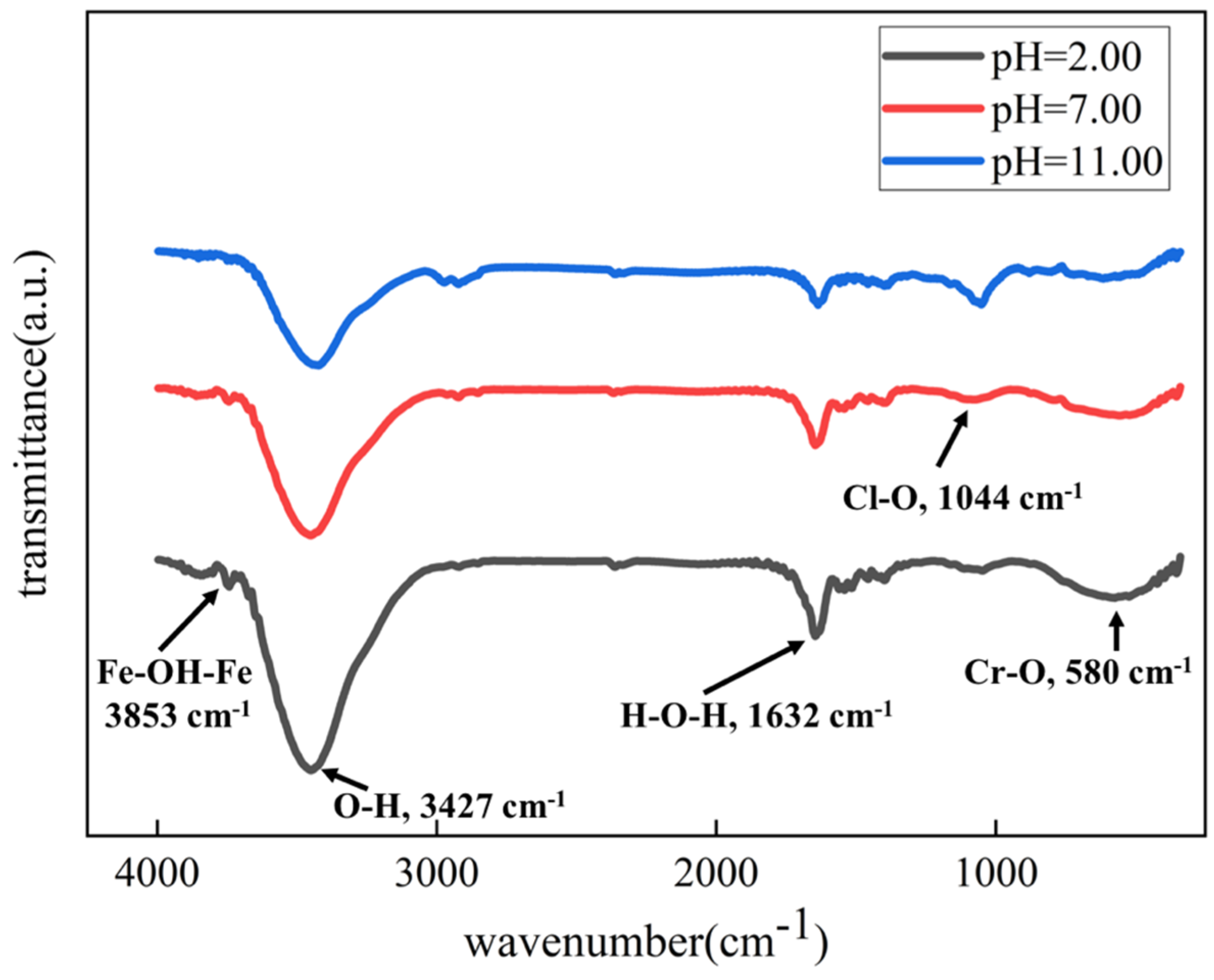
3.5. Ex Situ Observation of the Morphologies and Corrosion Products
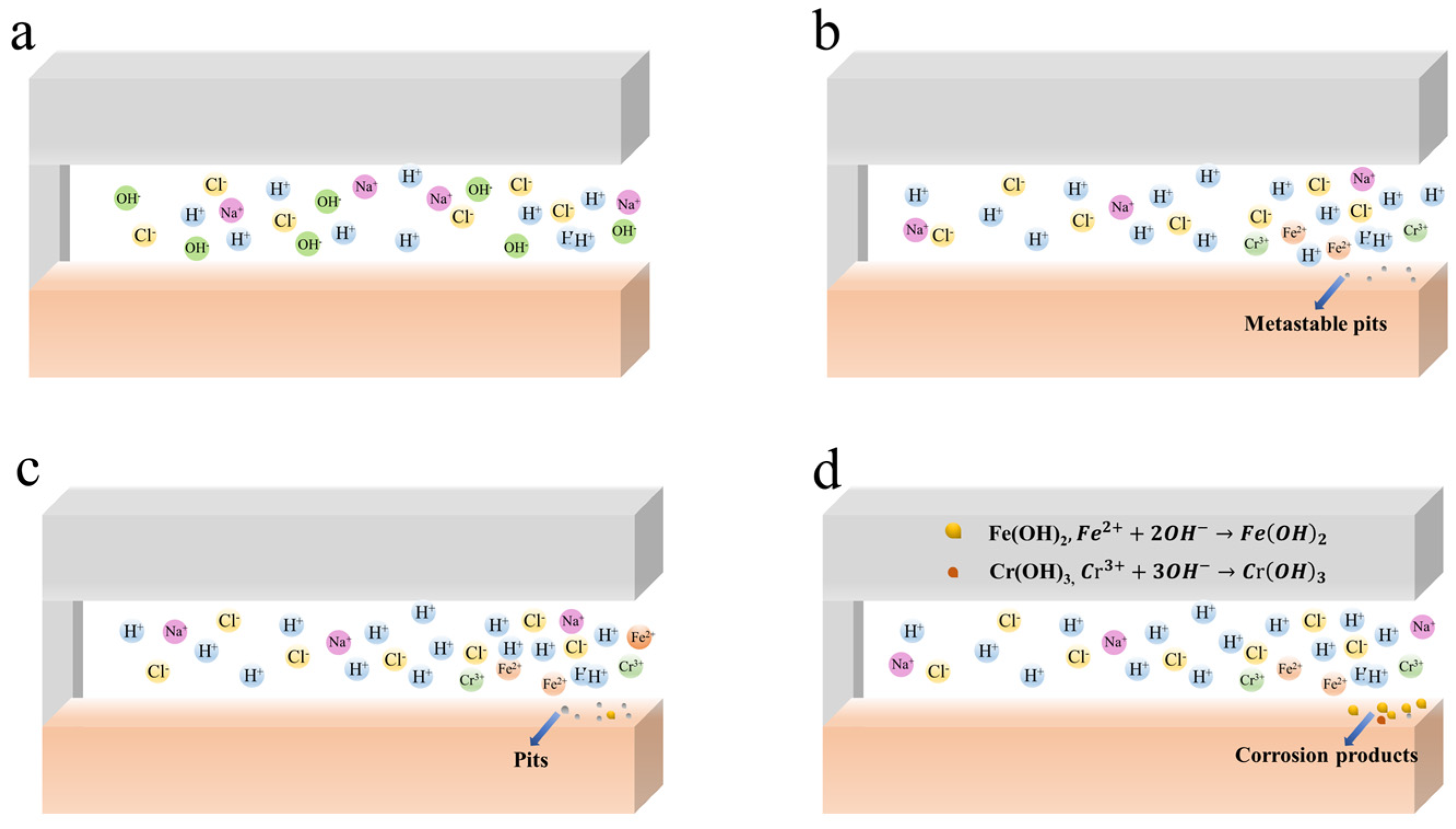
4. Discussion
4.1. The CC Mechanism of 201-SS in pH = 2.00 Solution
4.2. The CC Mechanism of 201-SS in pH = 7.00 and 11.00 Solutions
5. Conclusions
- (1)
- The sample is most seriously corroded in the pH = 2.00 solution, while it is scarcely damaged in the pH = 11.00 solution. The internal erosion area grows with the drop of pH value;
- (2)
- There is a clear radial pH distribution along the crevice wall. The biggest pH drop is observed at the opening, followed by the middle and the bottom areas;
- (3)
- The evolution of CC of 201-SS in various pH solutions is different. There is no incubation phase in the pH = 2.00 solution. In contrast, an incubation phase is observed in the CC development of 201-SS in the pH = 7.00 and 11.00 solutions;
- (4)
- The decrease in pH value inside results in a negative shift of OCP and the initiation of CC of 201-SS. The increased anodic dissolution rate in the acidic solution accelerates the breakdown of passive film within the gap and stimulates the spread of crevice corrosion;
- (5)
- The CC mechanism of 201-SS in various pH solutions can be well described by the combination of CCST and IRRT. The initiation and spreading process of CC is correlated with the passivation state of metal and the pH value inside.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mali, S.A.; Zhu, D.K.; Liu, Y.P.; Gilbert, J.L. Fretting crevice corrosion of 316 L stainless steel in physiological phosphate buffered saline: Load, potential and alloy counterface effects. Tribol. Int. 2021, 164, 107198. [Google Scholar] [CrossRef]
- Aoki, S.; Engelberg, D.L. Time-lapse observation of crevice corrosion in grade 2205 duplex stainless steel. Materials 2023, 16, 5300. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.P.; Tsai, K.C.; Huang, J.Y. The effect of deposited dust on SCC and crevice corrosion of AISI 304L stainless steel in saline environment. Materials 2021, 14, 6834. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, A.; Erning, J. Susceptibility of 304 stainless steel to crevice corrosion in electrochemically active fluids. Corros.-Us 2020, 76, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Dedavid, B.A.; Santos, C.A.; Lopes, N.F.; Fraccaro, C.; Pagartanidis, T.; Lovatto, L.P. Crevice corrosion on stainless steels in oil and gas industry: A review of techniques for evaluation, critical environmental factors and dissolved oxygen. Eng. Fail. Anal. 2023, 144, 106955. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Cui, Z.Y.; Cui, H.Z.; Wang, X.; Li, Y.Z. The effect of applied stress on the crevice corrosion of 304 stainless steel in 3.5 wt% NaCl solution. Corros. Sci. 2022, 196, 110039. [Google Scholar] [CrossRef]
- Li, G.; Yin, D.; Pang, J.R. Investigation of failure behavior of HRB335 steel due to crevice corrosion in concrete ssing electrochemical method. Int. J. Electrochem. Sci. 2021, 16, 211151. [Google Scholar] [CrossRef]
- Zeng, H.T.; Yang, Y.; Liu, L.L.; Li, M.C. Pitting and crevice corrosion evolution characteristics of 2205 duplex stainless steel in hot concentrated seawater. J. Solid. State. Electr. 2021, 25, 1555–1565. [Google Scholar] [CrossRef]
- Nose, K.; Miyamoto, K.; Yoshinobu, T. Estimation of Potential distribution during crevice corrosion through analysis of I-V curves obtained by LAPS. Sensors 2020, 20, 2873. [Google Scholar] [CrossRef]
- Aoyama, T.; Kato, C. Introduction of Cu2+ to the inside of the crevice by chelation and its effect on crevice corrosion of Type 316L stainless steel. Corros. Sci. 2023, 210, 110850. [Google Scholar] [CrossRef]
- Miyamoto, K.-I.; Hiramitsu, R.; Werner, C.F.; Yoshinobu, T. Simultaneous in situ imaging of pH and surface roughening during the progress of crevice corrosion of stainless steel. Sensors 2022, 22, 2246. [Google Scholar] [CrossRef] [PubMed]
- Vankeerberghen, M. Critical characteristic dimension or geometry for determining the susceptibility of a crevice to crevice corrosion. Corrosion 2004, 60, 707–717. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Zhao, J.; Shakouri, M.; Bobaru, F. A peridynamic model for crevice corrosion damage. Electrochim. Acta 2022, 401, 139512. [Google Scholar] [CrossRef]
- Shojaei, E.; Mirjalili, M.; Moayed, M.H. The influence of the crevice induced IR drop on polarization measurement of localized corrosion behavior of 316L stainless steel. Corros. Sci. 2019, 156, 96–105. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Park, Y.-S.; Hwang, W.-S. Initiation modeling of crevice corrosion in 316L stainless steels. J. Mater. Process. Tech. 2000, 103, 206–217. [Google Scholar] [CrossRef]
- Cho, K.; Picking, H.W. The Role of Chloride Ions in the IR & IR * criterion for crevice corrosion in ion. J. Electrochem. Soc. 1991, 138, L56–L58. [Google Scholar]
- Sridhar, N.; Dunn, D.S. Effect of applied potential on changes in solution chemistry inside crevices on type 304L stainless steel and alloy 825. Corros.-Us 1994, 50, 857–872. [Google Scholar] [CrossRef]
- Shaw, B.; Moran, P.; Gartland, P.J.C.S. The role of ohmic potential drop in the initiation of crevice corrosion on alloy 625 in seawater. Corros. Sci. 1991, 32, 707–719. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.Y.; Wu, W.; Li, X.G.; Zhao, J.B. Crack growth behaviour of E690 steel in artificial seawater with various pH values. Corros. Sci. 2020, 164, 108336. [Google Scholar] [CrossRef]
- Dong, L.J.; Zhang, X.L.; Li, Y.F.; Wang, Q.Y.; Liu, L.; Zheng, H.B. Effect of thiosulphate/H2S on crevice corrosion behaviour of P110 carbon steel in CO2− saturated solution. Corros. Eng. Sci. Technol. 2020, 55, 253–267. [Google Scholar] [CrossRef]
- Ning, F.Q.; Tan, J.B.; Wu, X.Q. Effects of 405 stainless steel on crevice corrosion behavior of Alloy 690 in high-temperature pure water. J. Mater. Sci. Technol. 2020, 47, 76–87. [Google Scholar] [CrossRef]
- Souza, R.C.; Santos, B.A.F.; Gonçalves, M.C.; Júnior, E.M.; Simões, T.A.; Oliveira, J.R.; Vaz, G.L.; Caldeira, L.; Gomes, J.A.C.P.; Bueno, A.H. The role of temperature and H2S (thiosulfate) on the corrosion products of API X65 carbon steel exposed to sweet environment. J. Petrol. Sci. Eng. 2019, 180, 78–88. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, X.; Zhang, G.A. Corrosion behaviour of 13Cr stainless steel under stress and crevice in 3.5 wt.% NaCl solution. Corros. Sci. 2020, 163, 108290. [Google Scholar] [CrossRef]
- Xu, W.; Deng, Y.; Zhang, B.; Zhang, J.; Peng, Z.; Hou, B.; Duan, J. Crevice corrosion of U75V high-speed rail steel with varying crevice gap size by in-situ monitoring. J. Mater. Res. Technol. 2022, 16, 1856–1874. [Google Scholar] [CrossRef]
- Heppner, K.L.; Evitts, R.W.; Postlethwaite, J. Effect of the crevice gap on the initiation of crevice corrosion in passive metals. Corrosion 2004, 60. [Google Scholar] [CrossRef]
- Jakobsen, P.; Maahn, E. Temperature and potential dependence of crevice corrosion of AISI 316 stainless steel. Corros. Sci. 2001, 43, 1693–1709. [Google Scholar] [CrossRef]
- Chen, D.X.; Han, E.H.; Wu, X.Q. Effects of crevice geometry on corrosion behavior of 304 stainless steel during crevice corrosion in high temperature pure water. Corros. Sci. 2016, 111, 518–530. [Google Scholar] [CrossRef]
- Mu, J.; Li, Y.Z.; Wang, X. Crevice corrosion behavior of X70 steel in NaCl solution with different pH. Corros. Sci. 2021, 182, 109310. [Google Scholar] [CrossRef]
- Cuesta, A.; Morales-Cantero, A.; De la Torre, A.G.; Aranda, M.A.G. Recent advances in C-S-H nucleation aeeding for improving cement performances. Materials 2023, 16, 1462. [Google Scholar] [CrossRef]
- Lee, K.M.; Marsh, Z.M.; Crenshaw, E.P.; Tohgha, U.N.; Ambulo, C.P.; Wolf, S.M.; Carothers, K.J.; Limburg, H.N.; McConney, M.E.; Godman, N.P. Recent advances in electro-optic response of polymer-stabilized cholesteric liquid crystals. Materials 2023, 16, 2248. [Google Scholar] [CrossRef]
- Cai, G.; Delgado, T.; Richard, C.; Viana, B. ZGSO apinel nanoparticles with dual emission of NIR persistent luminescence for anti-counterfeiting applications. Materials 2023, 16, 1132. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, X.; Tang, X.; Hao, Q.; Chen, M. Micro Spectrometers based on materials nanoarchitectonics. Materials 2023, 16, 2253. [Google Scholar] [CrossRef] [PubMed]
- Santana, J.J.; Izquierdo, J.; Souto, R.M. Uses of scanning electrochemical microscopy (SECM) for the characterization with spatial and chemical resolution of thin surface layers and coating systems applied on metals: A review. Coatings 2022, 12, 637. [Google Scholar] [CrossRef]
- Filotás, D.; Izquierdo, J.; Fernández-Pérez, B.M.; Nagy, L.; Nagy, G.; Souto, R.M. Contributions of microelectrochemical scanning techniques for the efficient detection of localized corrosion processes at the cut edges of polymer-coated galvanized steel. Molecules 2022, 27, 2167. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.M.P.; Izquierdo, J.; Milagre, M.X.; Antunes, R.A.; Souto, R.M.; Costa, I. Development of an Al3+ ion-selective microelectrode for the potentiometric microelectrochemical monitoring of corrosion sites on 2098-T351 aluminum alloy surfaces. Electrochim. Acta 2022, 415, 140260. [Google Scholar] [CrossRef]
- Asserghine, A.; Medvidovic-Kosanovic, M.; Stankovic, A.; Nagy, L.; Souto, R.M.; Nagy, G. A scanning electrochemical microscopy characterization of the localized corrosion reactions occurring on nitinol in saline solution after anodic polarization. Sens. Actuators B-Chem. 2020, 321, 128610. [Google Scholar] [CrossRef]
- Al-Jeda, M.; Mena-Morcillo, E.; Chen, A.C. Micro-sized pH sensors based on scanning electrochemical probe microscopy. Micromachines 2022, 13, 2143. [Google Scholar] [CrossRef]
- Cieslik, J.; Ryl, M.; Zielinski, A.; Ficek, M.; Dec, B.; Darowicki, K.; Bogdanowicz, R. High-temperature oxidation of heavy boron-doped diamond electrodes: Microstructural and electrochemical performance modification. Materials 2020, 13, 964. [Google Scholar]
- Zhu, Z.; Zhang, Q.; Liu, P.; Zhang, J.; Cao, F. Quasi-simultaneous electrochemical/chemical imaging of local Fe2+ and pH distributions on 316 L stainless steel surface. J. Electroanal. Chem. 2020, 871, 114107. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, X.; Ye, Z.; Zhang, J.; Cao, F.; Zhang, J. A fabrication of iridium oxide film pH micro-sensor on Pt ultramicroelectrode and its application on in-situ pH distribution of 316L stainless steel corrosion at open circuit potential. Sens. Actuators B Chem. 2018, 255, 1974–1982. [Google Scholar] [CrossRef]
- Bai, Y.H.; Zhu, Z.J.; Zhang, H.; Zhang, Q.H.; Du, H.W.; Wang, J.Y.; Cao, F.H. In-situ radial pH monitoring inside the crevice of stainless steel combined with potentiometric scanning electrochemical microscopy. Electrochim. Acta 2023, 467, 143109. [Google Scholar] [CrossRef]
- Tefashe, U.M.; Dauphin-Ducharme, P.; Danaie, M.; Cano, Z.P.; Kish, J.R.; Botton, G.A.; Mauzeroll, J. Localized corrosion behavior of AZ31B magnesium alloy with an electrodeposited poly(3,4-ethylenedioxythiophene) coating. J. Electrochem. Soc. 2015, 162, C536–C544. [Google Scholar] [CrossRef]
- Cristoforetti, A.; Izquierdo, J.; Souto, R.M.; Deflorian, F.; Fedel, M.; Rossi, S. In-situ measurement of electrochemical activity related to filiform corrosion in organic coated steel by scanning vibrating electrode technique and scanning micropotentiometry. Corros. Sci. 2024, 227, 111669. [Google Scholar] [CrossRef]
- da Silva, R.M.P.; Izquierdo, J.; Milagre, M.X.; Betancor-Abreu, A.M.; Costa, I.; Souto, R.M. Use of amperometric and potentiometric probes in scanning electrochemical microscopy for the spatially-resolved monitoring of severe localized corrosion sites on aluminum alloy 2098-T315. Sensors 2021, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
- Asserghine, A.; Filotás, D.; Németh, B.; Nagy, L.; Nagy, G. Potentiometric scanning electrochemical microscopy for monitoring the pH distribution during the self-healing of passive titanium dioxide layer on titanium dental root implant exposed to physiological buffered (PBS) medium. Electrochem. Commun. 2018, 95, 1–4. [Google Scholar] [CrossRef]






| Elements | Fe | Cr | Ni | Mn | Si | C | P | S |
|---|---|---|---|---|---|---|---|---|
| Content | 77.24 | 14.09 | 5.25 | 1.49 | 1.43 | 0.25 | 0.13 | 0.12 |
| pH | 2.00 | 7.00 | 11.00 |
|---|---|---|---|
| Ecorr/V | −0.5257 | −0.4432 | −0.3872 |
| icorr/A·cm2 | 7.68 × 10−4 | 4.52 × 10−5 | 2.44 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Zhang, H.; Bai, Y.; Liu, P.; Yuan, H.; Wang, J.; Cao, F. Crevice Corrosion Behavior of 201 Stainless Steel in NaCl Solutions with Different pH Values by In Situ Monitoring. Materials 2024, 17, 1158. https://doi.org/10.3390/ma17051158
Zhu Z, Zhang H, Bai Y, Liu P, Yuan H, Wang J, Cao F. Crevice Corrosion Behavior of 201 Stainless Steel in NaCl Solutions with Different pH Values by In Situ Monitoring. Materials. 2024; 17(5):1158. https://doi.org/10.3390/ma17051158
Chicago/Turabian StyleZhu, Zejie, Hang Zhang, Yihan Bai, Pan Liu, Haoran Yuan, Jiangying Wang, and Fahe Cao. 2024. "Crevice Corrosion Behavior of 201 Stainless Steel in NaCl Solutions with Different pH Values by In Situ Monitoring" Materials 17, no. 5: 1158. https://doi.org/10.3390/ma17051158
APA StyleZhu, Z., Zhang, H., Bai, Y., Liu, P., Yuan, H., Wang, J., & Cao, F. (2024). Crevice Corrosion Behavior of 201 Stainless Steel in NaCl Solutions with Different pH Values by In Situ Monitoring. Materials, 17(5), 1158. https://doi.org/10.3390/ma17051158







