Durability Performance of Basalt Fiber-Reinforced Concrete Subjected to Sulfate–Magnesium Combined Attack
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
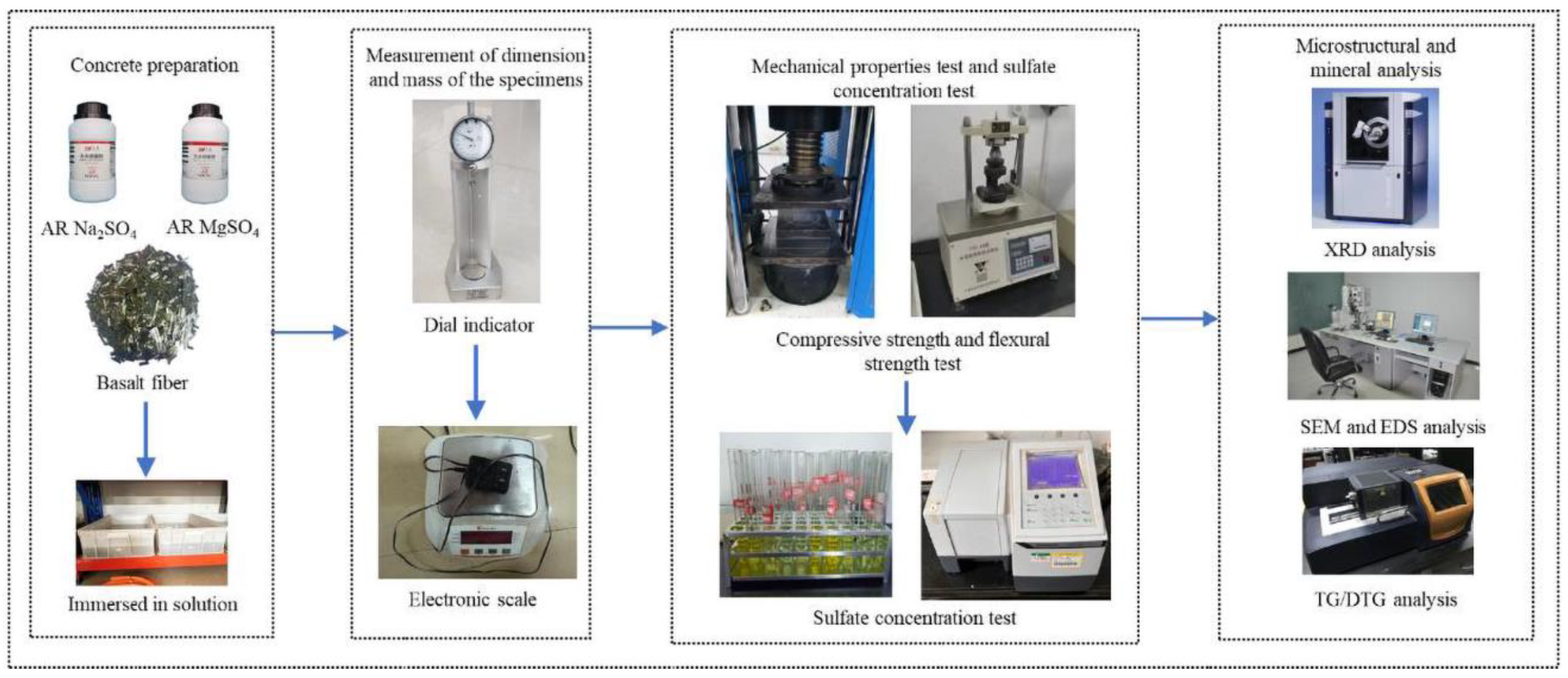
2.2. Testing Methods
2.2.1. Specimen and Solution Preparation
2.2.2. Dimension and Mass Change
2.2.3. Compressive and Flexural Strength
2.2.4. Microstructural and Mineral Analysis
2.2.5. Sulfate Concentration
3. Results
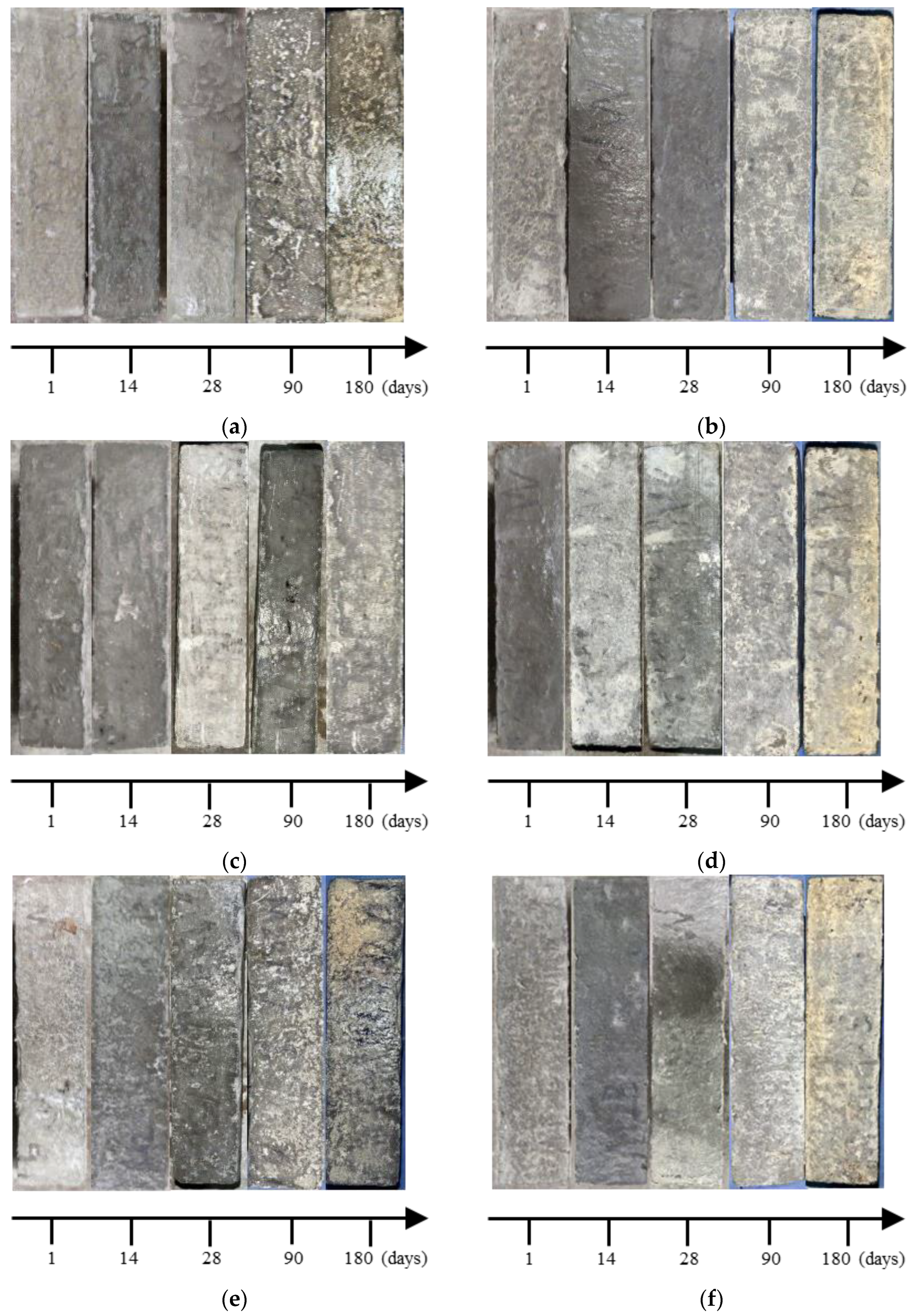
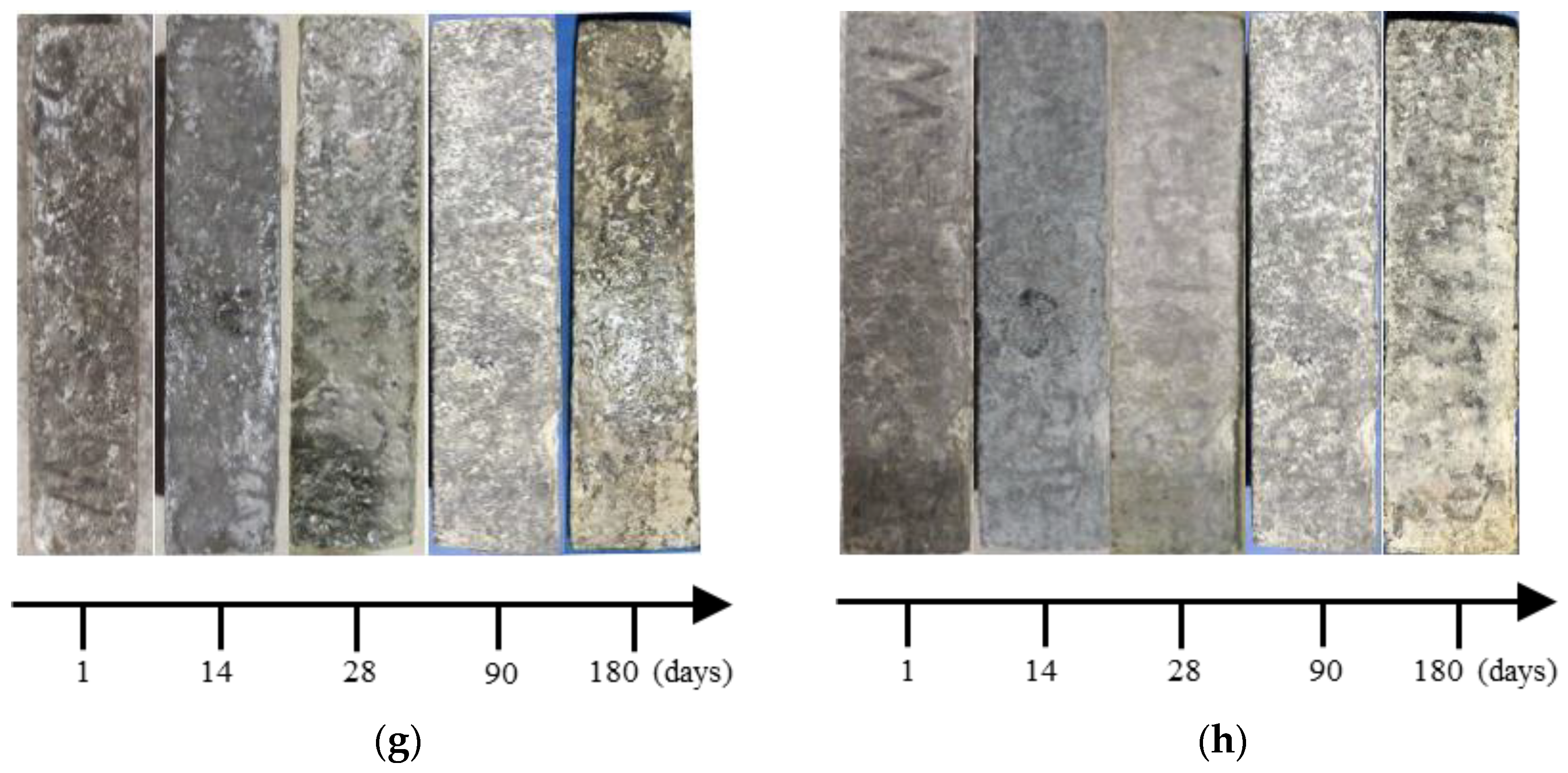
3.1. Appearance Change
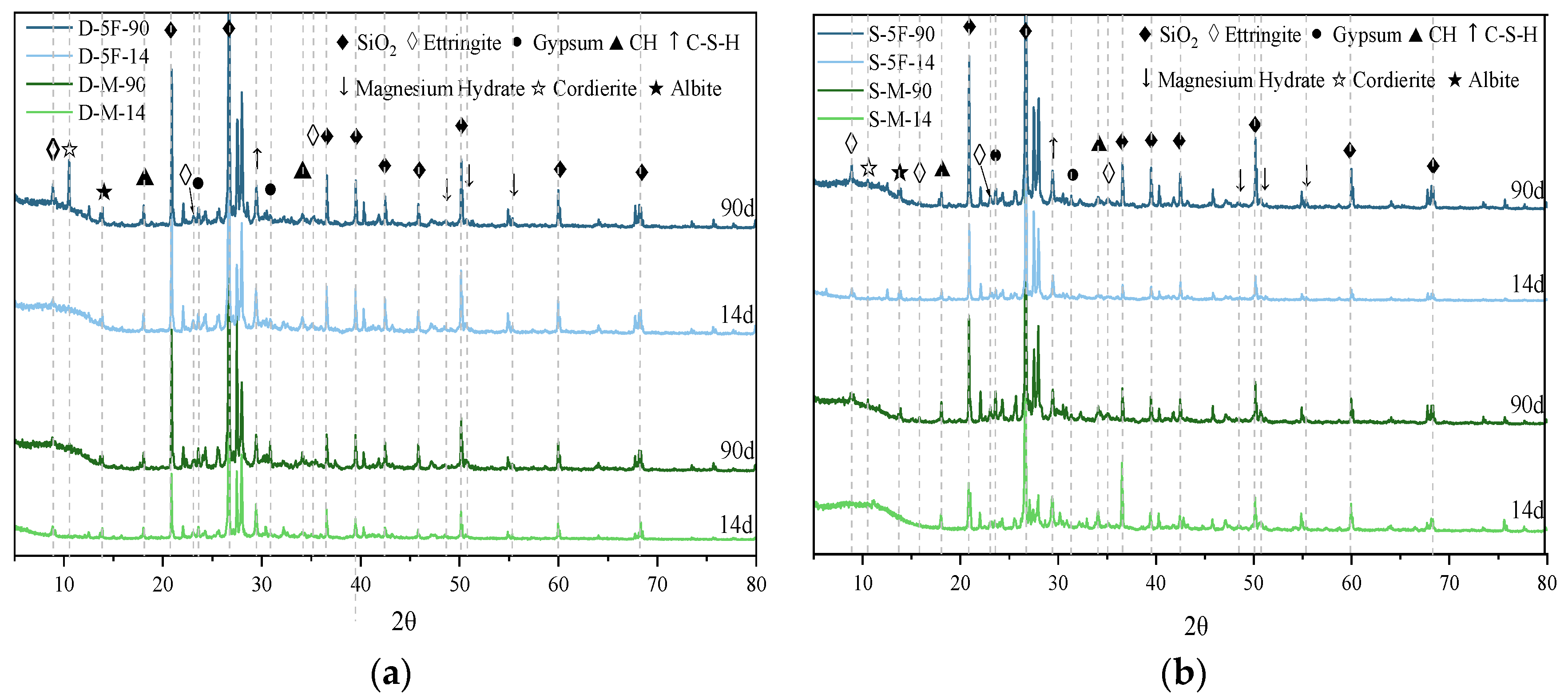
3.2. Microstructure and Mineral Changes
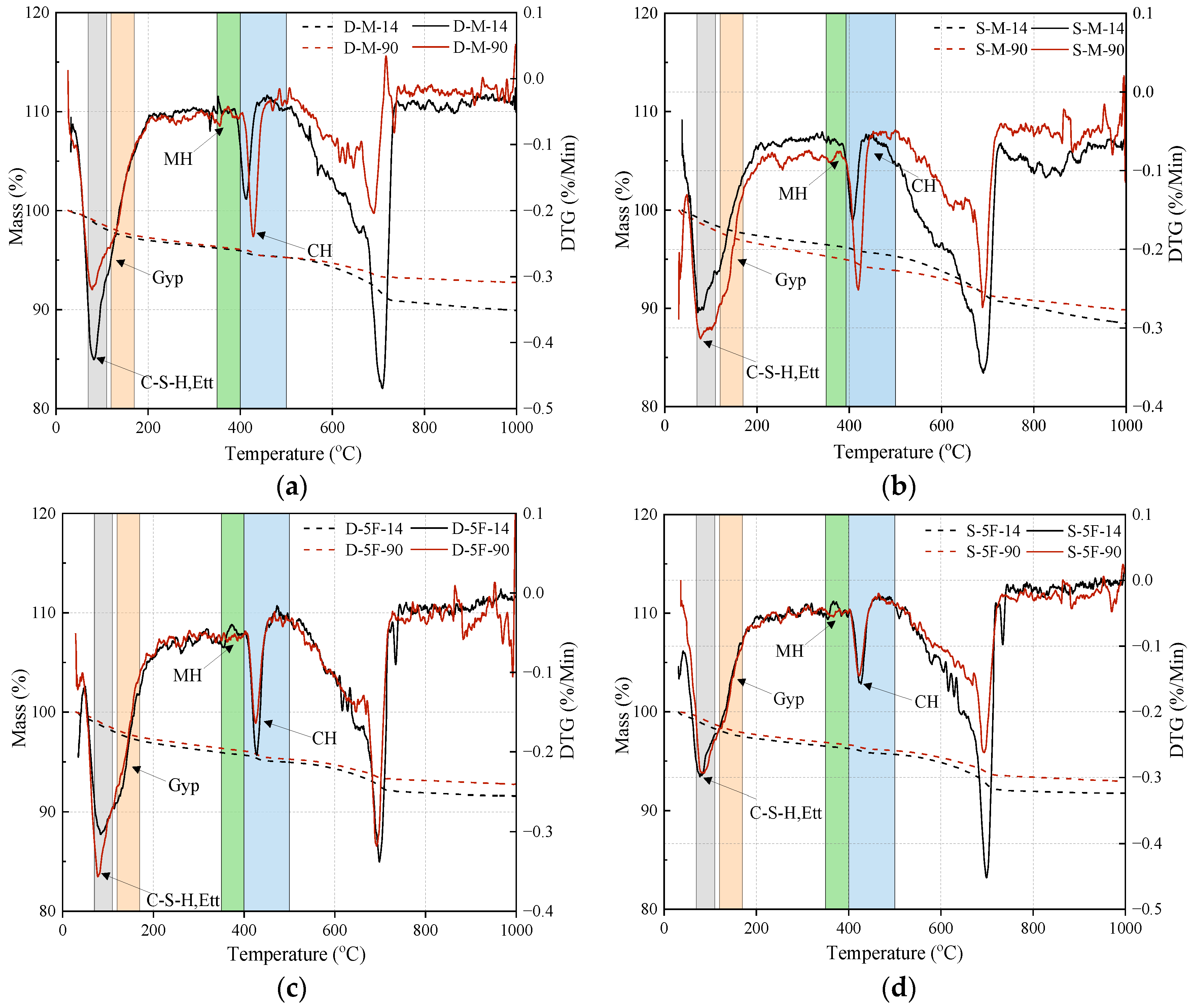
3.2.1. Mineral Changes
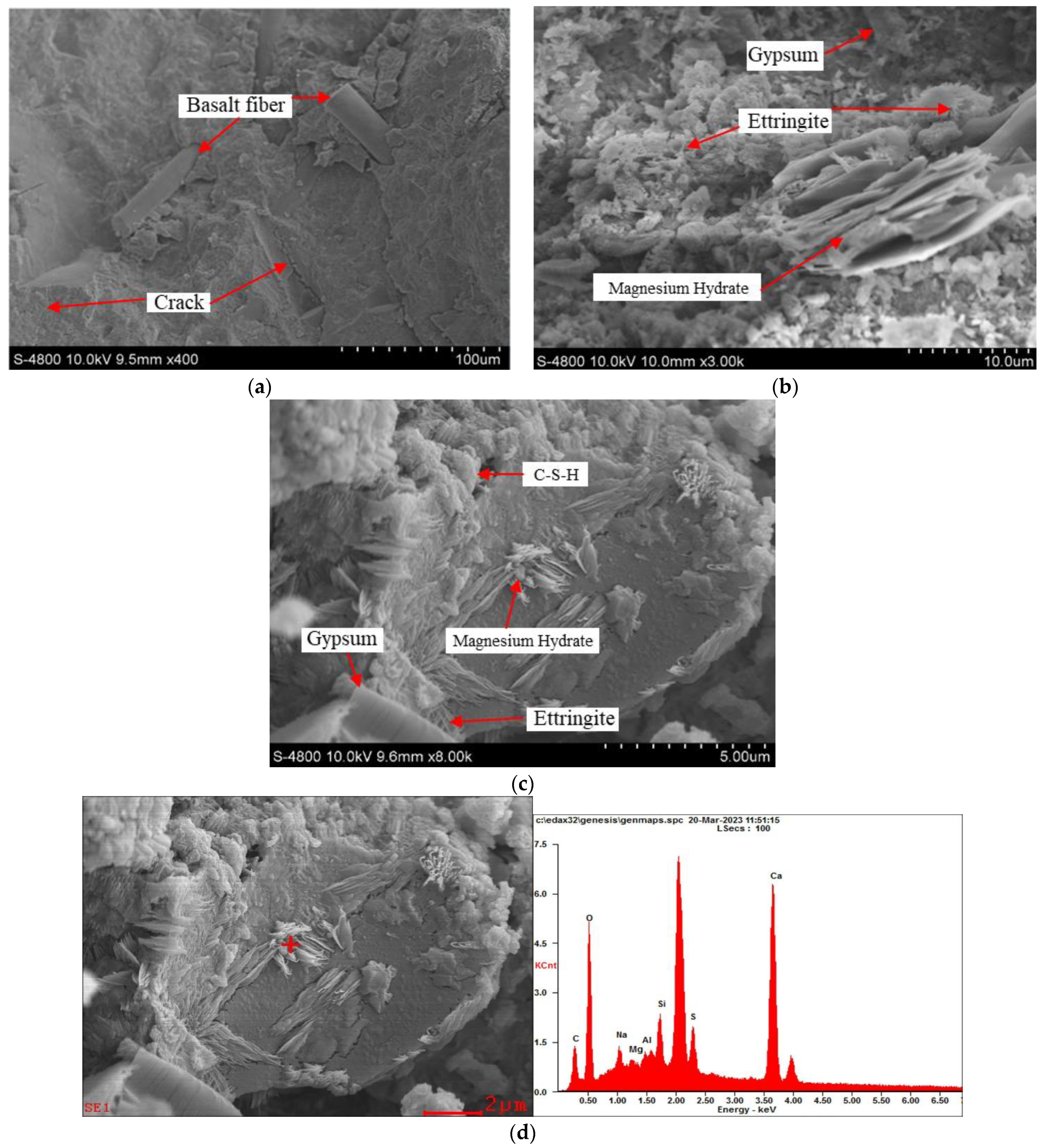
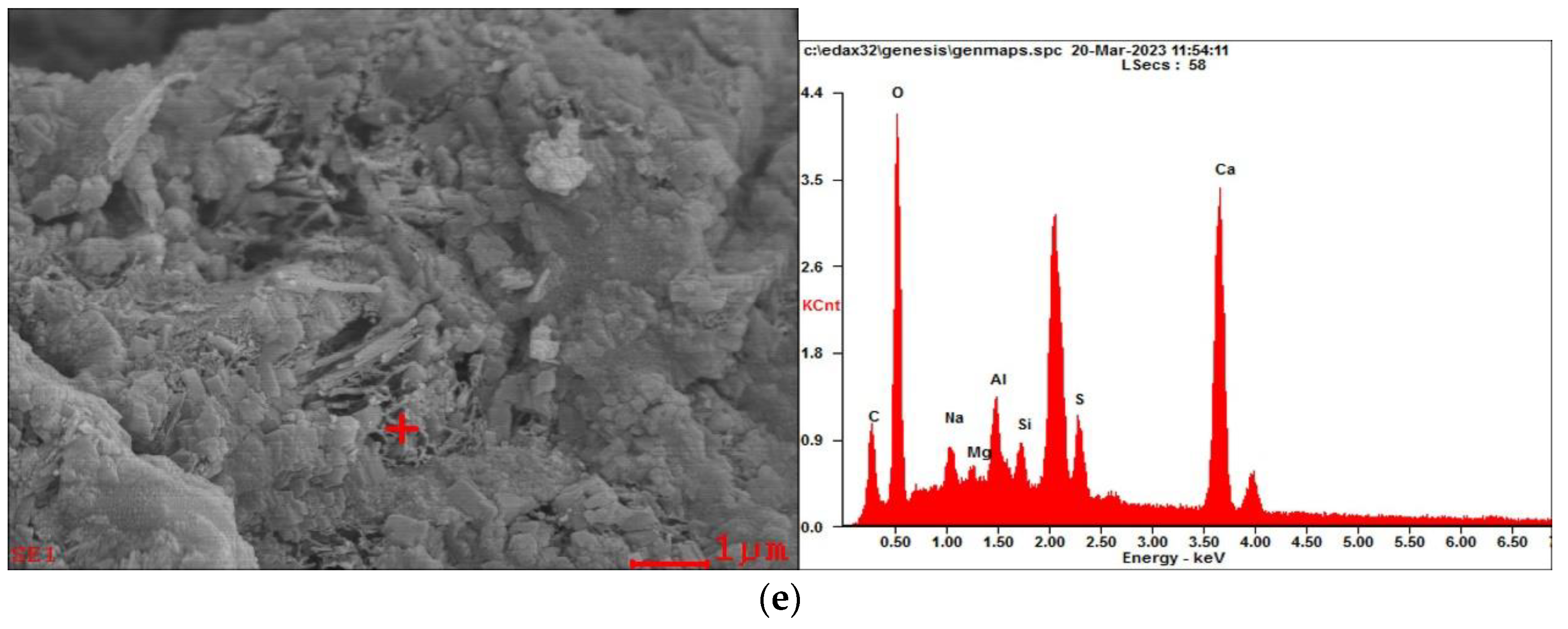
3.2.2. Microstructures
3.3. Dimension and Mass Changes
3.3.1. Dimension Change
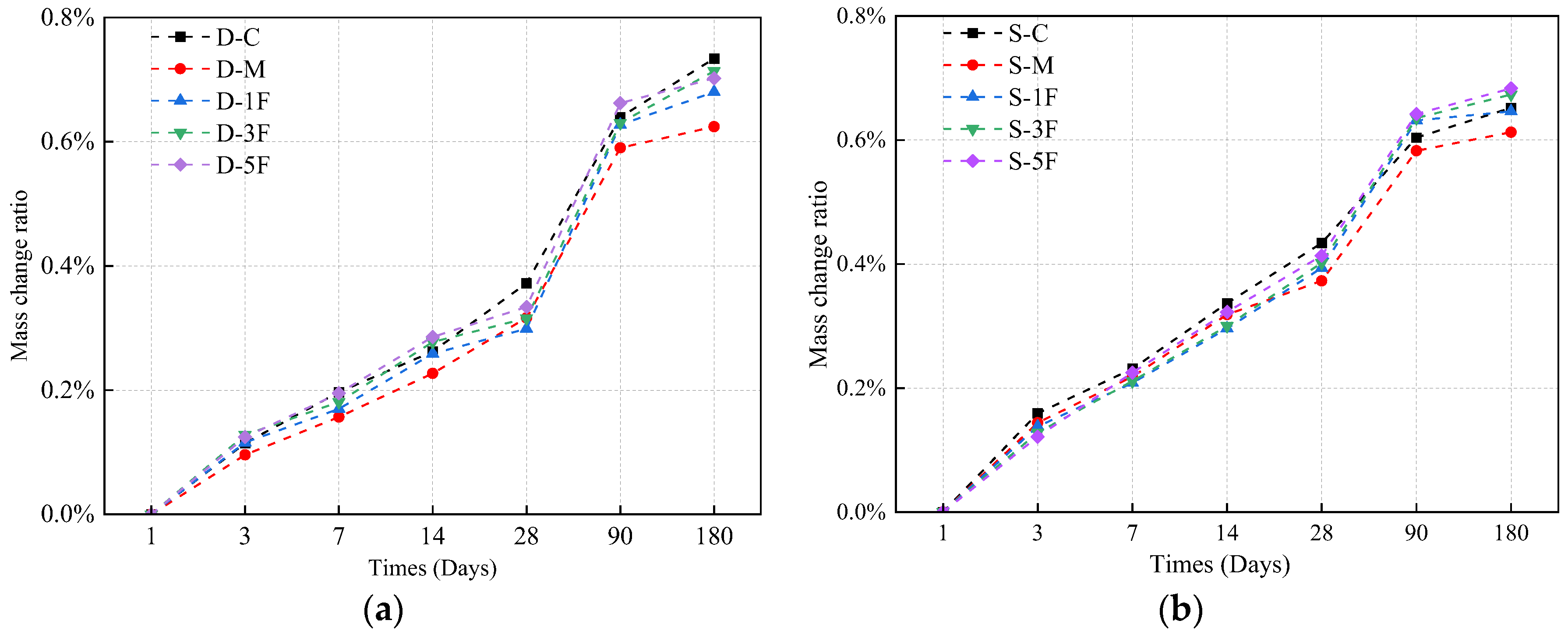
3.3.2. Mass Change
3.4. Mechanical Properties
3.4.1. Compressive Strength
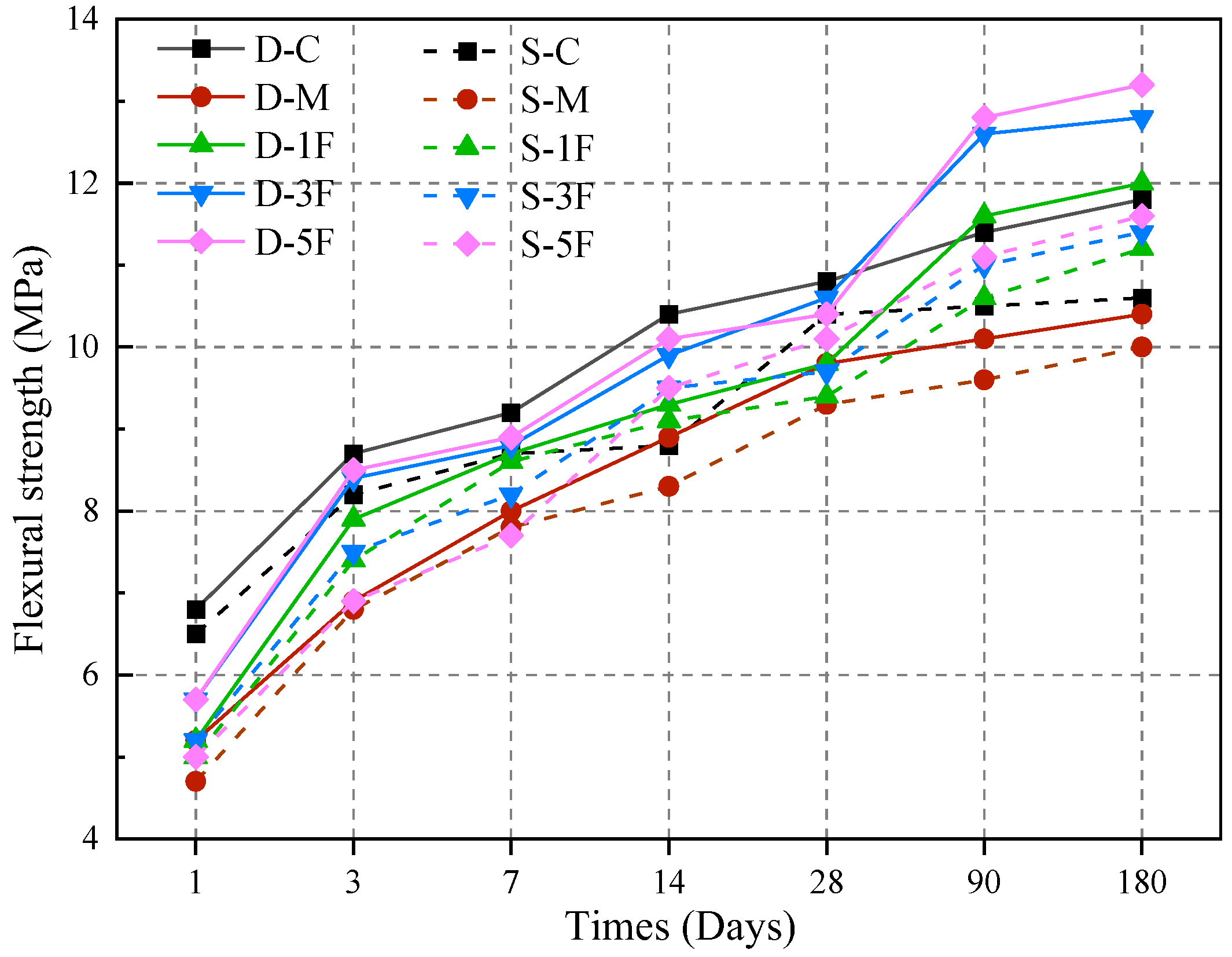
3.4.2. Flexural Strength
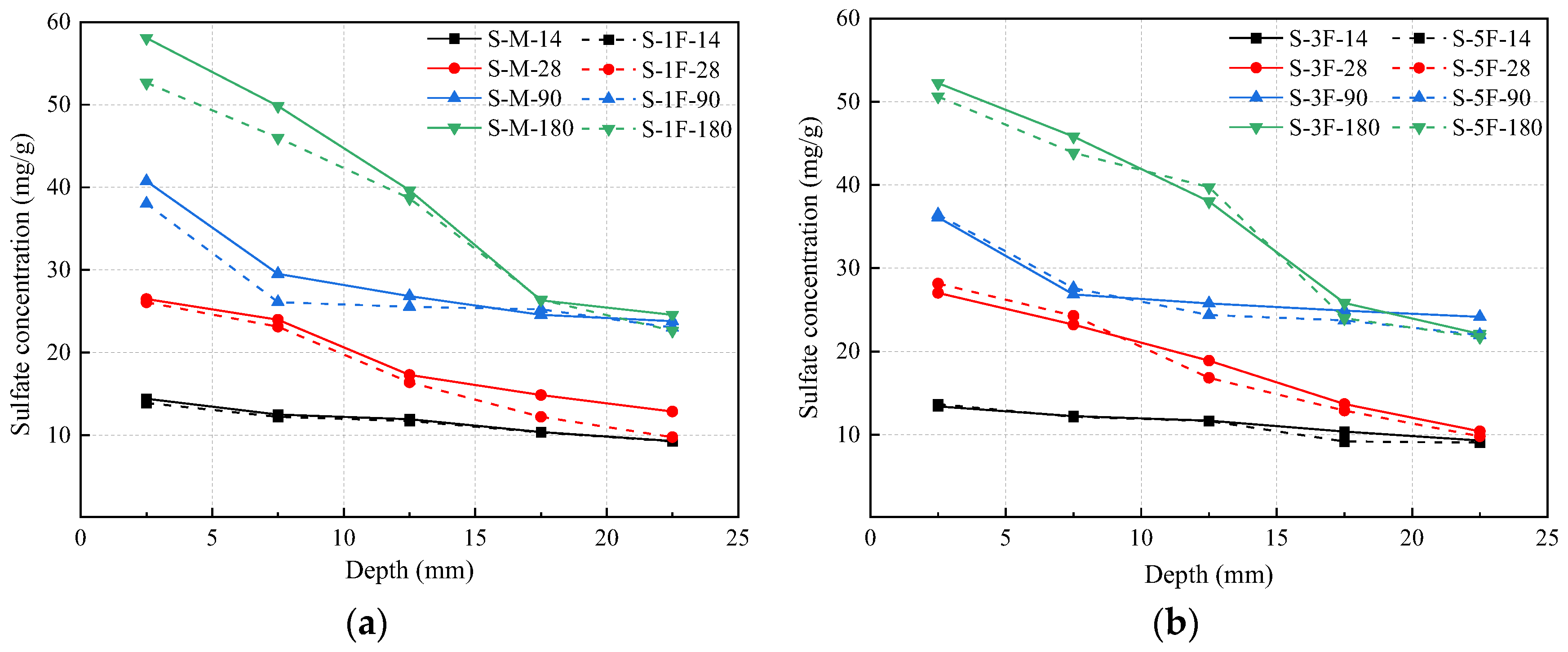
3.5. Sulfate Concentration
4. Discussion
5. Conclusions
- Corrosion products induced by magnesium primarily affect the strength instead of causing the expansion. Under the combined attack of external sulfate–internal magnesium, the specimen exhibits more severe degradation and relatively poor mechanical performance.
- After premixing with BF, the physical and mechanical properties of cast-in situ concrete are improved. BF mainly exerts the effects of strengthening and improving crack resistance. For strengthening, BF fills in the original defects and enhances the load-bearing capacity of the specimens. In terms of crack resistance, it restricts the development of cracks and reduces stress concentration at the crack tip.
- A 0.5% content of BF results in the most significant improvement in the properties of specimens. Under the attack of internal magnesium salt, both the compressive and flexural strength of these samples are higher than the control samples at 180 days. When exposed to external sulfate–internal magnesium combined attack for a long period, the flexural strength of specimens with 0.5% BF increased by 16.2%.
- Moreover, there is no available model for the development of strength under the external sulfate–internal magnesium combined attack. In the follow-up research, we will focus on carrying out statistical tests and parameter analysis to build applicable models. We will continue to research the performance of basalt fiber-reinforced concrete with different lengths and contents of BF, aiming to provide more references for the application of BF in concrete.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, A.A.; Zhang, X.; Yang, R.W.; Wang, C.L. The deterioration mechanisms of hardened cement paste exposed to combined action of cyclic wetting-drying, salt attack and carbonation. Constr. Build. Mater. 2023, 366, 130148. [Google Scholar] [CrossRef]
- Pasupathy, K.; Cheema, D.; Sanjayan, J. Durability performance of fly ash-based geopolymer concrete buried in saline environment for 10 years. Constr. Build. Mater. 2021, 281, 122596. [Google Scholar] [CrossRef]
- He, R.; Zheng, S.; Gan, V.J.L.; Wang, Z.D.; Fang, J.H.; Shao, Y. Damage mechanism and interfacial transition zone characteristics of concrete under sulfate erosion and Dry-Wet cycles. Constr. Build. Mater. 2020, 255, 119340. [Google Scholar] [CrossRef]
- Ye, H.L.; Chen, Z.; Huang, L. Mechanism of sulfate attack on alkali-activated slag: The role of activator composition. Cem. Concr. Res. 2019, 125, 105868. [Google Scholar] [CrossRef]
- Bednarska, D.; Koniorczyk, M.; Steiger, M. Identification of various salt crystallization and water freezing patterns induced by temperature variation from Na2SO4-H2O system confined in porous materials. Constr. Build. Mater. 2022, 347, 128540. [Google Scholar] [CrossRef]
- Bassuoni, M.T.; Rahman, M.M. Response of concrete to accelerated physical salt attack exposure. Cem. Concr. Res. 2016, 79, 395–408. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 29, 1613–1624. [Google Scholar] [CrossRef]
- Flatt, R.J.; Scherer, G.W. Hydration and crystallization pressure of sodium sulfate: A critical review. Mater. Res. Soc. Symp. Proc. 2002, 712, 29–34. [Google Scholar] [CrossRef]
- Wang, Y.; Ueda, T.; Gong, F.; Zhang, D.W. Meso-scale mechanical deterioration of mortar due to sodium chloride attack. Cem. Concr. Compos. 2019, 96, 163–173. [Google Scholar] [CrossRef]
- Tan, L.; Wang, F.; Liang, M.; Wang, X.L.; Das, R.; Mao, D.Q.; Luo, Y. Antibiotic resistance genes attenuated with salt accumulation in saline soil. J. Hazard. Mater. 2019, 374, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Karakoç, M.B.; Türkmen, I.; Maraş, M.M.; Kantarci, F.; Demirboga, R. Sulfate resistance of ferrochrome slag based geopolymer concrete. Ceram. Int. 2016, 42, 1254–1260. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Goff, F.; Pochard, I.; Dauzeres, A. Effect of magnesium on calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2017, 97, 61–72. [Google Scholar] [CrossRef]
- De Weerdt, K.; Bernard, E.; Kunther, W.; Pedersen, M.T.; Lothenbach, B. Phase changes in cementitious materials exposed to saline solutions. Cem. Concr. Res. 2023, 165, 107071. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Yu, X.H.; Shen, X.Y.; Geng, G.Q.; Lothenbach, B. The physiochemical alterations of calcium silicate hydrate (C-S-H) under magnesium attack. Cem. Concr. Res. 2022, 160, 106901. [Google Scholar] [CrossRef]
- Siad, H.; Lachemi, M.; Bernard, S.K.; Sahmaran, M.; Hossain, A. Assessment of the long-term performance of SCC incorporating different mineral admixtures in a magnesium sulphate environment. Constr. Build. Mater. 2015, 80, 141–154. [Google Scholar] [CrossRef]
- Pinto, S.R.; da Luz, C.A.; Munhoz, G.S.; Medeiros, R.A. Durability of phosphogypsum-based supersulfated cement mortar against external attack by sodium and magnesium sulfate. Cem. Concr. Res. 2020, 136, 106172. [Google Scholar] [CrossRef]
- Tan, Y.S.; Yu, H.F.; Ma, H.Y.; Zhang, Y.Q.; Wu, C.Y. Study on the micro-crack evolution of concrete subjected to stress corrosion and magnesium sulfate. Constr. Build. Mater. 2017, 141, 453–460. [Google Scholar] [CrossRef]
- Wu, J.; Wei, J.X.; Huang, H.L.; Hu, J.; Yu, Q.J. Effect of multiple ions on the degradation in concrete subjected to sulfate attack. Constr. Build. Mater. 2020, 259, 119846. [Google Scholar] [CrossRef]
- Neville, A. The confused world of sulphate attack on concrete, Review. Cem. Concr. Res. 2004, 34, 1275–1296. [Google Scholar] [CrossRef]
- Li, X.S.; Shui, Z.H.; Yu, R.; Wang, X.P. Magnesium induced hydration kinetics of ultra-high performance concrete (UHPC) served in marine environment: Experiments and modelling. Constr. Build. Mater. 2019, 224, 1056–1068. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.; Olek, J. Differentiating seawater and groundwater sulfate attack in Portland cement mortars. Cem. Concr. Res. 2006, 36, 2132–2137. [Google Scholar] [CrossRef]
- Xie, F.; Li, J.P.; Zhao, G.W.; Wang, C.; Wang, Y.H.; Zhou., P. Experimental investigations on the durability and degradation mechanism of cast-in-situ recycled aggregate concrete under chemical sulfate attack. Constr. Build. Mater. 2021, 297, 123771. [Google Scholar] [CrossRef]
- Shao, W.; Qin, F.L.; Shi, D.D.; Soomro, M.A. Horizontal bearing characteristic and seismic fragility analysis of CFRP composite pipe piles subject to chloride corrosion. Comput. Geotech. 2024, 166, 105977. [Google Scholar] [CrossRef]
- Chao, Z.M.; Wang, H.Y.; Hu, S.Y.; Wang, M.; Xu, S.K.; Zhang, W.B. Permeability and porosity of light-weight concrete with plastic waste aggregate: Experimental study and machine learning modelling. Constr. Build. Mater. 2024, 411, 134465. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Hu, X.; Shi, C.J.; Zhang, Z.H.; Zhu, D.J. A review on seawater sea-sand concrete: Mixture proportion, hydration, microstructure and properties. Constr. Build. Mater. 2021, 295, 123602. [Google Scholar] [CrossRef]
- Wang, X.F.; Dong, C.Y.; Xu, S.Y.; Song, Q.; Ren, J.; Zhu, J.H. Influence of seawater and sea sand on early-age performance and cracking sensitivity of concrete. J. Build. Eng. 2023, 79, 107811. [Google Scholar] [CrossRef]
- Guo, M.H.; Hu, B.; Xing, F.; Zhou, X.Q.; Sun, M.; Sui, L.L.; Zhou, Y.W. Characterization of the mechanical properties of eco-friendly concrete made with untreated sea sand and seawater based on statistical analysis. Constr. Build. Mater. 2020, 234, 117339. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, Z.R.; Wang, Z.H.; He, J.Q.; Niu, D.T. Erosion behavior of ions in lining concrete incorporating fly ash and silica fume under the combined action of load and flowing groundwater containing composite salt. Case Stud. Constr. Mater. 2022, 17, e01659. [Google Scholar] [CrossRef]
- Das, S.; Sobuz, M.; Tam, V.; Akid, A.M.; Sutan, N.M.; Rahman, F.M. Effects of incorporating hybrid fibres on rheological and mechanical properties of fibre reinforced concrete. Constr. Build. Mater. 2020, 262, 120561. [Google Scholar] [CrossRef]
- Banthia, N.; Sappakittipakorn, M. Toughness enhancement in steel fiber reinforced concrete through fiber hybridization. Cem. Concr. Res. 2007, 37, 1366–1372. [Google Scholar] [CrossRef]
- Siddika, A.; Mamun, M.A.A.; Alyousef, R.; Amran, Y.H.M. Strengthening of reinforced concrete beams by using fiber-reinforced polymer composites: A review. J. Build. Eng. 2019, 25, 100798. [Google Scholar] [CrossRef]
- Nasera, M.Z.; Hawilehb, R.A.; Abdallab, J.A. Fiber-reinforced polymer composites in strengthening reinforced concrete structures: A critical review. Eng. Struct. 2019, 198, 109542. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Zhang, Y.; Zhuo, J.B.; Zhang, Y.M.; Wan, C. A review of the mechanical properties and durability of basalt fiber-reinforced concrete. Constr. Build. Mater. 2022, 359, 12936. [Google Scholar] [CrossRef]
- Dilbas, H.; Çakır, Ö. Influence of basalt fiber on physical and mechanical properties of treated recycled aggregate concrete. Constr. Build. Mater. 2020, 254, 119216. [Google Scholar] [CrossRef]
- Khan, M.; Cao, M.; Chu, S.H.; Ali, M. Properties of hybrid steel-basalt fiber reinforced concrete exposed to different surrounding conditions. Constr. Build. Mater. 2022, 322, 126340. [Google Scholar] [CrossRef]
- Alzeebaree, R.; Cevik, A.; Mohammedameen, A.; Nis, A.; Gulsan, M.E. Mechanical performance of FRP-confined geopolymer concrete under seawater attack. Adv. Struct. Eng. 2019, 23, 1055–1073. [Google Scholar] [CrossRef]
- Kaya, Y.; Biricik, O.; Bayqra, S.; Mardani, A. Effect of fibre type and utilisation rate on dimensional stability and frost resistance of pavement mortar mixture. Int. J. Pavement Eng. 2023, 24, 2154351. [Google Scholar] [CrossRef]
- Wang, Z.S.; Xing, L.X.; Zhao, K. Study on corrosion resistance and mechanical property degradation of basalt fiber reinforced concrete under magnesium sulfate erosion environment. Chin. J. Appl. Mech. 2020, 37, 134–141. [Google Scholar] [CrossRef]
- Wu, F.; Yu, Q.; Liu, C.; Brouwers, H.; Wang, L.; Liu, D. Effect of fiber type and content on performance of bio-based concrete containing heat-treated apricot shell. Mater. Struct. 2020, 53, 137. [Google Scholar] [CrossRef]
- Chen, W.F.; Huang, B.; Yuan, Y.X.; Deng, M. Deterioration process of concrete exposed to internal sulfate attack. Materials 2020, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Mehran, K.; Cao, M.L.; Xie, C.P.; Ali, M. Hybrid fiber concrete with different basalt fiber length and content. Struct. Concr. 2021, 23, 346–364. [Google Scholar] [CrossRef]
- Li, T.H.; Zhu, Z.; Wu, T.; Ren, G.Z.; Zhao, G.W. A potential way for improving the dispersivity and mechanical properties of dispersive soil using calcined coal gangue. J. Mater. Res. Technol. 2024, 29, 3049–3062. [Google Scholar] [CrossRef]
- Chang, H.L.; Jin, Z.Q.; Wang, P.G.; Wang, J.H.; Liu, J. Comprehensive resistance of fair-faced concrete suffering from sulfate attack under marine environments. Constr. Build. Mater. 2021, 277, 122312. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Cau, D.C.; Chlique, C.; Dauzeres, A.; Pochard, I. Magnesium and calcium silicate hydrates, Part I: Investigation of the possible magnesium incorporation in calcium silicate hydrate (C-S-H) and of the calcium in magnesium silicate hydrate (M-S-H). Appl. Geochem. 2018, 89, 229–242. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Ji, Y.; She, W.; Yang, L.; Liu, G. A comparable study on the deterioration of limestone powder blended cement under sodium sulfate and magnesium sulfate attack at a low temperature. Constr. Build. Mater. 2020, 243, 118279. [Google Scholar] [CrossRef]
- Wu, Q.; Yoshikawa, N.; Zhai, H.Z. Composite forming simulation of a three-dimensional representative model with random fiber distribution. Comput. Mater. Sci. 2020, 182, 109780. [Google Scholar] [CrossRef]
- Zhao, G.W.; Guo, M.Z.; Li, S.M.; Li, S.M.; Weng, X.L.; Ding, S.J.; Han, F.Z.; Wang, H.R. Influence of Ca2+ on early degradation of cast-in-situ mortar induced by sulfate-magnesium multiple combined attack. Materials 2022, 15, 5752. [Google Scholar] [CrossRef]
- Yi, Y.; Zhu, D.J.; Guo, Z.C.; Zhang, Z.H.; Shi, C.J. A review on the deterioration and approaches to enhance the durability of concrete in the marine environment. Cem. Concr. Compos. 2020, 113, 103695. [Google Scholar] [CrossRef]
- Cai, Y.; Xuan, D.; Hou, P.; Shi, J.; Poon, C.S. Effect of seawater as mixing water on the hydration behaviour of tricalcium aluminate. Cem. Concr. Res. 2021, 149, 106565. [Google Scholar] [CrossRef]
- Kim, G.; Im, S.; Jee, H.; Suh, H.; Cho, S.; Kanematsu, M.; Morooka, S.; Koyama, T.; Machida, A. Effect of magnesium silicate hydrate (M-S-H) formation on the local atomic arrangements and mechanical properties of calcium silicate hydrate (C-S-H): In situ X-ray scattering study. Cem. Concr. Res. 2022, 159, 106869. [Google Scholar] [CrossRef]
- Suma, M.; Santhanam, M.; Rahul, A. The effect of specimen size on deterioration due to external sodium sulphate attack in full immersion studies. Cem. Concr. Compos. 2020, 114, 103806. [Google Scholar] [CrossRef]
- Metalssi, O.; Ragoug, R.; Barberon, F.; De Lacaillerie, J.; Roussel, N.; Divet, L.; Torrenti, J. Effect of an early-age exposure on the degradation mechanisms of cement paste under external sulfate attack. Materials 2023, 16, 6013. [Google Scholar] [CrossRef] [PubMed]


| Components of Cement | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | TiO 2 | Na2O | Cl |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 67.21 | 18.48 | 5.64 | 3.63 | 2.40 | 0.75 | 0.68 | 0.51 | 0.31 | 0.08 |
| Physical properties of basalt fiber | Length | Diameter | Tensile strength | Elastic modulus | Density | |||||
| Index | 12 mm | 17 µm | 3000–4800 MPa | 90–100 GPa | 2.80 gꞏcm−3 | |||||
| Specimens in Distilled Water | Specimens in Sulfate Solutions | Premixed Salts | Volume of BF |
|---|---|---|---|
| D-C | S-C | No premixed salts | 0 |
| D-M | S-M | 3% MgSO4 | 0 |
| D-1F | S-1F | 3% MgSO4 | 0.1% |
| D-3F | S-3F | 3% MgSO4 | 0.3% |
| D-5F | S-5F | 3% MgSO4 | 0.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Wang, C.; Hu, Y.; Zhao, G. Durability Performance of Basalt Fiber-Reinforced Concrete Subjected to Sulfate–Magnesium Combined Attack. Materials 2024, 17, 1128. https://doi.org/10.3390/ma17051128
Fan H, Wang C, Hu Y, Zhao G. Durability Performance of Basalt Fiber-Reinforced Concrete Subjected to Sulfate–Magnesium Combined Attack. Materials. 2024; 17(5):1128. https://doi.org/10.3390/ma17051128
Chicago/Turabian StyleFan, Henghui, Cheng Wang, Yiqi Hu, and Gaowen Zhao. 2024. "Durability Performance of Basalt Fiber-Reinforced Concrete Subjected to Sulfate–Magnesium Combined Attack" Materials 17, no. 5: 1128. https://doi.org/10.3390/ma17051128
APA StyleFan, H., Wang, C., Hu, Y., & Zhao, G. (2024). Durability Performance of Basalt Fiber-Reinforced Concrete Subjected to Sulfate–Magnesium Combined Attack. Materials, 17(5), 1128. https://doi.org/10.3390/ma17051128






