Myriophyllum Biochar-Supported Mn/Mg Nano-Composites as Efficient Periodate Activators to Enhance Triphenyl Phosphate Removal from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
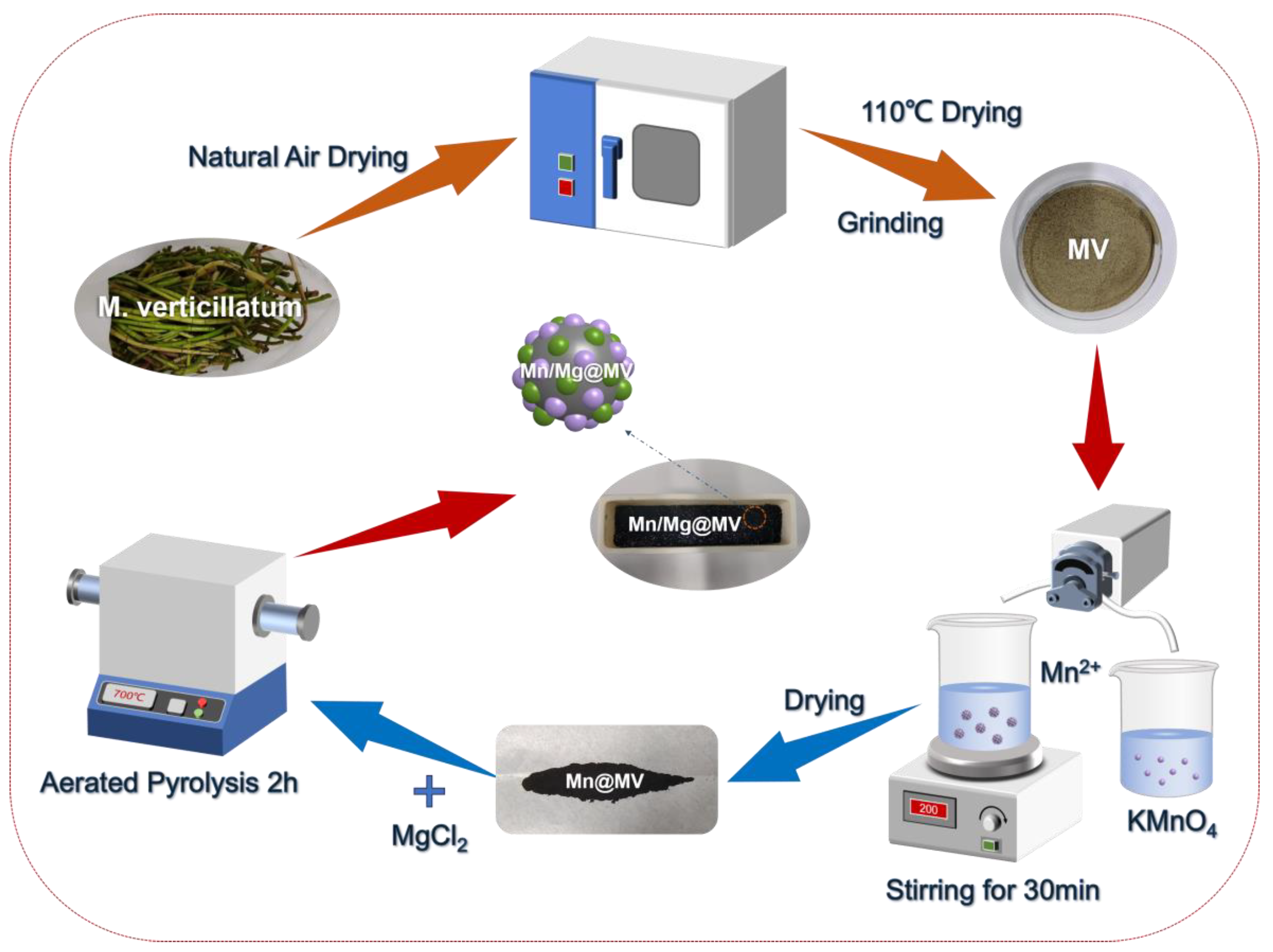
2.2. Synthesis of Mn/Mg@MV
2.3. Batch Removal Experiments
2.4. Characterization and Analytical Methods
2.5. DFT Calculations
3. Results and Discussion
3.1. Synthesis and Characterization of Mn/Mg@MV
3.2. Performance of Mn and Mg Bimetallic-Doped Myriophyllum verticillatum Biochar-Activated PI for TPhP Degradation
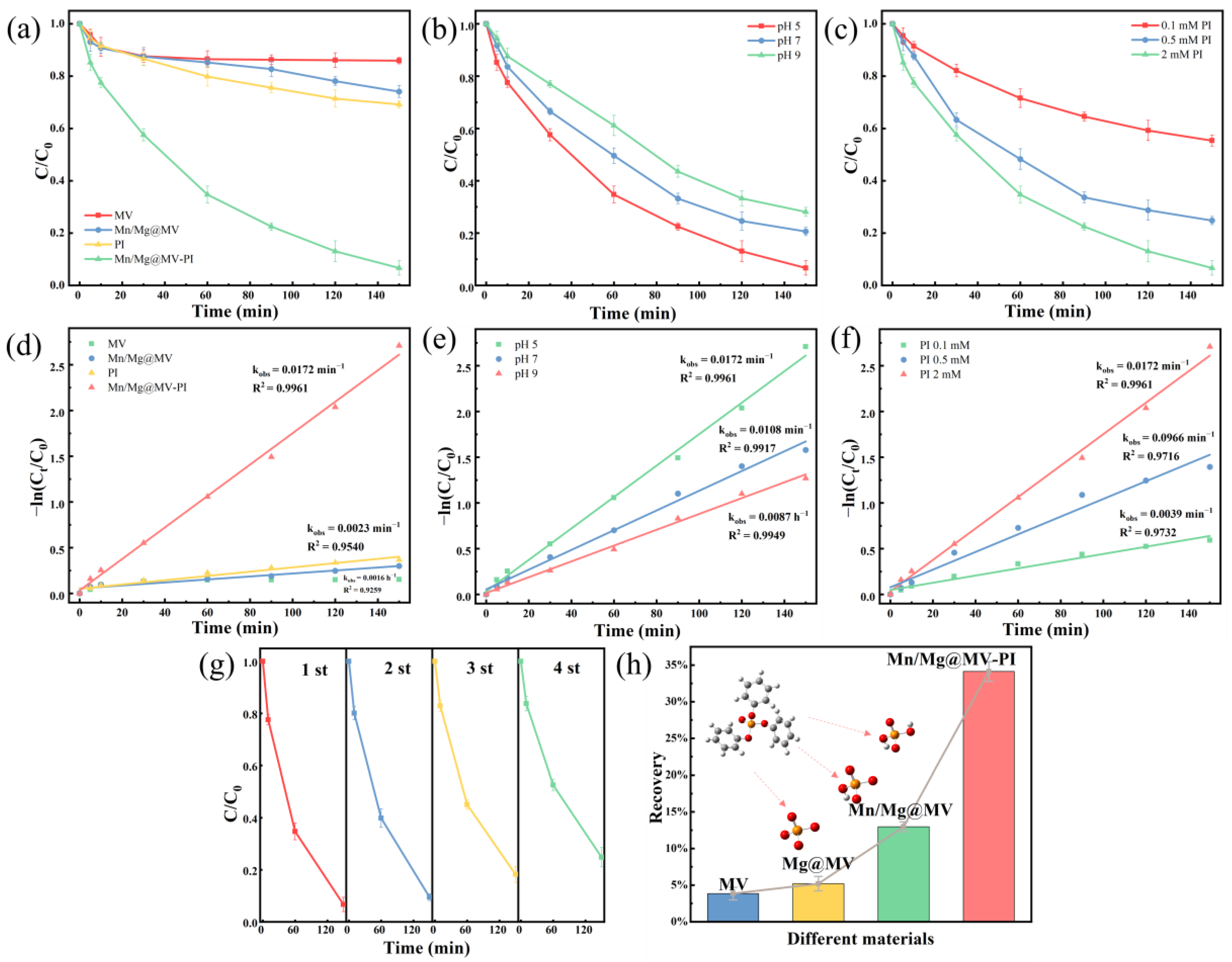
3.2.1. TPhP Removal Performance of Different Materials
3.2.2. Effect of Initial pH and Initial PI Dose
3.2.3. Evaluation of Reusability and Removal of Inorganic Phosphorus Degradation Residue
3.3. Mechanism Analysis
3.3.1. FTIR–XPS coupling analysis before and after degradation of TPhP by Mn/Mg@MV-PI
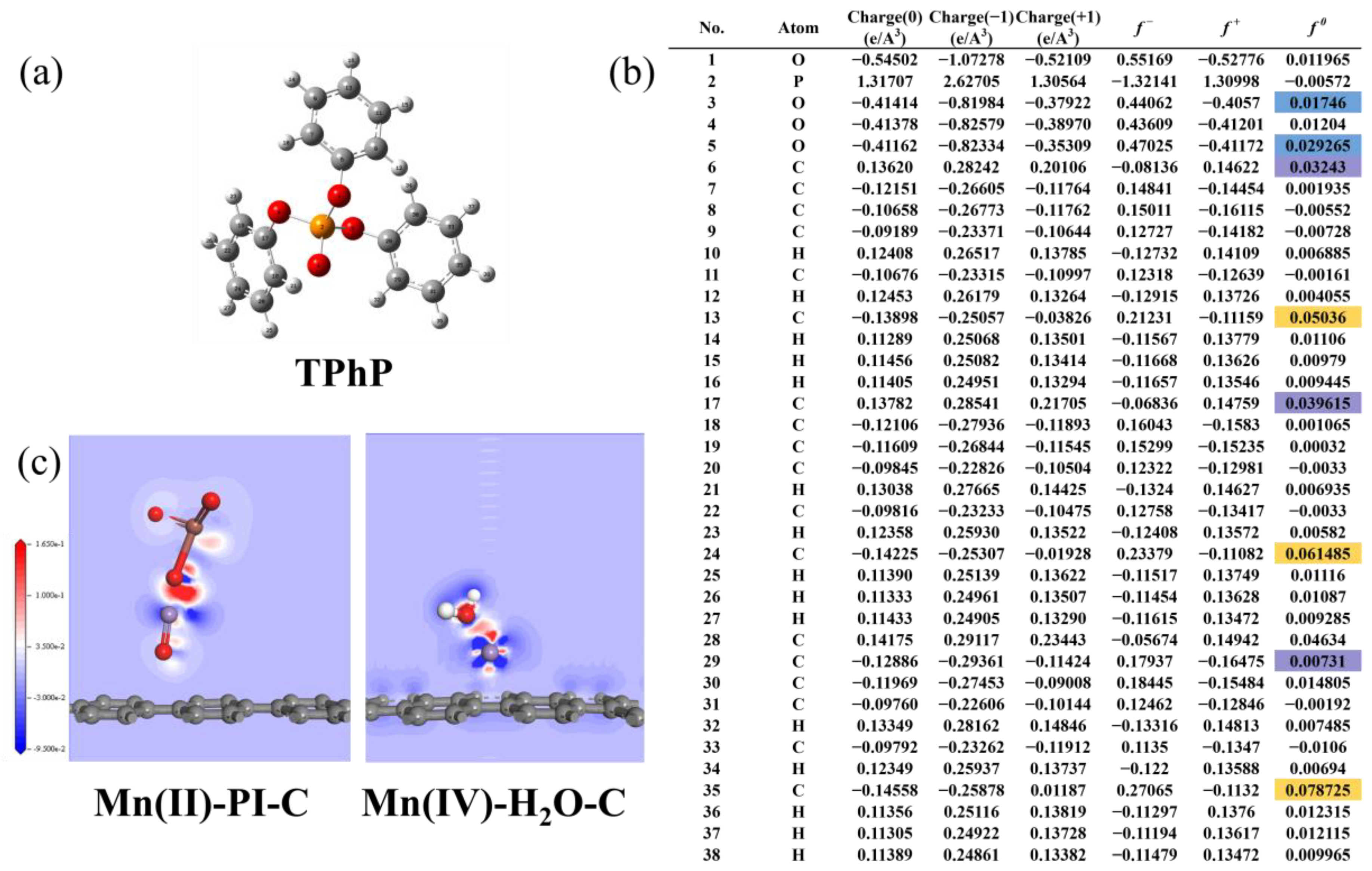
3.3.2. Fukui Function for Susceptible Site Prediction and Electron Transfer Pathway DFT Calculations
3.3.3. Possible Mechanisms of PI Activation on Mn/Mg@MV and the Degradation Pathway of TPhP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Y.; Stubbings, W.A.; Abdallah, M.A.-E.; Cline-Cole, R.; Harrad, S. Formal waste treatment facilities as a source of halogenated flame retardants and organophosphate esters to the environment: A critical review with particular focus on outdoor air and soil. Sci. Total Environ. 2022, 807, 150747. [Google Scholar] [CrossRef]
- Gbadamosi, M.R.; Abdallah, M.A.-E.; Harrad, S. A critical review of human exposure to organophosphate esters with a focus on dietary intake. Sci. Total Environ. 2021, 771, 144752. [Google Scholar] [CrossRef]
- Fabiańska, M.J.; Kozielska, B.; Konieczyński, J.; Bielaczyc, P. Occurrence of organic phosphates in particulate matter of the vehicle exhausts and outdoor environment—A case study. Environ. Pollut. 2019, 244, 351–360. [Google Scholar] [CrossRef]
- Xiao, K.; Lu, Z.; Yang, C.; Zhao, S.; Zheng, H.; Gao, Y.; Kaluwin, C.; Liu, Y.; Cai, M. Occurrence, distribution and risk assessment of organophosphate ester flame retardants and plasticizers in surface seawater of the West Pacific. Mar. Pollut. Bull. 2021, 170, 112691. [Google Scholar] [CrossRef]
- Estill, C.F.; Mayer, A.; Slone, J.; Chen, I.C.; Zhou, M.; La Guardia, M.J.; Jayatilaka, N.; Ospina, M.; Calafat, A. Assessment of triphenyl phosphate (TPhP) exposure to nail salon workers by air, hand wipe, and urine analysis. Int. J. Hyg. Environ. Health 2021, 231, 113630. [Google Scholar] [CrossRef]
- Pantelaki, I.; Voutsa, D. Occurrence and removal of organophosphate esters in municipal wastewater treatment plants in Thessaloniki, Greece. Environ. Res. 2022, 214, 113908. [Google Scholar] [CrossRef]
- Gopal, G.; Alex, S.A.; Chandrasekaran, N.; Mukherjee, A. A review on tetracycline removal from aqueous systems by advanced treatment techniques. RSC Adv. 2020, 10, 27081–27095. [Google Scholar] [CrossRef] [PubMed]
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Manganese oxides at different oxidation states for heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2013, 142–143, 729–735. [Google Scholar] [CrossRef]
- Yun, E.-T.; Park, S.-W.; Shin, H.J.; Lee, H.; Kim, D.-W.; Lee, J. Peroxymonosulfate activation by carbon-encapsulated metal nanoparticles: Switching the primary reaction route and increasing chemical stability. Appl. Catal. B Environ. 2020, 279, 119360. [Google Scholar] [CrossRef]
- Cristale, J.; Dantas, R.F.; De Luca, A.; Sans, C.; Esplugas, S.; Lacorte, S. Role of oxygen and DOM in sunlight induced photodegradation of organophosphorous flame retardants in river water. J. Hazard. Mater. 2017, 323, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Feng, Y.; Wang, Z.; Liu, G.; Lv, W. Degradation of triphenyl phosphate (TPhP) by CoFe2O4-activated peroxymonosulfate oxidation process: Kinetics, pathways, and mechanisms. Sci. Total Environ. 2019, 681, 331–338. [Google Scholar] [CrossRef]
- Hou, R.; Luo, X.; Liu, C.; Zhou, L.; Wen, J.; Yuan, Y. Enhanced degradation of triphenyl phosphate (TPHP) in bioelectrochemical systems: Kinetics, pathway and degradation mechanisms. Environ. Pollut. 2019, 254, 113040. [Google Scholar] [CrossRef]
- Marcińczyk, M.; Oleszczuk, P. Biochar and engineered biochar as slow- and controlled-release fertilizers. J. Clean. Prod. 2022, 339, 130685. [Google Scholar] [CrossRef]
- Chabi, N.; Baghdadi, M.; Sani, A.H.; Golzary, A.; Hosseinzadeh, M. Removal of tetracycline with aluminum boride carbide and boehmite particles decorated biochar derived from algae. Bioresour. Technol. 2020, 316, 123950. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.; Obaeed, G.L.O.; Sulkarnaev, F.; Buchkina, N.; Gubin, A.; Yurtaev, A. Effect of biochar aging in agricultural soil on its wetting properties and surface structure. Biochar 2023, 5, 75. [Google Scholar] [CrossRef]
- Sochacki, A.; Lebrun, M.; Minofar, B.; Pohořelý, M.; Vithanage, M.; Sarmah, A.K.; Böserle Hudcová, B.; Buchtelík, S.; Trakal, L. Adsorption of common greywater pollutants and nutrients by various biochars as potential amendments for nature-based systems: Laboratory tests and molecular dynamics. Environ. Pollut. 2024, 343, 123203. [Google Scholar] [CrossRef]
- Viswanthan, S.P.; Neelamury, S.P.; Parakkuzhiyil, S.; Njazhakunnathu, G.V.; Sebastian, A.; Padmakumar, B.; Ambatt, T.P. Removal efficiency of methylene blue from aqueous medium using biochar derived from Phragmites karka, a highly invasive wetland weed. Biomass Convers. Biorefinery 2022, 12, 3257–3273. [Google Scholar] [CrossRef]
- Kahkeci, J.; Aoudi, B.; Sánchez-Montes, I.; Gamal El-Din, M. Engineered biochar supported bismuth tungstate: Unveiling the influence of precursor concentrations and biochar dosage for the solar photocatalysis of 1,3-diphenylguanidine in secondary municipal effluent. Chem. Eng. J. 2024, 483, 149142. [Google Scholar] [CrossRef]
- Masud, M.A.A.; Annamalai, S.; Shin, W.S. Remediation of ciprofloxacin in soil using peroxymonosulfate activated by ball-milled seaweed kelp biochar: Performance, mechanism, and phytotoxicity. Chem. Eng. J. 2023, 465, 142908. [Google Scholar] [CrossRef]
- Monisha, R.S.; Mani, R.L.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Green remediation of pharmaceutical wastes using biochar: A review. Environ. Chem. Lett. 2022, 20, 681–704. [Google Scholar] [CrossRef]
- Dung, N.T.; Thao, V.D.; Thao, N.P.; Thuy, C.T.M.; Nam, N.H.; Ngan, L.V.; Lin, K.-Y.A.; Khiem, T.C.; Huy, N.N. Turning peroxymonosulfate activation into singlet oxygen-dominated pathway for ofloxacin degradation by co-doping N and S into durian peel-derived biochar. Chem. Eng. J. 2024, 483, 149099. [Google Scholar] [CrossRef]
- Leichtweis, J.; Carissimi, E.; Hagemann, U.; Ulbricht, M.; Fischer, L. NiFe2O4/biochar decorated porous polymer membranes for the flow-through photo-Fenton degradation of tetracycline. Chem. Eng. J. 2023, 477, 147203. [Google Scholar] [CrossRef]
- Peng, Y.; Luo, Y.; Li, Y.; Azeem, M.; Li, R.; Feng, C.; Qu, G.; Ali, E.F.; Hamouda, M.A.; Hooda, P.S.; et al. Effect of corn pre-puffing on the efficiency of MgO-engineered biochar for phosphorus recovery from livestock wastewater: Mechanistic investigations and cost benefit analyses. Biochar 2023, 5, 26. [Google Scholar] [CrossRef]
- Yu, Y.; Zhong, Z.; Guo, H.; Yu, Y.; Zheng, T.; Li, H.; Chang, Z. Biochar–goethite composites inhibited/enhanced degradation of triphenyl phosphate by activating persulfate: Insights on the mechanism. Sci. Total Environ. 2023, 858, 159940. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Speed, D.; Siriwardena, D.; Fernando, S.; Thagard, S.M.; Holsen, T.M. Comparison of hydrogen peroxide-based advanced oxidation processes for the treatment of azole-containing industrial wastewater. Chem. Eng. J. 2021, 425, 131785. [Google Scholar] [CrossRef]
- Sukhatskiy, Y.; Shepida, M.; Sozanskyi, M.; Znak, Z.; Gogate, P.R. Periodate-based advanced oxidation processes for wastewater treatment: A review. Sep. Purif. Technol. 2023, 304, 122305. [Google Scholar] [CrossRef]
- Chadi, N.E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. H2O2/periodate (IO4−): A novel advanced oxidation technology for the degradation of refractory organic pollutants. Environ. Sci. Water Res. Technol. 2019, 5, 1113–1123. [Google Scholar] [CrossRef]
- Chakraborty, B.; Gan-Or, G.; Raula, M.; Gadot, E.; Weinstock, I.A. Design of an inherently-stable water oxidation catalyst. Nat. Commun. 2018, 9, 4896. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- López, Y.C.; Viltres, H.; Gupta, N.K.; Acevedo-Peña, P.; Leyva, C.; Ghaffari, Y.; Gupta, A.; Kim, S.; Bae, J.; Kim, K.S. Transition metal-based metal–organic frameworks for environmental applications: A review. Environ. Chem. Lett. 2021, 19, 1295–1334. [Google Scholar] [CrossRef]
- Song, Q.; Feng, Y.; Liu, G.; Lv, W. Degradation of the flame retardant triphenyl phosphate by ferrous ion-activated hydrogen peroxide and persulfate: Kinetics, pathways, and mechanisms. Chem. Eng. J. 2019, 361, 929–936. [Google Scholar] [CrossRef]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient degradation method of emerging organic pollutants in marine environment using UV/periodate process: Case of chlorazol black. Mar. Pollut. Bull. 2018, 126, 557–564. [Google Scholar] [CrossRef]
- Ewais, H.A. Kinetics and mechanism of the oxidation of a ternary complex of cobalt(II) involving nitrilotriacetate and benzoate by periodate in acetate medium. Evidence for manganese(II) as a catalyst. J. Coord. Chem. 2009, 62, 940–950. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hasan, M.R.; Abd Hamid, S.B.; Marlina Samsudin, E.; Zain, S.M.; Khalid, K. Catalytic pretreatment of biochar residues derived from lignocellulosic feedstock for equilibrium studies of manganese, Mn(ii) cations from aqueous solution. RSC Adv. 2015, 5, 6345–6356. [Google Scholar] [CrossRef]
- Liu, D.; Guo, A.; Qi, Y.; Ji, Z.; Li, H.; Cao, X.; Zhang, Z.; Zhang, X.; Wu, K.; Cai, A. Activation of persulfate by magnetic Mg/Mn–layered double oxide–doped biochar composite for ciprofloxacin removal and bacterial inactivation. Sep. Purif. Technol. 2024, 329, 125322. [Google Scholar] [CrossRef]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- Xi, H.; Jiang, H.; Zhao, D.; Zhang, A.H.; Fan, B.; Yang, Y.; Zhang, J. Highly selective adsorption of phosphate from high-salinity water environment using MgO-loaded and sodium alginate-immobilized bentonite beads. J. Clean. Prod. 2021, 313, 127773. [Google Scholar] [CrossRef]
- Kang, M.W.; Yibeltal, M.; Kim, Y.H.; Oh, S.J.; Lee, J.C.; Kwon, E.E.; Lee, S.S. Enhancement of soil physical properties and soil water retention with biochar-based soil amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef] [PubMed]
- Piash, M.I.; Iwabuchi, K.; Itoh, T. Synthesizing biochar-based fertilizer with sustained phosphorus and potassium release: Co-pyrolysis of nutrient-rich chicken manure and Ca-bentonite. Sci. Total Environ. 2022, 822, 153509. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Xue, Y.; Inyang, M. Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem. Eng. J. 2012, 210, 26–32. [Google Scholar] [CrossRef]
- Gonçalves, M.A.F.; da Silva, B.R.S.; Nobre, J.R.C.; Batista, B.L.; da Silva Lobato, A.K. Biochar Mitigates the Harmful Effects of Drought in Soybean Through Changes in Leaf Development, Stomatal Regulation, and Gas Exchange. J. Soil Sci. Plant Nutr. 2024, 1–12. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Mandal, J.; Sharma, P.K.; Mondal, D.; Wood, M.D.; Hutchinson, S.M.; Kirby, J.; Srivastava, P. Meta-Analysis of Biochar as an Amendment for Arsenic Mitigation in Paddy Soils. Curr. Pollut. Rep. 2024, 1–14. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Michalak, I.; Baśladyńska, S.; Mokrzycki, J.; Rutkowski, P. Biochar from A Freshwater Macroalga as A Potential Biosorbent for Wastewater Treatment. Water 2019, 11, 1390. [Google Scholar] [CrossRef]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Baghernejad, M. Determining organo-chemical composition of sugarcane bagasse-derived biochar as a function of pyrolysis temperature using proximate and Fourier transform infrared analyses. J. Therm. Anal. Calorim. 2019, 138, 331–342. [Google Scholar] [CrossRef]
- Peng, P.; Lang, Y.-H.; Wang, X.-M. Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 2016, 90, 225–233. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, L.; Zhang, X.; Li, J.; Li, Y.; Peng, Y.; Luo, Y.; Li, R.; Gao, B.; Hamouda, M.A.; et al. Bio-assembled MgO-coated tea waste biochar efficiently decontaminates phosphate from water and kitchen waste fermentation liquid. Biochar 2023, 5, 22. [Google Scholar] [CrossRef]
- Hu, J.; Gong, H.; Liu, X.; Luo, J.; Zhu, N. Target-prepared sludge biochar-derived synergistic Mn and N/O induces high-performance periodate activation for reactive iodine radicals generation towards ofloxacin degradation. J. Hazard. Mater. 2023, 460, 132362. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, S.Y.; Jung, M.; Lee, K.; Nah, Y.-C.; Attia, N.F.; Oh, H. Efficient synthetic approach for nanoporous adsorbents capable of pre- and post-combustion CO2 capture and selective gas separation. J. CO2 Util. 2021, 45, 101404. [Google Scholar] [CrossRef]
- Isa, S.A.; Hafeez, M.A.; Singh, B.K.; Kwon, S.Y.; Choung, S.; Um, W. Efficient mercury sequestration from wastewaters using palm kernel and coconut shell derived biochars. Environ. Adv. 2022, 8, 100196. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Z.; Yang, K.; Yu, Z.; Zuo, Y.; Cheng, L.; Wang, Y.; Sun, H.; Yu, G.; Zhang, C.; et al. Periodate activated by different crystalline phases MnO2 for profound oxidation tetracycline hydrochloride: Oxygen vacancy-dominated active pivots and mechanism. Sep. Purif. Technol. 2022, 301, 122022. [Google Scholar] [CrossRef]
- Saini, K.; Kaushik, R.D.; Singh, J.; Manila; Chawla, M. An approach to the activation energy, thermodynamics, and flow injection kinetics of degradation of 2,4-dimethylaniline. Sustain. Energy Technol. Assess. 2022, 53, 102454. [Google Scholar] [CrossRef]
- Hu, J.; Zou, Y.; Li, Y.; Yu, Z.; Bao, Y.; Lin, L.; Li, B.; Li, X.-Y. Periodate activation by atomically dispersed Mn on carbon nanotubes for the production of iodate radicals and rapid degradation of sulfadiazine. Chem. Eng. J. 2023, 472, 144862. [Google Scholar] [CrossRef]
- Zhang, K.; Ye, C.; Lou, Y.; Yu, X.; Feng, M. Promoting selective water decontamination via boosting activation of periodate by nanostructured Ru-supported Co3O4 catalysts. J. Hazard. Mater. 2023, 442, 130058. [Google Scholar] [CrossRef]
- Parvizi, T.; Parsa, J.B. High-efficient photocatalytic fuel cell integrated with periodate activation for electricity production by degradation of refractory organics. J. Power Sources 2021, 484, 229264. [Google Scholar] [CrossRef]
- Shi, W.; Wang, H.; Yan, J.; Shan, L.; Quan, G.; Pan, X.; Cui, L. Wheat straw derived biochar with hierarchically porous structure for bisphenol A removal: Preparation, characterization, and adsorption properties. Sep. Purif. Technol. 2022, 289, 120796. [Google Scholar] [CrossRef]
- Duan, X.; Zhao, T.; Niu, B.; Wei, Z.; Li, G.; Zhang, Z.; Cheng, J.; Hao, Z. Simultaneously Constructing Active Sites and Regulating Mn–O Strength of Ru-Substituted Perovskite for Efficient Oxidation and Hydrolysis Oxidation of Chlorobenzene. Adv. Sci. 2023, 10, 2205054. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, J.J.; Dangal, P.; Lomnicki, S.; Roy, A.D.; Park, J.-H. A novel sugarcane residue-derived bimetallic Fe/Mn-biochar composite for activation of peroxymonosulfate in advanced oxidation process removal of azo dye: Degradation behavior and mechanism. J. Water Process Eng. 2024, 58, 104740. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B.; Song, Y. Facile synthesis of sludge-derived MnOx-N-biochar as an efficient catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2019, 255, 117765. [Google Scholar] [CrossRef]
- Liu, S.; Ji, J.; Yu, Y.; Huang, H. Facile synthesis of amorphous mesoporous manganese oxides for efficient catalytic decomposition of ozone. Catal. Sci. Technol. 2018, 8, 4264–4273. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Yan, Y.; Ma, R.; Zhang, X.; Wang, J.; Shen, Y.; Ullah, H.; Lu, L. Removal of organophosphorus flame retardant by biochar-coated nZVI activating persulfate: Synergistic mechanism of adsorption and catalytic degradation. Environ. Pollut. 2023, 331, 121880. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Chen, R.; Song, Y.; Shen, Y.; Song, F.; He, B.; Jiang, X.; Yin, Y.; Wang, W. Myriophyllum Biochar-Supported Mn/Mg Nano-Composites as Efficient Periodate Activators to Enhance Triphenyl Phosphate Removal from Wastewater. Materials 2024, 17, 1118. https://doi.org/10.3390/ma17051118
Xie H, Chen R, Song Y, Shen Y, Song F, He B, Jiang X, Yin Y, Wang W. Myriophyllum Biochar-Supported Mn/Mg Nano-Composites as Efficient Periodate Activators to Enhance Triphenyl Phosphate Removal from Wastewater. Materials. 2024; 17(5):1118. https://doi.org/10.3390/ma17051118
Chicago/Turabian StyleXie, Hanyun, Runhua Chen, Yuxia Song, Yan Shen, Fengming Song, Bo He, Xiaomei Jiang, Yifan Yin, and Wenming Wang. 2024. "Myriophyllum Biochar-Supported Mn/Mg Nano-Composites as Efficient Periodate Activators to Enhance Triphenyl Phosphate Removal from Wastewater" Materials 17, no. 5: 1118. https://doi.org/10.3390/ma17051118
APA StyleXie, H., Chen, R., Song, Y., Shen, Y., Song, F., He, B., Jiang, X., Yin, Y., & Wang, W. (2024). Myriophyllum Biochar-Supported Mn/Mg Nano-Composites as Efficient Periodate Activators to Enhance Triphenyl Phosphate Removal from Wastewater. Materials, 17(5), 1118. https://doi.org/10.3390/ma17051118





