Study of the Cold Curing Characteristics of Isocyanate-Modified Asphalt
Abstract
1. Introduction
2. Experiments
2.1. Raw Materials
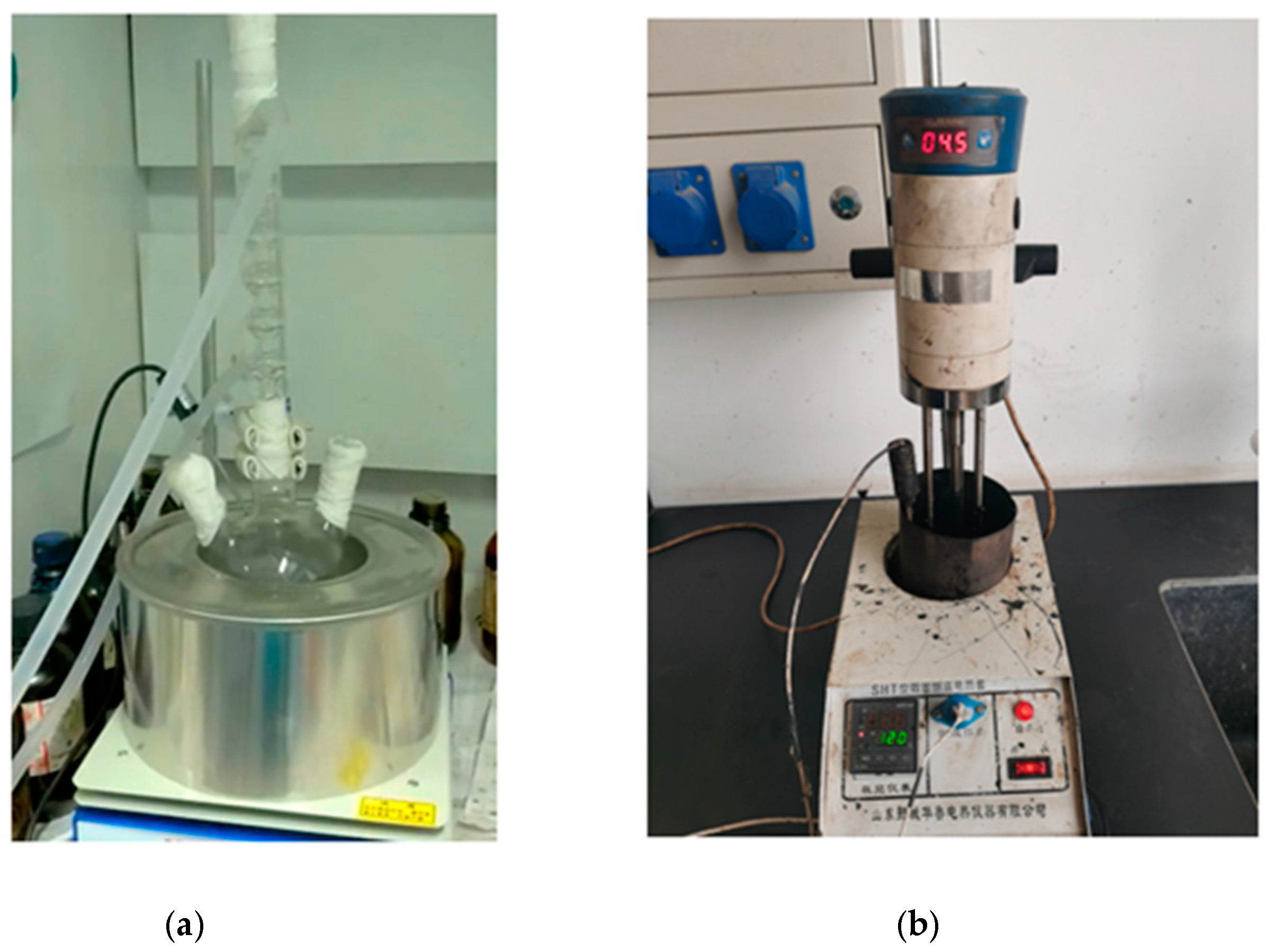
2.2. Preparation Method of MDI-Modified Asphalt
2.3. Technical Properties of MDI-Modified Asphalt
- (1)
- As the MDI content increases, the softening point of MDI-modified asphalt gradually decreases, while the ductility increases. This indicates that the addition of MDI worsens the high-temperature performance of the asphalt but enhances its low-temperature performance.
- (2)
- The penetration value of modified asphalt is inversely proportional to the amount of rock asphalt added. With an increase in the rock asphalt content, the penetration value of modified asphalt gradually decreases. Additionally, as the rock asphalt content increases, the high-temperature performance of MDI asphalt improves: the penetration value decreases gradually, and the softening point increases. This suggests that the addition of rock asphalt has a positive impact on enhancing the high-temperature performance of the modified asphalt.
- (3)
- Based on the trend of the penetration, softening point, and ductility indicators of MDI-modified asphalt with varying rock asphalt contents, it was found that when the rock asphalt content is 15% and the MDI-modified asphalt content is 15%, the comprehensive performance of the modified asphalt is relatively balanced. Therefore, this study selected a mixture of 15% rock asphalt and 15% MDI-modified asphalt as the research subject.
3. Molecular Dynamics Simulation
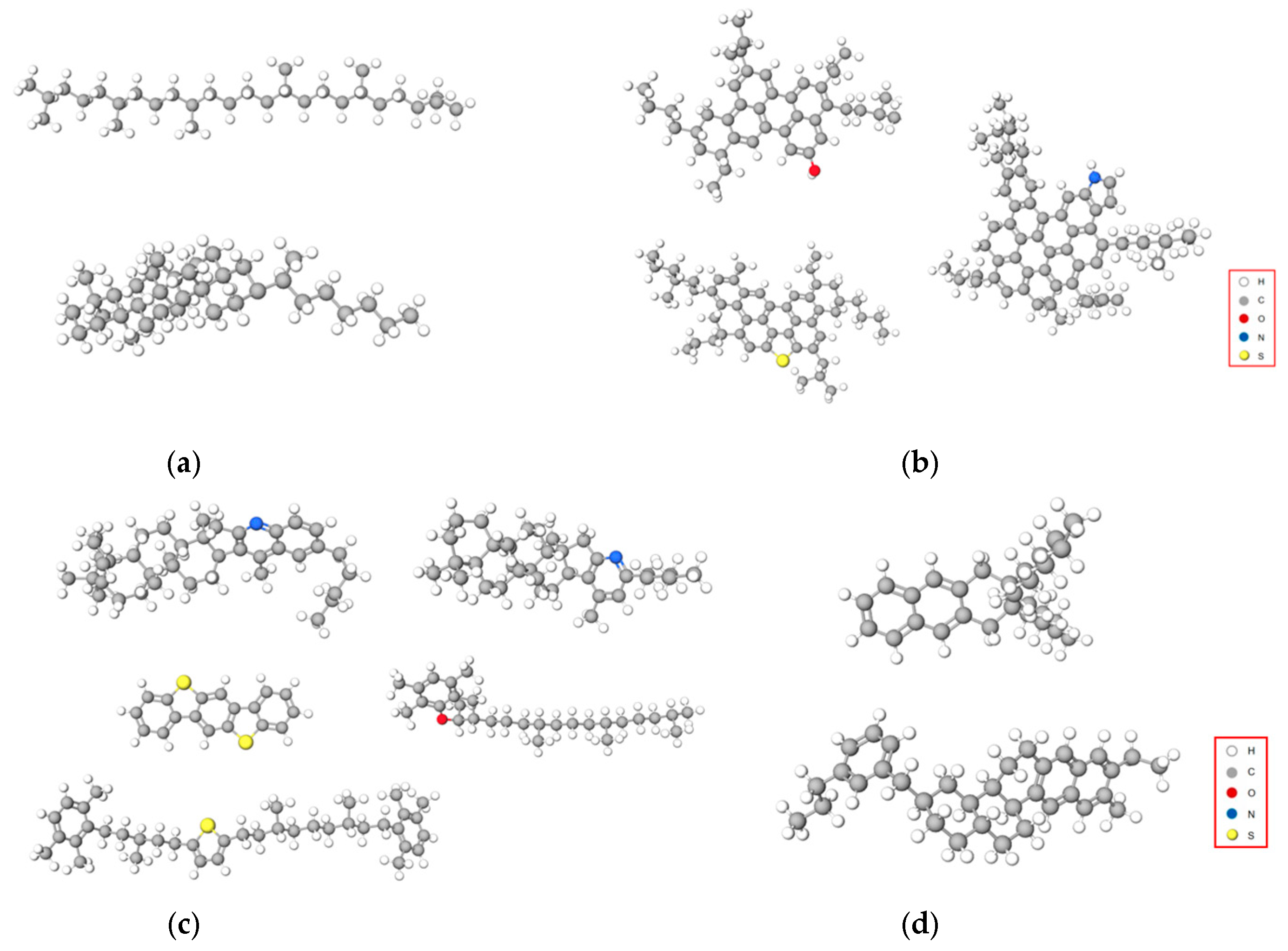
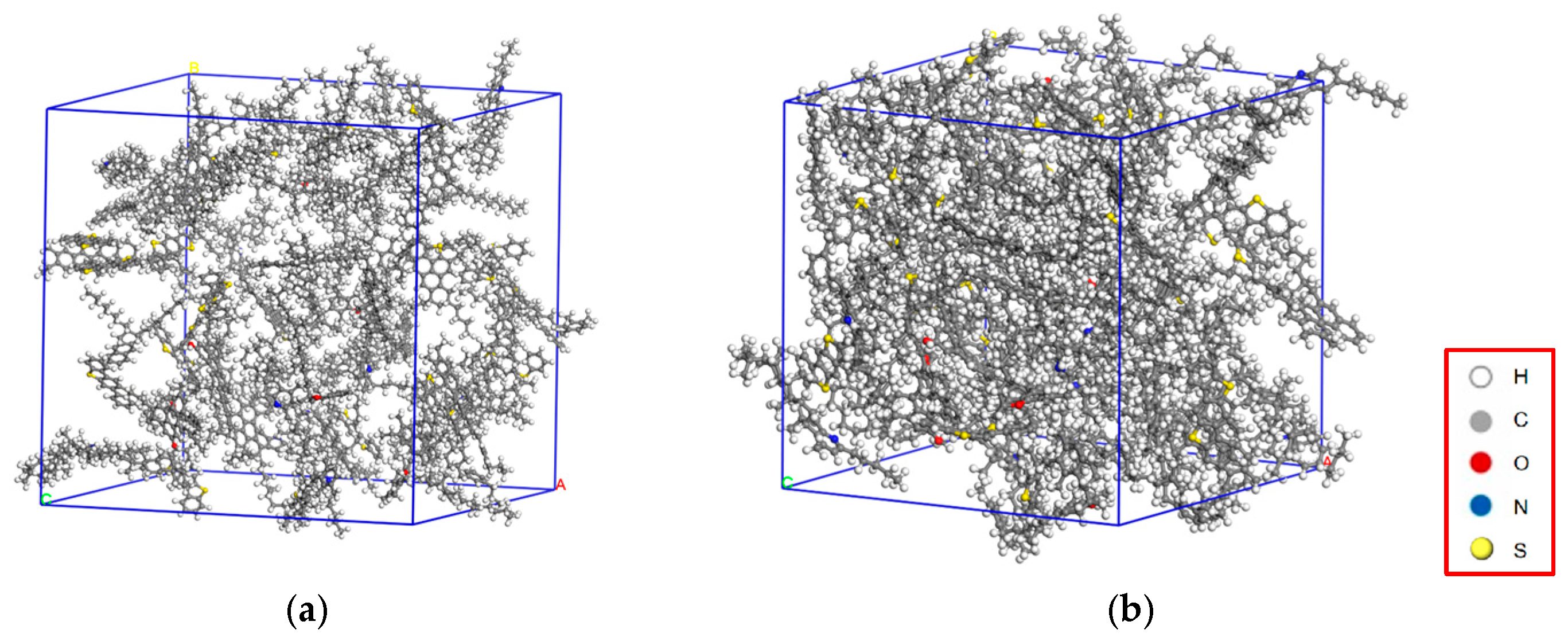
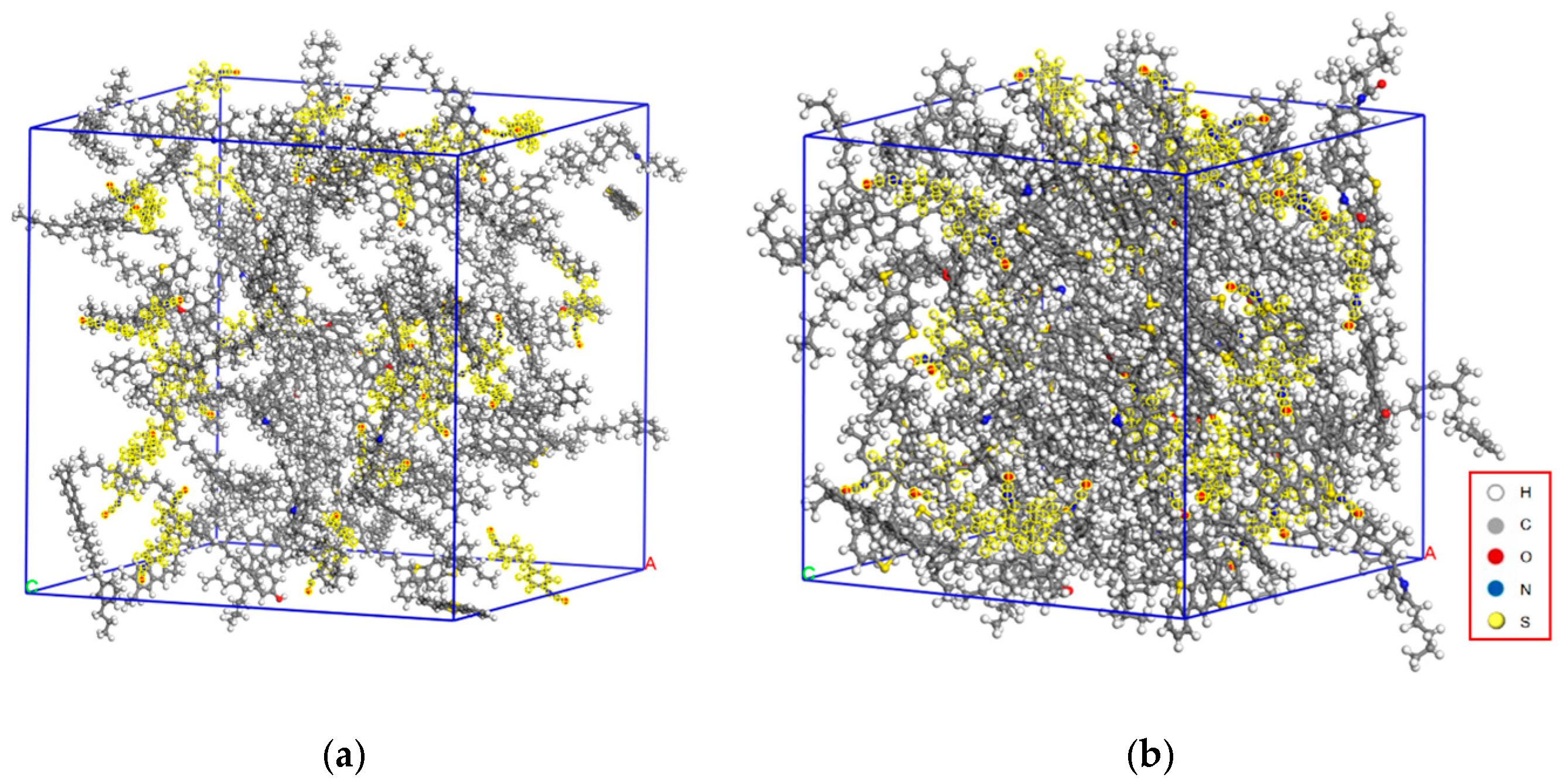
3.1. Molecular Modeling of Asphalt Components and MDI-Modified Asphalt
3.2. Analysis and Discussion
3.2.1. Density Analysis
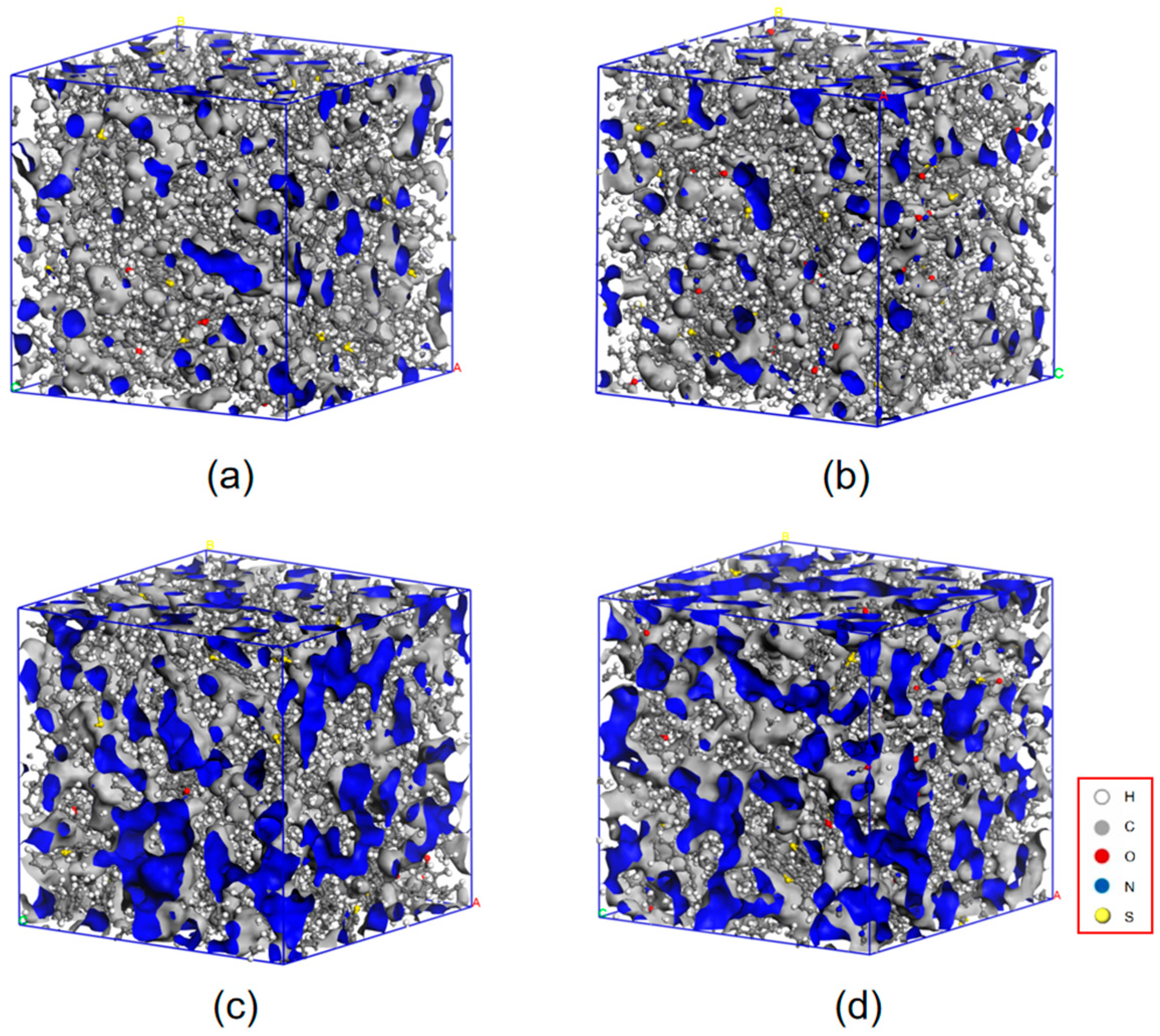
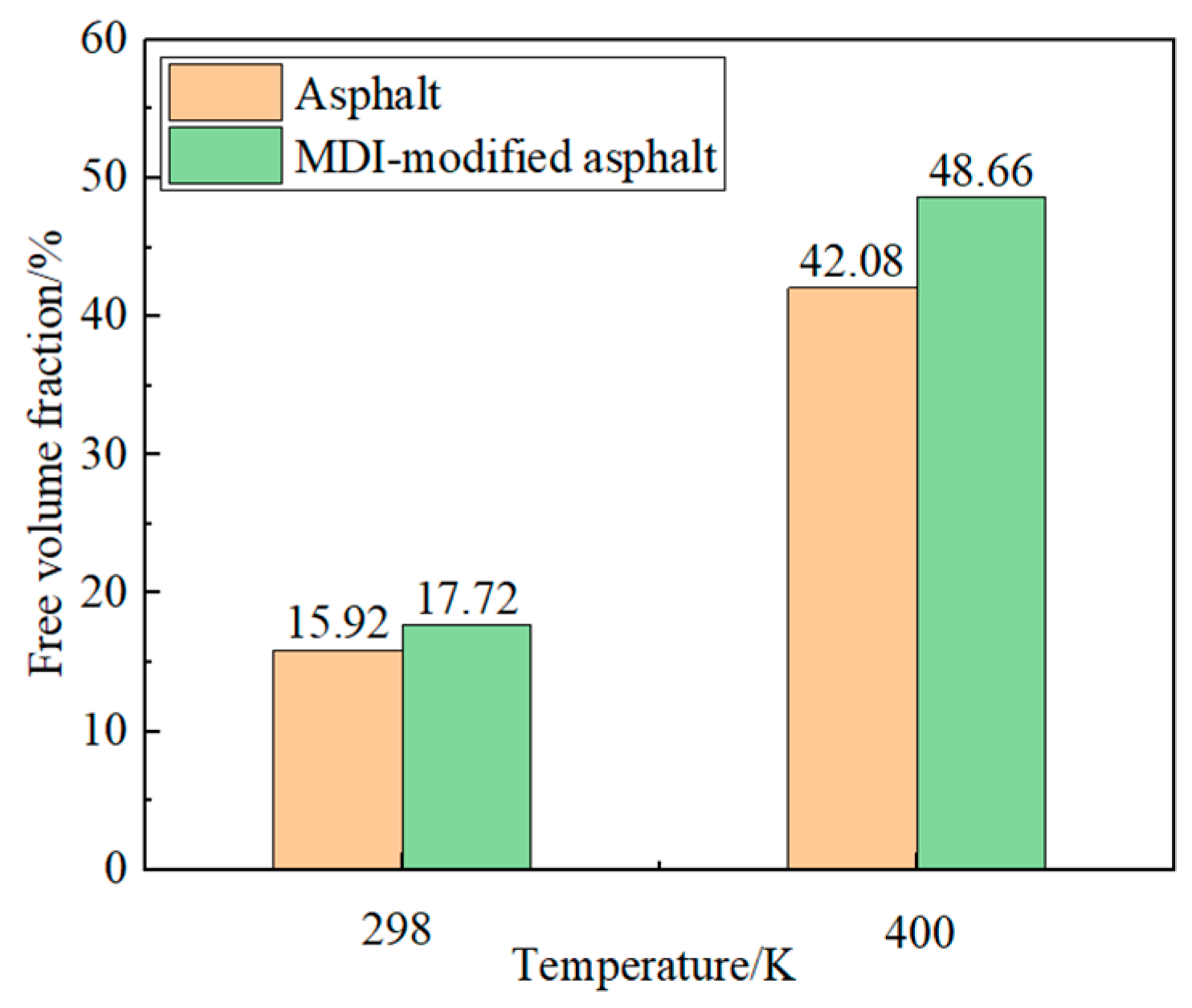
3.2.2. Free Volume Analysis
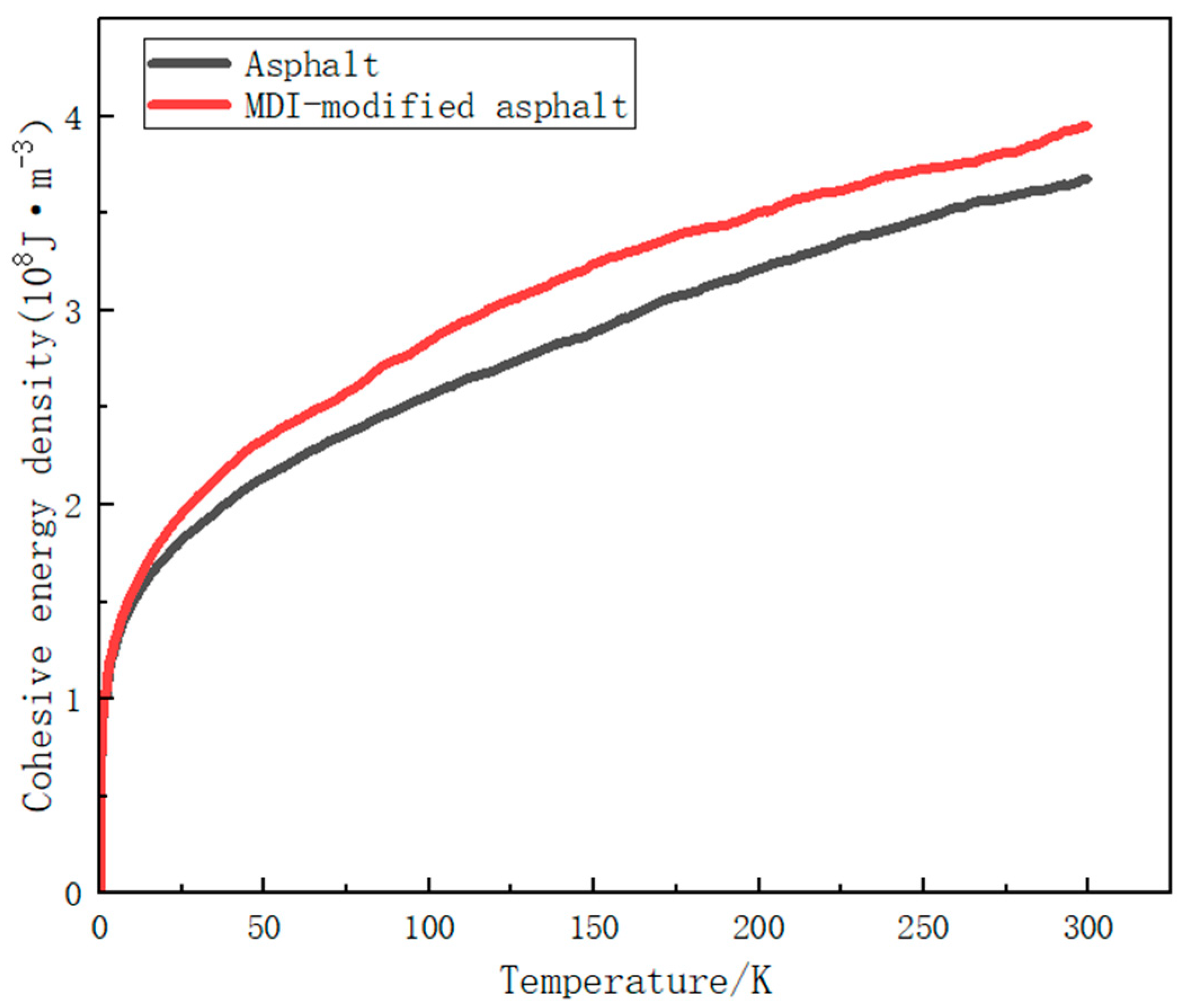
3.2.3. Cohesive Energy Density
3.2.4. Glass Transition Temperature
4. Asphalt Mixture Performance
4.1. Marshall Stability
4.2. High-Temperature Performance
4.3. Water Damage Resistance
5. Conclusions
- (1)
- Due to the potential decrease in the softening point and compromised high-temperature performance caused by the addition of MDI, rock asphalt was employed for further improvement. Following tests and analyses of various blends of MDI-modified asphalt with different incorporation levels of rock asphalt, a mixture of 15% rock asphalt and 15% MDI-modified asphalt demonstrated a more balanced overall performance.
- (2)
- Utilizing Materials Studio software, an MDI-modified asphalt model was simulated, and its fundamental properties, such as density, free volume, cohesive energy density, and glass transition temperature, were evaluated. The results indicate that the MDI-modified asphalt exhibits enhanced resistance to compression, durability, and cracking, and stability. These improvements contribute to enhanced curing performance, ultimately improving pavement stability and longevity, thereby reducing pavement damage.
- (3)
- Taking LB-13 gradation as an example, MDI-modified cold-mixed asphalt was prepared. The mechanical properties, high-temperature performance, and resistance to water damage were tested according to China’s specifications. The results indicate that the stability of the MDI-modified cold-mixed asphalt prepared here exceeds the requirements for hot-mixed asphalt (AC) specified in the Chinese standards. High-temperature rutting tests and resistance to water damage demonstrated that the prepared MDI-modified cold-mixed asphalt performs notably better in resisting rutting at high temperatures and water damage, consistent with the hot-mixed asphalt mixture.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulyman, M.; Sienkiewicz, M.; Haponiuk, J. Asphalt pavement material improvement: A review. Int. J. Environ. Sci. Dev. 2014, 5, 444. [Google Scholar] [CrossRef]
- Upadhya, A.; Thakur, M.; Sihag, P.; Kumar, R.; Kumar, S.; Afeeza, A.; Afzal, A.; Saleel, C.A. Modelling and prediction of binder content using latest intelligent machine learning algorithms in carbon fiber reinforced asphalt concrete. Alex. Eng. J. 2023, 65, 131–149. [Google Scholar] [CrossRef]
- Joumblat, R.; Al Basiouni Al Masri, Z.; Al Khateeb, G.; Elkordi, A.; El Tallis, A.R.; Absi, J. State-of-the-Art Review on Permanent Deformation Characterization of Asphalt Concrete Pavements. Sustainability 2023, 15, 1166. [Google Scholar] [CrossRef]
- Zarei, S.; Alae, M.; Ouyang, J.; Zhao, Y. Rutting and surface-initiated cracking mechanisms of semi-flexible pavements with cement asphalt emulsion pastes. Int. J. Pavement Eng. 2023, 24, 2024187. [Google Scholar] [CrossRef]
- Haghighatpour, P.J.; Aliha, M.R.M. The influence of recycled asphalt pavement materials on low and intermediate fracture behavior of asphalt concrete (AC) after exposing to different thawing and freezing periods. Eng. Fract. Mech. 2023, 293, 109715. [Google Scholar] [CrossRef]
- Herrington, P.R.; Henning, T.F.P. Dependence of carbonyl, sulphoxide and hydroxyl functional group formation on oxygen concentration during the oxidation of roading bitumen. Constr. Build. Mater. 2023, 372, 130731. [Google Scholar] [CrossRef]
- Pahlavan, F.; Gholipour, A.; Zhou, T.; Fini, E.H. Cleaner Asphalt Production by Suppressing Emissions Using Phenolic Compounds. ACS Sustain. Chem. Eng. 2023, 11, 2737–2751. [Google Scholar] [CrossRef]
- Feng, P.; Wang, X.; Shao, L.; Wang, H. Viscosity Property of Methylene Diphenyl Diisocyanate Modified Asphalt Based on Molecular Dynamics Simulation. In Proceedings of the CICTP 2021, 21st COTA International Conference of Transportation Professionals, Xi’an, China, 16–19 December 2021; pp. 1657–1668. [Google Scholar]
- Li, T.; Guo, Z.; Lu, G.; Liang, D.; Luo, S.; Hong, B.; Wang, D.; Oeser, M. Experimental investigations and quantum chemical calculations of methylene diphenyl diisocyanate (MDI)-based chemically modified bitumen and its crosslinking behaviours. Fuel 2022, 321, 124084. [Google Scholar] [CrossRef]
- Salahshoori, I.; Jorabchi, M.N.; Asghari, M.; Ghasemi, S.; Wohlrab, S. Insights into the morphology and gas separation characteristics of methylene diisocyanate (MDI)-functionalized nanoTiO2 polyurethane: Quantum mechanics and molecular simulations studies. J. Mater. Res. Technol. 2023, 23, 1862–1886. [Google Scholar] [CrossRef]
- Srivastava, I.; Kotia, A.; Ghosh, S.K.; Ali, M.K.A. Recent advances of molecular dynamics simulations in nanotribology. J. Mol. Liq. 2021, 335, 116154. [Google Scholar] [CrossRef]
- Rucker, G.; Zhang, L. Comparison of the Interaction and Structure of Lignin in Pure Systems and in Asphalt Media by Molecular Dynamics Simulations. Biomacromolecules 2023, 25, 626–643. [Google Scholar] [CrossRef]
- Jeyranpour, F.; Alahyarizadeh, G.H.; Minuchehr, A. The thermo-mechanical properties estimation of fullerene-reinforced resin epoxy composites by molecular dynamics simulation–A comparative study. Polymer 2016, 88, 9–18. [Google Scholar] [CrossRef]
- Silva, H.S.; Sodero, A.C.R.; Bouyssiere, B.; Carrier, H.; Korb, J.-P.; Alfarra, A.; Vallverdu, G.; Bégué, D.; Baraille, I. Molecular Dynamics Study of Nanoaggregation in Asphaltene Mixtures: Effects of the N, O, and S Heteroatoms. Energy Fuels 2016, 30, 5656–5664. [Google Scholar] [CrossRef]
- Su, M.; Si, C.; Zhang, Z.; Zhang, H. Molecular dynamics study on influence of Nano-ZnO/SBS on physical properties and molecular structure of asphalt binder. Fuel 2019, 263, 116777. [Google Scholar] [CrossRef]
- Yu, C.-H.; Hu, K.; Chen, G.-X.; Chang, R.; Wang, Y. Molecular dynamics simulation and microscopic observation of compatibility and interphase of composited polymer modified asphalt with carbon nanotubes. J. Zhejiang Univ. A 2021, 22, 528–546. [Google Scholar] [CrossRef]
- Dalhat, M.A.; Al-Adham, K. Review on laboratory preparation processes of polymer modified asphalt binder. J. Traffic Transp. Eng. 2023. [Google Scholar] [CrossRef]
- Ma, L.; Salehi, H.S.; Jing, R.; Erkens, S.; Vlugt, T.J.; Moultos, O.A.; Greenfield, M.L.; Varveri, A. Water diffusion mechanisms in bitumen studied through molecular dynamics simulations. Constr. Build. Mater. 2023, 409, 133828. [Google Scholar] [CrossRef]
- dos Santos, R.N.G.; de Almeida Lima, E.R.; Paredes, M.L.L. Solubility of asphaltenes samples in polar and apolar synthetic mixtures: Experimental and modeling. Braz. J. Chem. Eng. 2023, 40, 585–597. [Google Scholar] [CrossRef]
- Ahmadi, M.; Clarke, M.; Chen, Z. The effect of bitumen molecular fractions on diffusivity and rheology of bitumen under high-temperature conditions: Molecular dynamics (MD) simulation study. Can. J. Chem. Eng. 2023, 101, 1150–1161. [Google Scholar] [CrossRef]
- Chávez-Valencia, L.; Alonso, E.; Manzano, A.; Pérez, J.; Contreras, M.; Signoret, C. Improving the compressive strengths of cold-mix asphalt using asphalt emulsion modified by polyvinyl acetate. Constr. Build. Mater. 2007, 21, 583–589. [Google Scholar] [CrossRef]
- Geng, L.; Xu, Q.; Yu, X.; Jiang, C.; Zhang, Z.; Li, C. Laboratory performance evaluation of a cold patching asphalt material containing cooking waste oil. Constr. Build. Mater. 2020, 246, 117637. [Google Scholar] [CrossRef]
- Sena Neto, P.G.; Amorim, E.F.; Ingunza, M.P.D. Analysis of cold asphalt concrete mixtures using construction and demolition wastes. Matéria 2019, 24, e12529. [Google Scholar]
- Shirzad, S.; Idris, I.I.; Hassan, M.; Mohammad, L.N. Self-Healing Capability and Mechanical Properties of Asphalt Mixtures Prepared with Light-Activated Polyurethane Prepolymer Modified Asphalt Binder. Transp. Res. Rec. 2023, 03611981221138522. [Google Scholar] [CrossRef]
- Ting, J.H.; Khare, E.; DeBellis, A.; Orr, B.; Jourdan, J.S.; Martín-Martínez, F.J.; Jin, K.; Malonson, B.L.; Buehler, M.J. Role of Methylene Diphenyl Diisocyanate (MDI) Additives on SBS-Modified Asphalt with Improved Thermal Stability and Mechanical Performance. Energy Fuels 2021, 35, 17629–17641. [Google Scholar] [CrossRef]
- JTG E20-2011; Standard Test Methods of Bitumen and Bituminous Mixtures for Highway Engineering. Ministry of Transport of the People’s Republic of China: Beijing, China, 2011.
- Rahman, M.M.; Hasneen, A.; Jo, N.J.; Kim, H.I.; Lee, W.-K. Properties of Waterborne Polyurethane Adhesives with Aliphatic and Aromatic Diisocyanates. J. Adhes. Sci. Technol. 2011, 25, 2051–2062. [Google Scholar] [CrossRef]
- Wirts, M.; Grunwald, D.; Schulze, D.; Uhde, E.; Salthammer, T. Time course of isocyanate emission from curing polyurethane adhesives. Atmos. Environ. 2003, 37, 5467–5475. [Google Scholar] [CrossRef]
- Fallah, F.; Khabaz, F.; Kim, Y.-R.; Kommidi, S.R.; Haghshenas, H.F. Molecular dynamics modeling and simulation of bituminous binder chemical aging due to variation of oxidation level and saturate-aromatic-resin-asphaltene fraction. Fuel 2019, 237, 71–80. [Google Scholar] [CrossRef]
- Pan, J.; Tarefder, R.A. Investigation of asphalt aging behaviour due to oxidation using molecular dynamics simulation. Mol. Simul. 2016, 42, 667–678. [Google Scholar] [CrossRef]
- Tan, Y.; Xie, J.; Song, J.; Xu, J.; Li, X. Interfacial interaction behavior of recycled asphalt pavement: Molecular dynamics simulation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132194. [Google Scholar] [CrossRef]
- Sjöblom, J.; Simon, S.; Xu, Z. Model molecules mimicking asphaltenes. Adv. Colloid Interface Sci. 2015, 218, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yadykova, A.Y.; Strelets, L.A.; Ilyin, S.O. Infrared spectral classification of natural bitumens for their rheological and thermophysical characterization. Molecules 2023, 28, 2065. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- JTG F40-2004; Technical Specifications for Construction of Highway Asphalt Pavements. Ministry of Transport of the People’s Republic of China: Beijing, China, 2004.
- Ilyin, S.O.; Yadykova, A.Y. Eco-friendly bitumen binders from heavy crude oil and a relaxation approach to predicting their resistance to rutting and cracking. J. Clean. Prod. 2024, 434, 139942. [Google Scholar] [CrossRef]




| Technical Properties | Experimental Value | Test Method (JTG E20-2011) |
|---|---|---|
| Penetration value, 1/10 mm (25 °C, 100 g, 5 s) | 80.7 | T0604 |
| Softening point, °C (R&B) | 47 | T0606 |
| 135 °C Kinematic viscosity, mPa·s | 346.3 | T0619 |
| 5 °C Ductility, mm | 89 | T0605 |
| Testing Items | Indicators |
|---|---|
| Natural asphalt content, % | 28 ± 3 |
| Trichloroethylene solubility, % | 25~31 |
| Density, g/cm3 | 1.42 |
| Flash point, °C | ≥230 |
| Heating loss, % | <1.2 |
| Moisture content,% | <2 |
| Maximum particle size, mm | <0.3 |
| Raw Material | Mass Fraction | Function |
|---|---|---|
| Asphalt | 282 g | - |
| MDI | 24 g | Modification plasticizer |
| Petroleum Resin | 20 g | Enhances the flexibility of asphalt |
| Setting Accelerator | 4 g | Increases bonding strength |
| Ethylene Glycol | 40 mL | Improves compatibility with other components |
| Kerosene | 40 mL | Dilutes asphalt |
| Cellulose | 6 g | Enhances stability of the mixture |
| Item | Penetration Value (mm) (25 °C, 100 g, 5 s) | Softening Point (°C) | 5 °C Ductility (mm) |
|---|---|---|---|
| 10% MDI + petroleum asphalt | - | 43.9 | 140 |
| 15% MDI + petroleum asphalt | - | 36.8 | 180 |
| 20% MDI + petroleum asphalt | - | 14.8 | 210 |
| 10% rock asphalt + petroleum asphalt | 65 | 49.7 | 47 |
| 15% rock asphalt + petroleum asphalt | 55 | 54.7 | 38 |
| 20% rock asphalt + petroleum asphalt | 52 | 57.4 | 25 |
| 10% rock asphalt + 15% MDI-modified asphalt | 137 | 34 | 219 |
| 15% rock asphalt + 15% MDI-modified asphalt | 83 | 48.4 | 67.5 |
| 20% rock asphalt + 15% MDI-modified asphalt | 76 | 49.9 | 44 |
| Test method (JTG E20-2011) | T0604 | T0606 | T0605 |
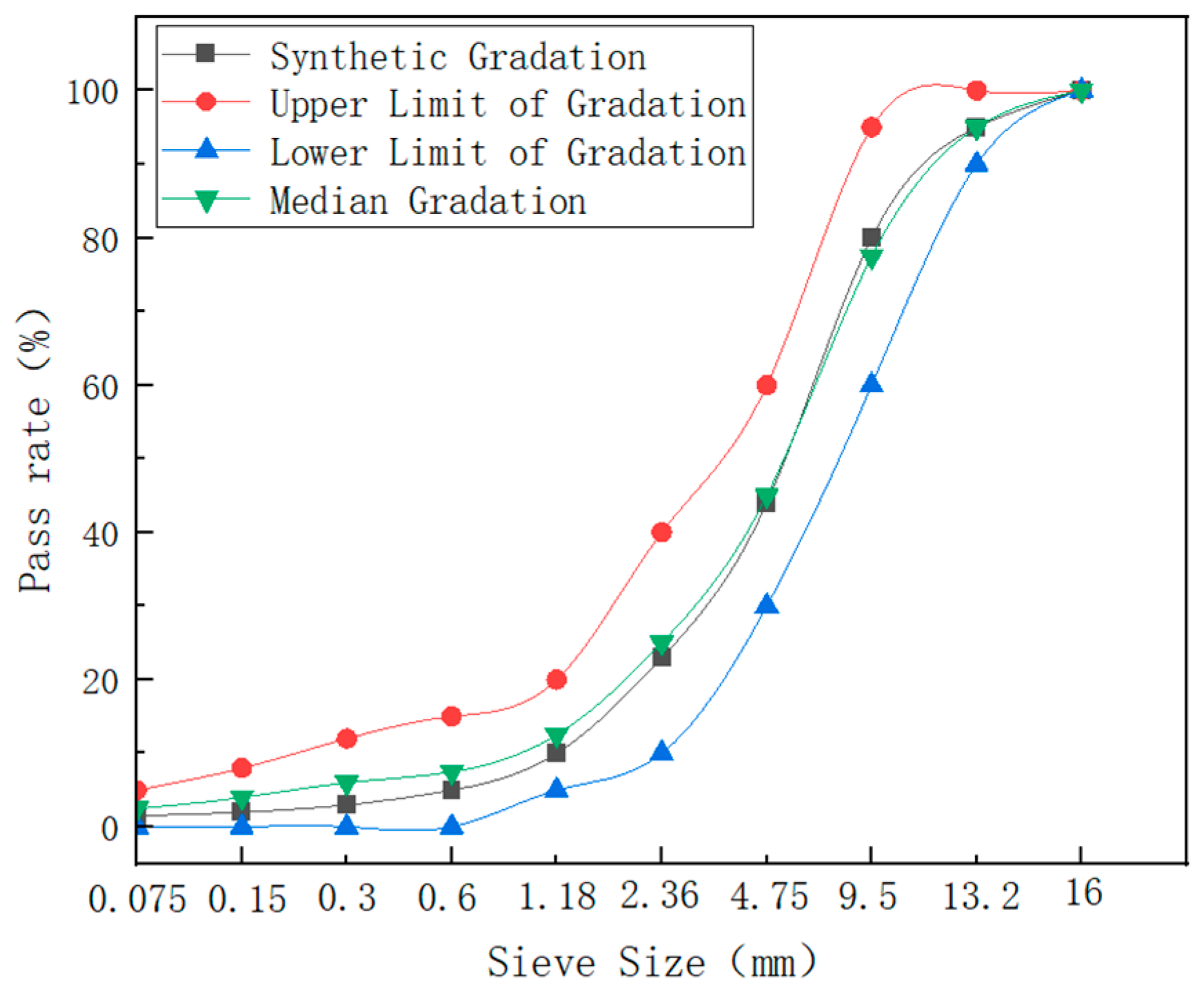
| Item | Cumulative Passing Rates at Different Sieve Sizes (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 13.2 | 9.5 | 4.75 | 2.36 | 1.18 | 0.6 | 0.3 | 0.15 | 0.075 | |
| Synthetic Gradation (%) | 100 | 95 | 80 | 44 | 23 | 10 | 5 | 3 | 2 | 1.5 |
| Upper Limit of Gradation (%) | 100 | 100 | 95 | 60 | 40 | 20 | 15 | 12 | 8 | 5 |
| Lower Limit of Gradation (%) | 100 | 90 | 60 | 30 | 10 | 5 | 0 | 0 | 0 | 0 |
| Median Gradation (%) | 100 | 95 | 77.5 | 45 | 25 | 12.5 | 7.5 | 6 | 4 | 2.5 |
| Item | Stability (kN) | Flow Value (mm) |
|---|---|---|
| MDI-Modified Asphalt Mixture with 15% MDI + 0% Rock Asphalt + 0% Diluent | 4.23 | 3.670 |
| MDI-Modified Asphalt Mixture with 15% MDI + 15% Rock Asphalt + 10% Diluent | 8.46 | 2.251 |
| MDI-Modified Asphalt Mixture with 15% MDI + 15% Rock Asphalt + 15% Diluent | 6.22 | 2.791 |
| Required Value for CMA (Low-Grade Highway) | >3 | 2–4 |
| Required Value for HMA (AC) | 8 (Expressway); 5 (Others) | 2–4.5 |
| Test method (JTG E20-2011) | T0709 | |
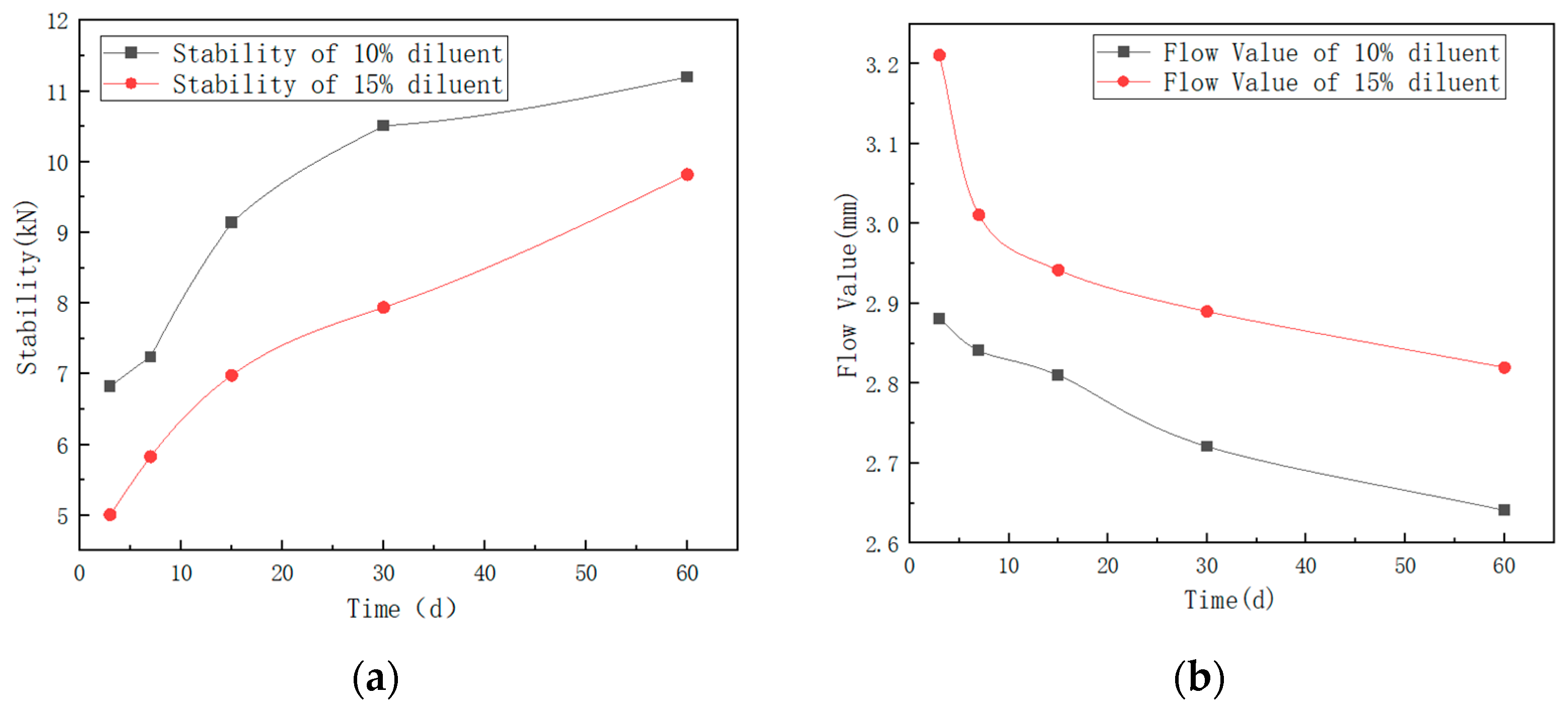
| Item | Time | |||||
|---|---|---|---|---|---|---|
| 3 Days | 7 Days | 15 Days | 30 Days | 60 Days | ||
| MDI-Modified Asphalt Mixture with 15% MDI + 15% Rock Asphalt + 10% Diluent | Stability (kN) | 6.82 | 7.24 | 9.14 | 10.51 | 11.20 |
| Flow Value (mm) | 2.881 | 2.841 | 2.810 | 2.721 | 2.641 | |
| MDI-Modified Asphalt Mixture with 15% MDI + 15% Rock Asphalt + 15% Diluent | Stability (kN) | 5.01 | 5.83 | 6.98 | 7.94 | 9.82 |
| Flow Value (mm) | 3.211 | 3.011 | 2.942 | 2.890 | 2.820 | |
| Item | Dynamic Stability (60 °C, 0.7 MPa) (Cycles/mm) |
|---|---|
| MDI-Modified Asphalt Mixture with 15% MDI + 15% Rock Asphalt + 10% Diluent | 1568 |
| Required value for HMA (AC) | ≥800 |
| Test method (JTG E20-2011) | T0719 |
| Item | Residual Stability |
|---|---|
| MDI-Modified Asphalt Mixture with 15% MDI + 15% Rock Asphalt + 10% Diluent | 87 |
| Standard value for HMA (AC) | ≥80 |
| Test method (JTG E20-2011) | T0709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Peng, M.; Yang, X.; Qi, Y.; Xu, B. Study of the Cold Curing Characteristics of Isocyanate-Modified Asphalt. Materials 2024, 17, 1048. https://doi.org/10.3390/ma17051048
Zhou C, Peng M, Yang X, Qi Y, Xu B. Study of the Cold Curing Characteristics of Isocyanate-Modified Asphalt. Materials. 2024; 17(5):1048. https://doi.org/10.3390/ma17051048
Chicago/Turabian StyleZhou, Changhong, Mingli Peng, Xue Yang, Yating Qi, and Bin Xu. 2024. "Study of the Cold Curing Characteristics of Isocyanate-Modified Asphalt" Materials 17, no. 5: 1048. https://doi.org/10.3390/ma17051048
APA StyleZhou, C., Peng, M., Yang, X., Qi, Y., & Xu, B. (2024). Study of the Cold Curing Characteristics of Isocyanate-Modified Asphalt. Materials, 17(5), 1048. https://doi.org/10.3390/ma17051048







