A Sustainable Banana Peel Activated Carbon for Removing Pharmaceutical Pollutants from Different Waters: Production, Characterization, and Application †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biomass and Activation
2.3. Characterization
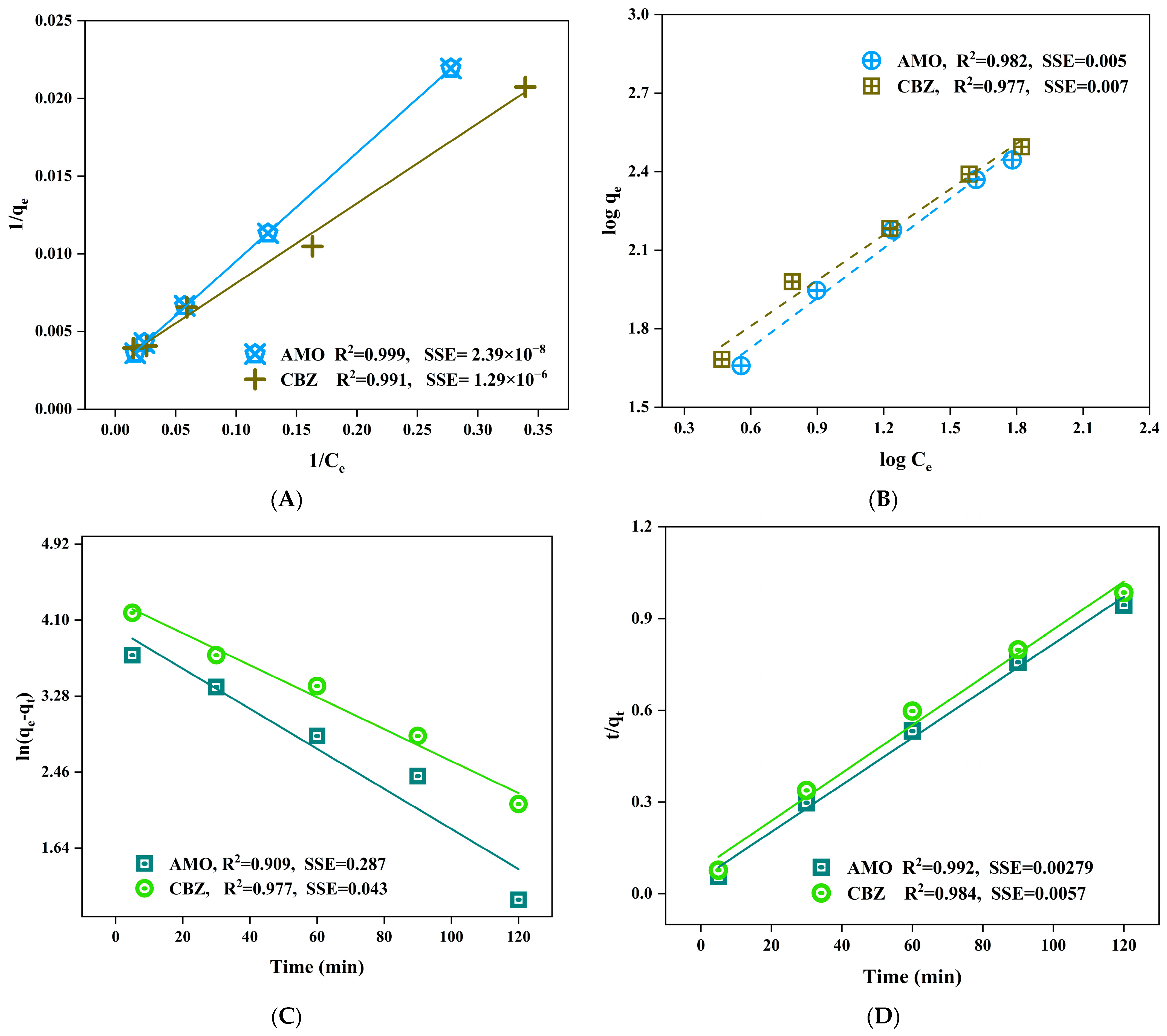
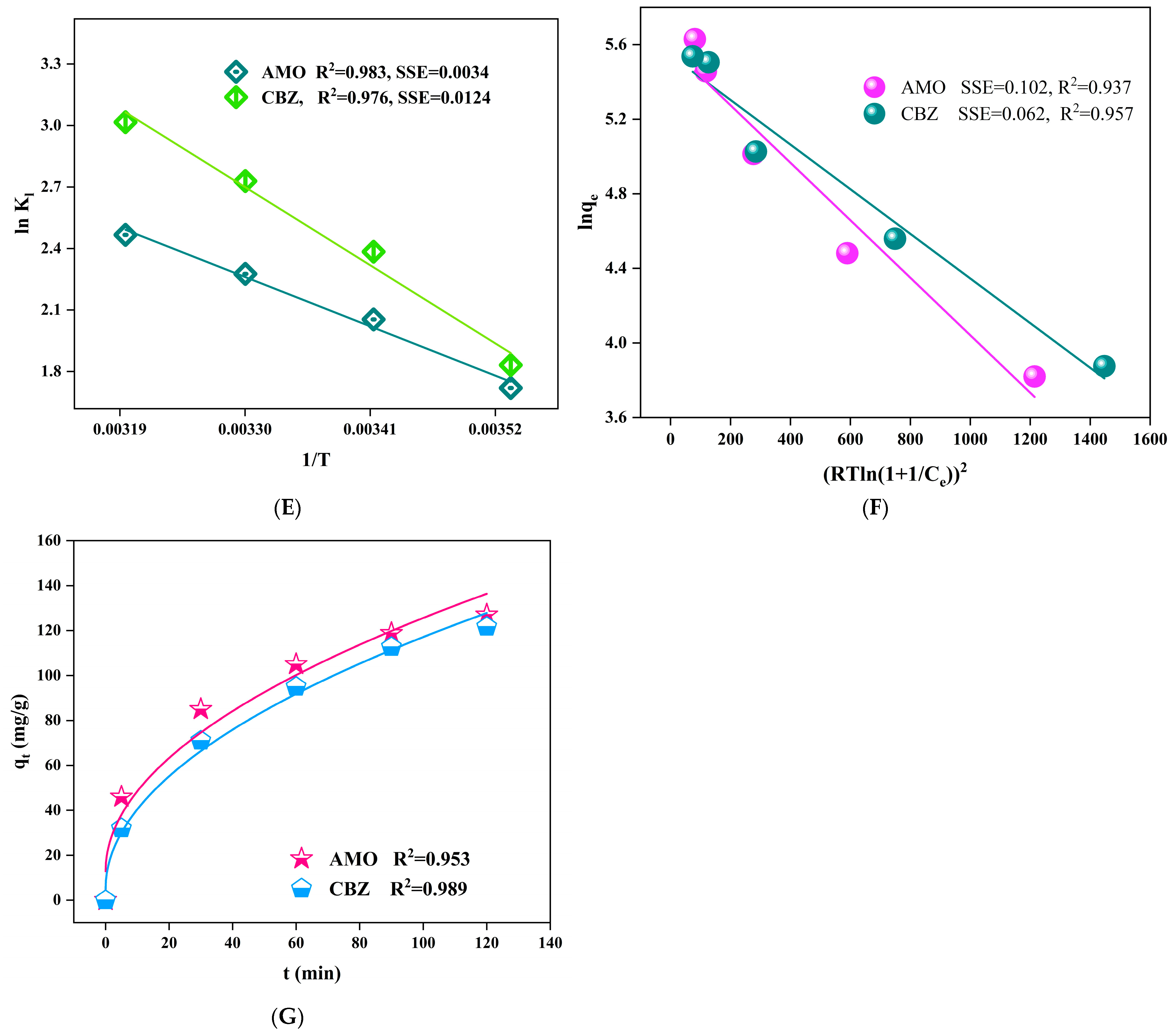
2.4. Adsorption Experiments, Kinetic, Equilibrium Studies and Thermodynamics
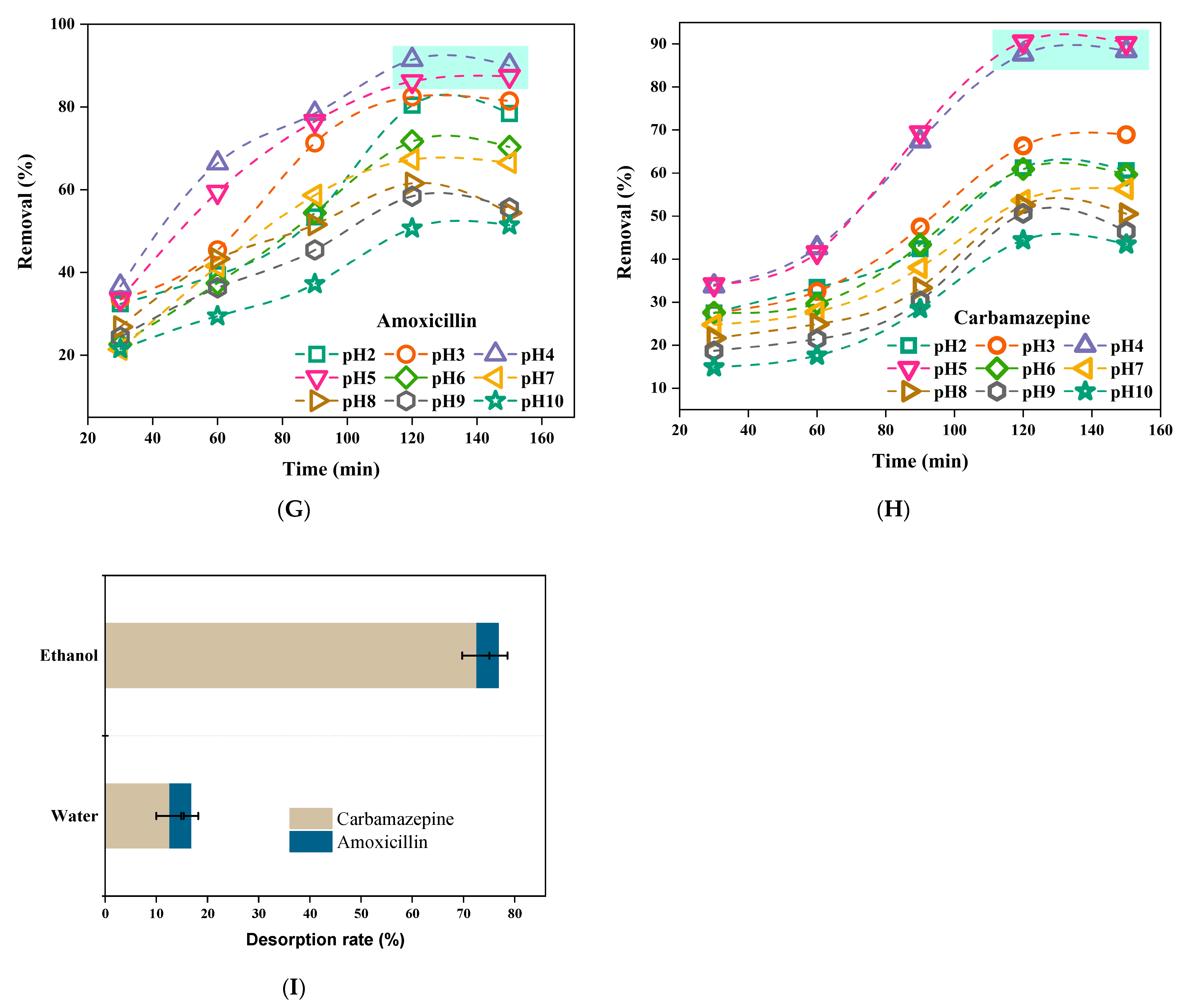
2.5. Desorption Experiments
3. Results and Discussion
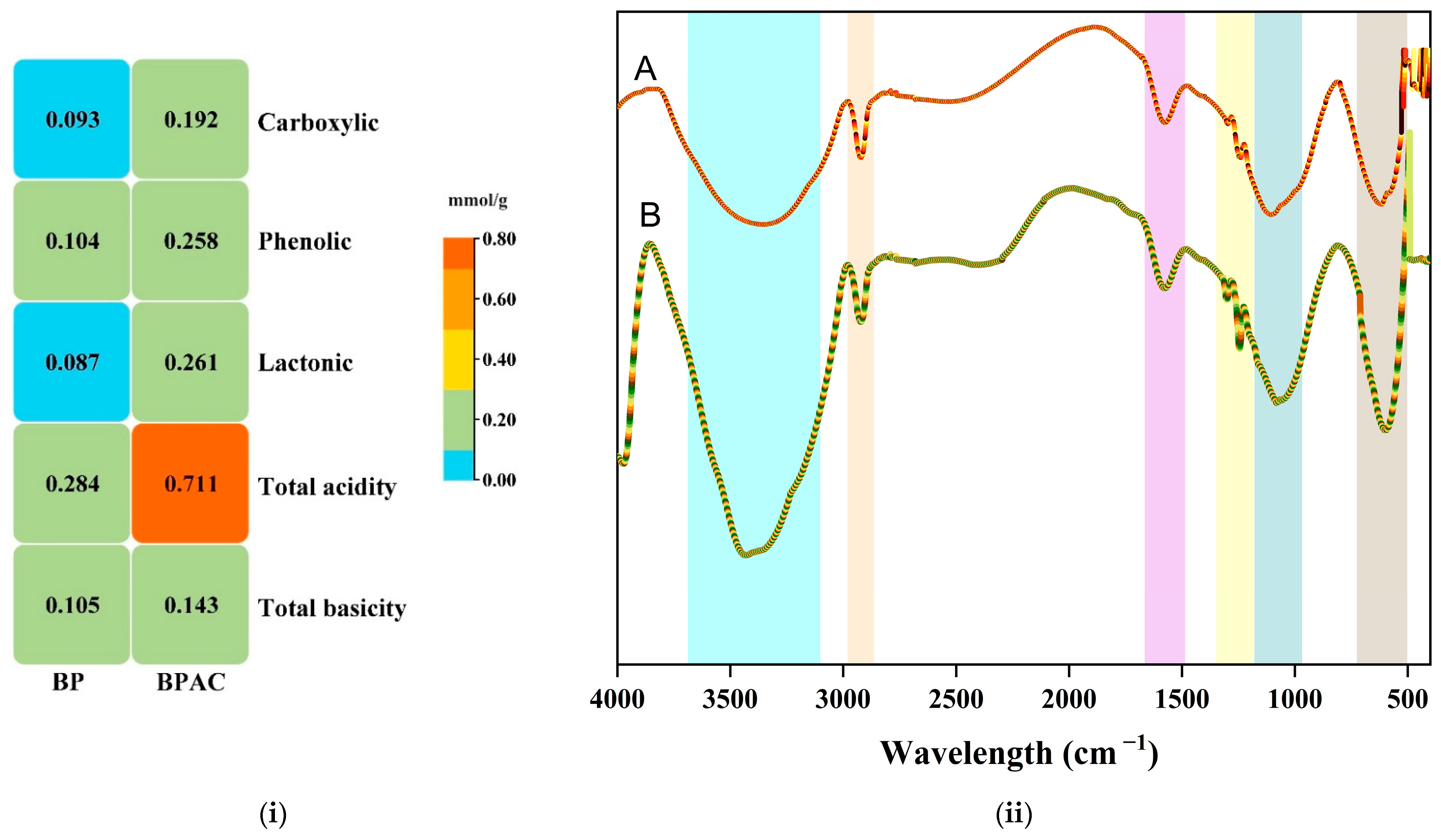
3.1. Characterization
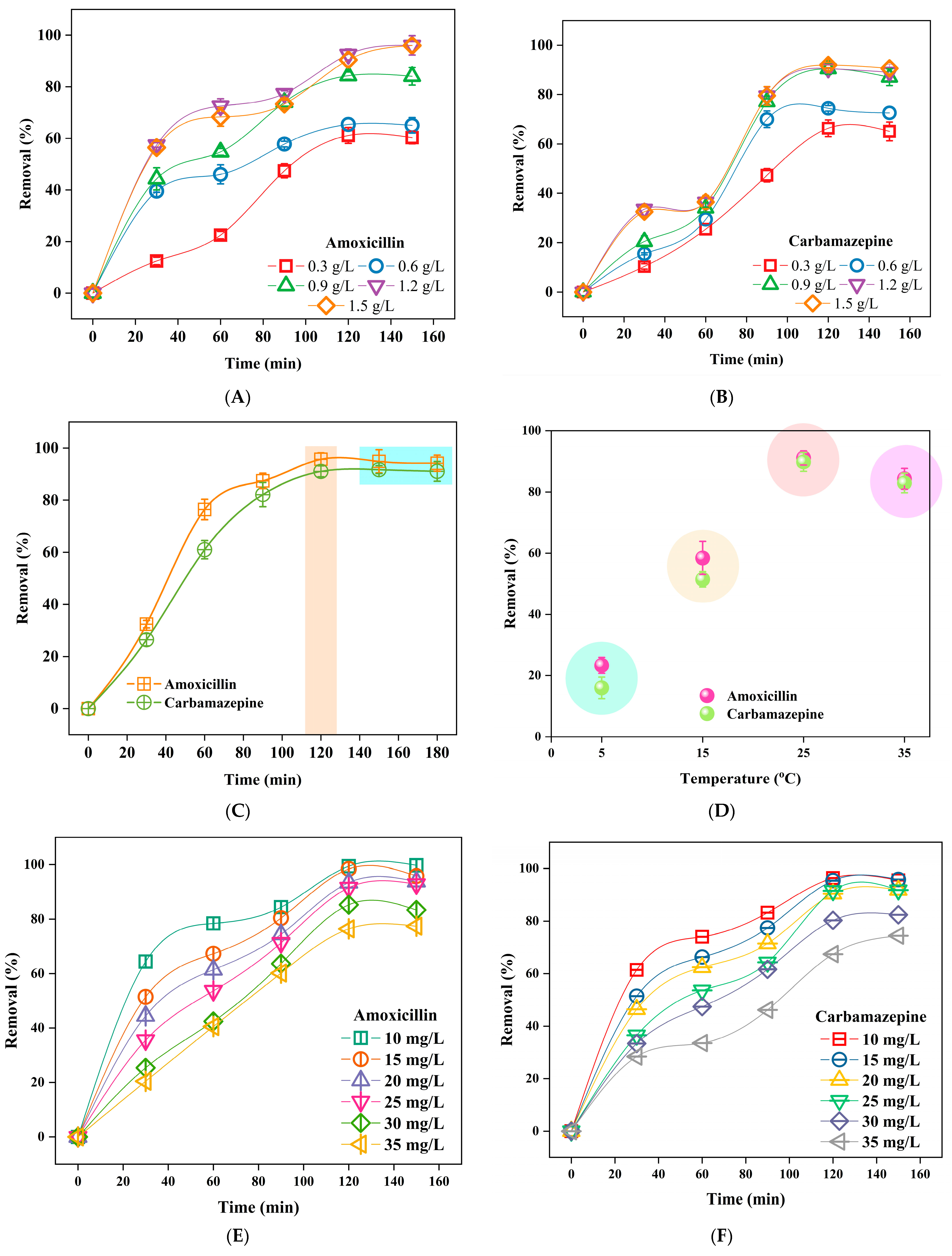
3.2. Influence of Adsorption Parameters
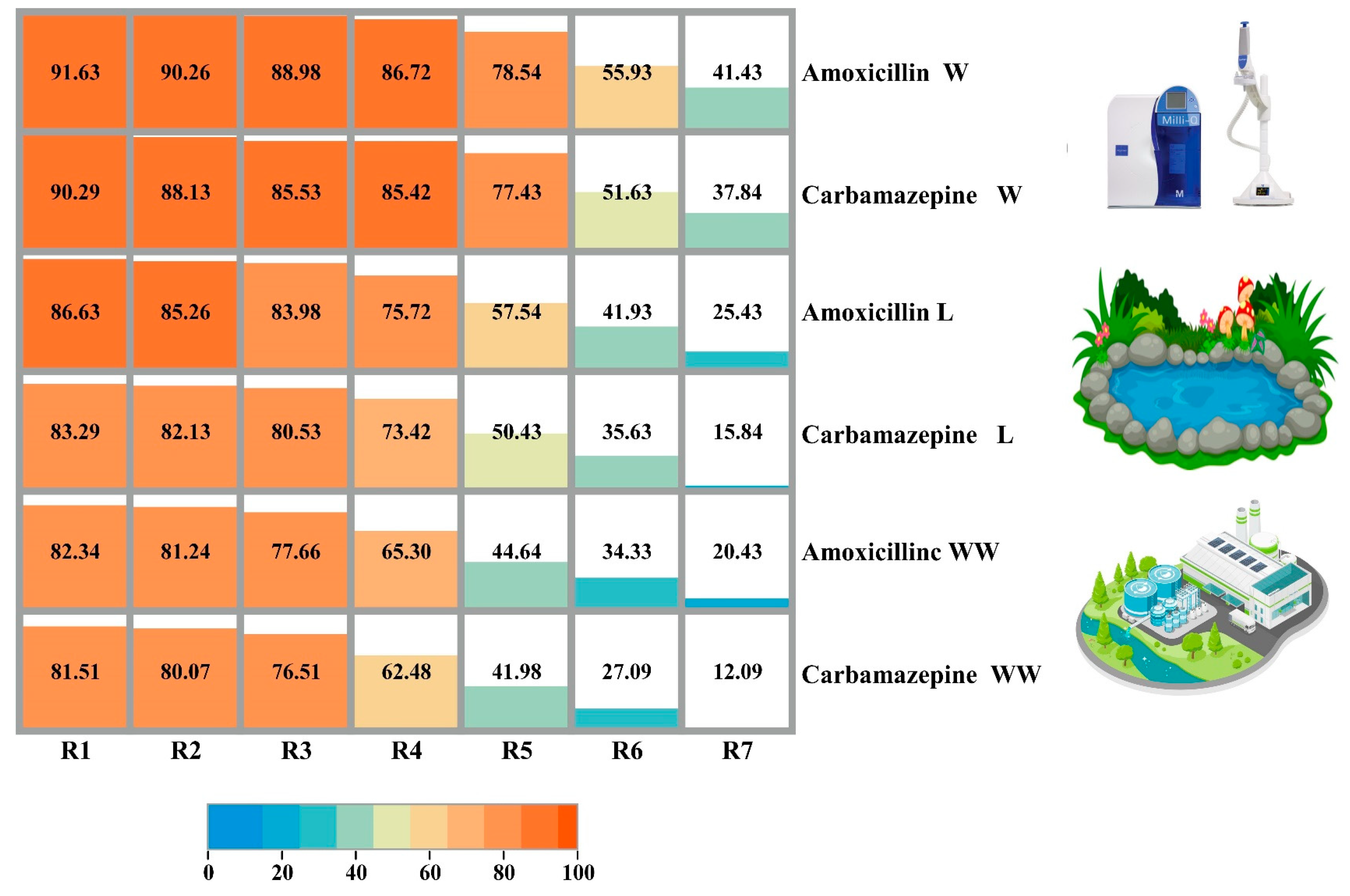
3.3. Adsorbent Reusability with Real Samples Application
3.4. Adsorbent Reusability with Real Samples Application
4. Spent BPAC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, S.; Sarkar, S.; Singh, P. Water pollution in rural areas: Primary sources, associated health issues, and remedies. In Water Resources Management for Rural Development; Elsevier: Amsterdam, The Netherlands, 2024; pp. 15–28. [Google Scholar]
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: A review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Caban, M.; Stepnowski, P. How to decrease pharmaceuticals in the environment? A review. Environ. Chem. Lett. 2021, 19, 3115–3138. [Google Scholar] [CrossRef]
- Madhura, L.; Singh, S.; Kanchi, S.; Sabela, M.; Bisetty, K.; Inamuddin. Nanotechnology-based water quality management for wastewater treatment. Environ. Chem. Lett. 2019, 17, 65–121. [Google Scholar] [CrossRef]
- Femina Carolin, C.; Senthil Kumar, P.; Janet Joshiba, G.; Vinoth Kumar, V. Analysis and removal of pharmaceutical residues from wastewater using membrane bioreactors: A review. Environ. Chem. Lett. 2021, 19, 329–343. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Jos, A.; Repetto, G.; Rios, J.C.; Hazen, M.J.; Molero, M.L.; Del Peso, A.; Salguero, M.; Fernández-Freire, P.; Pérez-Martín, J.M.; Cameán, A. Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol. Vitro 2003, 17, 525–532. [Google Scholar] [CrossRef] [PubMed]
- PQM, Promoting the Quality of Medicines (PQM). Product Information Report: Amoxicillin; U.S. Pharmacopeial Convention: Rockville, MD, USA, 2017; Available online: https://www.usp-pqm.org/sites/default/files/pqms/article/amoxicillin-pir-jul2018.pdf (accessed on 7 January 2024).
- Gupta, S.; Singh, A.; Sharma, T.; Kaur, R.; Khandelwal, V.; Rawat, K.D.; Pathak, S.; Sharma, M.K.; Singh, J.; Shah, M.P.; et al. Applications of ultrafiltration, nanofiltration, and reverse osmosis in pharmaceutical wastewater treatment. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–49. [Google Scholar]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Waris, R.F.; Farooqi, I.H. Different advanced oxidation processes for the abatement of pharmaceutical compounds. Int. J. Environ. Sci. Technol. 2024, 21, 2325–2338. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A new Cd (II)-based coordination polymer for efficient photocatalytic removal of organic dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green sorbents from agricultural wastes: A review of sustainable adsorption materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Sarker, A.; Ahmmed, R.; Ahsan, S.M.; Rana, J.; Ghosh, M.K.; Nandi, R. A comprehensive review of food waste valorization for the sustainable management of global food waste. Sustain. Food Technol. 2024, 2, 48–69. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.A.; Freitas, O.M. The use of algae and fungi for removal of pharmaceuticals by bioremediation and biosorption processes: A review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Danish, M. Prospects of banana waste utilization in wastewater treatment: A review. J. Environ. Manag. 2018, 206, 330–348. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). Crops and Livestock Products. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 3 January 2024).
- Ahmed, M.S.; Attia, T.; Abd El-Wahab, A.A.; Elgamsy, R.; Abd El-latif, M.H. Assessment of the physical properties of banana pseudo stem/ABS composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 634, p. 012023. [Google Scholar]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive decontamination of rhodamine-B from water using banana peel powder: A biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a biosorbent for the decontamination of water pollutants. A Review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Gupta, H.; Gupta, B. Adsorption of polycyclic aromatic hydrocarbons on banana peel activated carbon. Desalin. Water Treat. 2016, 57, 9498–9509. [Google Scholar] [CrossRef]
- Ingole, R.S.; Lataye, D.H.; Dhorabe, P.T. Adsorption of phenol onto banana peels activated carbon. KSCE J. Civ. Eng. 2017, 21, 100–110. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef]
- Delgado, N.; Capparelli, A.; Navarro, A.; Marino, D. Pharmaceutical emerging pollutants removal from water using powdered activated carbon: Study of kinetics and adsorption equilibrium. J. Environ. Manag. 2019, 236, 301–308. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Dai, Y. Phosphoric acid activation of cow dung biochar for adsorbing enrofloxacin in water: Icing on the cake. Environ. Pollut. 2024, 341, 122887. [Google Scholar] [CrossRef]
- Bakar, N.A.; Othman, N.; Yunus, Z.M.; Altowayti, W.A.H.; Al-Gheethi, A.; Asharuddin, S.M.; Tahir, M.; Fitriani, N.; Mohd-Salleh, S.N.A. Nipah (Musa Acuminata Balbisiana) banana peel as a lignocellulosic precursor for activated carbon: Characterization study after carbonization process with phosphoric acid impregnated activated carbon. Biomass Convers. Biorefinery 2021, 13, 11085–11098. [Google Scholar] [CrossRef]
- Daniel, L.S.; Rahman, A.; Hamushembe, M.N.; Kapolo, P.; Uahengo, V.; Jonnalagadda, S.B. The production of activated carbon from Acacia erioloba seedpods via phosphoric acid activation method for the removal of methylene blue from water. Bioresour. Technol. Rep. 2023, 23, 101568. [Google Scholar] [CrossRef]
- Al-Sareji, O.J.; Meiczinger, M.; Salman, J.M.; Al-Juboori, R.A.; Hashim, K.S.; Somogyi, V.; Jakab, M. Ketoprofen and aspirin removal by laccase immobilized on date stones. Chemosphere 2023, 311, 137133. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3174-04; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D3173-03; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D 3175-07; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2007.
- Al-Sareji, O.J.; Meiczinger, M.; Al-Juboori, R.A.; Grmasha, R.A.; Andredaki, M.; Somogyi, V.; Idowu, I.A.; Stenger-Kovács, C.; Jakab, M.; Lengyel, E.; et al. Efficient removal of pharmaceutical contaminants from water and wastewater using immobilized laccase on activated carbon derived from pomegranate peels. Sci. Rep. 2023, 13, 11933. [Google Scholar] [CrossRef] [PubMed]
- ASTM D3176; Standard Practice for Ultimate Analysis of Coal and Coke, 1–2. ASTM International: Conshohocken, PA, USA, 2015.
- Al-sareji, O.J.; Meiczinger, M.; Somogyi, V.; Al-Juboori, R.A.; Grmasha, R.A.; Stenger-Kovács, C.; Jakab, M.; Hashim, K.S. Removal of emerging pollutants from water using enzyme-immobilized activated carbon from coconut shell. J. Environ. Chem. Eng. 2023, 11, 109803. [Google Scholar] [CrossRef]
- Grmasha, R.A.; Al-Sareji, O.J.; Meiczinger, M.; Stenger-Kovács, C.; Al-Juboori, R.A.; Jakab, M.; Lengyel, E.; Somogyi, V.; Khan, M.A.; Hashim, K.S. A sustainable nano-hybrid system of laccase@ M-MWCNTs for multifunctional PAHs and PhACs removal from water, wastewater, and lake water. Environ. Res. 2024, 246, 118097. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Al-Sareji, O.J.; Grmasha, R.A.; Meiczinger, M.; Al-Juboori, R.A.; Somogyi, V.; Stenger-Kovács, C.; Hashim, K.S. A sustainable and highly efficient fossil-free carbon from olive stones for emerging contaminants removal from different water matrices. Chemosphere 2024, 351, 141189. [Google Scholar] [CrossRef]
- Nguyen, C.; Do, D.D. The Dubinin–Radushkevich equation and the underlying microscopic adsorption description. Carbon 2001, 39, 1327–1336. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.M.; Rahim, E.A.; Fouzy, A.S.M. Preparation and characterization of activated carbon from agricultural wastes and their ability to remove chlorpyrifos from water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sarswat, A.; Pittman, C.U., Jr.; Mlsna, T.; Mohan, D. Sustainable low-concentration arsenite [As (III)] removal in single and multicomponent systems using hybrid iron oxide–biochar nanocomposite adsorbents—A mechanistic study. ACS Omega 2020, 5, 2575–2593. [Google Scholar] [CrossRef]
- Yang, W.; Lu, C.; Liang, B.; Yin, C.; Lei, G.; Wang, B.; Zhou, X.; Zhen, J.; Quan, S.; Jing, Y. Removal of Pb (II) from Aqueous Solution and Adsorption Kinetics of Corn Stalk Biochar. Separations 2023, 10, 438. [Google Scholar] [CrossRef]
- Baharum, N.A.; Nasir, H.M.; Ishak, M.Y.; Isa, N.M.; Hassan, M.A.; Aris, A.Z. Highly efficient removal of diazinon pesticide from aqueous solutions by using coconut shell-modified biochar. Arab. J. Chem. 2020, 13, 6106–6121. [Google Scholar] [CrossRef]
- Agboola, O.S.; Bello, O.S. Enhanced adsorption of ciprofloxacin from aqueous solutions using functionalized banana stalk. Biomass Convers. Biorefinery 2020, 12, 5463–5478. [Google Scholar] [CrossRef]
- Yasim, N.S.E.M.; Ismail, Z.S.; Zaki, S.M.; Azis, M.F.A. Adsorption of Cu, As, Pb and Zn by banana trunk. Malays. J. Anal. Sci. 2016, 20, 187–196. [Google Scholar] [CrossRef]
- Ainane, T.; Khammour, F.; Talbi, M.; Elkouali, M.H. A novel bio-adsorbent of mint waste for dyes remediation in aqueous environments: Study and modeling of isotherms for removal of methylene Blue. Orient. J. Chem. 2014, 30, 1183–1189. [Google Scholar] [CrossRef]
- Çelebi, H.; Şimşek, İ.; Bahadir, T.; Tulun, Ş. Use of banana peel for the removal of boron from aqueous solutions in the batch adsorption system. Int. J. Environ. Sci. Technol. 2023, 20, 161–176. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rashid, R.A.; Ishak, M.A.M.; Ismail, K. Adsorptive removal of methylene blue by chemically treated cellulosic waste banana (Musa sapientum) peels. J. Taibah Univ. Sci. 2018, 12, 809–819. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A. Preparation and characterization of raw and carbon from banana peel by microwave activation: Application in citric acid adsorption. J. Environ. Chem. Eng. 2015, 3, 2435–2447. [Google Scholar] [CrossRef]
- Castro, R.S.; Caetano, L.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.U.; Castro, G.R. Banana peel applied to the solid phase extraction of copper and lead from river water: Preconcentration of metal ions with fruit waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Olivares-Marín, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Porous structure of activated carbon prepared from cherry stones by chemical activation with phosphoric acid. Energy Fuels 2007, 21, 2942–2949. [Google Scholar] [CrossRef]
- Farahani, M.; Abdullah, S.R.S.; Hosseini, S.; Shojaeipour, S.; Kashisaz, M. Adsorption-based cationic dyes using the carbon active sugarcane bagasse. Procedia Environ. Sci. 2011, 10, 203–208. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Muhammad, D.; Arumugasamy, S.K. An experimental and modelling approach to produce biochar from banana peels through pyrolysis as potential renewable energy resources. Model. Earth Syst. Environ. 2020, 6, 115–128. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Singh, V.K.; Alexandre-Franco, M.; Pittman, C.U., Jr. Development of magnetic activated carbon from almond shells for trinitrophenol removal from water. Chem. Eng. J. 2011, 172, 1111–1125. [Google Scholar] [CrossRef]
- Qu, D. Studies of the activated carbons used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef]
- Tong, W.; Liu, Q.; Ren, S.; Zhou, J.; Zhang, T.; Yang, C. Effect of pyrolysis temperature on pine sawdust chars and their gasification reactivity mechanism with CO2. Asia-Pac. J. Chem. Eng. 2018, 13, e2256. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Pittman, C.U., Jr.; Mohan, D. Ciprofloxacin and acetaminophen sorption onto banana peel biochars and Environmental; process parameter influences. Environ. Res. 2021, 201, 111218. [Google Scholar] [CrossRef] [PubMed]
- Pusponegoro, I.H.; Mujiyo, M.; Suntoro, S.; Herawati, A.; Widijanto, H. Planning of banana plant development based on the land conservation aspect in Jenawi District. J. Degrad. Min. Lands Manag. 2018, 5, 1319. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Clean. Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Guimaraes, J.L.; Frollini, E.; Da Silva, C.G.; Wypych, F.; Satyanarayana, K.G. Characterization of banana, sugarcane bagasse and sponge gourd fibers of Brazil. Ind. Crops Prod. 2009, 30, 407–415. [Google Scholar] [CrossRef]
- Shao, Z.; Wu, S.; Gao, Y.; Liu, X.; Dai, Y. Two-step pyrolytic preparation of biochar for the adsorption study of tetracycline in water. Environ. Res. 2024, 242, 117566. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Ledesma, B.; Román, S.; Bonelli, P.R.; Cukierman, A.L. Development and characterization of activated hydrochars from orange peels as potential adsorbents for emerging organic contaminants. Bioresour. Technol. 2015, 183, 221–228. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Zhang, H.; Li, R.; Hu, X.; Shahab, A.; Elnaggar, A.Y.; Alrefaei, A.F.; AlmutairiI, M.H.; Ali, E. Effective removal of methylene blue and crystal violet by low-cost biomass derived from eucalyptus: Characterization, experiments, and mechanism investigation. Environ. Technol. Innov. 2024, 33, 103459. [Google Scholar] [CrossRef]
- Mohamed, H.S.; Soliman, N.; Abdelrheem, D.A.; Ramadan, A.A.; Elghandour, A.H.; Ahmed, S.A. Adsorption of Cd2+ and Cr3+ ions from aqueous solutions by using residue of Padina gymnospora waste as promising low-cost adsorbent. Heliyon 2019, 5, e01287. [Google Scholar] [CrossRef]
- Hodúr, C.; Bellahsen, N.; Mikó, E.; Nagypál, V.; Šereš, Z.; Kertész, S. The adsorption of ammonium nitrogen from milking parlor wastewater using pomegranate peel powder for sustainable water, resources, and waste management. Sustainability 2020, 12, 4880. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hassanzadeh, A.; Kokhdan, S.N. Multiwalled carbon nanotubes as adsorbents for the kinetic and equilibrium study of the removal of alizarin red S and morin. J. Chem. Eng. Data 2011, 56, 2511–2520. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Binupriya, A.R.; Kavitha, D.; Yun, S.E. Kinetic and isothermal studies on liquid-phase adsorption of 2,4-dichlorophenol by palm pith carbon. Bioresour. Technol. 2007, 98, 866–873. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Sillanpää, M. Optimizing the removal of pharmaceutical drugs Carbamazepine and Dorzolamide from aqueous solutions using mesoporous activated carbons and multi-walled carbon nanotubes. J. Mol. Liq. 2017, 238, 379–388. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Pulicharla, R.; Rouissi, T.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Pine-wood derived nanobiochar for removal of carbamazepine from aqueous media: Adsorption behavior and influential parameters. Arab. J. Chem. 2019, 12, 5292–5301. [Google Scholar] [CrossRef]
- Jafari, K.; Heidari, M.; Rahmanian, O. Wastewater treatment for Amoxicillin removal using magnetic adsorbent synthesized by ultrasound process. Ultrason. Sonochem. 2018, 45, 248–256. [Google Scholar] [CrossRef] [PubMed]
- xing Zha, S.; Zhou, Y.; Jin, X.; Chen, Z. The removal of amoxicillin from wastewater using organobentonite. J. Environ. Manag. 2013, 129, 569–576. [Google Scholar]
- Laksaci, H.; Belhamdi, B.; Khelifi, O.; Khelifi, A.; Trari, M. Elimination of amoxicillin by adsorption on coffee waste based activated carbon. J. Mol. Struct. 2023, 1274, 134500. [Google Scholar] [CrossRef]
- Moussavi, G.; Alahabadi, A.; Yaghmaeian, K.; Eskandari, M. Preparation, characterization and adsorption potential of the NH4Cl-induced activated carbon for the removal of amoxicillin antibiotic from water. Chem. Eng. J. 2013, 217, 119–128. [Google Scholar] [CrossRef]
- Qin, Q.; Wu, X.; Chen, L.; Jiang, Z.; Xu, Y. Simultaneous removal of tetracycline and Cu (II) by adsorption and coadsorption using oxidized activated carbon. RSC Adv. 2018, 8, 1744–1752. [Google Scholar] [CrossRef]
- Chakraborty, P.; Show, S.; Banerjee, S.; Halder, G. Mechanistic insight into sorptive elimination of ibuprofen employing bidirectional activated biochar from sugarcane bagasse: Performance evaluation and cost estimation. J. Environ. Chem. Eng. 2018, 6, 5287–5300. [Google Scholar] [CrossRef]
- Ho, Y.S.; Huang, C.T.; Huang, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochem. 2002, 37, 1421–1430. [Google Scholar] [CrossRef]
- Al-sareji, O.J.; Abdulzahra, M.A.; Hussein, T.S.; Shlakaa, A.S.; Karhib, M.M.; Meiczinger, M.; Grmasha, R.A.; Al-Juboori, R.A.; Somogyi, V.; Domokos, E.; et al. Removal of pharmaceuticals from water using laccase immobilized on orange peels waste-derived activated carbon. Water 2023, 15, 3437. [Google Scholar] [CrossRef]
- Azzam, A.B.; Tokhy, Y.A.; Farida, M.; Younes, A.A. Construction of porous biochar decorated with NiS for the removal of ciprofloxacin antibiotic from pharmaceutical wastewaters. J. Water Process Eng. 2022, 49, 103006. [Google Scholar] [CrossRef]
- Silva, C.R.; Gomes, T.F.; Andrade, G.C.; Monteiro, S.H.; Dias, A.C.; Zagatto, E.A.; Tornisielo, V.L. Banana peel as an adsorbent for removing atrazine and ametryne from waters. J. Agric. Food Chem. 2013, 61, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.S. Thorium removal from wastewater using Banana peel and employment of Waste Residue. Adv. Nat. Appl. Sci. 2013, 7, 336–344. [Google Scholar]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total. Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Rosine, G.M.L.; Kaur, S.; Hegde, K.; Brar, S.K.; Drogui, P.; Verma, M. Novel biomaterials from citric acid fermentationas biosorbents for removal of metals from waste chromated copper arsenate wood leachates. Int. Biodeterior. Biodegrad. 2017, 119, 147–154. [Google Scholar] [CrossRef]
- Fernández-González, R.; Martín-Lara, M.; Moreno, J.; Blázquez, G.; Calero, M. Effective removal of zinc from industrial platingwastewater using hydrolyzed olive cake: Scale-up and preparation of zinc-Based biochar. J. Clean. Prod. 2019, 227, 634–644. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Hilal, N. Pharmaceuticals removal from wastewater: Ultrasound technology and its potential amalgamation with membrane processes. J. Water Process Eng. 2023, 53, 103810. [Google Scholar] [CrossRef]





| Equation | Parameter | Reference | |
|---|---|---|---|
| Langmuir | KL is the Langmuir constant (L/mg) and qm is maximum adsorption on BPAC (mg/g) | [39] | |
| Freundlich | qe is the pharmaceuticals absorbed per unit adsorbent at equilibrium (mg/g), Kf and n are Freundlich adsorption isotherm. constants and Ce is equilibrium adsorbate concentration (mg/L) | [40] | |
| Pseudo-first-order (PFO) | K1 is PFO constant rate (min−1) and qt is pharmaceuticals absorbed at time t (mg/g) | [41] | |
| pseudo-second-order kinetic (PSO) | K2 is PSO constant rate (g mg−1min −1) | [42] | |
| Gibbs free energy (ΔG°), standard entropy (ΔS°) and standard enthalpy (ΔH°) | R is the universal gas constant (8.314 J/mol K), ΔG° is Gibbs free energy, T is temperature (K), ΔS° is standard entropy, ΔH° is standard enthalpy | [43] | |
| Dubinin–Radushkevich (D–R) | Bd is the adsorption constant quantifies the average free energy (mol2/J2), qd is the maximum adsorption capacity (mg/g) T is the temperature (Kelvin) R is the universal gas constant (8.314 J/K/mol) | [44] | |
| Intra-particle diffusion | qt = kdiff t0.5 + I | Kdiff is the intra-particle diffusion constant (mg g−1 min−0.5), I is the IPD model’s boundary layer constant (mg g−1) | [45] |
| Compound | Matrix | Adsorbent | Removal Efficiency (%) | Reference |
|---|---|---|---|---|
| Phenol (50 mg/L) | Double-distilled water | Banana peel AC | 83 | [25] |
| Phenol (500 mg/L) | Double-distilled water | Banana peel AC | 60 | [25] |
| Citric acid | Double-distilled water | Microwave char banana peel | 88 | [55] |
| Citric acid | Double-distilled water | Raw banana peel | 86 | [55] |
| Rhodamine-B | Double-distilled water | Raw banana peel | 81.07 | [22] |
| Atrazine | River and treated waters | Banana peel | >90 | [86] |
| Ametryn | River and treated waters | Banana peel | >90 | [86] |
| Thorium | Wastewater | Banana peel | 95.34 | [87] |
| Amoxicillin and carbamazepine | MQ water | Banana peel AC | 91.63 and 90.29 | Current study |
| Lake water | 86.63 and 83.29 | |||
| Wastewater | 82.34 and 81.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-sareji, O.J.; Grmasha, R.A.; Meiczinger, M.; Al-Juboori, R.A.; Somogyi, V.; Hashim, K.S. A Sustainable Banana Peel Activated Carbon for Removing Pharmaceutical Pollutants from Different Waters: Production, Characterization, and Application. Materials 2024, 17, 1032. https://doi.org/10.3390/ma17051032
Al-sareji OJ, Grmasha RA, Meiczinger M, Al-Juboori RA, Somogyi V, Hashim KS. A Sustainable Banana Peel Activated Carbon for Removing Pharmaceutical Pollutants from Different Waters: Production, Characterization, and Application. Materials. 2024; 17(5):1032. https://doi.org/10.3390/ma17051032
Chicago/Turabian StyleAl-sareji, Osamah J., Ruqayah Ali Grmasha, Mónika Meiczinger, Raed A. Al-Juboori, Viola Somogyi, and Khalid S. Hashim. 2024. "A Sustainable Banana Peel Activated Carbon for Removing Pharmaceutical Pollutants from Different Waters: Production, Characterization, and Application" Materials 17, no. 5: 1032. https://doi.org/10.3390/ma17051032
APA StyleAl-sareji, O. J., Grmasha, R. A., Meiczinger, M., Al-Juboori, R. A., Somogyi, V., & Hashim, K. S. (2024). A Sustainable Banana Peel Activated Carbon for Removing Pharmaceutical Pollutants from Different Waters: Production, Characterization, and Application. Materials, 17(5), 1032. https://doi.org/10.3390/ma17051032








