Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review
Abstract
1. Introduction
1.1. Additive Manufacturing
1.2. The Significance of Precision Manufacturing in the Biomedical Sector
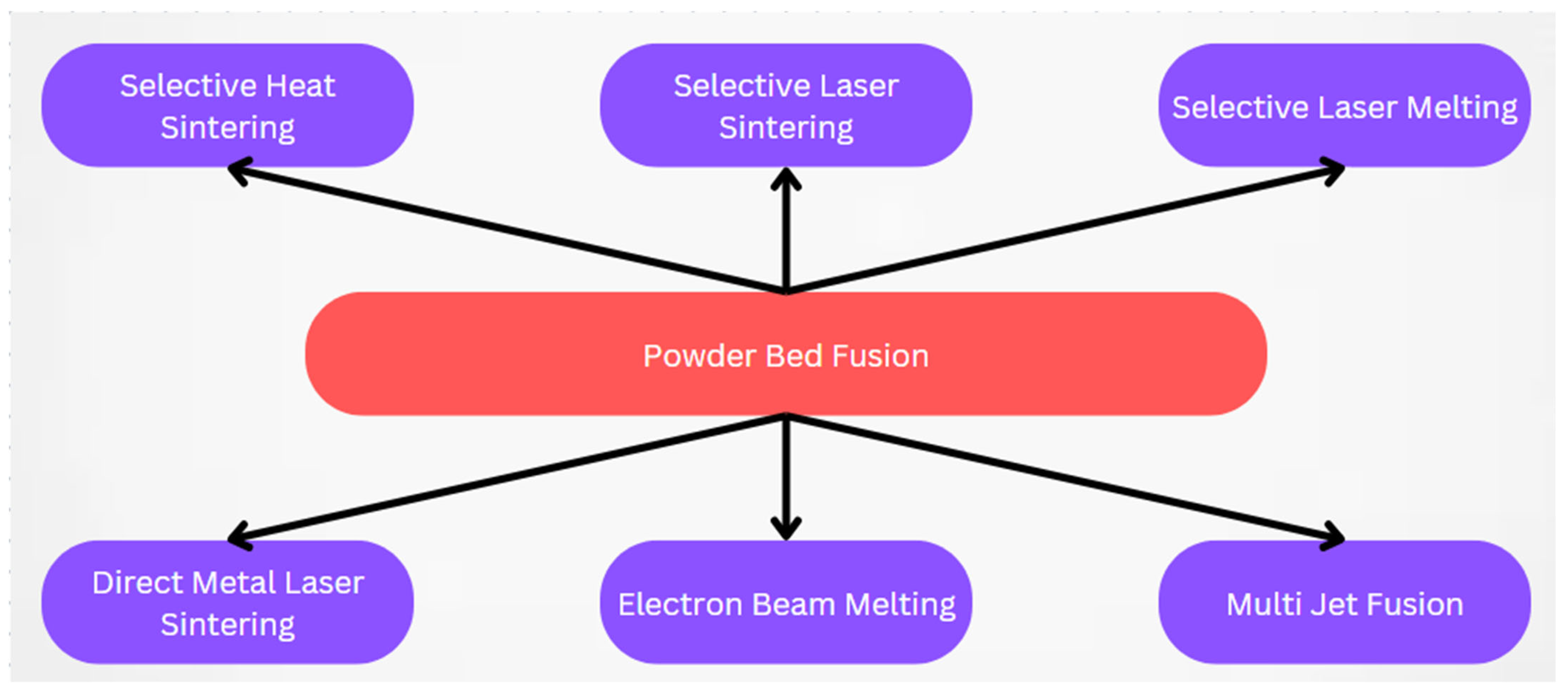
2. PBF 3D Printing
2.1. Overview
2.1.1. Selective Heat Sintering
2.1.2. Selective Laser Sintering
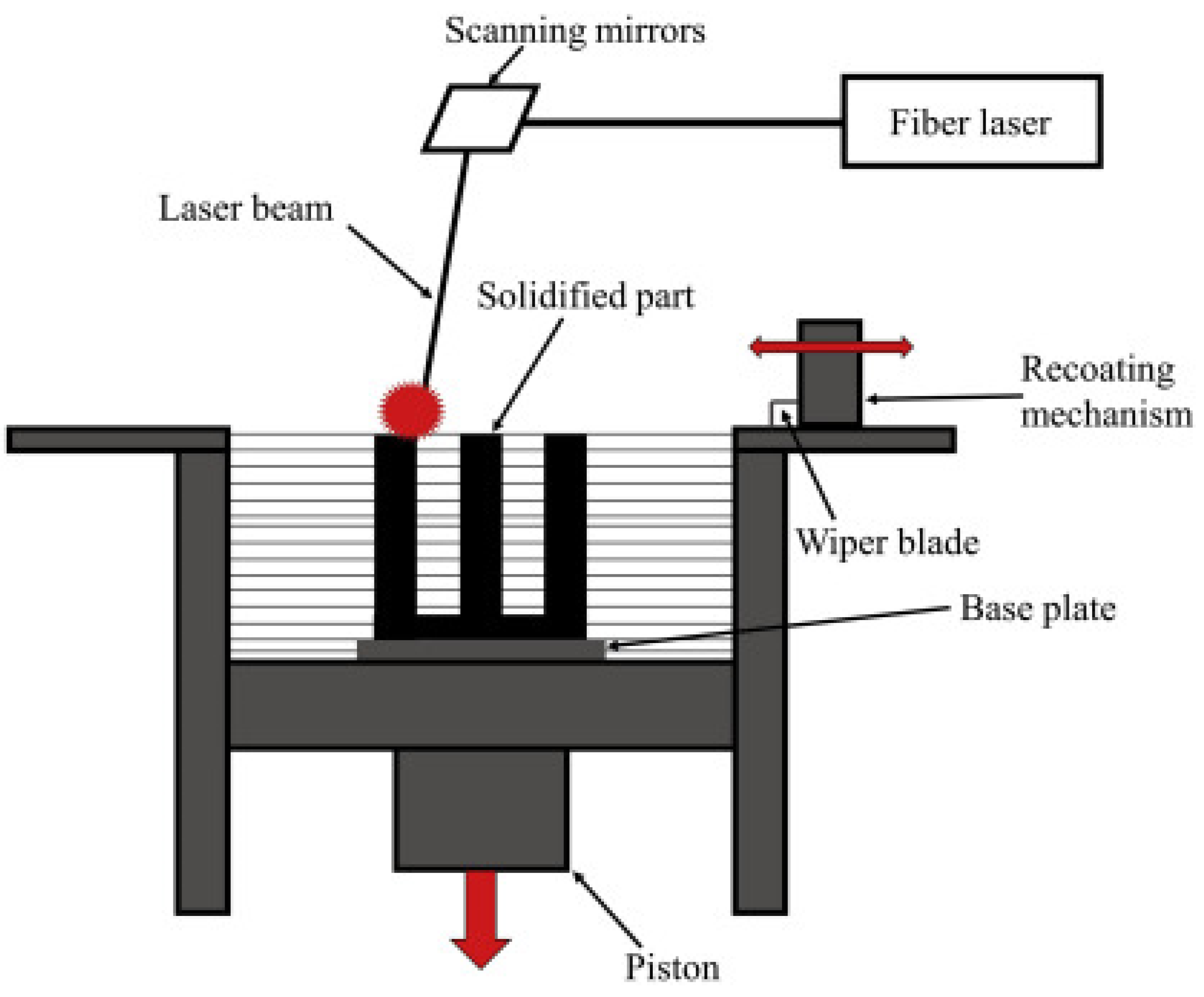
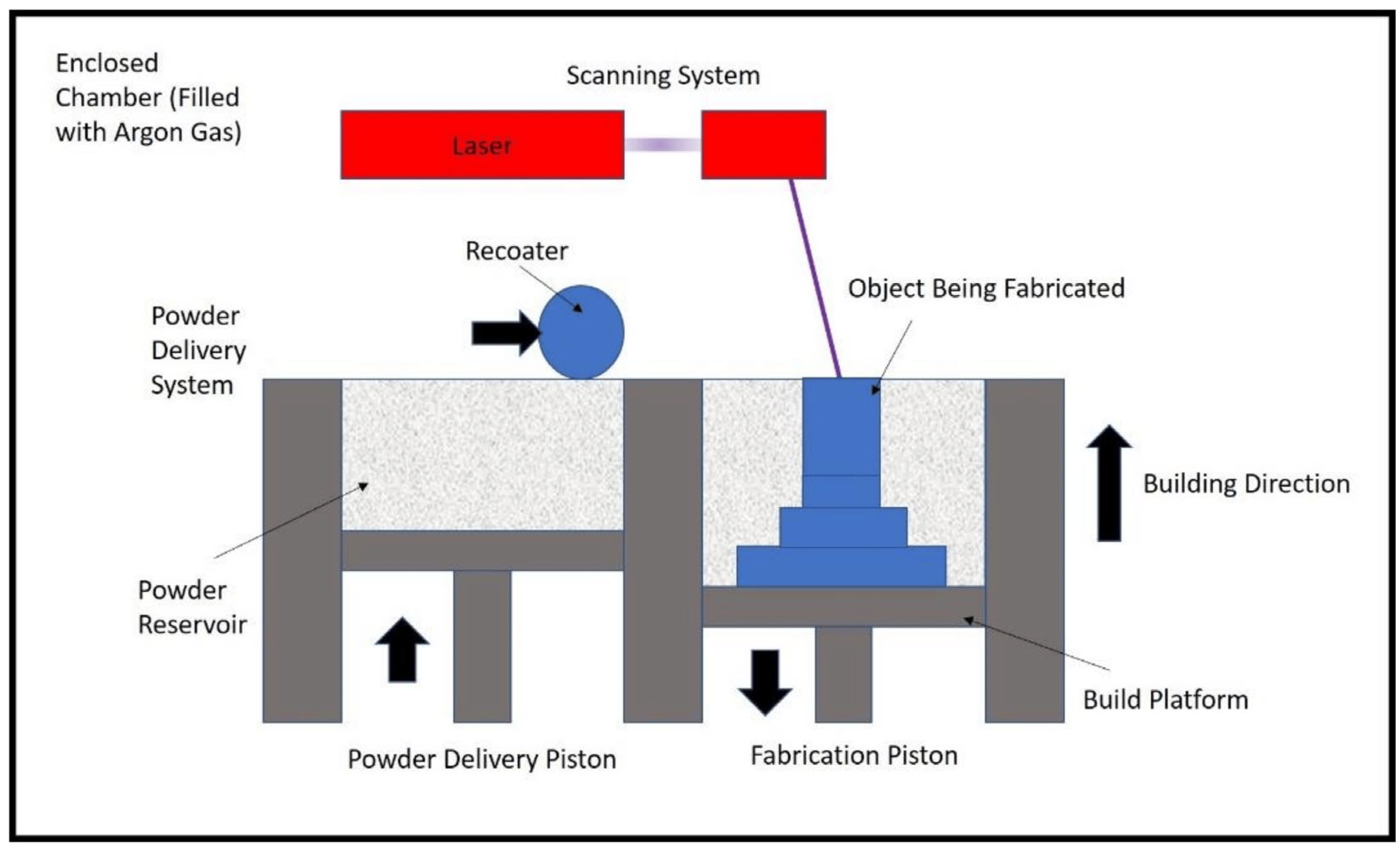
2.1.3. Selective Laser Melting
2.1.4. Direct Metal Laser Sintering
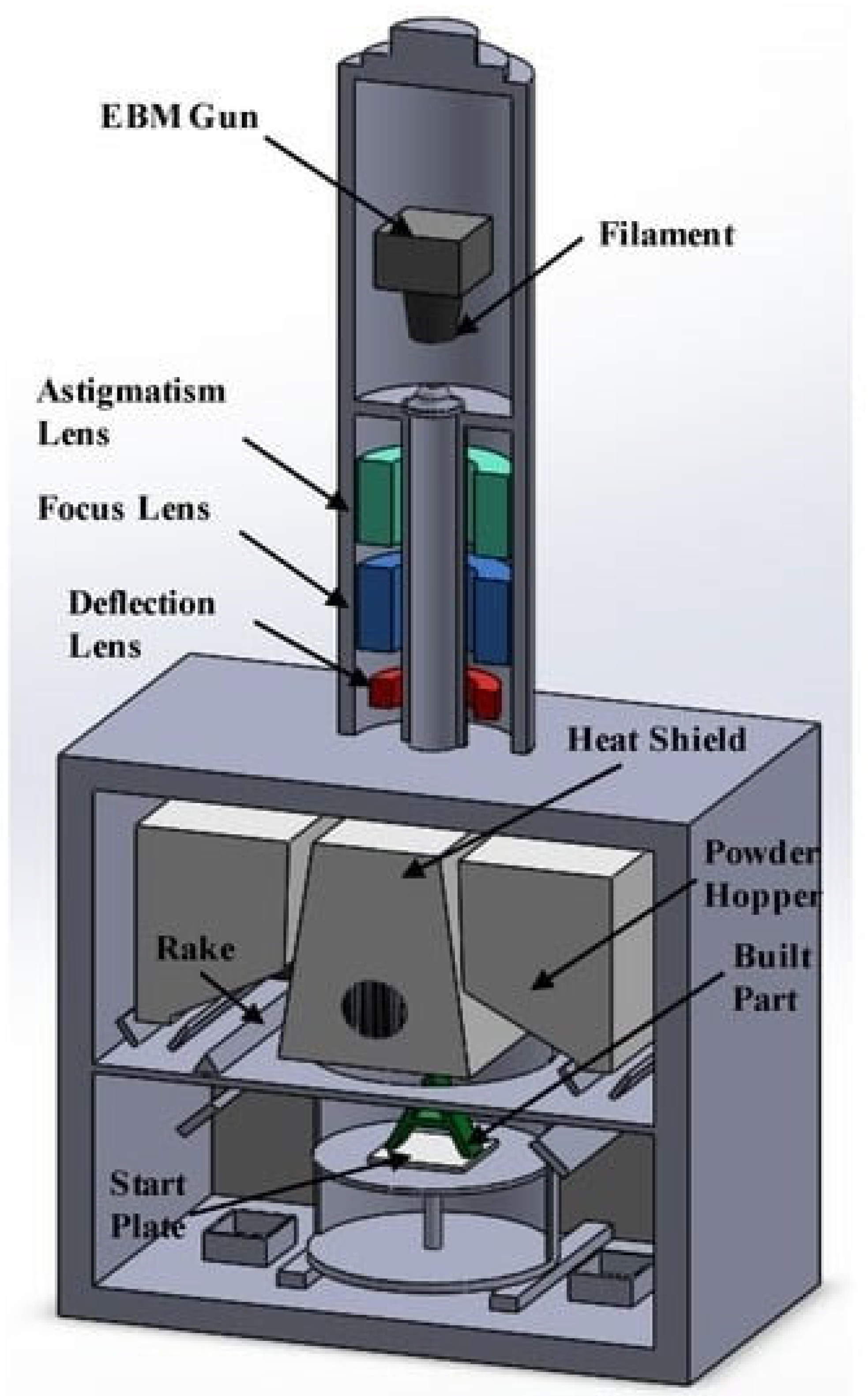
2.1.5. Electron Beam Melting
2.1.6. Multi-Jet Fusion
2.2. Materials Used in PBF
Materials Used in Biomedical Implants
3. Application of PBF 3D Printing in the Biomedical Field
3.1. Biomedical Implants
3.2. Factors Considered for 3D Printing of Biomedical Implants
3.2.1. Implant Design and Material Selection
3.2.2. Surface Finish
3.2.3. Accuracy and Precision
3.2.4. Sterility and Cleanliness
3.2.5. Testing and Quality Control
4. Advantages of PBF 3D Printing in Precision Manufacturing
4.1. High Degree of Precision
4.2. Complex Geometries
4.3. Reduced Waste
4.4. Quick Iteration
4.5. Short Lead Times
4.6. Reduced Assembly
4.7. No Tooling Cost Required
4.8. Inventory Reduction
5. Challenges and Limitations
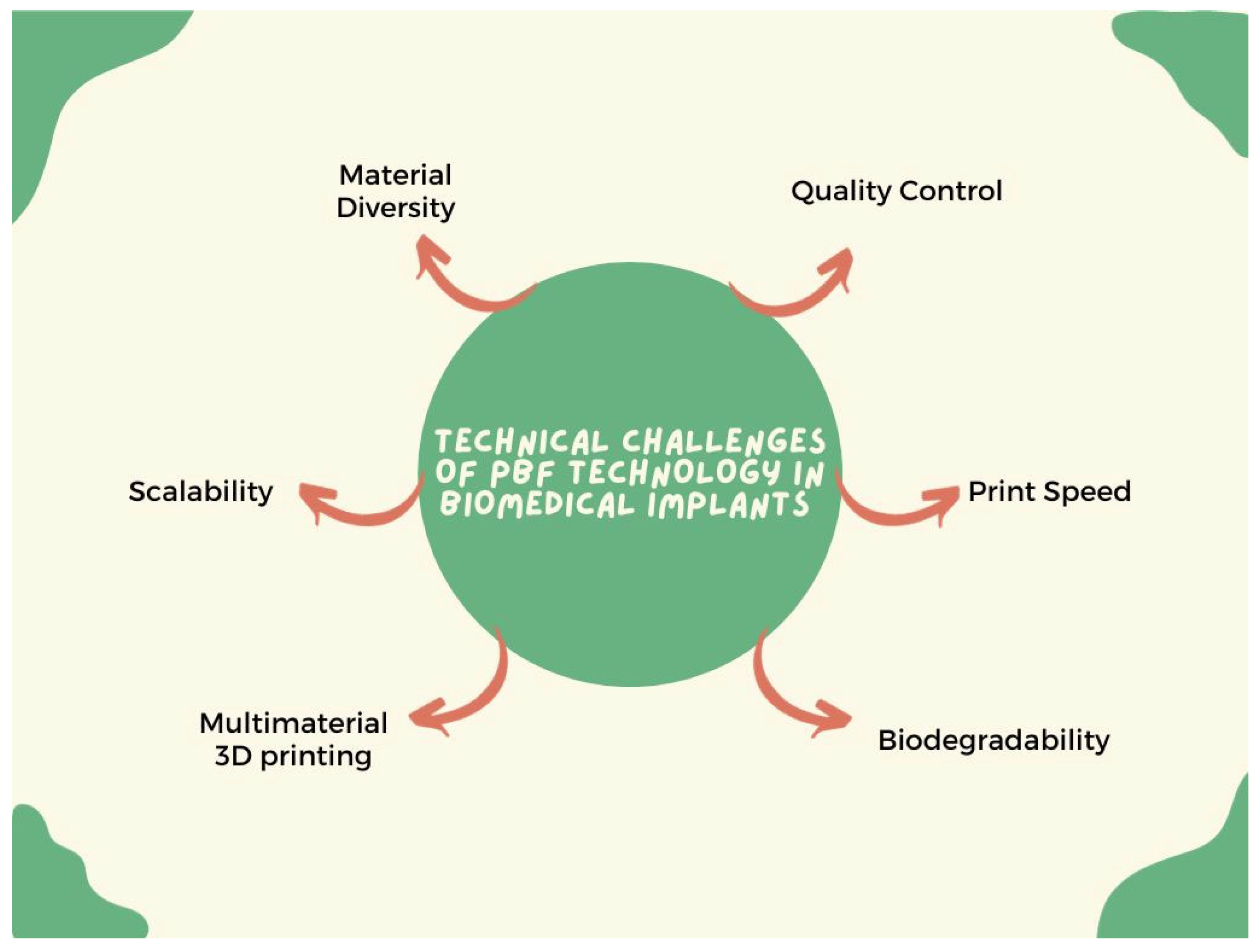
5.1. Technical Challenges
5.1.1. Material Diversity
5.1.2. Biodegradability
5.1.3. Multi-Material 3D Printing
5.1.4. Printing Speed
5.1.5. Scalability
5.1.6. Quality Control
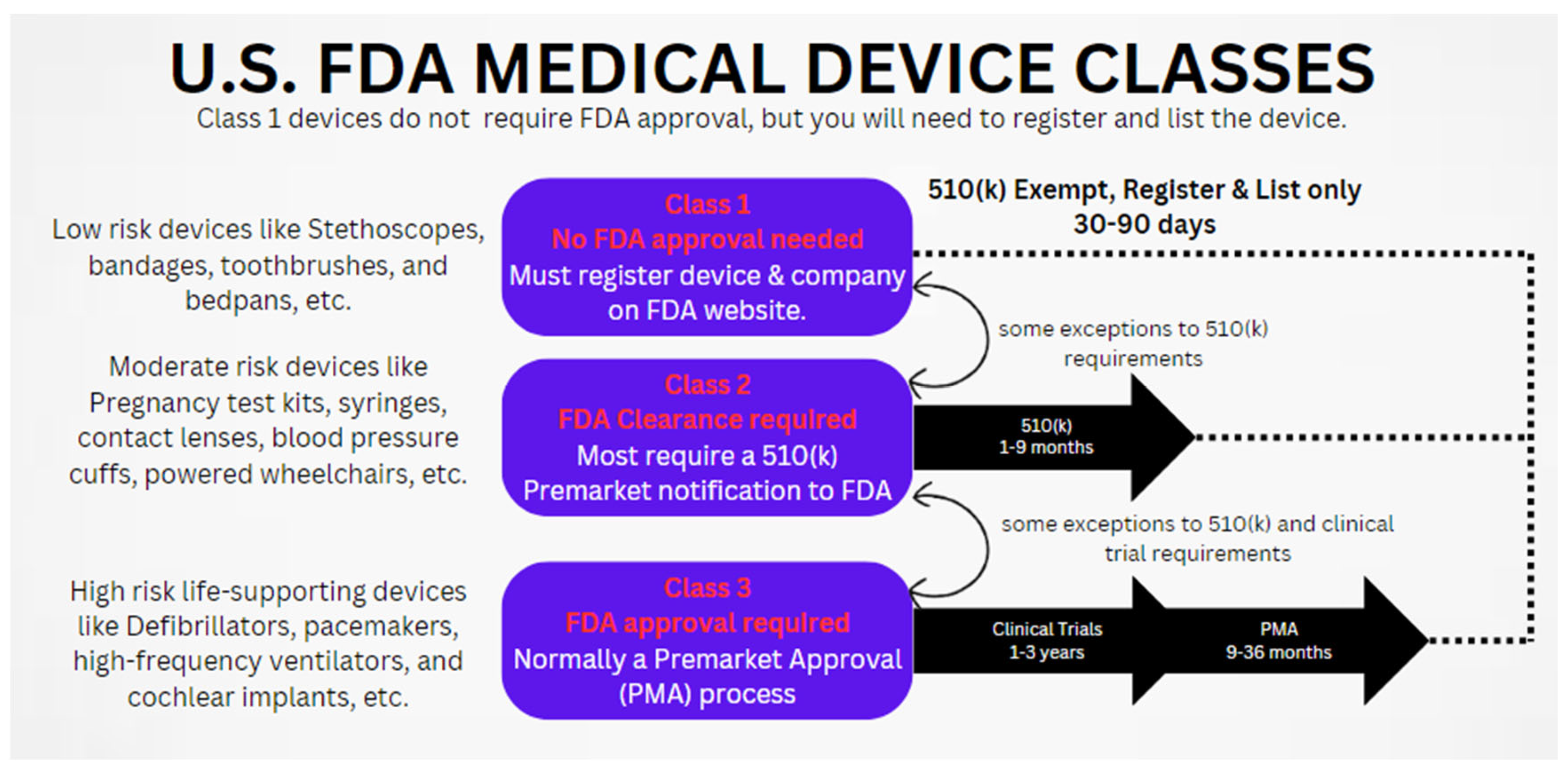
5.2. Regulatory and Safety Concerns
6. Future Prospects and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three dimensional |
| AM | additive manufacturing |
| AlSi10Mg | aluminum–silicon–magnesium alloy |
| BIC | bone-to-implant contact |
| Co-Cr-based alloy | cobalt–chromium-based alloy |
| Co-Cr-Mo | cobalt–chromium–molybdenum |
| CPR | cardiopulmonary resuscitation |
| DMLS | direct metal laser sintering |
| EBM | electron beam melting |
| FDA | Food and Drug Administration |
| H-ECMR | hybrid electrochemical-assisted magnetorheological |
| HP | Hewlett-Packard |
| LPBF | laser powder bed fusion |
| MJF | multi-jet fusion |
| NiTi | nitinol |
| PBF | powder bed fusion |
| PMA | pre-market approval |
| SEM | scanning electron microscopy |
| SHS | selective heat sintering |
| SLM | selective laser melting |
| SLM–SPS | selective laser melting–spark plasma sintering |
| SLS | selective laser sintering |
| SS316L | stainless steel 316L |
| Ti-6Al-4V | titanium–6% aluminum–4% vanadium alloy |
References
- Liu, J.; Wen, P. Metal Vaporization and Its Influence during Laser Powder Bed Fusion Process. Mater. Des. 2022, 215, 110505. [Google Scholar] [CrossRef]
- ISO/ASTM 52900:2021; Additive Manufacturing—General Principles—Fundamentals and Vocabulary. International Organization for Standardization: Geneva, Switzerland; ASTM International: West Conshohocken, PA, USA, 2021.
- Baek, I.; Kwon, O.; Lim, C.-M.; Park, K.Y.; Bae, C.-J. 3D PEEK Objects Fabricated by Fused Filament Fabrication (FFF). Materials 2022, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Adugna, Y.W.; Akessa, A.D.; Lemu, H.G. Overview Study on Challenges of Additive Manufacturing for a Healthcare Application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1201, 012041. [Google Scholar] [CrossRef]
- Kumar, N.; Ukey, P.D.; Francis, V.; Singh, R.P.; Sahu, S. Plastic Pellets. In Polymers for 3D Printing; Elsevier: Amsterdam, The Netherlands, 2022; pp. 307–323. [Google Scholar]
- Ravi, T.; Ranganathan, R.; Ramesh, S.P.; Dandotiya, D.S. 3D Printed Personalized Orthotic Inserts Using Photogrammetry and FDM Technology. In Fused Deposition Modeling Based 3D Printing; Springer: Berlin/Heidelberg, Germany, 2021; pp. 349–361. [Google Scholar]
- Meglioli, M.; Naveau, A.; Macaluso, G.M.; Catros, S. 3D Printed Bone Models in Oral and Cranio-Maxillofacial Surgery: A Systematic Review. 3D Print. Med. 2020, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, F.; Farshbaf, M.; Dahri, M.; Masjedi, M.; Maleki, R.; Amini, F.; Wirth, J.; Moharamzadeh, K.; Weber, F.E.; Tayebi, L. 3D Printing of Dental Prostheses: Current and Emerging Applications. J. Compos. Sci. 2023, 7, 80. [Google Scholar] [CrossRef]
- Aguilar-de-Leyva, Á.; Linares, V.; Casas, M.; Caraballo, I. 3D Printed Drug Delivery Systems Based on Natural Products. Pharmaceutics 2020, 12, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A.; Zhang, Q. 3D Printing Method for Bone Tissue Engineering Scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef]
- Subramanian, B.; Das, P.; Biswas, S.; Roy, A.; Basak, P. Polymers for Additive Manufacturing and 4D-Printing for Tissue Regenerative Applications. In Advances in Biomedical Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 159–182. [Google Scholar]
- Huang, S.; Wei, H.; Li, D. Additive Manufacturing Technologies in the Oral Implant Clinic: A Review of Current Applications and Progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef]
- Patel, P.; Dhal, K.; Gupta, R.; Tappa, K.; Rybicki, F.J.; Ravi, P. Medical 3D Printing Using Desktop Inverted Vat Photopolymerization: Background, Clinical Applications, and Challenges. Bioengineering 2023, 10, 782. [Google Scholar] [CrossRef]
- Milton, L.A.; Viglione, M.S.; Ong, L.J.Y.; Nordin, G.P.; Toh, Y.-C. Vat Photopolymerization 3D Printed Microfluidic Devices for Organ-on-a-Chip Applications. Lab Chip 2023, 23, 3537–3560. [Google Scholar] [CrossRef]
- Wilts, E.M.; Gula, A.; Davis, C.; Chartrain, N.; Williams, C.B.; Long, T.E. Vat Photopolymerization of Liquid, Biodegradable PLGA-Based Oligomers as Tissue Scaffolds. Eur. Polym. J. 2020, 130, 109693. [Google Scholar] [CrossRef]
- Xu, X.; Awad, A.; Robles-Martinez, P.; Gaisford, S.; Goyanes, A.; Basit, A.W. Vat Photopolymerization 3D Printing for Advanced Drug Delivery and Medical Device Applications. J. Control. Release 2021, 329, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Muthuram, N.; Sriram Madhav, P.; Keerthi Vasan, D.; Mohan, M.E.; Prajeeth, G. A Review of Recent Literatures in Poly Jet Printing Process. Mater. Today Proc. 2022, 68, 1906–1920. [Google Scholar] [CrossRef]
- Kjar, A.; Huang, Y. Application of Micro-Scale 3D Printing in Pharmaceutics. Pharmaceutics 2019, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Sing, S.L.; Zhou, M.; Yeong, W.Y. 3D Bioprinting Processes: A Perspective on Classification and Terminology. Int. J. Bioprinting 1970, 4, 151. [Google Scholar] [CrossRef] [PubMed]
- Otton, J.M.; Birbara, N.S.; Hussain, T.; Greil, G.; Foley, T.A.; Pather, N. 3D Printing from Cardiovascular CT: A Practical Guide and Review. Cardiovasc. Diagn. Ther. 2017, 7, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder Jet 3D Printing—Process Parameters, Materials, Properties, Modeling, and Challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, Y.; Zhang, J.; Shi, Y.; Wang, L. Recent Advances in Additive Manufacturing Technology for Bone Tissue Engineering Scaffolds. Int. J. Adv. Manuf. Technol. 2020, 108, 3591–3606. [Google Scholar] [CrossRef]
- Polygenis, T.T. Binder Jetting: A Comprehensive Guide to the Additive Manufacturing Process. Available online: https://www.wevolver.com/article/binder-jetting-a-comprehensive-guide-to-the-additive-manufacturing-process (accessed on 19 January 2024).
- Mostafaei, A.; Stevens, E.L.; Ference, J.J.; Schmidt, D.E.; Chmielus, M. Binder Jetting of a Complex-Shaped Metal Partial Denture Framework. Addit. Manuf. 2018, 21, 63–68. [Google Scholar] [CrossRef]
- Park, S.; Deng, K.; Fu, K.K. Additive Manufacturing Including Laser-Based Manufacturing. In Sustainable Manufacturing Processes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 285–311. [Google Scholar]
- Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Wu, J.; Duan, S.; Wang, X.; Hong, X.; Han, X.; Li, C.; Kang, D.; Wang, Z.; et al. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics 2022, 14, 2589. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical Applications of Powder-Based Binder Jet 3D Printing Process—A Review. Adv. Drug Deliv. Rev. 2021, 177, 113943. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, S.S.; Singh, J. 3D Printing of Biomaterials for Biomedical Applications: A Review. Int. J. Interact. Des. Manuf. 2023, 1–22. [Google Scholar] [CrossRef]
- Dzogbewu, T.C.; de Beer, D. Powder Bed Fusion of Multimaterials. J. Manuf. Mater. Process. 2023, 7, 15. [Google Scholar] [CrossRef]
- Gradl, P.R.; Tinker, D.C.; Ivester, J.; Skinner, S.W.; Teasley, T.; Bili, J.L. Geometric Feature Reproducibility for Laser Powder Bed Fusion (L-PBF) Additive Manufacturing with Inconel 718. Addit. Manuf. 2021, 47, 102305. [Google Scholar] [CrossRef]
- Zhou, Y.; Ning, F.; Zhang, P.; Sharma, A. Geometrical, Microstructural, and Mechanical Properties of Curved-Surface AlSi10Mg Parts Fabricated by Powder Bed Fusion Additive Manufacturing. Mater. Des. 2021, 198, 109360. [Google Scholar] [CrossRef]
- Nouri, A.; Rohani Shirvan, A.; Li, Y.; Wen, C. Additive Manufacturing of Metallic and Polymeric Load-Bearing Biomaterials Using Laser Powder Bed Fusion: A Review. J. Mater. Sci. Technol. 2021, 94, 196–215. [Google Scholar] [CrossRef]
- Küng, V.E.; Scherr, R.; Markl, M.; Körner, C. Multi-Material Model for the Simulation of Powder Bed Fusion Additive Manufacturing. Comput. Mater. Sci. 2021, 194, 110415. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Tuma, D.; Vaneker, T.; Afrasiabi, M.; Bambach, M.; Gibson, I. Multimaterial Powder Bed Fusion Techniques. Rapid Prototyp. J. 2022, 28, 1–19. [Google Scholar] [CrossRef]
- Wasti, S.; Adhikari, S. Use of Biomaterials for 3D Printing by Fused Deposition Modeling Technique: A Review. Front. Chem. 2020, 8, 315. [Google Scholar] [CrossRef]
- Choong, Y.Y.C. Additive Manufacturing for Digital Transformation. In Digital Manufacturing; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–182. [Google Scholar]
- Bidare, P.; Abdullah, R.; Jiménez, A.; Essa, K. Powder Reusability in Metal Binder Jetting. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2023, 095440892211477. [Google Scholar] [CrossRef]
- Salmi, M. Comparing Additive Manufacturing Processes for Distributed Manufacturing. IFAC-PapersOnLine 2022, 55, 1503–1508. [Google Scholar] [CrossRef]
- Rehman, M.; Yanen, W.; Mushtaq, R.T.; Ishfaq, K.; Zahoor, S.; Ahmed, A.; Kumar, M.S.; Gueyee, T.; Rahman, M.M.; Sultana, J. Additive Manufacturing for Biomedical Applications: A Review on Classification, Energy Consumption, and Its Appreciable Role since COVID-19 Pandemic. Prog. Addit. Manuf. 2022, 8, 1007–1041. [Google Scholar] [CrossRef]
- da Silva, L.R.R.; Sales, W.F.; Campos, F.d.A.R.; de Sousa, J.A.G.; Davis, R.; Singh, A.; Coelho, R.T.; Borgohain, B. A Comprehensive Review on Additive Manufacturing of Medical Devices. Prog. Addit. Manuf. 2021, 6, 517–553. [Google Scholar] [CrossRef]
- Zhang, X.; Liou, F. Introduction to Additive Manufacturing. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–31. [Google Scholar]
- Louvis, E.; Fox, P.; Sutcliffe, C.J. Selective Laser Melting of Aluminium Components. J. Mater. Process. Technol. 2011, 211, 275–284. [Google Scholar] [CrossRef]
- Selective Heat Sintering. Available online: https://en.wikipedia.org/wiki/Selective_heat_sintering (accessed on 29 January 2024).
- All About Selective Heat Sintering 3D Printing. Available online: https://www.thomasnet.com/articles/custom-manufacturing-fabricating/selective-heat-sintering-shs-3d-printing/ (accessed on 29 January 2024).
- Selective Heat Sintering, SHS. Available online: https://www.manufacturingguide.com/en/selective-heat-sintering-shs (accessed on 29 January 2024).
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Powder Bed Fusion|Additive Manufacturing Research Group|Loughborough University. Available online: https://www.lboro.ac.uk/research/amrg/about/the7categoriesofadditivemanufacturing/powderbedfusion/ (accessed on 29 January 2024).
- Song, Y.; Ghafari, Y.; Asefnejad, A.; Toghraie, D. An Overview of Selective Laser Sintering 3D Printing Technology for Biomedical and Sports Device Applications: Processes, Materials, and Applications. Opt. Laser Technol. 2024, 171, 110459. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Wu, B.; Brandon, N.P. Electrical Conductivity and Porosity in Stainless Steel 316L Scaffolds for Electrochemical Devices Fabricated Using Selective Laser Sintering. Mater. Des. 2016, 106, 51–59. [Google Scholar] [CrossRef]
- Grossin, D.; Montón, A.; Navarrete-Segado, P.; Özmen, E.; Urruth, G.; Maury, F.; Maury, D.; Frances, C.; Tourbin, M.; Lenormand, P.; et al. A Review of Additive Manufacturing of Ceramics by Powder Bed Selective Laser Processing (Sintering/Melting): Calcium Phosphate, Silicon Carbide, Zirconia, Alumina, and Their Composites. Open Ceram. 2021, 5, 100073. [Google Scholar] [CrossRef]
- Snodderly, K.; Cunningham, A.; Zipin, N.; Sung, M.K.; Di Prima, M.; Porter, D. Effect of Lattice Orientation on Compressive Properties of Selective Laser Sintered Nylon Lattice Coupons. Mech. Mater. 2023, 183, 104686. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, W.; Yu, S.; Tian, Y.; Zhou, K. Selective Laser Sintering of Carbon Nanotube–Coated Thermoplastic Polyurethane: Mechanical, Electrical, and Piezoresistive Properties. Compos. Part C Open Access 2022, 7, 100212. [Google Scholar] [CrossRef]
- Mahmoud, M.; Huitorel, B.; Fall, A. Rheology and Agglomeration Behavior of Semi-Crystalline Polyamide Powders for Selective Laser Sintering: A Comparative Study of PA11 and PA12 Formulations. Powder Technol. 2024, 433, 119279. [Google Scholar] [CrossRef]
- Munir, K.S.; Li, Y.; Wen, C. Metallic Scaffolds Manufactured by Selective Laser Melting for Biomedical Applications. In Metallic Foam Bone; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–23. [Google Scholar]
- Gan, X.; Fei, G.; Wang, J.; Wang, Z.; Lavorgna, M.; Xia, H. Powder Quality and Electrical Conductivity of Selective Laser Sintered Polymer Composite Components. In Structure and Properties of Additive Manufactured Polymer Components; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–185. [Google Scholar]
- Najmon, J.C.; Raeisi, S.; Tovar, A. Review of Additive Manufacturing Technologies and Applications in the Aerospace Industry. In Additive Manufacturing for the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 7–31. [Google Scholar]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Bataille, B.; Aubert, A.; Leclercq, L.; Rossi, J.-C.; Soulairol, I. Selective Laser Sintering of Solid Oral Dosage Forms with Copovidone and Paracetamol Using a CO2 Laser. Pharmaceutics 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Yasa, E. Selective Laser Melting. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 77–120. [Google Scholar]
- Yan, C. Foreword. In Selective Laser Sintering Additive Manufacturing Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. xi–xiii. [Google Scholar]
- Shi, W.; Dong, L.; Zhang, X.; Li, J.; Xie, C.; Yan, T.; Liu, Y. Simulation and Experimental Study of the Hole-Making Process of Ti-6Al-4V Titanium Alloy for Selective Laser Melting. J. Manuf. Process. 2023, 106, 223–239. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y. New Insights into the Evolution of TiB Whisker and TiC Particle during Selective Laser Melting of Titanium Matrix Composites. Mater. Sci. Eng. A 2023, 877, 145200. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, H.s.; Zhou, J.; Wang, W.; Xi, S.X.; Yuan, Y. Effect of Boron Element on Microstructure and Mechanical Properties of 316L Stainless Steel Manufactured by Selective Laser Melting. J. Mater. Res. Technol. 2023, 26, 3744–3755. [Google Scholar] [CrossRef]
- Bibili Nzengue, A.G.; Mpofu, K.; Mathe, N.R.; Muvunzi, R. Optimising a Processing Window for the Production of Aluminium Silicon-12 Samples via Selective Laser Melting. J. Mater. Res. Technol. 2024, 28, 1062–1073. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Rahman, M.H.; Slater, E.; Patel, R.; Evangelista, C.; Austin, E.; Tompkins, E.; McCarroll, A.; Rajak, D.K.; Menezes, P.L. Powder Bed Fusion–Based Additive Manufacturing: SLS, SLM, SHS, and DMLS. In Tribology of Additively Manufactured Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–37. [Google Scholar]
- Neikov, O.D. Powders for Additive Manufacturing Processing. In Handbook of Non-Ferrous Metal Powders; Elsevier: Amsterdam, The Netherlands, 2019; pp. 373–399. [Google Scholar]
- Jiao, L.; Chua, Z.; Moon, S.; Song, J.; Bi, G.; Zheng, H. Femtosecond Laser Produced Hydrophobic Hierarchical Structures on Additive Manufacturing Parts. Nanomaterials 2018, 8, 601. [Google Scholar] [CrossRef]
- Cook, P.S.; Phua, A.; Davies, C.H.J.; Delaney, G.W. Modelling the Influences of Powder Layer Depth and Particle Morphology on Powder Bed Fusion Using a Coupled DEM-CFD Approach. Powder Technol. 2023, 429, 118927. [Google Scholar] [CrossRef]
- Schniedenharn, M.; Wiedemann, F.; Schleifenbaum, J.H. Visualization of the Shielding Gas Flow in SLM Machines by Space-Resolved Thermal Anemometry. Rapid Prototyp. J. 2018, 24, 1296–1304. [Google Scholar] [CrossRef]
- Munir, K.; Biesiekierski, A.; Wen, C.; Li, Y. Selective Laser Melting in Biomedical Manufacturing. In Metallic Biomaterials Processing and Medical Device Manufacturing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 235–269. [Google Scholar]
- Harun, W.S.W.; Kadirgama, K.; Samykano, M.; Ramasamy, D.; Ahmad, I.; Moradi, M. Mechanical Behavior of Selective Laser Melting-Produced Metallic Biomaterials. In Mechanical Behaviour of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–116. [Google Scholar]
- Rahmati, S. Direct Rapid Tooling. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 303–344. [Google Scholar]
- Jessy Michla, J.R.; Ravikumar, B.; Ram Prabhu, T.; Siengchin, S.; Arul Kumar, M.; Rajini, N. Effect of Nitriding on Mechanical and Microstructural Properties of Direct Metal Laser Sintered 17-4PH Stainless Steel. J. Mater. Res. Technol. 2022, 19, 2810–2821. [Google Scholar] [CrossRef]
- Dwivedi, A.; Khurana, M.K.; Bala, Y.G. Heat-Treated Nickel Alloys Produced Using Laser Powder Bed Fusion-Based Additive Manufacturing Methods: A Review. Chin. J. Mech. Eng. Addit. Manuf. Front. 2023, 2, 100087. [Google Scholar] [CrossRef]
- Jaivignesh, M.; Suresh Babu, A.; Arumaikkannu, G. In-Vitro Analysis of Titanium Cellular Structures Fabricated by Direct Metal Laser Sintering. Mater. Today Proc. 2020, 22, 2372–2377. [Google Scholar] [CrossRef]
- Anthony Xavior, M.; Ashwath, P.; Batako, A.; Jeyapandiarajan, P.; Joel, J.; Anbalagan, A. Processing and Characterization of Aluminium Alloy 6061 Graphene Composite Printed by Direct Metal Laser Sintering. Mater. Today Proc. 2023, in press. [CrossRef]
- Martinho, P.G. Rapid Manufacturing and Tooling. In Design and Manufacturing of Plastics Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 381–456. [Google Scholar]
- DSourced Direct Metal Laser Sintering: Everything to Know about DMLS 3D Printing—3DSourced. Available online: https://www.3dsourced.com/guides/direct-metal-laser-sintering-dmls/ (accessed on 30 January 2024).
- Direct Metal Laser Sintering (DMLS) 3D Printing Service|Fathom. Available online: https://fathommfg.com/direct-metal-laser-sintering-dmls (accessed on 30 January 2024).
- Mugeshwaran, A. Overview on DMLS Process for 3D Printing and Its Applications in Space Industry|Blog|EDS Technologies. Available online: https://edstechnologies.com/blog/overview-on-dmls-process-for-3d-printing-and-its-applications-in-space-industry (accessed on 30 January 2024).
- What Is DMLS 3D Printing?|Metal 3D Printing. Available online: https://hlhrapid.com/knowledge/what-is-dmls-3d-printing (accessed on 30 January 2024).
- Franchitti, S.; Borrelli, R.; Pirozzi, C.; Carrino, L.; Polini, W.; Sorrentino, L.; Gazzerro, A. Investigation on Electron Beam Melting: Dimensional Accuracy and Process Repeatability. Vacuum 2018, 157, 340–348. [Google Scholar] [CrossRef]
- Körner, C. Additive Manufacturing of Metallic Components by Selective Electron Beam Melting—A Review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Sun, J.; Shu, W.; Yang, H.; Ngan, A.H.W.; Huang, Y. Tensile Properties of Ti6.5Al2Zr1Mo1V Titanium Alloy Fabricated via Electron Beam Selective Melting at High Temperature. Mater. Sci. Eng. A 2023, 888, 145806. [Google Scholar] [CrossRef]
- Roos, S.; Barbera Flichi, F.; Ortiz-Membrado, L.; Botero Vega, C.A.; Jiménez-Piqué, E.; Rännar, L.-E. Assessing the Viability of High-Frequency Spot Melting for Super Duplex Stainless Steel 2507 via Electron Beam Powder Bed Fusion. J. Mater. Res. Technol. 2023, 27, 5720–5728. [Google Scholar] [CrossRef]
- Wolf, T.; Fu, Z.; Körner, C. Selective Electron Beam Melting of an Aluminum Bronze: Microstructure and Mechanical Properties. Mater. Lett. 2019, 238, 241–244. [Google Scholar] [CrossRef]
- Toh, W.; Wang, P.; Tan, X.; Nai, M.; Liu, E.; Tor, S. Microstructure and Wear Properties of Electron Beam Melted Ti-6Al-4V Parts: A Comparison Study against As-Cast Form. Metals 2016, 6, 284. [Google Scholar] [CrossRef]
- Galati, M.; Iuliano, L. A Literature Review of Powder-Based Electron Beam Melting Focusing on Numerical Simulations. Addit. Manuf. 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Koh, Z.H.; Chen, K.; Du, H.; Zeng, J.; Zhou, K. Long-Term Ageing Effect on Mechanical Properties of Polyamide 12 Printed by Multi-Jet-Fusion. Int. J. Mech. Sci. 2023, 256, 108513. [Google Scholar] [CrossRef]
- Chen, A.Y.-J.; Chen, A.; Fitzhugh, A.; Hartman, A.; Kaiser, P.; Nwaogwugwu, I.; Zeng, J.; Gu, G.X. Multi Jet Fusion Printed Lattice Materials: Characterization and Prediction of Mechanical Performance. Mater. Adv. 2023, 4, 1030–1040. [Google Scholar] [CrossRef]
- What Is Multi Jet Fusion(MJF)3D Printing. Available online: https://jlcpcb.com/help/article/372-What-is-Multi-Jet-Fusion(MJF)3D-Printing (accessed on 30 January 2024).
- A Beginner’s Guide to MJF 3D Printing: Understanding the Process. Available online: https://www.rapidmade.com/3d-printing/a-beginners-guide-to-mjf-3d-printing-understanding-the-multi-jet-fusion-process (accessed on 30 January 2024).
- Lee, K.P.M.; Kajtaz, M. Experimental Characterisation and Finite Element Modelling of Polyamide-12 Fabricated via Multi Jet Fusion. Polymers 2022, 14, 5258. [Google Scholar] [CrossRef] [PubMed]
- Adach, M.; Sokołowski, P.; Piwowarczyk, T.; Nowak, K. Study on Geometry, Dimensional Accuracy and Structure of Parts Produced by Multi Jet Fusion. Materials 2021, 14, 4510. [Google Scholar] [CrossRef] [PubMed]
- Pandelidi, C.; Lee, K.P.M.; Kajtaz, M. Effects of Polyamide-11 Powder Refresh Ratios in Multi-Jet Fusion: A Comparison of New and Used Powder. Addit. Manuf. 2021, 40, 101933. [Google Scholar] [CrossRef]
- Deshmukh, K.; Muzaffar, A.; Kovářík, T.; Křenek, T.; Ahamed, M.B.; Pasha, S.K.K. Fundamentals and Applications of 3D and 4D Printing of Polymers: Challenges in Polymer Processing and Prospects of Future Research. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 527–560. [Google Scholar]
- Mizuno, M.; Sugita, K.; Do, K.; Ishimoto, T.; Nakano, T.; Araki, H. Stability of Vacancies in β-Type Ti-15Mo-5Zr-3Al Alloy Fabricated via Laser Powder Bed Fusion. Addit. Manuf. Lett. 2023, 7, 100162. [Google Scholar] [CrossRef]
- Depboylu, F.N.; Yasa, E.; Poyraz, Ö.; Minguella-Canela, J.; Korkusuz, F.; De los Santos López, M.A. Titanium Based Bone Implants Production Using Laser Powder Bed Fusion Technology. J. Mater. Res. Technol. 2022, 17, 1408–1426. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Takezawa, A.; du Plessis, A.; Takata, N.; Krakhmalev, P.; Kobashi, M.; Yadroitsava, I.; Yadroitsev, I. Topology Optimization and Characterization of Ti6Al4V ELI Cellular Lattice Structures by Laser Powder Bed Fusion for Biomedical Applications. Mater. Sci. Eng. A 2019, 766, 138330. [Google Scholar] [CrossRef]
- Ju, J.; Zan, R.; Shen, Z.; Wang, C.; Peng, P.; Wang, J.; Sun, B.; Xiao, B.; Li, Q.; Liu, S.; et al. Remarkable Bioactivity, Bio-Tribological, Antibacterial, and Anti-Corrosion Properties in a Ti-6Al-4V-XCu Alloy by Laser Powder Bed Fusion for Superior Biomedical Implant Applications. Chem. Eng. J. 2023, 471, 144656. [Google Scholar] [CrossRef]
- Ishimoto, T.; Suganuma, R.; Nakano, T. Tailoring the Crystallographic Texture of Biomedical Metastable β-Type Ti-Alloy Produced via Laser Powder Bed Fusion Using Temperature-Field Simulations. Mater. Lett. 2023, 349, 134835. [Google Scholar] [CrossRef]
- Huang, S.; Sing, S.L.; de Looze, G.; Wilson, R.; Yeong, W.Y. Laser Powder Bed Fusion of Titanium-Tantalum Alloys: Compositions and Designs for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 108, 103775. [Google Scholar] [CrossRef] [PubMed]
- Fereiduni, E.; Mahmoud, D.; Balbaa, M.; Elbestawi, M. Laser Powder Bed Fusion of Hydroxyapatite Functionalized Ti-6Al-4V Bi-Material with Potential Biomedical Applications. Mater. Lett. 2022, 326, 132973. [Google Scholar] [CrossRef]
- Rehman, M.; Wang, Y.; Ishfaq, K.; Mushtaq, R.T.; Kumar, M.S.; Yang, H. Potential Assessment in Laser Powder Bed Fusion of Bionic Porous Ti Scaffolds Concerning Compressive Behavior, Porosity, and Surface Roughness. J. Manuf. Process. 2023, 95, 461–478. [Google Scholar] [CrossRef]
- Jam, A.; du Plessis, A.; Lora, C.; Raghavendra, S.; Pellizzari, M.; Benedetti, M. Manufacturability of Lattice Structures Fabricated by Laser Powder Bed Fusion: A Novel Biomedical Application of the Beta Ti-21S Alloy. Addit. Manuf. 2022, 50, 102556. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kermanpur, A.; Kharaziha, M. The Effects of Pore Size and Heat Treatment on Compression and Corrosion Behaviors of Ti–6Al–4V Sheet-Based Gyroid Implants Fabricated by Laser Powder-Bed Fusion Process. J. Mater. Res. Technol. 2023, 26, 7707–7721. [Google Scholar] [CrossRef]
- Anuar, A.; Guraya, T.; Chen, Z.W.; Ramezani, M.; San Sebastián-Ormazabal, M. Effect of Build Direction Dependent Grain Structure on Fatigue Crack Growth of Biomedical Co–29Cr–6Mo Alloy Processed by Laser Powder Bed Fusion. J. Mech. Behav. Biomed. Mater. 2021, 123, 104741. [Google Scholar] [CrossRef]
- Jiang, W.; An, X.; Xiao, C.; Ni, S.; Song, M. Effects of Heat Treatment on the Microstructure and Properties of a Face-Centered Cubic CoCrMoW Alloy Prepared via Laser Powder Bed Fusion. J. Alloys Compd. 2023, 963, 171212. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Lou, D.; Xia, W.; Fang, X. Microstructural Features of Biomedical Cobalt–Chromium–Molybdenum (CoCrMo) Alloy from Powder Bed Fusion to Aging Heat Treatment. J. Mater. Sci. Technol. 2020, 45, 146–156. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Wang, S.; Xiang, Z.; Shen, X.; Huang, S.; Yang, Q. Mechanical and Functional Properties of HfH2-Decorated NiTi Shape Memory Alloy Fabricated by Laser Powder-Bed Fusion. J. Alloys Compd. 2022, 913, 165296. [Google Scholar] [CrossRef]
- Obeidi, M.A. Achieving High Quality Nitinol Parts with Minimised Input Thermal Energy by Optimised Pulse Wave Laser Powder Bed Fusion Process. Results Mater. 2022, 14, 100279. [Google Scholar] [CrossRef]
- Safdel, A.; Torbati-Sarraf, H.; Elbestawi, M.A. Laser Powder Bed Fusion of Differently Designed NiTi Stent Structures Having Enhanced Recoverability and Superelasticity. J. Alloys Compd. 2023, 954, 170196. [Google Scholar] [CrossRef]
- Fink, A.; Fu, Z.; Körner, C. Functional Properties and Shape Memory Effect of Nitinol Manufactured via Electron Beam Powder Bed Fusion. Materialia 2023, 30, 101823. [Google Scholar] [CrossRef]
- Gatto, M.L.; Cerqueni, G.; Groppo, R.; Santecchia, E.; Tognoli, E.; Defanti, S.; Mattioli-Belmonte, M.; Mengucci, P. Improved Biomechanical Behavior of 316L Graded Scaffolds for Bone Tissue Regeneration Produced by Laser Powder Bed Fusion. J. Mech. Behav. Biomed. Mater. 2023, 144, 105989. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, A.; Stylianou, R.; Loizou, A.; Kim, D.; Liang, A.; Reed, P.; Constantinides, G.; Kyratsi, T. Effects of Process Parameters and Scan Strategy on the Microstructure and Density of Stainless Steel 316 L Produced via Laser Powder Bed Fusion. J. Alloy. Metall. Syst. 2023, 3, 100027. [Google Scholar] [CrossRef]
- Li, M.; Benn, F.; Derra, T.; Kröger, N.; Zinser, M.; Smeets, R.; Molina-Aldareguia, J.M.; Kopp, A.; LLorca, J. Microstructure, Mechanical Properties, Corrosion Resistance and Cytocompatibility of WE43 Mg Alloy Scaffolds Fabricated by Laser Powder Bed Fusion for Biomedical Applications. Mater. Sci. Eng. C 2021, 119, 111623. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.F.; Li, J.; Misra, R.D.K.; Yang, Y.F.; Xiao, S.Y.; Tian, Z.J. Mechanistic Understanding of Strengthening in a Novel MXene/AlSi10Mg Matrix Composite Processed by Laser Powder Bed Fusion. Mater. Sci. Eng. A 2023, 885, 145662. [Google Scholar] [CrossRef]
- Hanawa, T. Biocompatibility of Titanium from the Viewpoint of Its Surface. Sci. Technol. Adv. Mater. 2022, 23, 457–472. [Google Scholar] [CrossRef]
- Jiang, G.; Zhao, Z.; Xiao, G.; Li, S.; Chen, B.; Zhuo, X.; Zhang, J. Study of Surface Integrity of Titanium Alloy (TC4) by Belt Grinding to Achieve the Same Surface Roughness Range. Micromachines 2022, 13, 1950. [Google Scholar] [CrossRef]
- Hanawa, T. Titanium–Tissue Interface Reaction and Its Control with Surface Treatment. Front. Bioeng. Biotechnol. 2019, 7, 170. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Li, X.; Zhang, X.; Gong, X.; Zhu, Y.; Ren, Z.; Zhang, B.; Cheng, J. Biocompatibility and Osseointegration Properties of a Novel High Strength and Low Modulus β- Ti10Mo6Zr4Sn3Nb Alloy. Front. Bioeng. Biotechnol. 2023, 11, 1127929. [Google Scholar] [CrossRef]
- Odaira, T.; Xu, S.; Hirata, K.; Xu, X.; Omori, T.; Ueki, K.; Ueda, K.; Narushima, T.; Nagasako, M.; Harjo, S.; et al. Flexible and Tough Superelastic Co–Cr Alloys for Biomedical Applications. Adv. Mater. 2022, 34, e2202305. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, R.; Chen, D.; Qin, G.; Zhang, E. Novel CoCrWNi Alloys with Cu Addition: Microstructure, Mechanical Properties, Corrosion Properties and Biocompatibility. J. Alloys Compd. 2020, 824, 153924. [Google Scholar] [CrossRef]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Traxel, K.D.; Avila, J.D.; Mitra, I.; Bose, S. CoCr Alloys. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–269. [Google Scholar]
- Sathishkumar, M.; Kumar, C.P.; Ganesh, S.S.S.; Venkatesh, M.; Radhika, N.; Vignesh, M.; Pazhani, A. Possibilities, Performance and Challenges of Nitinol Alloy Fabricated by Directed Energy Deposition and Powder Bed Fusion for Biomedical Implants. J. Manuf. Process. 2023, 102, 885–909. [Google Scholar] [CrossRef]
- Ali, S.; Abdul Rani, A.M.; Baig, Z.; Ahmed, S.W.; Hussain, G.; Subramaniam, K.; Hastuty, S.; Rao, T.V.V.L.N. Biocompatibility and Corrosion Resistance of Metallic Biomaterials. Corros. Rev. 2020, 38, 381–402. [Google Scholar] [CrossRef]
- Andersen, P.J. Stainless Steels. In Biomaterials Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 249–255. [Google Scholar]
- Bekmurzayeva, A.; Duncanson, W.J.; Azevedo, H.S.; Kanayeva, D. Surface Modification of Stainless Steel for Biomedical Applications: Revisiting a Century-Old Material. Mater. Sci. Eng. C 2018, 93, 1073–1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef]
- Kumar, R.; Katyal, P. Effects of Alloying Elements on Performance of Biodegradable Magnesium Alloy. Mater. Today Proc. 2022, 56, 2443–2450. [Google Scholar] [CrossRef]
- Seetharaman, S.; Sankaranarayanan, D.; Gupta, M. Magnesium-Based Temporary Implants: Potential, Current Status, Applications, and Challenges. J. Funct. Biomater. 2023, 14, 324. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg Alloys for Orthopedic Implants—A Review. J. Magnes. Alloys 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Gong, J.; Wei, K.; Liu, M.; Song, W.; Li, X.; Zeng, X. Microstructure and Mechanical Properties of AlSi10Mg Alloy Built by Laser Powder Bed Fusion/Direct Energy Deposition Hybrid Laser Additive Manufacturing. Addit. Manuf. 2022, 59, 103160. [Google Scholar] [CrossRef]
- Estupiñan-López, F.; Gaona-Tiburcio, C.; Jáquez-Muñoz, J.; Zambrano-Robledo, P.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Delgado, A.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. A Comparative Study of Corrosion AA6061 and AlSi10Mg Alloys Produced by Extruded and Additive Manufacturing. Materials 2021, 14, 5793. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, X.; Zhao, X.; Yang, H.; Li, M.V. Additively Manufactured AlSi10Mg Ultrathin Walls: Microstructure and Nano-Mechanical Properties under Different Energy Densities and Interlayer Cooling Times. Mater. Sci. Eng. A 2022, 835, 142652. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D Printing Metal Implants in Orthopedic Surgery: Methods, Applications and Future Prospects. J. Orthop. Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, J.; Kang, L.; Tian, J.; Zhang, X.; Hu, J.; Huang, Y.; Liu, F.; Wang, H.; Wu, Z. An Overview of 3D Printed Metal Implants in Orthopedic Applications: Present and Future Perspectives. Heliyon 2023, 9, e17718. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, D.; Dou, W.; Wang, Y.; Yang, Y.; Wang, J.; Chen, J. Design and Manufacture of Bionic Porous Titanium Alloy Spinal Implant Based on Selective Laser Melting (SLM). Comput. Model. Eng. Sci. 2020, 124, 1099–1117. [Google Scholar] [CrossRef]
- Kityk, A.; Protsenko, V.; Danilov, F.; Pavlik, V.; Hnatko, M.; Šoltýs, J. Enhancement of the Surface Characteristics of Ti-Based Biomedical Alloy by Electropolishing in Environmentally Friendly Deep Eutectic Solvent (Ethaline). Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126125. [Google Scholar] [CrossRef]
- Murr, L.E. Strategies for Creating Living, Additively Manufactured, Open-Cellular Metal and Alloy Implants by Promoting Osseointegration, Osteoinduction and Vascularization: An Overview. J. Mater. Sci. Technol. 2019, 35, 231–241. [Google Scholar] [CrossRef]
- Murr, L.E. Open-Cellular Metal Implant Design and Fabrication for Biomechanical Compatibility with Bone Using Electron Beam Melting. J. Mech. Behav. Biomed. Mater. 2017, 76, 164–177. [Google Scholar] [CrossRef]
- Rahmani, R.; Kamboj, N.; Brojan, M.; Antonov, M.; Prashanth, K.G. Hybrid Metal-Ceramic Biomaterials Fabricated through Powder Bed Fusion and Powder Metallurgy for Improved Impact Resistance of Craniofacial Implants. Materialia 2022, 24, 101465. [Google Scholar] [CrossRef]
- Mali, S.A.; Zhu, D.; Liu, Y.; Gilbert, J.L. Fretting Crevice Corrosion of 316 L Stainless Steel in Physiological Phosphate Buffered Saline: Load, Potential and Alloy Counterface Effects. Tribol. Int. 2021, 164, 107198. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Shaikh, S.; Nahar, P.; Shaikh, S.; Sayed, A.J.; Ali, H.M. Current Perspectives of 3d Printing in Dental Applications. Braz. Dent. Sci. 2021, 24. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Design for Additive Manufacturing. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; pp. 399–435. [Google Scholar]
- Grover, T.; Pandey, A.; Kumari, S.T.; Awasthi, A.; Singh, B.; Dixit, P.; Singhal, P.; Saxena, K.K. Role of Titanium in Bio Implants and Additive Manufacturing: An Overview. Mater. Today Proc. 2020, 26, 3071–3080. [Google Scholar] [CrossRef]
- Olmo, J.A.-D.; Ruiz-Rubio, L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef]
- Brunello, G.; Sivolella, S.; Meneghello, R.; Ferroni, L.; Gardin, C.; Piattelli, A.; Zavan, B.; Bressan, E. Powder-Based 3D Printing for Bone Tissue Engineering. Biotechnol. Adv. 2016, 34, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Pitjamit, S.; Thunsiri, K.; Nakkiew, W.; Wongwichai, T.; Pothacharoen, P.; Wattanutchariya, W. The Possibility of Interlocking Nail Fabrication from FFF 3D Printing PLA/PCL/HA Composites Coated by Local Silk Fibroin for Canine Bone Fracture Treatment. Materials 2020, 13, 1564. [Google Scholar] [CrossRef]
- Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3D Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Alexander, P.G.; Ocasio-Nieves, B.D.; Yocum, L.; Lin, H.; Tuan, R.S. Pathogenesis of Osteoarthritis: Risk Factors, Regulatory Pathways in Chondrocytes, and Experimental Models. Biology 2020, 9, 194. [Google Scholar] [CrossRef]
- Dall’Ava, L.; Hothi, H.; Henckel, J.; Di Laura, A.; Shearing, P.; Hart, A. Comparative Analysis of Current 3D Printed Acetabular Titanium Implants. 3D Print. Med. 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, Y.; Li, F.; Wang, X.; Zhang, K.; Liu, Z.; Tian, H. A New 3D Printing Porous Trabecular Titanium Metal Acetabular Cup for Primary Total Hip Arthroplasty: A Minimum 2-Year Follow-up of 92 Consecutive Patients. J. Orthop. Surg. Res. 2020, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Rappe, K.S.; Ortiz-Hernandez, M.; Punset, M.; Molmeneu, M.; Barba, A.; Mas-Moruno, C.; Guillem-Marti, J.; Caparrós, C.; Rupérez, E.; Calero, J.; et al. On-Growth and In-Growth Osseointegration Enhancement in PM Porous Ti-Scaffolds by Two Different Bioactivation Strategies: Alkali Thermochemical Treatment and RGD Peptide Coating. Int. J. Mol. Sci. 2022, 23, 1750. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, H.; Zhang, N.; Zhang, M.; Cheng, C.-K. Femoral Stems with Porous Lattice Structures: A Review. Front. Bioeng. Biotechnol. 2021, 9, 772539. [Google Scholar] [CrossRef] [PubMed]
- Büssemaker, H.; Meinshausen, A.-K.; Bui, V.D.; Döring, J.; Voropai, V.; Buchholz, A.; Mueller, A.J.; Harnisch, K.; Martin, A.; Berger, T.; et al. Silver-Integrated EDM Processing of TiAl6V4 Implant Material Has Antibacterial Capacity While Optimizing Osseointegration. Bioact. Mater. 2024, 31, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Amato, K.N.; Li, S.J.; Tian, Y.X.; Cheng, X.Y.; Gaytan, S.M.; Martinez, E.; Shindo, P.W.; Medina, F.; Wicker, R.B. Microstructure and Mechanical Properties of Open-Cellular Biomaterials Prototypes for Total Knee Replacement Implants Fabricated by Electron Beam Melting. J. Mech. Behav. Biomed. Mater. 2011, 4, 1396–1411. [Google Scholar] [CrossRef]
- Hashmi, A.W.; Mali, H.S.; Meena, A.; Saxena, K.K.; Puerta, A.P.V.; Rao, U.S.; Buddhi, D.; Mohammed, K.A. Design and Modeling of Abrasive Flow Finishing of Freeform Surfaces of FDM Printed Femoral Component of Knee Implant Pattern. Int. J. Interact. Des. Manuf. 2023, 17, 2507–2526. [Google Scholar] [CrossRef]
- Markopoulos, A.; Galanis, N.; Karkalos, N.; Manolakos, D. Precision CNC Machining of Femoral Component of Knee Implant: A Case Study. Machines 2018, 6, 10. [Google Scholar] [CrossRef]
- Meena, M.; Dixit, N.; Sharma, V. Abrasive Flow Machining of Additively Manufactured Femoral Head of the Hip Joint. J. Mater. Eng. Perform. 2023, 1–13. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Hashmi, M.S.J. Implant Materials and Their Processing Technologies. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Xu, S.; Guo, Z.; Shen, Q.; Peng, Y.; Li, J.; Li, S.; He, P.; Jiang, Z.; Que, Y.; Cao, K.; et al. Reconstruction of Tumor-Induced Pelvic Defects with Customized, Three-Dimensional Printed Prostheses. Front. Oncol. 2022, 12, 935059. [Google Scholar] [CrossRef]
- Nakano, T.; Ishimoto, T. Powder-Based Additive Manufacturing for Development of Tailor-Made Implants for Orthopedic Applications. KONA Powder Part. J. 2015, 32, 75–84. [Google Scholar] [CrossRef]
- Rahmani, R.; Lopes, S.I.; Prashanth, K.G. Selective Laser Melting and Spark Plasma Sintering: A Perspective on Functional Biomaterials. J. Funct. Biomater. 2023, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Sheha, E.D.; Gandhi, S.D.; Colman, M.W. 3D Printing in Spine Surgery. Ann. Transl. Med. 2019, 7, S164. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kenan, S.; Cheng, M.; Cai, W.; Huang, W.; Yan, W. 3D-Printed Patient-Customized Artificial Vertebral Body for Spinal Reconstruction after Total En Bloc Spondylectomy of Complex Multi-Level Spinal Tumors. Int. J. Bioprinting 2022, 8, 576. [Google Scholar] [CrossRef] [PubMed]
- Intihar, U.; Železnik, J.; Brajlih, T.; Drstvenšek, I.; Hudak, R.; Antonič, M. Sternal Reconstruction Using 3D-Printed Titanium Custom-Made Prosthesis for Sternal Dehiscence After Cardiac Surgery. Heart Surg. Forum 2023, 26, E160–E163. [Google Scholar] [CrossRef] [PubMed]
- Balke, D.; Gupta, V.; Welter, S. Prospects of 3D-Printed Sternum Prostheses: A Review. J. Vis. Surg. 2020, 6, 10. [Google Scholar] [CrossRef]
- Dzian, A.; Živčák, J.; Penciak, R.; Hudák, R. Implantation of a 3D-Printed Titanium Sternum in a Patient with a Sternal Tumor. World J. Surg. Oncol. 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Van Horn, M.R.; Beard, R.; Wang, W.; Cunningham, B.W.; Mullinix, K.P.; Allall, M.; Bucklen, B.S. Comparison of 3D-Printed Titanium-Alloy, Standard Titanium-Alloy, and PEEK Interbody Spacers in an Ovine Model. Spine J. 2021, 21, 2097–2103. [Google Scholar] [CrossRef]
- Al-Zyoud, W.; Haddadin, D.; Hasan, S.A.; Jaradat, H.; Kanoun, O. Biocompatibility Testing for Implants: A Novel Tool for Selection and Characterization. Materials 2023, 16, 6881. [Google Scholar] [CrossRef]
- Nimmawitt, P.; Aliyu, A.A.A.; Lohwongwatana, B.; Arunjaroensuk, S.; Puncreobutr, C.; Mattheos, N.; Pimkhaokham, A. Understanding the Stress Distribution on Anatomic Customized Root-Analog Dental Implant at Bone-Implant Interface for Different Bone Densities. Materials 2022, 15, 6379. [Google Scholar] [CrossRef]
- Dank, A.; Aartman, I.H.A.; Wismeijer, D.; Tahmaseb, A. Effect of Dental Implant Surface Roughness in Patients with a History of Periodontal Disease: A Systematic Review and Meta-Analysis. Int. J. Implant Dent. 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.S.; Kapil, S.; Das, M. A Post Processing Technique to Achieve Nanofinishing for Functionality Enhancement of Ti-6Al-4V Femoral Head Fabricated by Laser Powder Bed Fusion. CIRP J. Manuf. Sci. Technol. 2023, 45, 99–112. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Hoppe, V.; Rusińska, M.; Gąsiorek, J.; Ziółkowski, G.; Dydak, K.; Czajkowska, J.; Junka, A. The Impact of EBM-Manufactured Ti6Al4V ELI Alloy Surface Modifications on Cytotoxicity toward Eukaryotic Cells and Microbial Biofilm Formation. Materials 2020, 13, 2822. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Saikia, R.; Das, A.; Das, D.; Islam, M.A.; Pramanik, P.; Parasar, A.; Borthakur, P.P.; Sarmah, P.; Saikia, M.; et al. 3D Printing in Biomedicine: Advancing Personalized Care through Additive Manufacturing. Explor. Med. 2023, 4, 1135–1167. [Google Scholar] [CrossRef]
- Inui, H.; Yamagami, R.; Kono, K.; Kawaguchi, K. What Are the Causes of Failure after Total Knee Arthroplasty? J. Jt. Surg. Res. 2023, 1, 32–40. [Google Scholar] [CrossRef]
- Revision (Re-Do) Knee Replacement—Adam Watson Orthopaedic Surgeon. Available online: https://watsonorthopaedics.com/home/health-professional/knee/revision-re-do-knee-replacement (accessed on 20 January 2024).
- Duda, T.; Raghavan, L.V. 3D Metal Printing Technology. IFAC-PapersOnLine 2016, 49, 103–110. [Google Scholar] [CrossRef]
- Mecheter, A.; Tarlochan, F.; Kucukvar, M. A Review of Conventional versus Additive Manufacturing for Metals: Life-Cycle Environmental and Economic Analysis. Sustainability 2023, 15, 12299. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. 3D Scanning Applications in Medical Field: A Literature-Based Review. Clin. Epidemiol. Glob. Health 2019, 7, 199–210. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Ul Haq, M.I. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of Cleaning and Sterilization on Titanium Implant Surface Properties and Cellular Response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef]
- Chen, Y.; Neff, M.; McEvoy, B.; Cao, Z.; Pezzoli, R.; Murphy, A.; Gately, N.; Jnr, M.H.; Rowan, N.J.; Devine, D.M. 3D Printed Polymers Are Less Stable than Injection Moulded Counterparts When Exposed to Terminal Sterilization Processes Using Novel Vaporized Hydrogen Peroxide and Electron Beam Processes. Polymer 2019, 183, 121870. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Evaluation of Sterilisation Techniques for 3D-Printed Implantable Devices. RPS Pharm. Pharmacol. Rep 2023, 2, rqad003. [Google Scholar] [CrossRef]
- Tapia-Guerrero, Y.S.; Del Prado-Audelo, M.L.; Borbolla-Jiménez, F.V.; Giraldo Gomez, D.M.; García-Aguirre, I.; Colín-Castro, C.A.; Morales-González, J.A.; Leyva-Gómez, G.; Magaña, J.J. Effect of UV and Gamma Irradiation Sterilization Processes in the Properties of Different Polymeric Nanoparticles for Biomedical Applications. Materials 2020, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, M.T.; Ozsahin, I.; Ozsahin, D.U. Evaluation of Sterilization Methods for Medical Devices. In Proceedings of the 2019 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 26 March–10 April 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 1–4. [Google Scholar]
- McEvoy, B.; Rowan, N.J. Terminal Sterilization of Medical Devices Using Vaporized Hydrogen Peroxide: A Review of Current Methods and Emerging Opportunities. J. Appl. Microbiol. 2019, 127, 1403–1420. [Google Scholar] [CrossRef] [PubMed]
- Colaço, R.; Serro, A.P. Sterilization Methods. In Hydrogels for Tissue Engineering and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2024; pp. 139–159. [Google Scholar]
- Limaye, N.; Veschini, L.; Coward, T. Assessing Biocompatibility & Mechanical Testing of 3D-Printed PEEK versus Milled PEEK. Heliyon 2022, 8, e12314. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.J. Sterilisation Techniques for Polymers. In Sterilisation of Biomaterials and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 151–211. [Google Scholar]
- LI, W.; ZHOU, J.; XU, Y. Study of the in Vitro Cytotoxicity Testing of Medical Devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Xometry, T. Powder Bed Fusion (PBF) Machines: Definition, Types, Limitations, Benefits, and How They Work. Available online: https://www.xometry.com/resources/3d-printing/powder-bed-fusion-pbf-machines (accessed on 20 January 2024).
- Wunderer, S. Powder Bed Fusion 3D Printing in Dental Applications—2oneLab GmbH. Available online: https://2onelab.com/en/learn/blog/powder-bed-fusion-3d-printing-in-dental-applications (accessed on 20 January 2024).
- Katz-Demyanetz, A.; Popov, V.V.; Kovalevsky, A.; Safranchik, D.; Koptyug, A. Powder-Bed Additive Manufacturing for Aerospace Application: Techniques, Metallic and Metal/Ceramic Composite Materials and Trends. Manuf. Rev. 2019, 6, 5. [Google Scholar] [CrossRef]
- Luo, H.; Du, Y. Mechanical Properties of Bulk Metallic Glasses Additively Manufactured by Laser Powder Bed Fusion: A Review. Materials 2023, 16, 7034. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.U.; Schiffer, A.; Kumar, S. Piezoresistive Behavior of MWCNT/PA12 Honeycomb Composites Processed via Selective Laser Sintering. J. Mater. Res. Technol. 2023, 26, 2319–2332. [Google Scholar] [CrossRef]
- Obadimu, S.O.; Kourousis, K.I. Load-Rate Effects on the in-Plane Compressive Behaviour of Additively Manufactured Steel 316L Honeycomb Structures. Eng. Struct. 2022, 273, 115063. [Google Scholar] [CrossRef]
- Pramanik, S.; Milaege, D.; Hoyer, K.-P.; Schaper, M. Additively Manufactured Novel Ti6Al7Nb Circular Honeycomb Cellular Solid for Energy Absorbing Applications. Mater. Sci. Eng. A 2022, 854, 143887. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Q.; Zhu, C.; Wang, F.; Feng, Q.; Song, J.; Setchi, R.; Ma, C.; Tao, R. Laser Powder Bed Fusion-Built Ti6Al4V Bone Scaffolds Composed of Sheet and Strut-Based Porous Structures: Morphology, Mechanical Properties, and Biocompatibility. Chinese J. Mech. Eng. Addit. Manuf. Front. 2022, 1, 100051. [Google Scholar] [CrossRef]
- Červinek, O.; Pettermann, H.; Todt, M.; Koutný, D.; Vaverka, O. Non-Linear Dynamic Finite Element Analysis of Micro-Strut Lattice Structures Made by Laser Powder Bed Fusion. J. Mater. Res. Technol. 2022, 18, 3684–3699. [Google Scholar] [CrossRef]
- Jung, S.; Kara, L.B.; Nie, Z.; Simpson, T.W.; Whitefoot, K.S. Is Additive Manufacturing an Environmentally and Economically Preferred Alternative for Mass Production? Environ. Sci. Technol. 2023, 57, 6373–6386. [Google Scholar] [CrossRef] [PubMed]
- Padasak, Z. A Guide to Mass Producing with Metal Additive Manufacturing. Available online: https://www.alphaprecisionpm.com/blog/metal-additive-manufacturing-for-mass-production (accessed on 11 October 2023).
- Chowdhury, S.; Yadaiah, N.; Prakash, C.; Ramakrishna, S.; Dixit, S.; Gupta, L.R.; Buddhi, D. Laser Powder Bed Fusion: A State-of-the-Art Review of the Technology, Materials, Properties & Defects, and Numerical Modelling. J. Mater. Res. Technol. 2022, 20, 2109–2172. [Google Scholar] [CrossRef]
- Khorasani, A.; Gibson, I.; Veetil, J.K.; Ghasemi, A.H. A Review of Technological Improvements in Laser-Based Powder Bed Fusion of Metal Printers. Int. J. Adv. Manuf. Technol. 2020, 108, 191–209. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, S.; Qian, W.; Zhang, H.; Zhu, H.; Chen, Q.; Zhang, Z.; Guo, F.; Wang, J.; Withers, P.J. Structural Integrity Issues of Additively Manufactured Railway Components: Progress and Challenges. Eng. Fail. Anal. 2023, 149, 107265. [Google Scholar] [CrossRef]
- Casini, M. Advanced Building Construction Methods. In Construction 4.0; Elsevier: Amsterdam, The Netherlands, 2022; pp. 405–470. [Google Scholar]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Powder Bed Fusion Processes. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; pp. 107–145. [Google Scholar]
- Gameros, A.; Lowth, S.; Axinte, D.; Nagy-Sochacki, A.; Craig, O.; Siller, H.R. State-of-the-Art in Fixture Systems for the Manufacture and Assembly of Rigid Components: A Review. Int. J. Mach. Tools Manuf. 2017, 123, 1–21. [Google Scholar] [CrossRef]
- Kim, S.; Moon, S.K. A Part Consolidation Design Method for Additive Manufacturing Based on Product Disassembly Complexity. Appl. Sci. 2020, 10, 1100. [Google Scholar] [CrossRef]
- Cuellar, J.S.; Smit, G.; Plettenburg, D.; Zadpoor, A. Additive Manufacturing of Non-Assembly Mechanisms. Addit. Manuf. 2018, 21, 150–158. [Google Scholar] [CrossRef]
- Attaran, M. The Rise of 3-D Printing: The Advantages of Additive Manufacturing over Traditional Manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Wojciechowski, P.; Wojciechowski, P. 3D Printing vs. Traditional Manufacturing: 10 Key Factors. Available online: https://zmorph3d.com/blog/3d-printing-vs-traditional-manufacturing-10-key-factors/#:~:text=Traditionalmanufacturingmethodssuchas,runsmaketheprocessuneconomical (accessed on 21 January 2024).
- Dev Singh, D.; Mahender, T.; Raji Reddy, A. Powder Bed Fusion Process: A Brief Review. Mater. Today Proc. 2021, 46, 350–355. [Google Scholar] [CrossRef]
- The Benefits of Designing for Powder Bed Fusion|Prima Additive. Available online: https://www.primaadditive.com/en/news/vision/benefits-designing-powder-bed-fusion#:~:text=ProbablythemainadvantageofPBFtechnology,tocreatecustomizedandhighlyoptimizedparts (accessed on 21 January 2024).
- Kulkarni, P.; Kumar, A.; Chate, G.; Dandannavar, P. Elements of Additive Manufacturing Technology Adoption in Small- and Medium-Sized Companies. Innov. Manag. Rev. 2021, 18, 400–416. [Google Scholar] [CrossRef]
- Popov, V.V.; Grilli, M.L.; Koptyug, A.; Jaworska, L.; Katz-Demyanetz, A.; Klobčar, D.; Balos, S.; Postolnyi, B.O.; Goel, S. Powder Bed Fusion Additive Manufacturing Using Critical Raw Materials: A Review. Materials 2021, 14, 909. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Koshy, P.; Alipal, J.; Kadir, M.H.A.; Lee, T.C. Challenges of 3D Printing Technology for Manufacturing Biomedical Products: A Case Study of Malaysian Manufacturing Firms. Heliyon 2020, 6, e03734. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.-S.; Kim, D.; Han, G.; Yoon, C.-B.; Jung, H.-D. Powder Based Additive Manufacturing for Biomedical Application of Titanium and Its Alloys: A Review. Biomed. Eng. Lett. 2020, 10, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Leigh, D.K.; Bourell, D. Powder Bed Fusion of Polymers. In Additive Manufacturing Processes; ASM International: West Conshohocken, PA, USA, 2020; pp. 52–57. [Google Scholar]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Powder Bed Fusion. In Additive Manufacturing Technologies; Springer International Publishing: Cham, Switzerland, 2021; pp. 125–170. [Google Scholar]
- Agueda, J.R.H.S.; Chen, Q.; Maalihan, R.D.; Ren, J.; da Silva, Í.G.M.; Dugos, N.P.; Caldona, E.B.; Advincula, R.C. 3D Printing of Biomedically Relevant Polymer Materials and Biocompatibility. MRS Commun. 2021, 11, 197–212. [Google Scholar] [CrossRef]
- Jeršovaitė, J.; Šarachovaitė, U.; Matulaitienė, I.; Niaura, G.; Baltriukienė, D.; Malinauskas, M. Biocompatibility Enhancement via Post-Processing of Microporous Scaffolds Made by Optical 3D Printer. Front. Bioeng. Biotechnol. 2023, 11, 1167753. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Nilawar, S.; Uddin, M.; Chatterjee, K. Surface Engineering of Biodegradable Implants: Emerging Trends in Bioactive Ceramic Coatings and Mechanical Treatments. Mater. Adv. 2021, 2, 7820–7841. [Google Scholar] [CrossRef]
- Behera, M.; Panemangalore, D.B.; Shabadi, R. Additively Manufactured Magnesium-Based Bio-Implants and Their Challenges. Trans. Indian Natl. Acad. Eng. 2021, 6, 917–932. [Google Scholar] [CrossRef]
- An, J.; Leong, K.F. Multi-Material and Multi-Dimensional 3D Printing for Biomedical Materials and Devices. Biomed. Mater. Devices 2023, 1, 38–48. [Google Scholar] [CrossRef]
- Yao, L.; Ramesh, A.; Xiao, Z.; Chen, Y.; Zhuang, Q. Multimetal Research in Powder Bed Fusion: A Review. Materials 2023, 16, 4287. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, F.; Farshidianfar, M.H.; Bakhshivash, S.; Gerlich, A.P.; Khajepour, A. Dissimilar Metals Deposition by Directed Energy Based on Powder-Fed Laser Additive Manufacturing. J. Manuf. Process. 2019, 43, 83–97. [Google Scholar] [CrossRef]
- Iftekar, S.F.; Aabid, A.; Amir, A.; Baig, M. Advancements and Limitations in 3D Printing Materials and Technologies: A Critical Review. Polymers 2023, 15, 2519. [Google Scholar] [CrossRef] [PubMed]
- Nudelis, N.; Mayr, P. Defect-Based Analysis of the Laser Powder Bed Fusion Process Using X-Ray Data. Int. J. Adv. Manuf. Technol. 2022, 123, 3223–3232. [Google Scholar] [CrossRef]
- Maplesden, P. Additive Manufacturing: Learn about Powder Bed Fusion. Available online: https://www.techtarget.com/searcherp/tip/Additive-manufacturing-Learn-about-powder-bed-fusion (accessed on 10 November 2023).
- Mazur, M.; Selvakannan, P. Laser Powder Bed Fusion—Principles, Challenges, and Opportunities. In Additive Manufacturing for Chemical Sciences and Engineering; Springer Nature Singapore: Singapore, 2022; pp. 77–108. [Google Scholar]
- Hendrixson, S. What Is Powder Bed Fusion 3D Printing? Available online: https://www.additivemanufacturing.media/kc/what-is-additive-manufacturing/articles/am-101-powder-bed-fusion-pbf (accessed on 21 January 2024).
- Dejene, N.D.; Lemu, H.G. Current Status and Challenges of Powder Bed Fusion-Based Metal Additive Manufacturing: Literature Review. Metals 2023, 13, 424. [Google Scholar] [CrossRef]
- Ravalji, J.M.; Raval, S.J. Review of Quality Issues and Mitigation Strategies for Metal Powder Bed Fusion. Rapid Prototyp. J. 2023, 29, 792–817. [Google Scholar] [CrossRef]
- Chadha, U.; Selvaraj, S.K.; Abraham, A.S.; Khanna, M.; Mishra, A.; Sachdeva, I.; Kashyap, S.; Dev, S.J.; Swatish, R.S.; Joshi, A.; et al. Powder Bed Fusion via Machine Learning-Enabled Approaches. Complexity 2023, 2023, 9481790. [Google Scholar] [CrossRef]
- Medical Devices—Angela N Johnson. Available online: https://angelanjohnson.com/medicaldevices/ (accessed on 6 October 2023).
- Amato, S.F. Regulatory Strategies for Biomaterials and Medical Devices in the USA: Classification, Design, and Risk Analysis. In Regulatory Affairs for Biomaterials and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–46. [Google Scholar]
- Tobin, J.J. Global Marketing Authorisation of Biomaterials and Medical Devices. In Regulatory Affairs for Biomaterials and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2015; pp. 93–114. [Google Scholar]
- Wreh, E. Premarket Notification [510(K)]. In Medical Device Regulation; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–89. [Google Scholar]
- Ashter, S.A. Classification of Medical Devices. In Applications of Polymers and Plastics in Medical Devices; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–44. [Google Scholar]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef]





| Material | Advantages |
|---|---|
| Titanium and its alloys | High degree of biocompatibility and minimal allergic response risk [118] |
| High strength-to-weight ratio and structural integrity [119] | |
| Corrosion resistance and ensures the longevity of the implant in the body [120] | |
| Good osseointegration encourages the formation and attachment of new bone [121] | |
| Co-Cr-based alloys | Useful for articulating surfaces owing to high wear resistance [122] |
| Excellent biocompatibility and corrosion resistance [123,124] | |
| A good fit for load-bearing implants, including hip and knee replacements [125] | |
| NiTi alloy | Dynamic implants with unique shape memory and superelasticity [126] |
| Biocompatible and corrosion resistant [126] | |
| Ideal for vascular implants, devices for orthodontics, and stents [126] | |
| Stainless steel | Biocompatibility and high resistance to corrosion [127] |
| The availability of several grades designed to meet implant requirements [128] | |
| Economical choice [129] | |
| Magnesium and its alloys | Lightweight and density is similar to that of bone [130] |
| Biodegradable; slowly metabolized by the body over time [131] | |
| Appropriate for utilization in temporary implantation scenarios, such as bone fixation devices [132,133] | |
| AlSi10Mg | The material exhibits favorable mechanical properties by effectively integrating both high strength and low density [134] |
| Corrosion resistant [135] | |
| High specific strength [136] |
| Implant | Material | Advantages | Applications | References |
|---|---|---|---|---|
| Spinal | Ti-6Al-4V; SS316L; Co-Cr | Biocompatible; mechanically strong; corrosion resistant | Customizable; improved fit and stability | [126,134,137] |
| Orthodontic brackets | Titanium | Highly customizable; reduced treatment time | Improved therapeutic outcomes | [146,147] |
| Trauma nails | Titanium (coated with bioactive coating) | High degree of functionality; promote bone regeneration; reduce infection | Osteoporosis treatment | [149,150,151] |
| Hip | Titanium | Natural joint feeling; reduced probability for requiring revision surgery | Improves patient experience and long-term outcomes | [152] |
| Cellular implant plugs for osteoarthritis | Titanium | Lightweight; replicate healthy joint tissues | Minimally invasive treatment; promote cartilage regeneration | [104] |
| Acetabular cup | Ti-6Al-4V | Customized fit; improved stability; bone ingrowth | Total hip replacement | [141,154,155,156,157] |
| Femoral appliance | Co-Cr-Mo | Customized; enhanced fit and functionality | Faster restoration of mobility after knee surgeries | [159,160,161,162,163] |
| Porous pelvic girdle | Ti-6Al-4V | Customized fit; stability; facilitates bone growth | Treatment for osteoporosis | [142,164,165] |
| Cranio-maxillofacial implant | Hybrid metal ceramic | Customized; aesthetically pleasing; improved osseointegration | Jaw, face, and skull reconstruction | [143,166] |
| Spinal spacers | Titanium | Correct spinal height and alignment | Reduced pain and improved mobility in spinal stenosis patients | [173] |
| Vertebral bodies | Titanium | Customizable; replace destroyed bone in spinal injuries | Spinal reconstruction | [167,168] |
| Sternal plates | Titanium | Customized, enhance healing process | Repair fractured chest bone | [169,170,171] |
| Factor | Description | Importance | Examples | References |
|---|---|---|---|---|
| Implant design and material selection | Matching function and biocompatibility | Crucial for long-term success and patient safety | Co-Cr alloy; titanium; PEEK polymers | [174] |
| Surface finish | Patient comfort and osseointegration | Affects bone–implant contact and infection risk | Rougher for dental implants; smoother for joint implants | [175,176] |
| Accuracy and precision | Proper fit and function | Avoids revision surgery and complications | High-resolution scanning; modern printing; quality control | [179,180,181,182,183,184,185] |
| Sterility and cleanliness | Prevents post-surgical infection | Essential for patient safety | Gamma irradiation; steam; hydrogen peroxide; ethylene oxide | [186,187,188,189,190,191,192] |
| Testing and quality control | Structural integrity and functionality | Guarantees safety and performance | Mechanical testing; imaging; cytotoxicity testing | [193,194,195] |
| Advantage | Description | Importance | Examples | References |
|---|---|---|---|---|
| High degree of precision | Tight tolerances and accurate parts | Minimizes assembly problems and improves functionality | Medical implants; aerospace components | [196,197] |
| Complex geometries | Create intricate internal structures and unique shapes | Impossible with traditional manufacturing processes | Lattices; honeycomb patterns | [200,201,202,203,204] |
| Reduced waste | Additive approach uses only needed material | Minimizes material costs and environmental impact | compared to subtractive manufacturing | [205] |
| Quick iteration | Rapid prototyping and design changes | Saves time and resources during development | No need for expensive tooling or setup | [206,207] |
| Short lead times | Faster production compared to traditional methods | Reduce time to market and improve production efficiency | Eliminate setup, tooling, and machining stages | [196,208] |
| Reduced assembly | Prints entire assemblies in one piece | Minimizes errors and weaknesses; improves product quality | Avoids joining multiple components and associated risks | [211,212] |
| No tooling cost required | Eliminates expensive dies, tools, and molds | Reduces manufacturing costs and increases flexibility | Creates complex parts that are impossible to create with traditional methods | [217,218] |
| Inventory reduction | Creates parts on demand, minimizing stock | Frees up space and resources; improves efficiency | Eliminates need for storing large quantities of parts | [220,221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshua, R.J.N.; Raj, S.A.; Hameed Sultan, M.T.; Łukaszewicz, A.; Józwik, J.; Oksiuta, Z.; Dziedzic, K.; Tofil, A.; Shahar, F.S. Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review. Materials 2024, 17, 769. https://doi.org/10.3390/ma17030769
Joshua RJN, Raj SA, Hameed Sultan MT, Łukaszewicz A, Józwik J, Oksiuta Z, Dziedzic K, Tofil A, Shahar FS. Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review. Materials. 2024; 17(3):769. https://doi.org/10.3390/ma17030769
Chicago/Turabian StyleJoshua, Rajan John Nekin, Sakthivel Aravind Raj, Mohamed Thariq Hameed Sultan, Andrzej Łukaszewicz, Jerzy Józwik, Zbigniew Oksiuta, Krzysztof Dziedzic, Arkadiusz Tofil, and Farah Syazwani Shahar. 2024. "Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review" Materials 17, no. 3: 769. https://doi.org/10.3390/ma17030769
APA StyleJoshua, R. J. N., Raj, S. A., Hameed Sultan, M. T., Łukaszewicz, A., Józwik, J., Oksiuta, Z., Dziedzic, K., Tofil, A., & Shahar, F. S. (2024). Powder Bed Fusion 3D Printing in Precision Manufacturing for Biomedical Applications: A Comprehensive Review. Materials, 17(3), 769. https://doi.org/10.3390/ma17030769












