Research Progress on Hygroscopic Agents for Atmospheric Water Harvesting Systems
Abstract
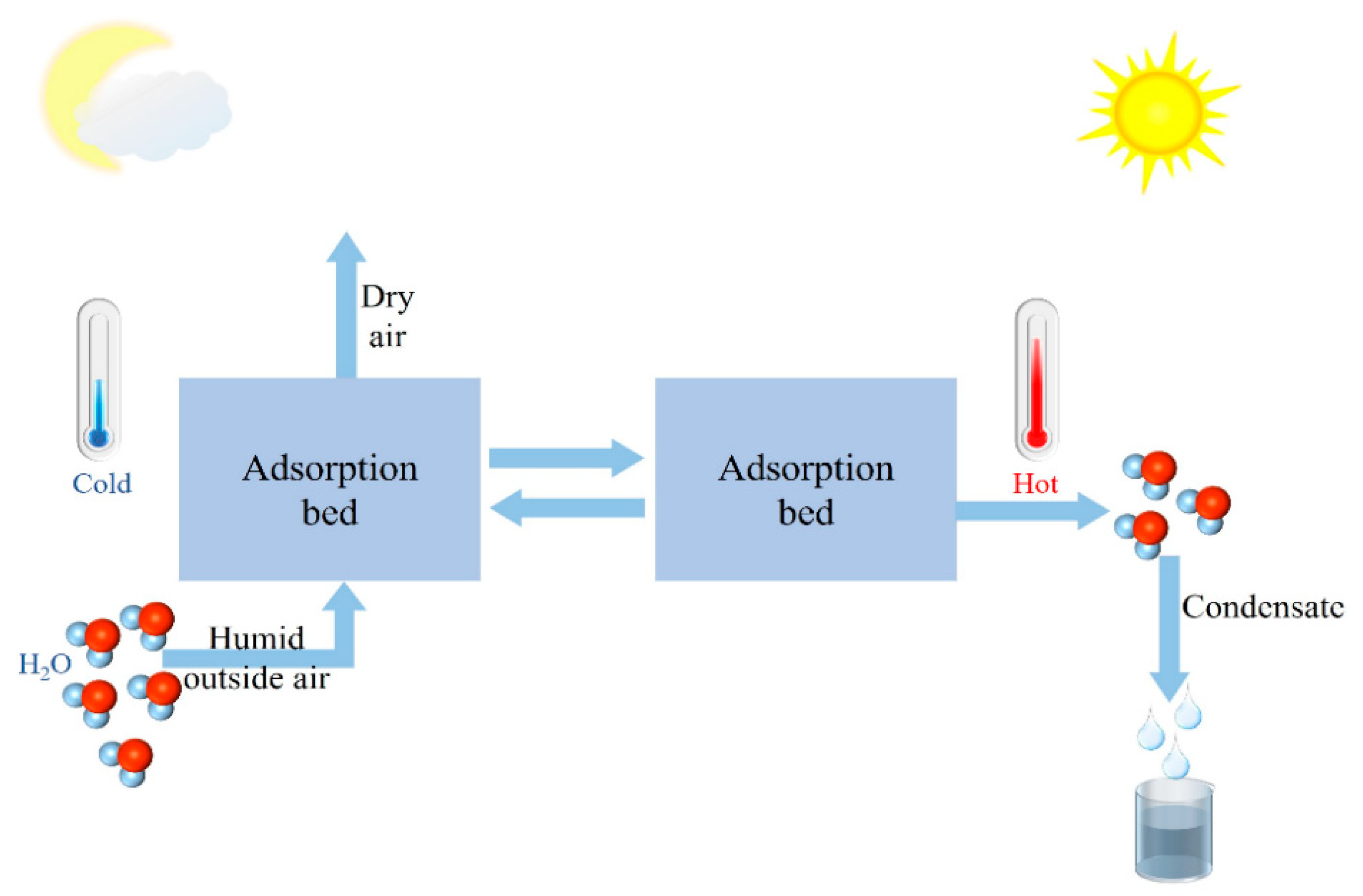
1. Introduction
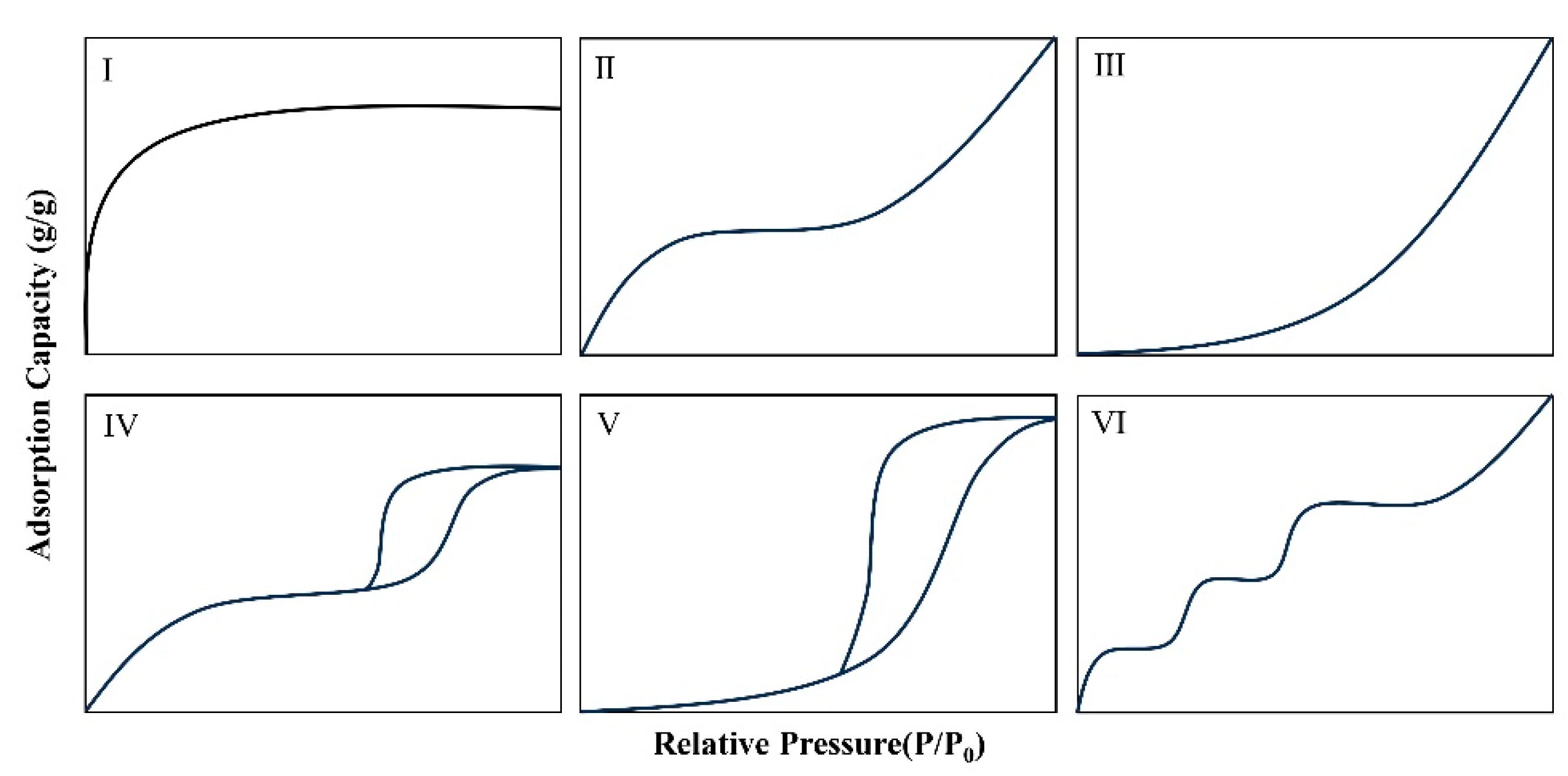
2. Adsorption Mechanisms and Isotherms
- Physical adsorption: Physical adsorption is the process by which water molecules attach to the surface of an adsorbent via van der Waals forces. This process is reversible, allowing the adsorbent to release the adsorbed water molecules. However, the amount of water that can be physically adsorbed is relatively small. Porous materials, such as activated carbon, zeolite, and silica gel, typically exhibit this type of adsorption. The larger pore space and specific surface area make it possible to adsorb more adsorbent substances under the intermolecular force. However, the amount of adsorption is still less compared to other adsorption mechanisms, but the adsorption rate is faster. Zhao et al. [38] prepared a composite hygroscopic agent and found that the difference in the adsorption mechanism between salt and zeolite resulted in different adsorption rates for different hygroscopic agents. Additionally, the intermolecular forces between some porous materials, such as zeolite and water molecules, are stronger, and the resolution temperature is higher.
- Chemisorption: Chemisorption refers to the formation of chemical bonds between water molecules and the surface of the adsorbent. For example, water molecules can form salt hydrates with salts such as calcium chloride (CaCl2). The strength of these chemical bonds surpasses that of intermolecular forces, making chemisorption stronger than physical adsorption. Consequently, the energy required to release the water molecules is higher, rendering the process less reversible.
- Condensed matter adsorption: Under conditions of high pressure or low temperature, water molecules may condense on the surface of the adsorbent to form droplets or water films. However, this adsorption process is generally difficult to occur because it requires a specific temperature or pressure range.
- Capillary condensation: Capillary condensation refers to the phenomenon where condensation occurs within a capillary tube due to the combined effect of capillary action and the condensation process. When water vapor is present inside the capillary tube, and the temperature of the tube falls below the saturation temperature of the water vapor, the vapor condenses into a liquid state. For instance, the P(SA+AA)-SPH and SPHC-5 gels prepared by Mittal et al. [39] have an extremely high density of interconnected pores that form capillary condensation, resulting in extremely high water vapor adsorption.
- Surface active sites: Specific molecules or atoms, such as amino groups and hydroxyl groups, which possess high adsorption or catalytic capacity, may be present on the surface of the adsorbent. For example, polymers encompass a wide range of hydrophilic groups, and their adsorption behavior can be influenced by modulating these active sites. Han et al. [40] loaded polypyrrole in mesoporous silica, which increased the number of hydrogen bonds with water vapor and synergized with LiCl to improve the moisture absorption of the material.
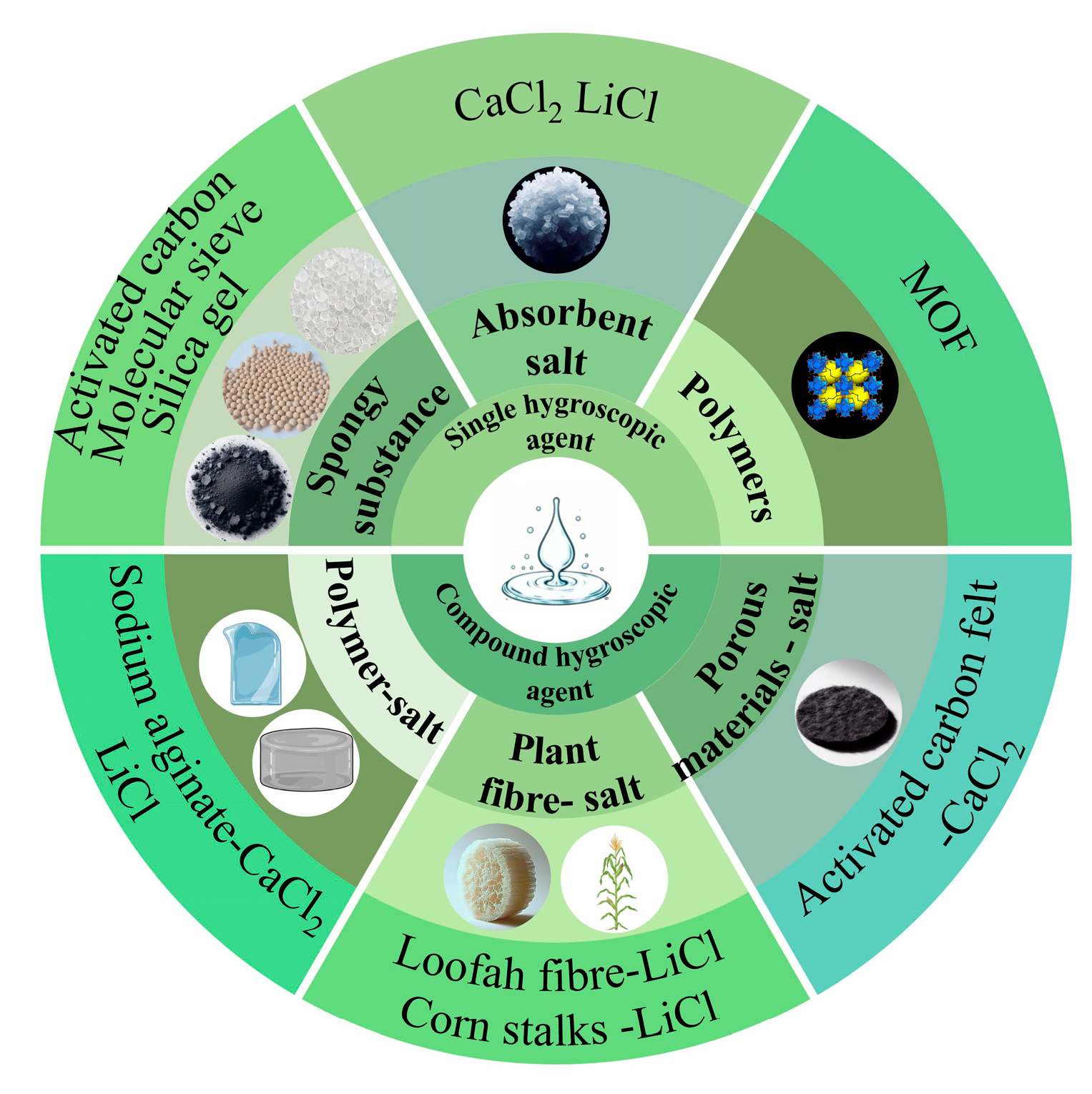
3. Moisture Absorber Classification and Performance
3.1. Single Hygroscopic Agent
3.1.1. Porous Materials
3.1.2. Hygroscopic Salts
3.1.3. Moisture-Absorbing Materials for MOFs
3.2. Compound Hygroscopic Agent
3.2.1. Porous Materials-Salt
3.2.2. MOFs Composite Hygroscopic Agents
3.2.3. Polymer-Based Composite Hygroscopic Agents
3.2.4. Plant Fiber–Salt Composite Hygroscopic Agents
| Material Classification | Absorbent | Hygroscopic Conditions | Maximum Moisture Absorption (g/g) | Desorption Conditions | Literature Sources |
|---|---|---|---|---|---|
| Spongy substance | 13X zeolite | 25 °C, RH = 50% | 0.21 | — | [45] |
| A3 zeolite | 25 °C, RH = 40% | 0.07 | Not desorbed below 60 °C | [46] | |
| Absorbent salt | LiCl | 25 °C, RH = 70% | 2.5 | Not desorbed below 80 °C | [79] |
| CaCl2 | 25 °C, RH = 70% | 2.0 | Not desorbed below 80 °C | [79] | |
| MOFs | MOF-801 | 25 °C, RH = 20% | 0.25 | — | [12] |
| PC-MOF | 25 °C, RH = 90% | 6.39 | 23 °C~65 °C | [65] | |
| Porous materials-salt | ACF-LiCl | 25 °C, RH = 70% | 2.9 | 80 °C | [69] |
| ACF-CaCl2 | 20 °C, RH = 70% | 1.7 | — | [58] | |
| Cured ACF-LiCl | 25 °C, P0/P = 0.9 | 1.2 | 77 °C, RH = 20% | [71] | |
| Silica gel-CaCl2 | 25 °C, RH = 70% | 0.5 | — | [56] | |
| 13X zeolite-LiCl | 25 °C, RH = 50% | 0.7 | — | [45] | |
| MOFs-salt | LiCl@MIL101(Cr) | 30 °C, RH = 30% | 0.77 | 76 °C~97 °C | [55] |
| CaCl2@ UiO-66_53 | 20 °C, RH = 90% | 2.59 | 370 K | [76] | |
| TUN-2/SA | 25 °C, RH = 40% | 0.23 | 100.2 °C | [77] | |
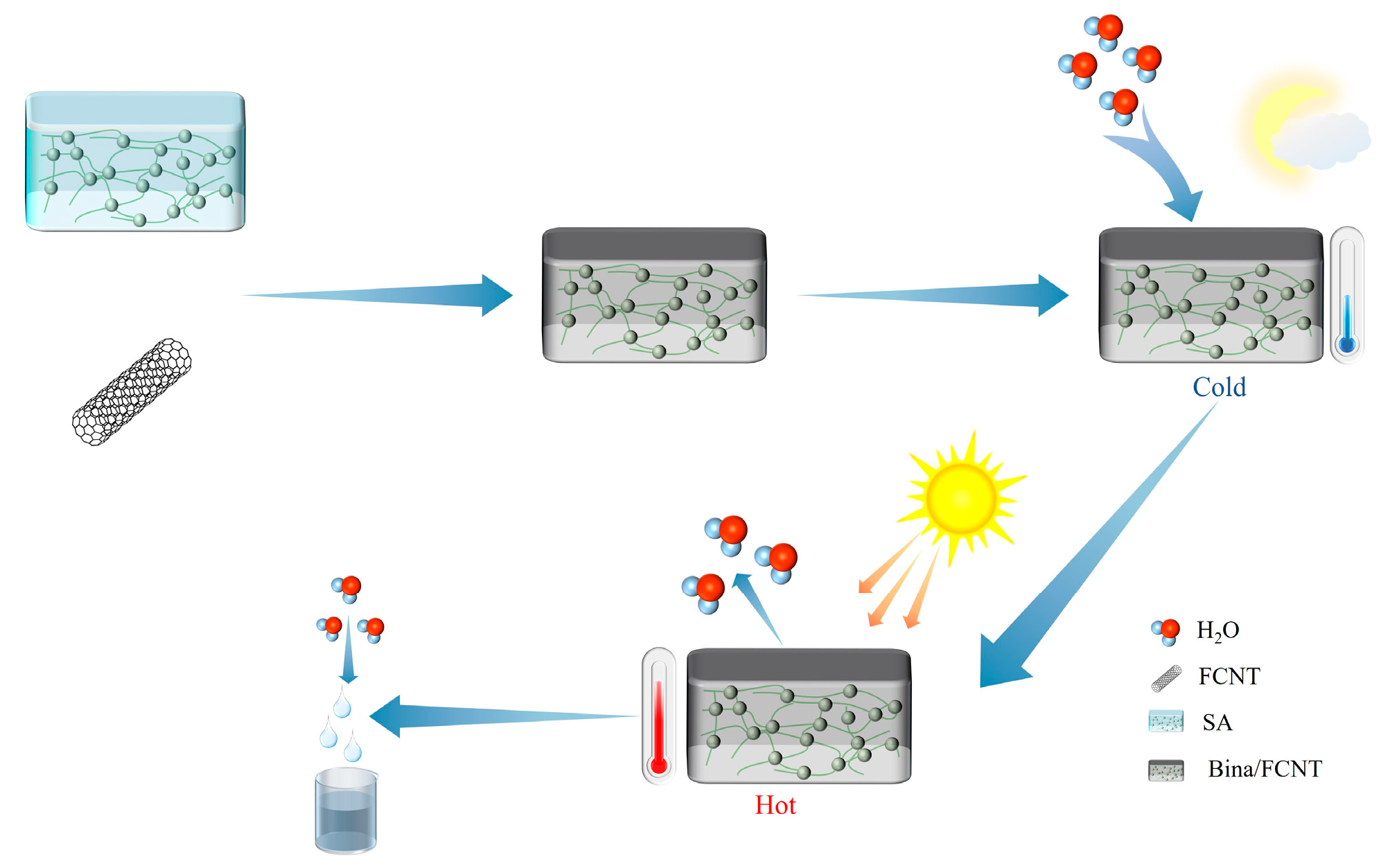
| Polymers | Bina/FCNT | 25 °C, RH = 70% | 5.6 | 80 °C | [79] |
| SMAG | 10~40 °C, RH = 90% | 6.7 | 40 °C~63 °C | [80] | |
| Vegetable fiber–salt | LBC@LiCl | 25 °C, RH = 40~90% | 0.81~2.47 | Bulk desorption at 60 °C | [81] |
| BCS | RH = 20~80% | 0.5~1.8 | Solar 1 kW/m2 resolution 90% | [84] |
3.3. Performance Evaluation Criteria
4. Factors Affecting and Improving Adsorption Performance
4.1. Influencing Factors
- Pore structure: The adsorption performance is significantly influenced by the pore structure type, specific surface area, pore diameter, and other pore structure properties of the adsorbent. Various types of pore structures affect the diffusion and transport of adsorbent molecules inside the adsorbent. A uniform pore structure is conducive to molecular diffusion. For example, carbon nanotubes doped in Bina/FCNT are conducive to maintaining the homogeneity and stability of the porous network structure, which provides a channel for the diffusion and transport of water vapor [79]. Pore volume refers to the volume size of the pores inside the adsorbent, which determines the adsorption capacity under specific conditions. Therefore, it is essential to study and enhance the adsorbent pore structure to improve the adsorption performance of the adsorbent.
- Surface properties: The surface properties of adsorbents, such as surface polarity, chemical composition, functional groups, and contact angle, directly influence the interaction between the adsorbent and adsorbate molecules, as well as adsorption selectivity, which in turn affects the amount and rate of adsorption. Surface functional groups are the most important surface properties that affect adsorption performance. The type, concentration, and spatial distribution of these functional groups significantly affect the adsorption behavior of water molecules on the adsorbent.
- Activation conditions: activation conditions have an important impact on the hygroscopic agent, such as zeolite molecular sieve, activated carbon, and other activation temperatures are high, after adsorption of water vapor in the sun irradiation is difficult to desorption, so the activation of the difficult conditions of the material used in the sun desorption of adsorption of the water collection system is difficult.
- Temperature: Temperature has a significant impact on the adsorption performance of adsorbents in two key areas: the adsorption process and the preparation of the adsorbent. During the adsorption process, temperature primarily affects the thermal motion of water molecules and the stability of clusters. It also influences the binding capacity of functional groups and water molecules, thereby affecting both the adsorption capacity and rate. In the context of adsorbent preparation, temperature induces changes in functional groups, surface charge, and contact angle, which in turn leads to significant modifications in the adsorption properties [90].
- Humidity: Changes in humidity cause shifts in the position of the adsorption equilibrium. As humidity increases, the adsorbent is more likely to reach its adsorption saturation point, resulting in a higher adsorption quantity and rate. Conversely, lower humidity levels correspond to a reduced adsorption capacity of the adsorbent.
- Photothermal conversion and heat transfer: The sorbent’s photothermal conversion and heat transfer capabilities can increase the material’s temperature when exposed to sunlight or other heat sources, thereby facilitating desorption. The increase in desorption promotes an increase in the number of adsorption cycles and, thus, an increase in the amount of water withdrawn. Therefore, heat and mass transfer capabilities are also crucial for hygroscopic agents.
- Water stability: In the process of moisture absorption, moisture absorbent will inevitably be in the environment containing a large amount of water. High water stability is the inevitable requirement of moisture absorbent [91]. High stability can maintain the pore structure and other properties of the hygroscopic agent, which is conducive to maintaining efficient hygroscopic performance and structural design.
4.2. Enhancement Methods
- Improvement of adsorbent pore structure: (1) Wet activation, i.e., the material is soaked and etched by liquid medicines to make the pore channels open and the specific surface area increase; (2) High-temperature treatment, which can make the material’s specific surface area, pore volume, etc. change by high-temperature treatment; (3) Material synthesis using template or template-free method to synthesize materials with specific structure or morphology, e.g., Marchesini et al. [92] synthesized a BN with a specific surface area of more than 1900 m2/g by the template-free method; (4) Composite, Zhao et al. [80] prepared a super-hygroscopic gel with uniform pore size by compositing.
- Surface modification: Using modification, changing the surface properties of the adsorbent, increasing functional groups, hydrophilic particles, and other hydrophilic groups can improve the adsorption performance, such as Entezari et al. [79] prepared gels with CaCl2, LiCl modification, so that the sodium ions in the sodium alginate gels and the exchange of more hydrophilic Ca2+, Li+, and thus improve the adsorption performance.
- Material composite: Composite adsorbents formed by combining two or more materials using specific methods can leverage the benefits of different adsorbents and effectively enhance the adsorption performance of the adsorbent.
- Photothermal conversion and heat transfer: The photothermal conversion and heat transfer capacity of the composite hygroscopic agent can be enhanced by adding materials with higher photothermal conversion and heat transfer capacity, increasing the desorption temperature of the material and improving the water collection efficiency. For instance, Entezari et al. [79] developed hygroscopic gels doped with carbon nanotubes that have a very high photothermal conversion capacity. In simulated solar experiments at 1 kW/m2, the material temperatures exceeded 70 °C, which increased the rate of water release.
- Adsorbent regeneration: To increase the actual amount of water produced, it is necessary to improve the adsorption and resolution conditions through the adsorption device and increase the number of cycles.
5. Future Directions
- Currently, most composites of porous materials, salts, and polymers are produced through impregnation, which is a time-consuming and inefficient process. Investigating the effects of simpler methods, such as milling, on composite adsorbent materials may be advantageous.
- Inorganic hygroscopic salts such as CaCl2 and LiCl, which are commonly used today, have strong hygroscopic properties. However, they are prone to deliquescence in high humidity environments and can corrode adsorption equipment. Therefore, exploring the impact of weakly corrosive organic acid salts in composite hygroscopic agents has become a significant trend.
- Designing hygroscopic agents that are customized to specific conditions based on the application environment can optimize their performance.
- Designing energy-efficient water collection equipment that matches the performance of the hygroscopic agent and the usage environment can increase the number of adsorption/desorption cycles of the material through the water collection device, thereby maximizing the amount of water produced.
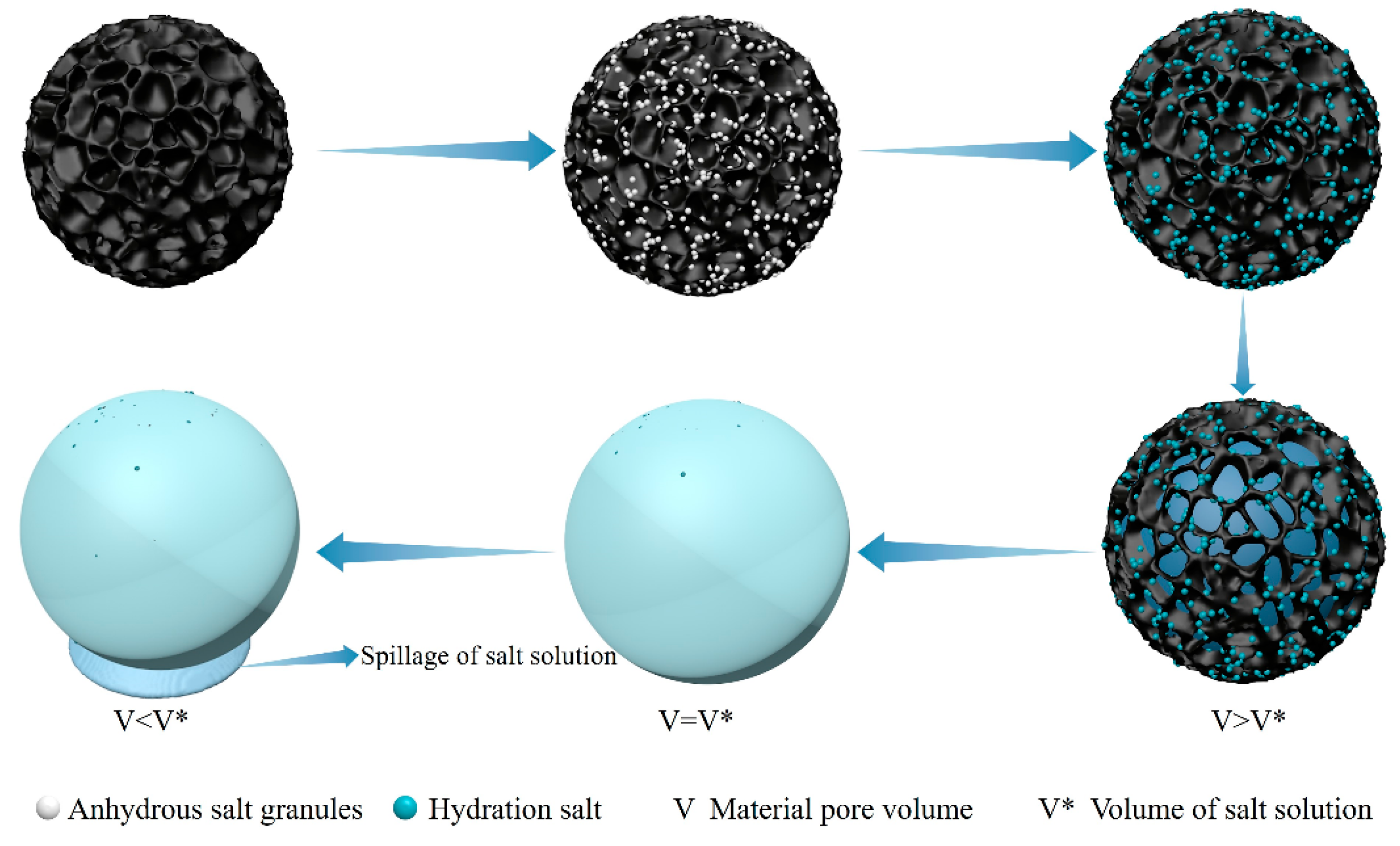
- Instead of limiting the amount of water vapor adsorbed per unit mass as an evaluation criterion in the past, the difference in the density of the material before and after moisture adsorption is used as part of the criterion for evaluating moisture adsorption performance, which in turn takes into account the effect of moisture-absorbing materials on the volume of the water-collecting device.
- This technology can be used for water recycling in agriculture and industry, not just for drinking water. The choice of moisture absorption materials and devices should be based on the water quality requirements.
- In the development of efficient hygroscopic agents, it is essential to conduct research on the long-term stability of materials, economy, sustainability, and the complexity of the synthesis process.
6. Conclusions
- Adsorption AWH is a simple, compact, adaptable, scalable, economical, low-energy, renewable, and clean water extraction technology with significant potential for application.
- Currently, the large-scale application of adsorption AWH is challenging. Performance indicators such as efficiency, economy, adaptability, cycling, and stability are not simultaneously satisfied, making the research and development of high-performance, balanced moisture-absorbing materials a current research focus. Performance indicators are not limited to moisture absorption per unit mass but also consider moisture absorption per unit volume.
- The study of moisture-absorbing materials should shift from single to composite materials, with a focus on utilizing low-cost light and heat conversion technologies or materials.
- Plant fibers are a natural material that has great potential for reducing costs and increasing environmental sustainability.
- AWH technology is not limited to providing access to potable water but can also be extended to industrial water conservation, facility-based agricultural water recycling, and agricultural irrigation.
- Moisture-absorbing materials should not be limited to fixed temperature and humidity. Instead, materials should be developed to adapt to different conditions depending on the environment in which they are used, maximizing material performance in the corresponding environment.
- The device and hygroscopic materials are inseparable. Microcontrollers and other control systems can be used to regulate sunlight exposure, which can increase the number of adsorption and desorption cycles and, thus, the actual amount of water pumped.
- Water extraction efficiency can be improved by using moisture-absorbing materials, optimizing system design, and enhancing condensation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Zarei, Z.; Karami, E.; Keshavarz, M. Co-production of knowledge and adaptation to water scarcity in developing countries. J. Environ. Manag. 2020, 262, 110283. [Google Scholar] [CrossRef] [PubMed]
- Tashtoush, B.; Alshoubaki, A.Y. Solar-off-grid atmospheric water harvesting system: Performance analysis and evaluation in diverse climate conditions. Sci. Total Environ. 2024, 906, 167804. [Google Scholar] [CrossRef]
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- Fessehaye, M.; Abdul-Wahab, S.A.; Savage, M.J.; Kohler, T.; Gherezghiher, T.; Hurni, H. Fog-water collection for community use. Renew. Sustain. Energy Rev. 2014, 29, 52–62. [Google Scholar] [CrossRef]
- Narayan, G.P.; Sharqawy, M.H.; Summers, E.K.; Lienhard, J.H.; Zubair, S.M.; Antar, M.A. The potential of solar-driven humidification–dehumidification desalination for small-scale decentralized water production. Renew. Sustain. Energy Rev. 2010, 14, 1187–1201. [Google Scholar] [CrossRef]
- Peter-Varbanets, M.; Zurbrugg, C.; Swartz, C.; Pronk, W. Decentralized systems for potable water and the potential of membrane technology. Water Res. 2009, 43, 245–265. [Google Scholar] [CrossRef]
- Loo, S.L.; Fane, A.G.; Krantz, W.B.; Lim, T.T. Emergency water supply: A review of potential technologies and selection criteria. Water Res. 2012, 46, 3125–3151. [Google Scholar] [CrossRef]
- Macedonio, F.; Drioli, E.; Gusev, A.A.; Bardow, A.; Semiat, R.; Kurihara, M. Efficient technologies for worldwide clean water supply. Chem. Eng. Process. Process Intensif. 2012, 51, 2–17. [Google Scholar] [CrossRef]
- Beysens, D. Estimating dew yield worldwide from a few meteo data. Atmos. Res. 2016, 167, 146–155. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Wahlgren, R.V. Atmospheric Water Vapour Processor Designs for Potable Water Production: A Review. Water Res. 2001, 35, 1–22. [Google Scholar] [CrossRef]
- Muselli, M.; Beysens, D.; Marcillat, J.; Milimouk, I.; Nilsson, T.R.; Louche, A. Dew water collector for potable water in Ajaccio (Corsica Island, France). Atmos. Res. 2002, 64, 297–312. [Google Scholar] [CrossRef]
- Clus, O.; Ortega, P.; Muselli, M.; Milimouk, I.; Beysens, D. Study of dew water collection in humid tropical islands. J. Hydrol. 2008, 361, 159–171. [Google Scholar] [CrossRef]
- Park, K.C.; Chhatre, S.S.; Srinivasan, S.; Cohen, R.E.; McKinley, G.H. Optimal design of permeable fiber network structures for fog harvesting. Langmuir 2013, 29, 13269–13277. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Moon, M.W.; Lim, H.; Kim, W.D.; Kim, H.Y. Water harvest via dewing. Langmuir 2012, 28, 10183–10191. [Google Scholar] [CrossRef]
- Park, K.C.; Kim, P.; Grinthal, A.; He, N.; Fox, D.; Weaver, J.C.; Aizenberg, J. Condensation on slippery asymmetric bumps. Nature 2016, 531, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rao, S.R.; Kapustin, E.A.; Zhao, L.; Yang, S.; Yaghi, O.M.; Wang, E.N. Adsorption-based atmospheric water harvesting device for arid climates. Nat. Commun. 2018, 9, 1191. [Google Scholar] [CrossRef]
- Zhou, P.L.; Zhu, Q.J.; Sun, X.X.; Liu, L.; Cai, Z.W.; Xu, J. Recent advances in MXene-based membrane for solar-driven interfacial evaporation desalination. Chem. Eng. J. 2023, 464, 142508. [Google Scholar] [CrossRef]
- Kandeal, A.W.; Joseph, A.; Elsharkawy, M.; Elkadeem, M.R.; Hamada, M.A.; Khalil, A.; Moustapha, M.E.; Sharshir, S.W. Research progress on recent technologies of water harvesting from atmospheric air: A detailed review. Sustain. Energy Technol. 2022, 52, 102000. [Google Scholar] [CrossRef]
- LaPotin, A.; Kim, H.; Rao, S.R.; Wang, E.N. Adsorption-Based Atmospheric Water Harvesting: Impact of Material and Component Properties on System-Level Performance. Acc. Chem. Res. 2019, 52, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Tu, R.; Hwang, Y. Reviews of atmospheric water harvesting technologies. Energy 2020, 201, 117630. [Google Scholar] [CrossRef]
- Kogan, B.; Trahtman, A. The moisture from the air as water resource in arid region: Hopes, doubts and facts. J. Arid. Environ. 2003, 53, 231–240. [Google Scholar] [CrossRef]
- Beysens, D.; Milimouk, I. The Case for Alternative Fresh Water Sources. Secheresse 2000, 11, 1–16. [Google Scholar]
- Jumikis, A.R. Aerial Wells: Secondary Sources of Water. Soil Sci. 1965, 100, 23–95. [Google Scholar] [CrossRef]
- Sadek, S.; Deng, S.; Zhao, J.; Zayed, M.E. Solar-powered adsorption-based atmospheric water harvesting systems: Principles, materials, performance analysis, and configurations. Sustain. Energy Technol. 2022, 54, 102874. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, H.; Zhao, F.; Yu, G. Atmospheric Water Harvesting: A Review of Material and Structural Designs. ACS Mater. Lett. 2020, 2, 671–684. [Google Scholar] [CrossRef]
- Ejeian, M.; Wang, R.Z. Adsorption-based atmospheric water harvesting. Joule 2021, 5, 1678–1703. [Google Scholar] [CrossRef]
- Bilal, M.; Sultan, M.; Morosuk, T.; Den, W.; Sajjad, U.; Aslam, M.M.A.; Shahzad, M.W.; Farooq, M. Adsorption-based atmospheric water harvesting: A review of adsorbents and systems. Int. Commun. Heat Mass Transf. 2022, 133, 105961. [Google Scholar] [CrossRef]
- Thavalengal, M.S.; Jamil, M.A.; Mehroz, M.; Xu, B.B.; Yaqoob, H.; Sultan, M.; Imtiaz, N.; Shahzad, M.W. Progress and Prospects of Air Water Harvesting System for Remote Areas: A Comprehensive Review. Energies 2023, 16, 2686. [Google Scholar] [CrossRef]
- Li, S.; Hernandez, S.; Salazar, N. Biopolymer-Based Hydrogels for Harvesting Water from Humid Air: A Review. Sustainability 2023, 15, 848. [Google Scholar] [CrossRef]
- Huang, X.; Qin, Q.; Ma, Q.; Wang, B. Atmospheric Water Harvesting with Metal-Organic Frameworks and Their Composites: From Materials to Devices. Water 2022, 14, 3487. [Google Scholar] [CrossRef]
- Ito, H.; Vallejos-Burgos, F.; Ono, Y.; Yoshimoto, M.; Kaneko, K.; Futamura, R.; Iiyama, T.; Matsumoto, A. Isotope effect on adsorption diffusivity of water molecules in hydrophobic carbon micropores. Carbon 2020, 168, 415–418. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Teshima, K.; Futamura, R.; Tanaka, H.; Neimark, A.V.; Kaneko, K. Structural mechanism of reactivation with steam of pitch-based activated carbon fibers. J. Colloid Interface Sci. 2020, 578, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zeng, Y.; Do, D.D.; Nicholson, D. An undulation theory for condensation in open end slit pores: Critical hysteresis temperature & critical hysteresis pore size. Phys. Chem. Chem. Phys. 2014, 16, 12362–12373. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Utilization of clay based super-porous hydrogel composites in atmospheric water harvesting. Appl. Clay Sci. 2022, 230, 106712. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Li, Q.; Wu, T.; Zhang, M.; Shi, Q. Water sorption on composite material “zeolite 13X modified by LiCl and CaCl2”. Microporous Mesoporous Mater. 2020, 299, 110109. [Google Scholar] [CrossRef]
- Mittal, H.; Alili, A.A.; Alhassan, S.M. Hybrid super-porous hydrogel composites with high water vapor adsorption capacity—Adsorption isotherm and kinetics studies. J. Environ. Chem. Eng. 2021, 9, 106611. [Google Scholar] [CrossRef]
- Han, X.; Zhong, L.; Zhang, L.; Zhu, L.; Zhou, M.; Wang, S.; Yu, D.; Chen, H.; Hou, Y.; Zheng, Y. Efficient Atmospheric Water Harvesting of Superhydrophilic Photothermic Nanocapsule. Small 2023, 19, e2303358. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76/77, 137–152. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Yang, F.; Zhang, N.; Cao, X. Inorganic composite sorbents for water vapor sorption: A research progress. Renew. Sustain. Energy Rev. 2016, 54, 761–776. [Google Scholar] [CrossRef]
- Broach, R.W.; Jan, D.-Y.; Lesch, D.A.; Kulprathipanja, S.; Roland, E.; Kleinschmit, P. Zeolites. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Pliekhov, O.; Pliekhova, O.; Arčon, I.; Bondino, F.; Magnano, E.; Mali, G.; Logar, N.Z. Study of water adsorption on EDTA dealuminated zeolite Y. Microporous Mesoporous Mater. 2020, 302, 110208. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Liu, T.; Wang, Z.Y.; Li, Q.W.; Wu, T.H.; Zhang, M. Preparation and characterization of composite adsorbent LiCl/zeolite 13X for adsorption system. Int. J. Environ. Sci. Technol. 2020, 18, 2693–2702. [Google Scholar] [CrossRef]
- Trapani, F.; Polyzoidis, A.; Loebbecke, S.; Piscopo, C.G. On the general water harvesting capability of metal-organic frameworks under well-defined climatic conditions. Microporous Mesoporous Mater. 2016, 230, 20–24. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, B.; Gao, Y.; Zhang, F.; Sun, Y. Experimental study on the adsorption and desorption performance of composite adsorbent activated carbon/calcium chloride. Int. J. Environ. Sci. Technol. 2022, 20, 5585–5596. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Shi, L.; Alsaedi, M.; Wang, P. Harvesting Water from Air: Using Anhydrous Salt with Sunlight. Environ. Sci. Technol. 2018, 52, 5398–5406. [Google Scholar] [CrossRef]
- Sögütoglu, L.-C.; Steiger, M.; Houben, J.; Biemans, D.; Fischer, H.R.; Donkers, P.; Huinink, H.; Adan, O.C.G. Understanding the Hydration Process of Salts: The Impact of a Nucleation Barrier. Cryst. Growth Des. 2019, 19, 2279–2288. [Google Scholar] [CrossRef]
- Vainio, E.; DeMartini, N.; Hupa, L.; Åmand, L.-E.; Richards, T.; Hupa, M. Hygroscopic Properties of Calcium Chloride and Its Role on Cold-End Corrosion in Biomass Combustion. Energy Fuels 2019, 33, 11913–11922. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, G.; Li, J.; Hu, X.; Xu, N.; Zhao, W.; Zhu, B.; Zhu, J. An Interfacial Solar Heating Assisted Liquid Sorbent Atmospheric Water Generator. Angew. Chem. Int. Ed. Engl. 2019, 58, 12054–12058. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, W.W.; Xie, S.T.; Pan, Q.W. Performance characterization and application of composite adsorbent LiCl@ACFF for moisture harvesting. Sci. Rep. 2021, 11, 14412. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wei, T.; Zhao, W.; Zhu, B.; Liu, G.; Wang, P.; Lin, Z.; Wang, X.; Li, X.; Zhang, X.; et al. An Interfacial Solar-Driven Atmospheric Water Generator Based on a Liquid Sorbent with Simultaneous Adsorption-Desorption. Adv. Mater. 2019, 31, e1903378. [Google Scholar] [CrossRef] [PubMed]
- Mastronardo, E.; Piperopoulos, E.; Palamara, D.; Frazzica, A.; Calabrese, L. Morphological Observation of LiCl Deliquescence in PDMS-Based Composite Foams. Appl. Sci. 2022, 12, 1510. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Chao, J.; Wu, S.; Yan, T.; Li, W.; Cao, B.; Wang, R. Efficient Solar-Driven Water Harvesting from Arid Air with Metal-Organic Frameworks Modified by Hygroscopic Salt. Angew. Chem. Int. Ed. Engl. 2020, 59, 5202–5210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Pore structure of new composite adsorbent SiO2·xH2O·yCaCl2 with high uptake of water from air. Sci. China Ser. E 2003, 46, 551. [Google Scholar] [CrossRef]
- Ye, H.; Yuan, Z.; Li, S.; Zhang, L. Activated carbon fiber cloth and CaCl2 composite sorbents for a water vapor sorption cooling system. Appl. Therm. Eng. 2014, 62, 690–696. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, R.Z.; Wang, L.W. Water vapor sorption performance of ACF-CaCl2 and silica gel-CaCl2 composite adsorbents. Appl. Therm. Eng. 2016, 100, 893–901. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Najafi, M.; Derakhshandeh, P.G.; Van der Voort, P. Metal- and covalent organic frameworks as catalyst for organic transformation: Comparative overview and future perspectives. Coord. Chem. Rev. 2022, 451, 214259. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. Engl. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Furukawa, H.; Gandara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal-organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal-Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018, 30, e1704304. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, G.; Meng, F.L.; Lu, W.; Abed, J.; Peh, C.K.N.; Gao, M.; Sargent, E.H.; Ho, G.W. Autonomous atmospheric water seeping MOF matrix. Sci. Adv. 2020, 6, eabc8605. [Google Scholar] [CrossRef] [PubMed]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.M.; Askalany, A.A.; Shea, A.D.; Dakkama, H.J.; Mahmoud, S.; Al-Dadah, R.; Kaialy, W. A state of the art of required techniques for employing activated carbon in renewable energy powered adsorption applications. Renew. Sustain. Energy Rev. 2017, 79, 503–519. [Google Scholar] [CrossRef]
- Yu, N.; Wang, R.Z.; Lu, Z.S.; Wang, L.W. Development and characterization of silica gel–LiCl composite sorbents for thermal energy storage. Chem. Eng. Sci. 2014, 111, 73–84. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R.Z. Extraordinary air water harvesting performance with three phase sorption. Mater. Today Energy 2019, 13, 362–373. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, R.Z.; Wang, L.W.; Liu, J.Y. A high efficient semi-open system for fresh water production from atmosphere. Energy 2017, 138, 542–551. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, J.Y.; Wang, R.Z.; Wang, L.W. Experimental research of composite solid sorbents for fresh water production driven by solar energy. Appl. Therm. Eng. 2017, 121, 941–950. [Google Scholar] [CrossRef]
- Simonova, I.A.; Freni, A.; Restuccia, G.; Aristov, Y.I. Water sorption on composite “silica modified by calcium nitrate”. Microporous Mesoporous Mater. 2009, 122, 223–228. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Katiyar, A.; Yadav, S.; Smirniotis, P.G.; Pinto, N.G. Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules. J. Chromatogr. A 2006, 1122, 13–20. [Google Scholar] [CrossRef]
- Rangnekar, N.; Mittal, N.; Elyassi, B.; Caro, J.; Tsapatsis, M. Zeolite membranes—A review and comparison with MOFs. Chem. Soc. Rev. 2015, 44, 7128–7154. [Google Scholar] [CrossRef]
- Garzón-Tovar, L.; Pérez-Carvajal, J.; Imaz, I.; Maspoch, D. Composite Salt in Porous Metal-Organic Frameworks for Adsorption Heat Transformation. Adv. Funct. Mater. 2017, 27, 1606424. [Google Scholar] [CrossRef]
- Wu, Q.; Su, W.; Li, Q.; Tao, Y.; Li, H. Enabling Continuous and Improved Solar-Driven Atmospheric Water Harvesting with Ti3C2-Incorporated Metal-Organic Framework Monoliths. ACS Appl. Mater. Interfaces 2021, 13, 38906–38915. [Google Scholar] [CrossRef] [PubMed]
- Almassad, H.A.; Abaza, R.I.; Siwwan, L.; Al-Maythalony, B.; Cordova, K.E. Environmentally adaptive MOF-based device enables continuous self-optimizing atmospheric water harvesting. Nat. Commun. 2022, 13, 4873. [Google Scholar] [CrossRef] [PubMed]
- Entezari, A.; Ejeian, M.; Wang, R. Super Atmospheric Water Harvesting Hydrogel with Alginate Chains Modified with Binary Salts. ACS Mater. Lett. 2020, 2, 471–477. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super Moisture-Absorbent Gels for All-Weather Atmospheric Water Harvesting. Adv. Mater. 2019, 31, e1806446. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhu, X.; Xu, Z.; Davis, R.A.; Liu, G.; Zhong, H.; Lin, X.; Dong, P.; Ye, M.; Shen, J. Loofah Sponge-Derived Hygroscopic Photothermal Absorber for All-Weather Atmospheric Water Harvesting. ACS Appl. Mater. Interfaces 2022, 14, 4680–4689. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Tudoroiu, E.-E.; Ionescu, D.-G.; Dinu-Pîrvu, C.-E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Zhou, Q.; Wang, M.; Wang, W.; Lv, Y.; Xiao, R.; Papavassiliou, D.V. Agricultural waste-derived moisture-absorber for all-weather atmospheric water collection and electricity generation. Nano Energy 2020, 74, 104922. [Google Scholar] [CrossRef]
- Solovyeva, M.V.; Krivosheeva, I.V.; Gordeeva, L.G.; Khudozhitkov, A.E.; Kolokolov, D.I.; Stepanov, A.G.; Ludwig, R. Salt Confined in MIL-101(Cr)-Tailoring the Composite Sorbents for Efficient Atmospheric Water Harvesting. ChemSusChem 2023, 16, e202300520. [Google Scholar] [CrossRef]
- Gado, M.G.; Ookawara, S. 3D-printed triply periodic minimal surface (TPMS) structures: Towards potential application of adsorption-based atmospheric water harvesting. Energy Convers. Manag. 2023, 297, 117729. [Google Scholar] [CrossRef]
- Laha, S.; Maji, T.K. Binary/Ternary MOF Nanocomposites for Multi-Environment Indoor Atmospheric Water Harvesting. Adv. Funct. Mater. 2022, 32, 2203093. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Solovyeva, M.V.; Sapienza, A.; Aristov, Y.I. Potable water extraction from the atmosphere: Potential of MOFs. Renew. Energy 2020, 148, 72–80. [Google Scholar] [CrossRef]
- Gado, M.G.; Nasser, M.; Hassan, A.A.; Hassan, H. Adsorption-based atmospheric water harvesting powered by solar energy: Comprehensive review on desiccant materials and systems. Process Saf. Environ. Prot. 2022, 160, 166–183. [Google Scholar] [CrossRef]
- Song, X.; Chen, C.; Zhou, H.; Shang, J.Y.; Ren, T.S. Effect of high-temperature treatment on water vapour sorption of montmorillonite. Geoderma 2023, 436, 116563. [Google Scholar] [CrossRef]
- Li, H.-J.; Cheng, L.; Sun, P.; Li, F.-F.; Qiu, J. Potential Analysis of Atmospheric Water Harvesting Technologies from the Perspective of “Trading-in Energy for Water”. Water 2023, 15, 878. [Google Scholar] [CrossRef]
- Marchesini, S.; McGilvery, C.M.; Bailey, J.; Petit, C. Template-Free Synthesis of Highly Porous Boron Nitride: Insights into Pore Network Design and Impact on Gas Sorption. ACS Nano 2017, 11, 10003–10011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, R.; Li, Y. Diversifying Water Sources with Atmospheric Water Harvesting to Enhance Water Supply Resilience. Sustainability 2022, 14, 7783. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Zhou, W.; Cui, W.; Qi, Z. Research Progress on Hygroscopic Agents for Atmospheric Water Harvesting Systems. Materials 2024, 17, 722. https://doi.org/10.3390/ma17030722
Bai Q, Zhou W, Cui W, Qi Z. Research Progress on Hygroscopic Agents for Atmospheric Water Harvesting Systems. Materials. 2024; 17(3):722. https://doi.org/10.3390/ma17030722
Chicago/Turabian StyleBai, Qi, Wanlai Zhou, Wenzhong Cui, and Zhiyong Qi. 2024. "Research Progress on Hygroscopic Agents for Atmospheric Water Harvesting Systems" Materials 17, no. 3: 722. https://doi.org/10.3390/ma17030722
APA StyleBai, Q., Zhou, W., Cui, W., & Qi, Z. (2024). Research Progress on Hygroscopic Agents for Atmospheric Water Harvesting Systems. Materials, 17(3), 722. https://doi.org/10.3390/ma17030722






