Advances in Electrochemical Energy Storage over Metallic Bismuth-Based Materials
Abstract
:1. Introduction
2. Fundamentals of Bismuth
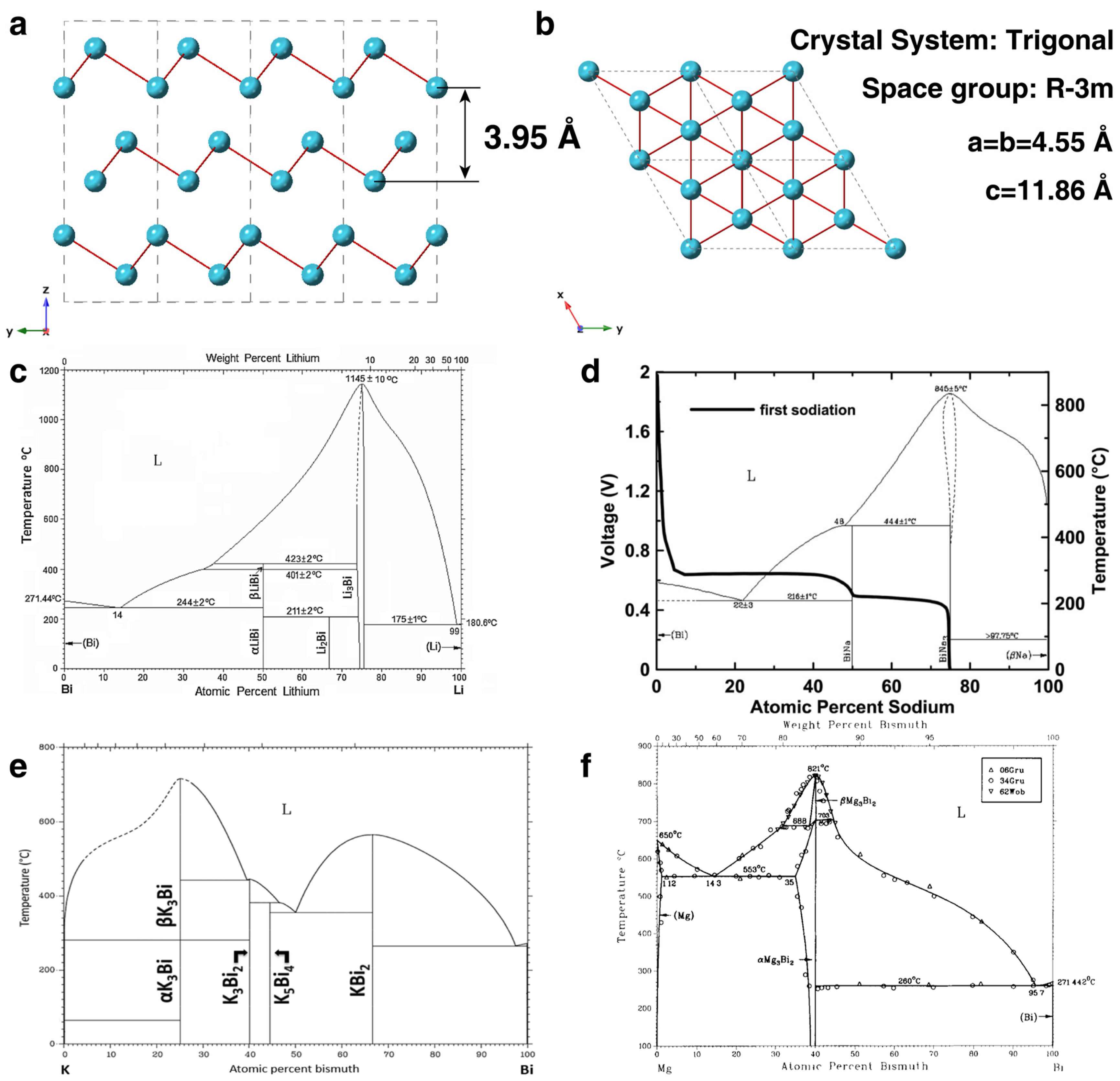
2.1. Crystal Structure of Bi
2.2. Physicochemical Properties of Bi
3. Application of Bi-Based Composites for Energy Storage Systems
3.1. Anode for Alkali Ion Batteries
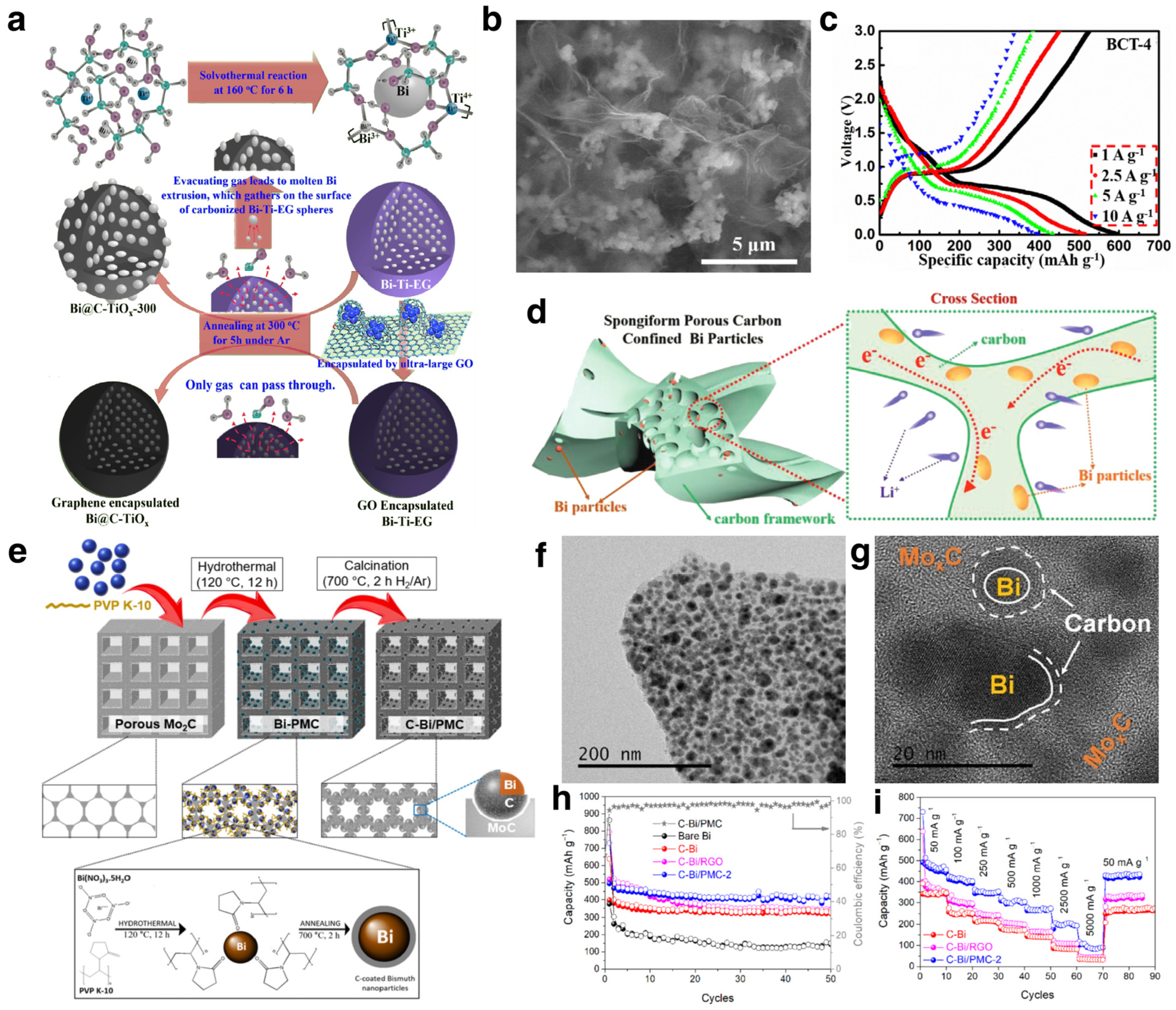
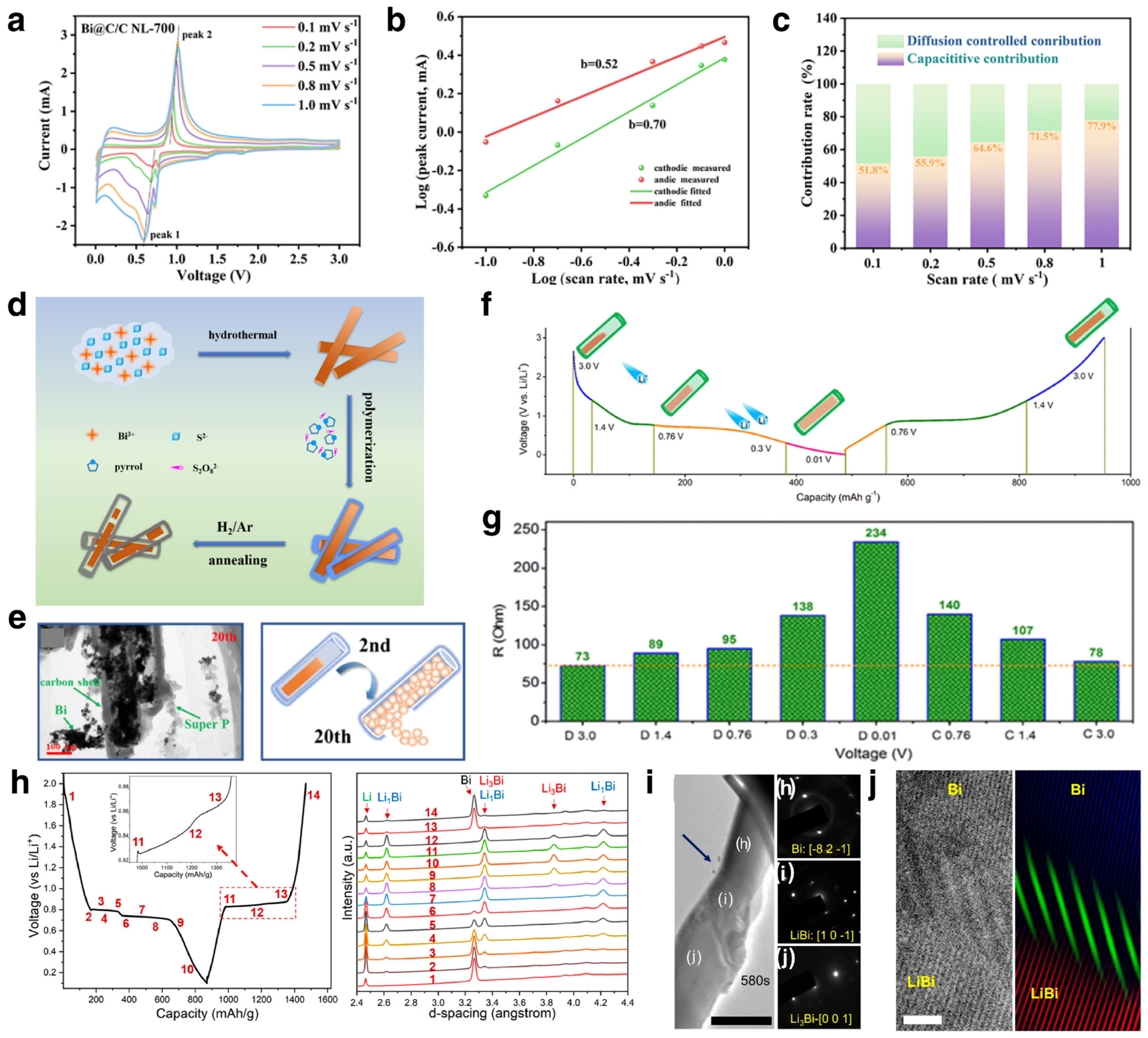
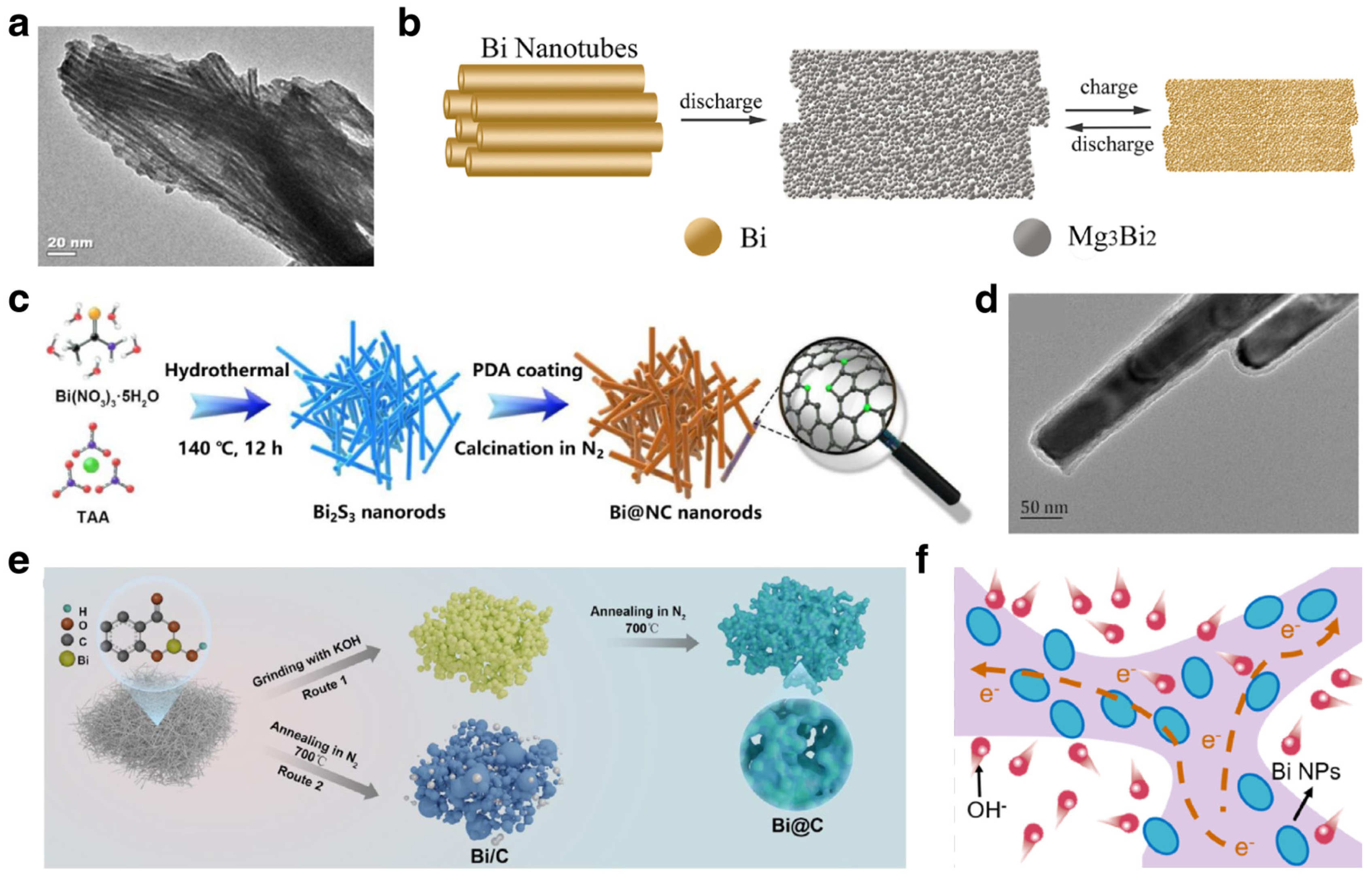
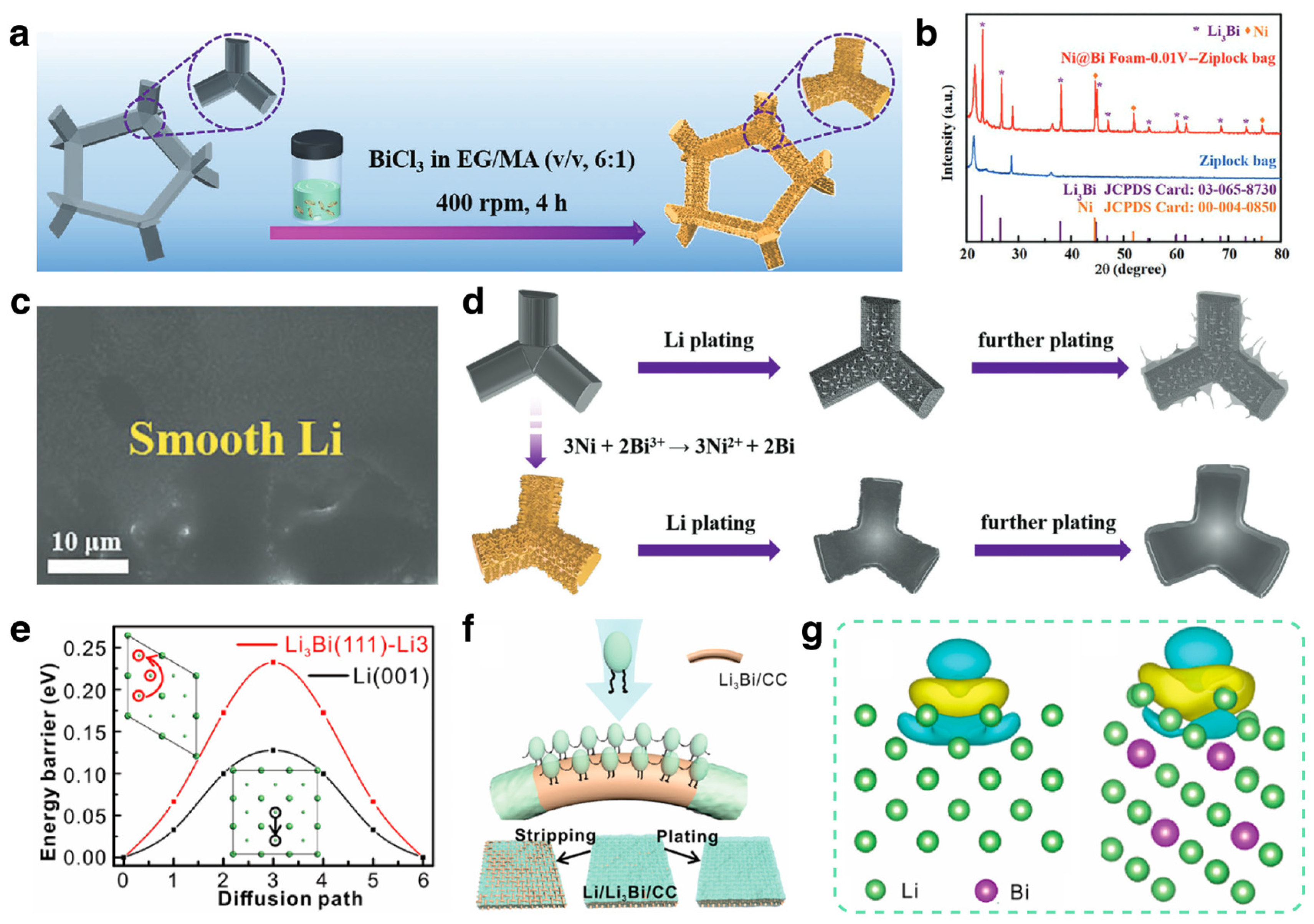
3.1.1. Li Ion Batteries
Modification Strategies
Mechanism Investigations
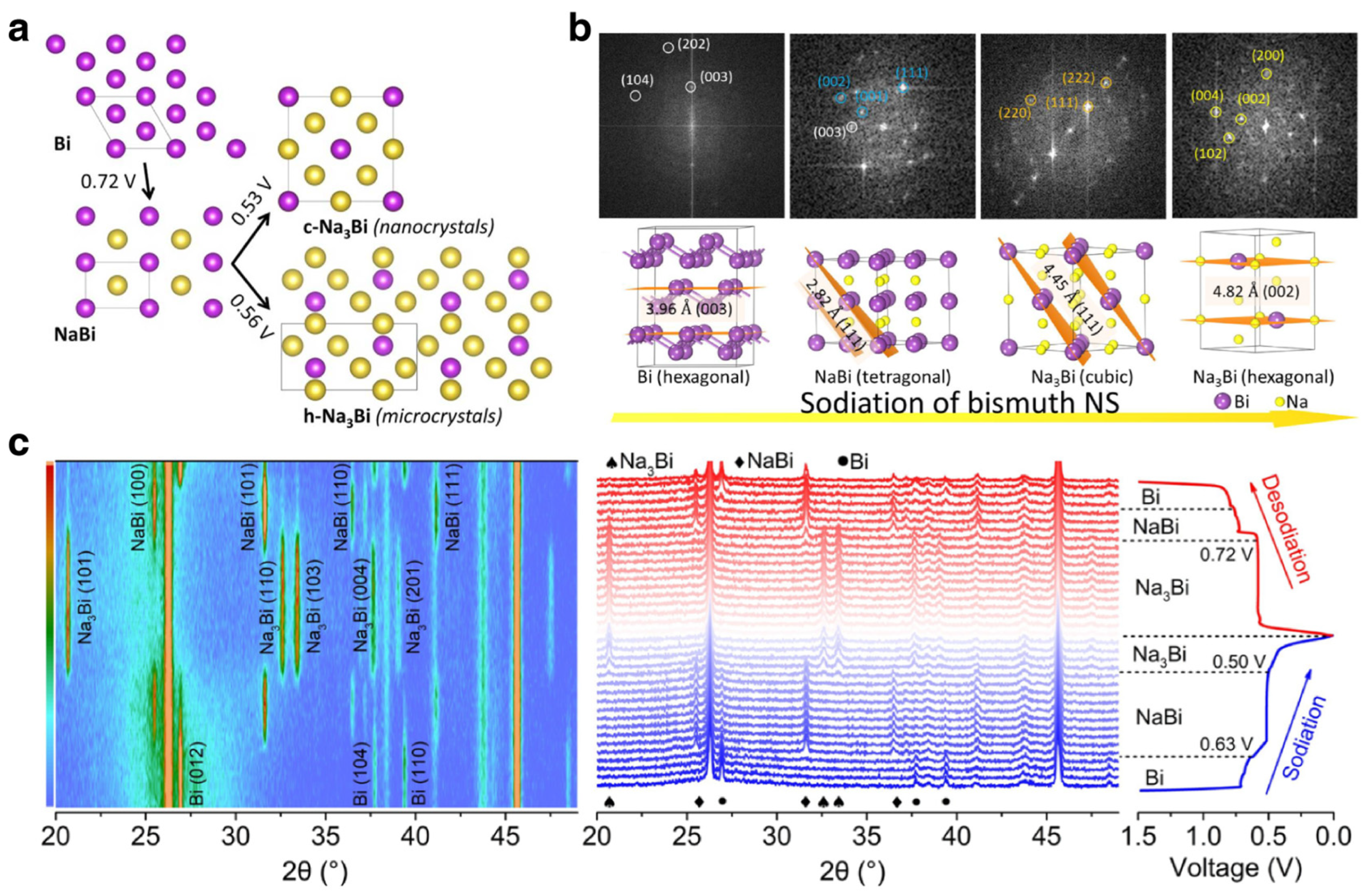
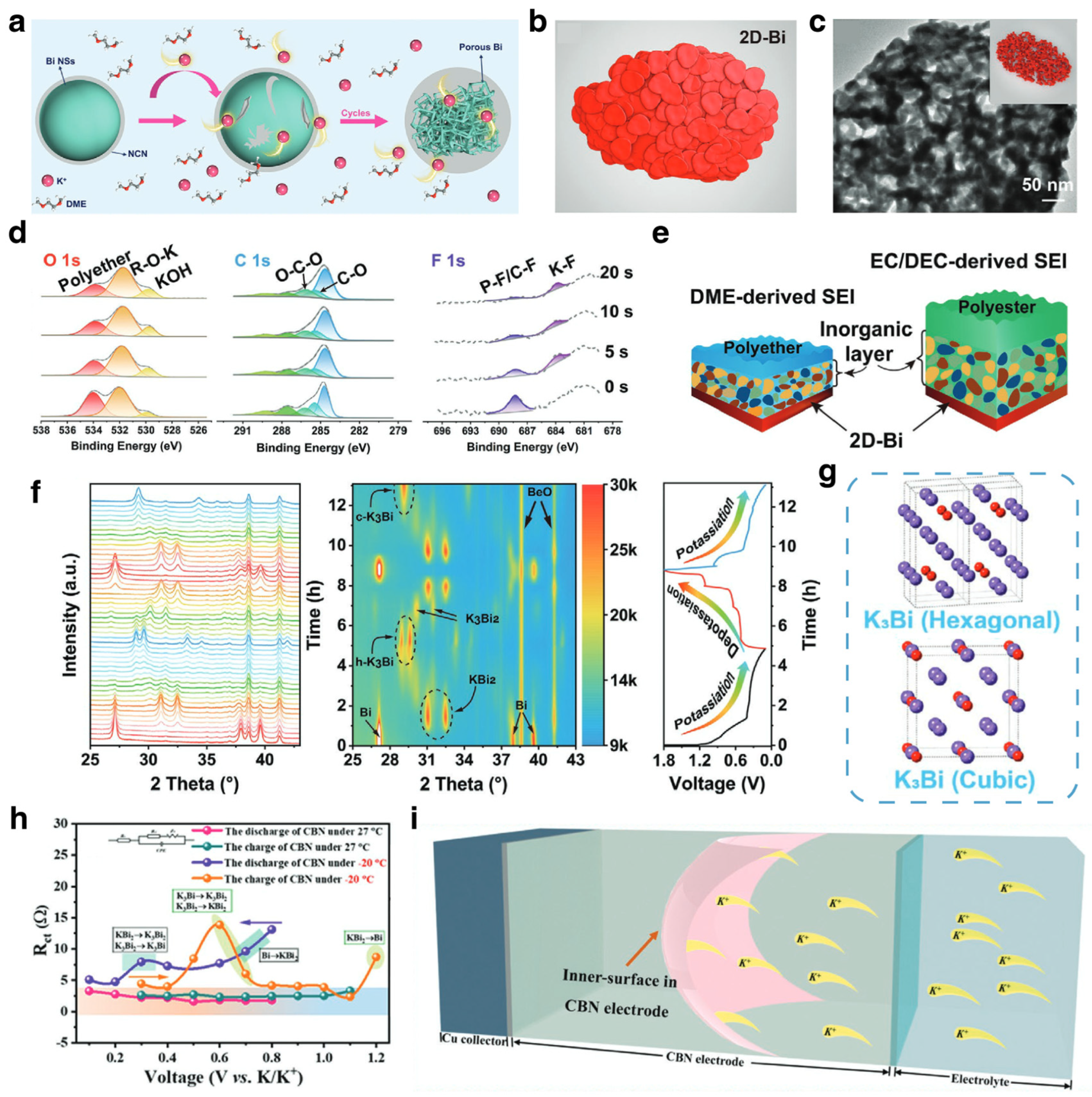
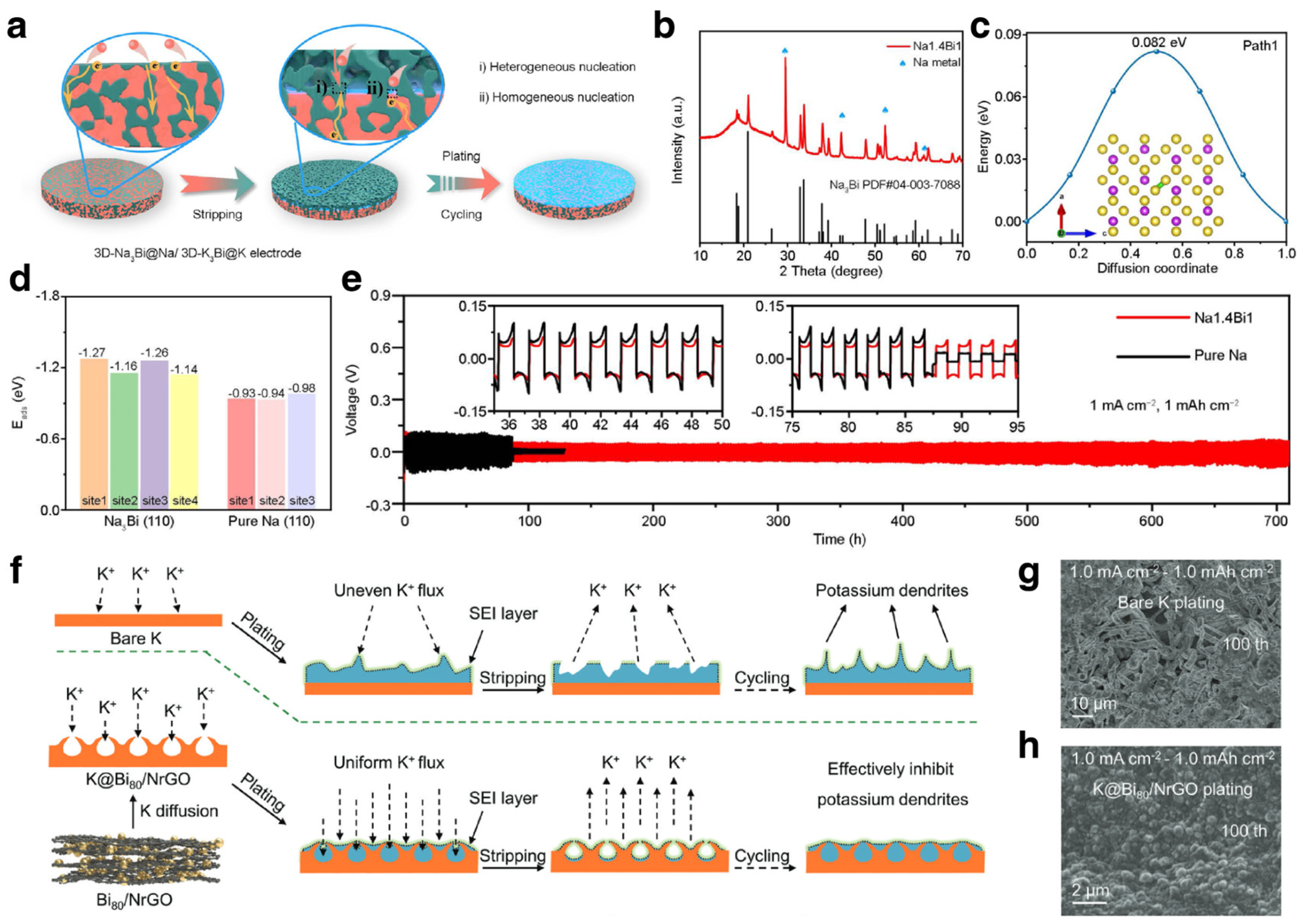
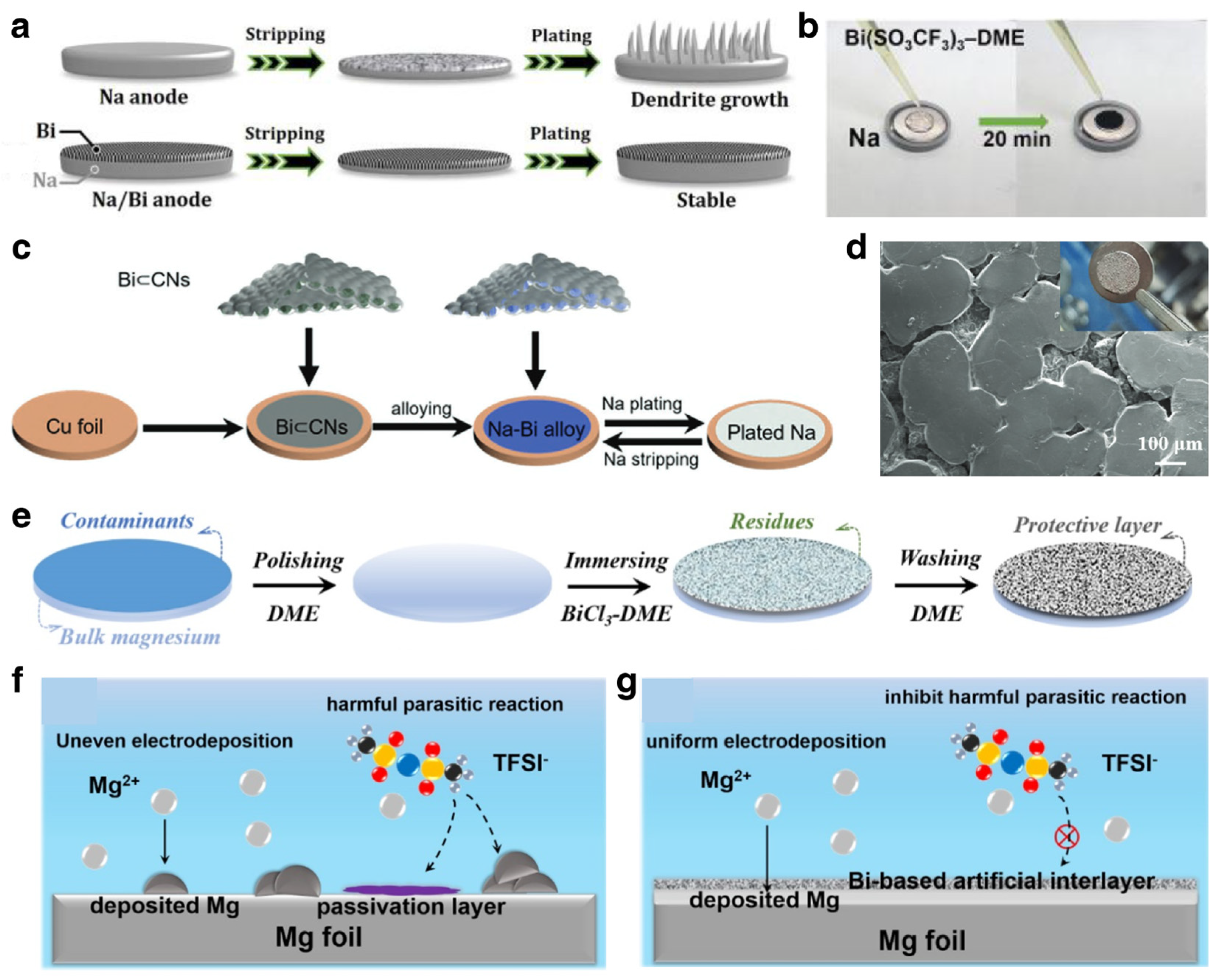
3.1.2. Na/K Ion Batteries
Modification Strategies for SIBs
Mechanism Investigations for SIBs
Modification Strategies and Mechanism Investigations for PIBs
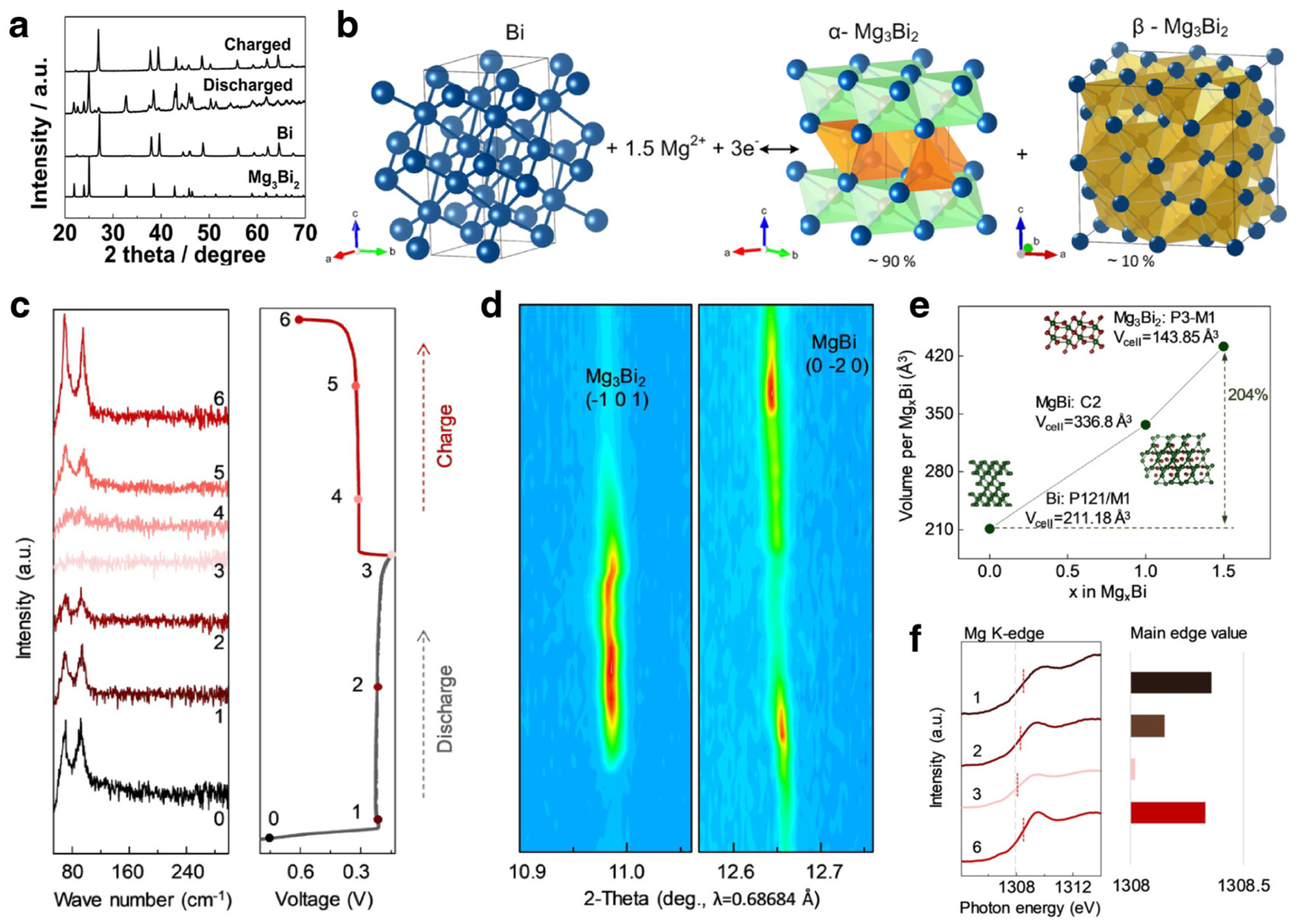
3.1.3. Mg Ion Batteries
Modification Strategies
Mechanism Investigations
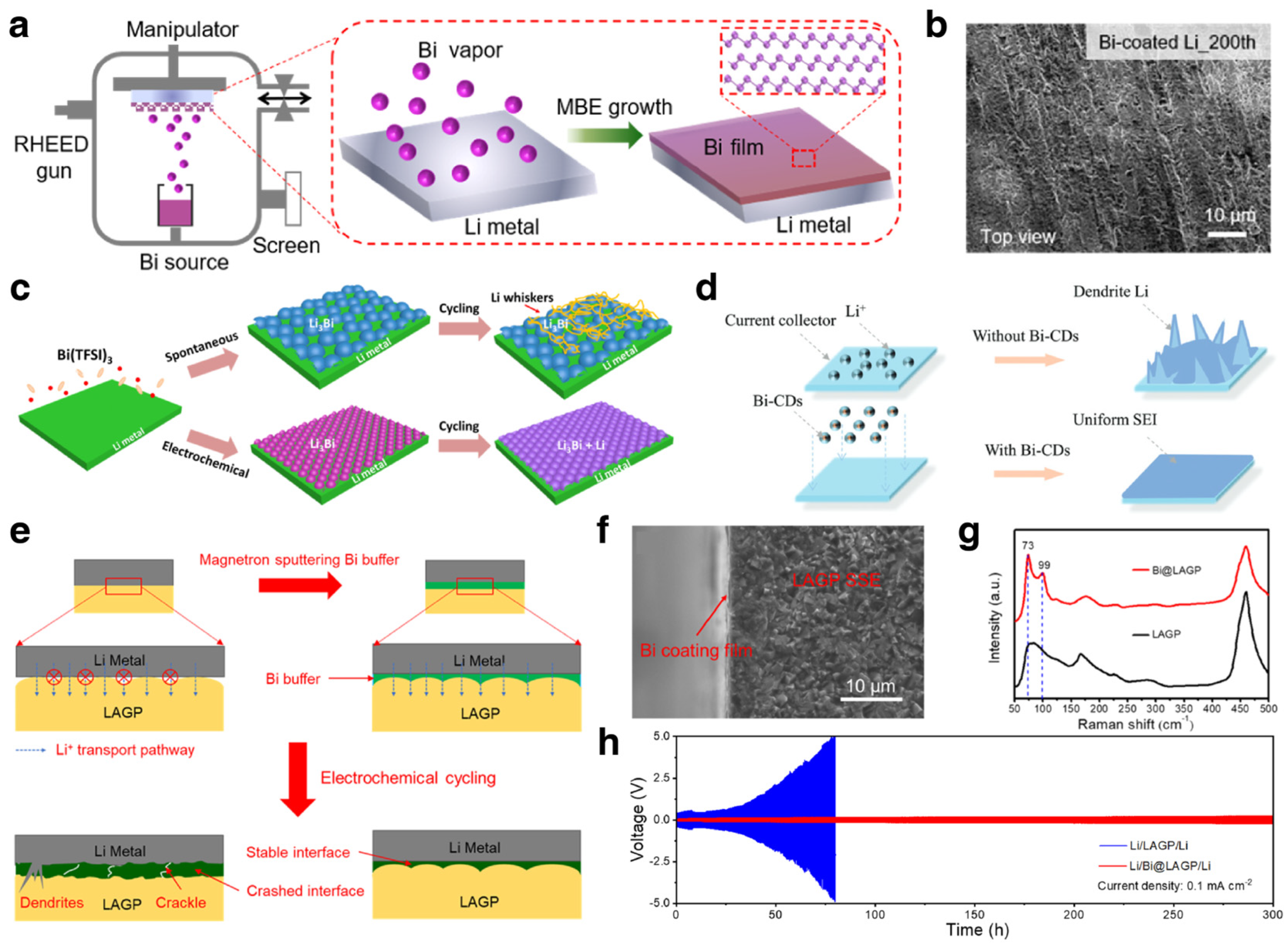
3.2. Modification for Alkali Metal Anodes
3.2.1. Electrode Engineering
3.2.2. Interlayer Engineering
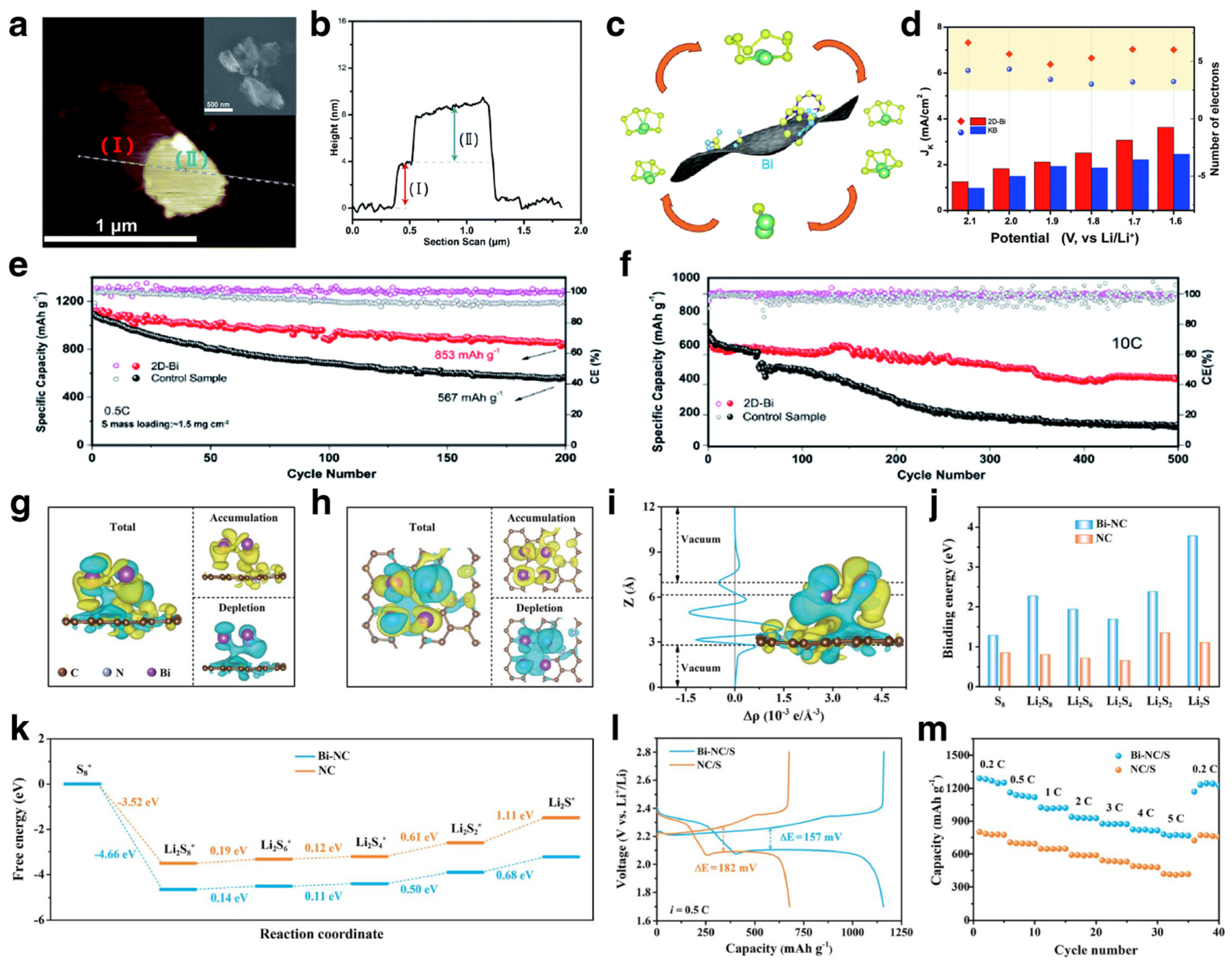
3.3. Host for Sulfur Cathodes
4. Conclusions and Perspectives
- Some electrochemical performance indicators for Bi-based anodes in alkali ion batteries are still not fully satisfactory, including initial coulombic efficiency, performance of high-loading electrodes and energy density of the full cells. More efforts in structural and electrode design should be devoted to addressing these issues for practical applications. As mentioned earlier, metallic Bi-based materials will undergo particle refinement and morphological evolution during the first charge/discharge process, which will expose abundant fresh surfaces and lead to the continuous generation of SEI films. Therefore, reasonable structural design and electrolyte optimization will be beneficial to improving the initial coulombic efficiency. The porous structure will improve the cycle and rate performance of the batteries, but will also lead to a reduction in energy density. Therefore, it is necessary to find the optimal porosity and build a suitable porous structure.
- The reaction mechanisms should be further investigated, including the morphological evolution mechanisms and alloying reaction mechanism. Judging from the published literature, metallic Bi-based materials tend to evolve into continuous porous structures after charge and discharge cycles. There is currently no clear explanation for this morphological evolution rule. The study of this rule will help to design more stable material structures. As for the final product and reaction pathways of the alloying reaction mechanism of Bi-based materials, there is currently no unified conclusion, and further research is needed.
- In the application of metallic Bi-based materials for alkali metal anodes and sulfur cathodes, Bi plays a role as functional additive rather than active material. Therefore, the content of Bi-based materials can be reduced as much as possible for maximal effect. Electrode engineering for alkali metal anodes is a promising research direction for the development of a suitable and stable 3D skeleton structure with nanosized Bi uniformly dispersed to induce homogeneous deposition of metal ions. Interfacial engineering plays an important role in the alkali metal anode. The ideal metal anode interface layer should be composed of inorganic components with high mechanical strength and organic components with highly elasticity. Therefore, hybrid organic–inorganic SEIs with Bi-based components hold promise for better alkali metal anodes.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, B.; Zhang, Q.; Pan, Z.; Li, L.; Li, C.; Ling, Y.; Wang, Z.; Chen, M.; Wang, Z.; Yao, Y.; et al. Freestanding Metal–Organic Frameworks and Their Derivatives: An Emerging Platform for Electrochemical Energy Storage and Conversion. Chem. Rev. 2022, 122, 10087–10125. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, X.; Zhan, F.; He, Q.; Wang, H.; Chen, J.; Wang, H.; Ren, X.; Chen, L. Toward Emerging Two-Dimensional Nickel-Based Materials for Electrochemical Energy Storage: Progress and Perspectives. Energy Storage Mater. 2022, 53, 79–135. [Google Scholar] [CrossRef]
- Hu, X.; Deng, X.; Wang, F.; Deng, Z.; Lin, X.; Teodorescu, R.; Pecht, M.G. A Review of Second-Life Lithium-Ion Batteries for Stationary Energy Storage Applications. Proc. IEEE 2022, 110, 735–753. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Zheng, Z. Toward Practical High-Energy and High-Power Lithium Battery Anodes: Present and Future. Adv. Sci. 2022, 9, 2105213. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Li, Z.; Yuan, X.; Wang, D.-Y. Toward a New Generation of Fire-Safe Energy Storage Devices: Recent Progress on Fire-Retardant Materials and Strategies for Energy Storage Devices. Small Methods 2022, 6, 2101428. [Google Scholar] [CrossRef] [PubMed]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode Materials for Lithium-Ion Batteries: A Review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.-Y.; Wang, Z.; Lv, W.; He, Y.-B.; Yang, Q.-H.; Kang, F. Revisiting the Roles of Natural Graphite in Ongoing Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2106704. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Xiang, J.; Wei, Y.; Zhong, Y.; Yang, Y.; Cheng, H.; Yuan, L.; Xu, H.; Huang, Y. Building Practical High-Voltage Cathode Materials for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2200912. [Google Scholar] [CrossRef]
- Hao, H.; Ma, Z.; Wang, A.; Xing, W.; Song, H.; Zhao, P.; Wei, J.; Zheng, S. Modeling and Assessing the Robustness of the Lithium Global Trade System against Cascading Failures. Resour. Policy 2023, 85, 103822. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Lim, M.K.; Chen, W.-Q.; Teng, L.; Wang, P.; Wang, H.; Zhang, C.; Yao, C.; Ghadimi, P. Critical Systemic Risk Sources in Global Lithium-Ion Battery Supply Networks: Static and Dynamic Network Perspectives. Renew. Sustain. Energy Rev. 2023, 173, 113083. [Google Scholar] [CrossRef]
- York, M.; Larson, K.; Harris, K.C.; Carmona, E.; Albertus, P.; Sharma, R.; Noked, M.; Strauss, E.; Ragones, H.; Golodnitsky, D. Recent Advances in Solid-State beyond Lithium Batteries. J. Solid State Electrochem. 2022, 26, 1851–1869. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, Y.; He, Q.; Deng, S.; Huang, Q.; Huang, S.; Yang, Y. Emerging Carbonyl Polymers as Sustainable Electrode Materials for Lithium-Free Metal-Ion Batteries. Energy Environ. Mater. 2022, 5, 1037–1059. [Google Scholar] [CrossRef]
- Chen, Q.; Cheng, X.; Long, H.; Rao, Y. A Short Review on Recent Progress of Bi/Semiconductor Photocatalysts: The Role of Bi Metal. Chin. Chem. Lett. 2020, 31, 2583–2590. [Google Scholar] [CrossRef]
- Wan, Y.; Zhou, H.; Zheng, M.; Huang, Z.-H.; Kang, F.; Li, J.; Lv, R. Oxidation State Modulation of Bismuth for Efficient Electrocatalytic Nitrogen Reduction to Ammonia. Adv. Funct. Mater. 2021, 31, 2100300. [Google Scholar] [CrossRef]
- Zhang, R.; Ji, L.; Kong, W.; Wang, H.; Zhao, R.; Chen, H.; Li, T.; Li, B.; Luo, Y.; Sun, X. Electrocatalytic N2-to-NH3 Conversion with High Faradaic Efficiency Enabled Using a Bi Nanosheet Array. Chem. Commun. 2019, 55, 5263–5266. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, X.; Ji, X.; Gao, T.; Hou, S.; Zhou, X.; Wang, L.; Wang, F.; Yang, C.; Chen, L.; et al. Intercalation of Bi Nanoparticles into Graphite Results in an Ultra-Fast and Ultra-Stable Anode Material for Sodium-Ion Batteries. Energy Environ. Sci. 2018, 11, 1218–1225. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Oh, J.A.S.; Zeng, K.; Lu, L. Recent Advances of Bismuth Based Anode Materials for Sodium-Ion Batteries. Mater. Technol. 2018, 33, 563–573. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, X.; Xing, Z.; Zhu, D.; Tian, W.; Guan, C.; Yang, Y.; Qin, W.; Wang, J.; Zhang, L.; et al. In Situ Formation of Hierarchical Bismuth Nanodots/Graphene Nanoarchitectures for Ultrahigh-Rate and Durable Potassium-Ion Storage. Small 2020, 16, 1905789. [Google Scholar] [CrossRef]
- Zhao, X.B.; Cao, G.S.; Lv, C.P.; Zhang, L.J.; Hu, S.H.; Zhu, T.J.; Zhou, B.C. Electrochemical Properties of Some Sb or Te Based Alloys for Candidate Anode Materials of Lithium-Ion Batteries. J. Alloys Compd. 2001, 315, 265–269. [Google Scholar] [CrossRef]
- Arthur, T.S.; Singh, N.; Matsui, M. Electrodeposited Bi, Sb and Bi1-xSbx Alloys as Anodes for Mg-Ion Batteries. Electrochem. Commun. 2012, 16, 103–106. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Bismuth: A New Anode for the Na-Ion Battery. Nano Energy 2015, 12, 88–95. [Google Scholar] [CrossRef]
- Zhang, Q.; Mao, J.; Pang, W.K.; Zheng, T.; Sencadas, V.; Chen, Y.; Liu, Y.; Guo, Z. Boosting the Potassium Storage Performance of Alloy-Based Anode Materials via Electrolyte Salt Chemistry. Adv. Energy Mater. 2018, 8, 1703288. [Google Scholar] [CrossRef]
- Yang, H.; Xu, R.; Yao, Y.; Ye, S.; Zhou, X.; Yu, Y. Multicore–Shell Bi@N-Doped Carbon Nanospheres for High Power Density and Long Cycle Life Sodium- and Potassium-Ion Anodes. Adv. Funct. Mater. 2019, 29, 1809195. [Google Scholar] [CrossRef]
- Cheng, X.; Li, D.; Wu, Y.; Xu, R.; Yu, Y. Bismuth Nanospheres Embedded in Three-Dimensional (3D) Porous Graphene Frameworks as High Performance Anodes for Sodium- and Potassium-Ion Batteries. J. Mater. Chem. A 2019, 7, 4913–4921. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Y.; Da, P.; Wu, H.; Xu, M.; Zheng, G. Indirect Growth of Mesoporous Bi@C Core-Shell Nanowires for Enhanced Lithium-Ion Storage. Nanoscale 2014, 6, 13236–13241. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, L.; Li, F.; Cheng, F.; Chen, J. Bulk Bismuth as a High-Capacity and Ultralong Cycle-Life Anode for Sodium-Ion Batteries by Coupling with Glyme-Based Electrolytes. Adv. Mater. 2017, 29, 1702212. [Google Scholar] [CrossRef]
- Zhang, H.; Ju, S.; Xia, G.; Yu, X. Identifying the Positive Role of Lithium Hydride in Stabilizing Li Metal Anode. Sci. Adv. 2022, 8, eabl8245. [Google Scholar] [CrossRef]
- Shen, X.; Shi, S.; Li, B.; Li, S.; Zhang, H.; Chen, S.; Deng, H.; Zhang, Q.; Zhu, J.; Duan, X. Lithiophilic Interphase Porous Buffer Layer toward Uniform Nucleation in Lithium Metal Anodes. Adv. Funct. Mater. 2022, 32, 2206388. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, Q.; Liu, Y.; Wang, Y.; Jiang, F.; Wang, N.; Bai, Z.; Dou, S. Molybdenum Chalcogenides Based Anode Materials for Alkali Metal Ions Batteries: Beyond Lithium Ion Batteries. Energy Storage Mater. 2022, 50, 308–333. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation. Nano-Micro Lett. 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Stalin, S.; Archer, L.A. Stabilizing Metal Battery Anodes through the Design of Solid Electrolyte Interphases. Joule 2021, 5, 1119–1142. [Google Scholar] [CrossRef]
- Zhang, S.-J.; You, J.-H.; He, Z.; Zhong, J.; Zhang, P.-F.; Yin, Z.-W.; Pan, F.; Ling, M.; Zhang, B.; Lin, Z. Scalable Lithiophilic/Sodiophilic Porous Buffer Layer Fabrication Enables Uniform Nucleation and Growth for Lithium/Sodium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2200967. [Google Scholar] [CrossRef]
- Rakov, D.A.; Chen, F.; Ferdousi, S.A.; Li, H.; Pathirana, T.; Simonov, A.N.; Howlett, P.C.; Atkin, R.; Forsyth, M. Engineering High-Energy-Density Sodium Battery Anodes for Improved Cycling with Superconcentrated Ionic-Liquid Electrolytes. Nat. Mater. 2020, 19, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Pastel, G.; Kuang, Y.; Xie, H.; Li, Y.; Liu, B.; Luo, W.; Chen, C.; Hu, L. 3D Wettable Framework for Dendrite-Free Alkali Metal Anodes. Adv. Energy Mater. 2018, 8, 1800635. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, R.; Dong, S.; Miao, X.; Zhang, Z.; Wang, C.; Yin, L. Alkali-Induced 3D Crinkled Porous Ti3C2 MXene Architectures Coupled with NiCoP Bimetallic Phosphide Nanoparticles as Anodes for High-Performance Sodium-Ion Batteries. Energy Environ. Sci. 2019, 12, 2422–2432. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, F.; Wang, Y.; Yao, Y.; Shao, Y.; Rui, X.; Wu, F.; Yu, Y. Heterogeneous Interfacial Layers Derived from the In Situ Reaction of CoF2 Nanoparticles with Sodium Metal for Dendrite-Free Na Metal Anodes. Adv. Energy Mater. 2022, 12, 2202323. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Ling, F.; Lu, G.; Huang, F.; Tao, X.; Wu, S.; Cheng, X.; Liu, F.; Li, D.; et al. Artificial Heterogeneous Interphase Layer with Boosted Ion Affinity and Diffusion for Na/K-Metal Batteries. Adv. Mater. 2022, 34, 2109439. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Pollard, T.P.; Fan, X.; Borodin, O.; Wang, C. Electrolyte Design for Li Metal-Free Li Batteries. Mater. Today 2020, 39, 118–126. [Google Scholar] [CrossRef]
- Kim, K.; Ma, H.; Park, S.; Choi, N.-S. Electrolyte-Additive-Driven Interfacial Engineering for High-Capacity Electrodes in Lithium-Ion Batteries: Promise and Challenges. ACS Energy Lett. 2020, 1537–1553. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, S.; Liu, B.; Wang, D.; Zhong, Y.; Zhang, X.; Wang, X.; Xia, X.; Gu, C.; Tu, J. Bi-Containing Electrolyte Enables Robust and Li Ion Conductive Solid Electrolyte Interphase for Advanced Lithium Metal Anodes. Front. Chem. 2019, 7, 952. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, Y.; Wei, X.; Song, L.; Tao, G.; Cao, X.; Wang, D.; Zhou, G.; Song, Y. Dual-Functional V2C MXene Assembly in Facilitating Sulfur Evolution Kinetics and Li-Ion Sieving toward Practical Lithium–Sulfur Batteries. Adv. Mater. 2023, 35, 2300771. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shaibani, M.; Gamot, T.D.; Wang, M.; Jovanović, P.; Dilusha Cooray, M.C.; Mirshekarloo, M.S.; Mulder, R.J.; Medhekar, N.V.; Hill, M.R.; et al. A Saccharide-Based Binder for Efficient Polysulfide Regulations in Li-S Batteries. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, W.-Q. A Review of Heteroatom Doped Materials for Advanced Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2107166. [Google Scholar] [CrossRef]
- Fan, K.; Huang, H. Two-Dimensional Host Materials for Lithium-Sulfur Batteries: A Review and Perspective. Energy Storage Mater. 2022, 50, 696–717. [Google Scholar] [CrossRef]
- Lei, K.; Wang, C.; Liu, L.; Luo, Y.; Mu, C.; Li, F.; Chen, J. A Porous Network of Bismuth Used as the Anode Material for High-Energy-Density Potassium-Ion Batteries. Angew. Chem. Int. Ed. 2018, 57, 1–6. [Google Scholar] [CrossRef]
- Feng, Y.; Rao, A.M.; Zhou, J.; Lu, B. Selective Potassium Deposition Enables Dendrite-Resistant Anodes for Ultrastable Potassium-Metal Batteries. Adv. Mater. 2023, 35, 2300886. [Google Scholar] [CrossRef]
- Cohn, A.P.; Metke, T.; Donohue, J.; Muralidharan, N.; Share, K.; Pint, C.L. Rethinking Sodium-Ion Anodes as Nucleation Layers for Anode-Free Batteries. J. Mater. Chem. A 2018, 6, 23875–23884. [Google Scholar] [CrossRef]
- Xu, H.; Yang, S.; Li, B. Ultrathin Bismuth Nanosheets as an Efficient Polysulfide Catalyst for High Performance Lithium–Sulfur Batteries. J. Mater. Chem. A 2019, 8, 149–157. [Google Scholar] [CrossRef]
- Ellis, L.D.; Wilkes, B.N.; Hatchard, T.D.; Obrovac, M.N. In Situ XRD Study of Silicon, Lead and Bismuth Negative Electrodes in Nonaqueous Sodium Cells. J. Electrochem. Soc. 2014, 161, A416. [Google Scholar] [CrossRef]
- Hofmann, P. The Surfaces of Bismuth: Structural and Electronic Properties. Prog. Surf. Sci. 2006, 81, 191–245. [Google Scholar] [CrossRef]
- Pavlyuk, V.; Sozanskyi, M.; Dmytriv, G.; Indris, S.; Ehrenberg, H. Amendment of the Li-Bi Phase Diagram Crystal and Electronic Structure of Li2Bi. J. Phase Equilib. Diffus. 2015, 36, 544–553. [Google Scholar] [CrossRef]
- Gabaudan, V.; Berthelot, R.; Stievano, L.; Monconduit, L. Inside the Alloy Mechanism of Sb and Bi Electrodes for K-Ion Batteries. J. Phys. Chem. C 2018, 122, 18266–18273. [Google Scholar] [CrossRef]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Bi-Mg (Bismuth-Magnesium) System. Bull. Alloy Phase Diagr. 1985, 6, 528–533. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wu, G.; Zhao, J. Facile and Efficient Synthesis of Bismuth Nanowires for Improved Photocatalytic Activity. Inorg. Chem. 2016, 55, 4897–4905. [Google Scholar] [CrossRef]
- Ko, J.K.; Halajko, A.; Parkinson, M.F.; Amatucci, G.G. Electronic Transport in Lithiated Iron and Bismuth Fluoride. J. Electrochem. Soc. 2014, 162, A149. [Google Scholar] [CrossRef]
- An, Y.; Tian, Y.; Wei, C.; Tao, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Dealloying: An Effective Method for Scalable Fabrication of 0D, 1D, 2D, 3D Materials and Its Application in Energy Storage. Nano Today 2021, 37, 101094. [Google Scholar] [CrossRef]
- Zhu, G.-L.; Zhao, C.-Z.; Huang, J.-Q.; He, C.; Zhang, J.; Chen, S.; Xu, L.; Yuan, H.; Zhang, Q. Fast Charging Lithium Batteries: Recent Progress and Future Prospects. Small 2019, 15, 1805389. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yuan, N.; Ding, J.; Qin, S.; Razal, J.M.; Wang, X.; Ge, S.; Gogotsi, Y. Extending the Low Temperature Operational Limit of Li-Ion Battery to −80 °C. Energy Storage Mater. 2019, 23, 383–389. [Google Scholar] [CrossRef]
- Yuan, T.; Tan, Z.; Ma, C.; Yang, J.; Ma, Z.-F.; Zheng, S. Challenges of Spinel Li4Ti5O12 for Lithium-Ion Battery Industrial Applications. Adv. Energy Mater. 2017, 7, 1601625. [Google Scholar] [CrossRef]
- Pan, S.; Han, J.; Wang, Y.; Li, Z.; Chen, F.; Guo, Y.; Han, Z.; Xiao, K.; Yu, Z.; Yu, M.; et al. Integrating SEI into Layered Conductive Polymer Coatings for Ultrastable Silicon Anodes. Adv. Mater. 2022, 34, 2203617. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Zhang, Z.; Wang, H.; Cao, Y.; Zhang, B.; Liu, X.; Mao, C.; Han, X.; Gong, H.; et al. Bi Works as a Li Reservoir for Promoting the Fast-Charging Performance of Phosphorus Anode for Li-Ion Batteries. Adv. Energy Mater. 2022, 12, 2103888. [Google Scholar] [CrossRef]
- Weppner, W.; Huggins, R.A. Electrochemical Investigation of the Chemical Diffusion, Partial Ionic Conductivities, and Other Kinetic Parameters in Li3Sb and Li3Bi. J. Solid State Chem. 1977, 22, 297–308. [Google Scholar] [CrossRef]
- Chai, W.; Yin, W.; Wang, K.; Ye, W.; Tang, B.; Rui, Y. Carbon-Coated Bismuth Nanospheres Derived from Bi-BTC as a Promising Anode Material for Lithium Storage. Electrochim. Acta 2019, 325, 134927. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, S.; Luo, W.; Wang, C.; Fan, X.; Zhou, N.; Wan, R.; Fang, D. Enhanced Electrochemical Properties of Bi Nanowires as Anode Materials in Lithium and Sodium Batteries. Curr. Nanosci. 2017, 13, 342–348. [Google Scholar] [CrossRef]
- Ramirez, G.; Halajko, A.; Amatucci, G.G. In Situ Derived Bi Alloys for High-Performance Li-Ion Batteries: Effect of Conversion Chemistry, Mesomatrix, and Electrolyte. J. Electrochem. Soc. 2022, 169, 080514. [Google Scholar] [CrossRef]
- Yang, F.; Yu, F.; Zhang, Z.; Zhang, K.; Lai, Y.; Li, J. Bismuth Nanoparticles Embedded in Carbon Spheres as Anode Materials for Sodium/Lithium-Ion Batteries. Chem. Eur. J. 2016, 22, 2333–2338. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Z.; Zhang, D.; Zuo, S.; Liu, J.; Zhu, M. Scalable One-Pot Synthesis of Hierarchical Bi@C Bulk with Superior Lithium-Ion Storage Performances. ACS Appl. Mater. Interfaces 2020, 12, 51478–51487. [Google Scholar] [CrossRef]
- Huang, Z.-D.; Lu, H.; Qian, K.; Fang, Y.-W.; Du, Q.-C.; He, Y.-B.; Masese, T.; Yang, X.-S.; Ma, Y.-W.; Huang, W. Interfacial Engineering Enables Bi@C-TiOx Microspheres as Superpower and Long Life Anode for Lithium-Ion Batteries. Nano Energy 2018, 51, 137–145. [Google Scholar] [CrossRef]
- Hong, W.; Wang, A.; Li, L.; Qiu, T.; Li, J.; Jiang, Y.; Zou, G.; Peng, H.; Hou, H.; Ji, X. Bi Dots Confined by Functional Carbon as High-Performance Anode for Lithium Ion Batteries. Adv. Funct. Mater. 2021, 31, 2000756. [Google Scholar] [CrossRef]
- Devina, W.; Setiadi Cahyadi, H.; Albertina, I.; Chandra, C.; Park, J.-H.; Yoon Chung, K.; Chang, W.; Kyu Kwak, S.; Kim, J. High-Energy-Density Carbon-Coated Bismuth Nanodots on Hierarchically Porous Molybdenum Carbide for Superior Lithium Storage. Chem. Eng. J. 2022, 432, 134276. [Google Scholar] [CrossRef]
- Sadan, M.K.; Song, E.; Yu, H.; Yun, J.; Kim, T.; Ahn, J.-H.; Cho, K.-K.; Ahn, H.-J. Extended Cycling Performance of Micron-Sized Bismuth Anodes for Lithium-Ion Batteries: Self-Healing of an Alloy-Type Anode for Lithium Batteries. J. Mater. Chem. A 2023, 11, 15466–15474. [Google Scholar] [CrossRef]
- Yin, H.; Li, Q.; Cao, M.; Zhang, W.; Zhao, H.; Li, C.; Huo, K.; Zhu, M. Nanosized-Bismuth-Embedded 1D Carbon Nanofibers as High-Performance Anodes for Lithium-Ion and Sodium-Ion Batteries. Nano Res. 2017, 10, 2156–2167. [Google Scholar] [CrossRef]
- Hong, W.; Ge, P.; Jiang, Y.; Yang, L.; Tian, Y.; Zou, G.; Cao, X.; Hou, H.; Ji, X. Yolk-Shell-Structured Bismuth@N-Doped Carbon Anode for Lithium-Ion Battery with High Volumetric Capacity. ACS Appl. Mater. Interfaces 2019, 11, 10829–10840. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, J.; Tang, Y.; Guo, J.; Lu, Z.; Liu, B.; Zhang, H.; Cao, Y. Bi@C Sandwiched Carbon Nanolayers Enables Remarkable Cyclability at High Current Density for Lithium-Ion Batteries. Appl. Surf. Sci. 2023, 613, 155996. [Google Scholar] [CrossRef]
- Yuan, Y.; Yao, W.; Yurkiv, V.; Liu, T.; Song, B.; Mashayek, F.; Shahbazian-Yassar, R.; Lu, J. Beyond Volume Variation: Anisotropic and Protrusive Lithiation in Bismuth Nanowire. ACS Nano 2020, 14, 15669–15677. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, J.; Wang, X.; Yue, L.; Wang, W.; Wang, B.; Shen, D.; Li, Y. Boosting Potassium Storage Kinetics, Stability, and Volumetric Performance of Honeycomb-Like Porous Red Phosphorus via In Situ Embedding Self-Growing Conductive Nano-Metal Networks. Adv. Funct. Mater. 2023, 33, 2209388. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, Z.; Yang, L.; Liang, G.; Zhang, R.; Cheng, Y.; Liu, J.; Zhang, J.; Che, R. Defect and Interface Engineered Tungsten Bronze Superstructure Anode toward Advanced Sodium Storage. Adv. Funct. Mater. 2023, 33, 2305342. [Google Scholar] [CrossRef]
- Wu, X.; Lan, X.; Hu, R.; Yao, Y.; Yu, Y.; Zhu, M. Tin-Based Anode Materials for Stable Sodium Storage: Progress and Perspective. Adv. Mater. 2022, 34, 2106895. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Liu, C.; Mi, L.; Chou, S.; Chen, W.; Shen, C. Recent Progress on the Alloy-Based Anode for Sodium-Ion Batteries and Potassium-Ion Batteries. Small 2021, 17, 1903194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Manthiram, A. High-Capacity, High-Rate Bi-Sb Alloy Anodes for Lithium-Ion and Sodium-Ion Batteries. Chem. Mater. 2015, 27, 3096–3101. [Google Scholar] [CrossRef]
- Liu, S.; Feng, J.; Bian, X.; Liu, J.; Xu, H. Advanced Arrayed Bismuth Nanorod Bundle Anode for Sodium-Ion Batteries. J. Mater. Chem. A 2016, 4, 10098–10104. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, Z.; Song, T. Bi@C Nanoplates Derived from (BiO)2CO3 as an Enhanced Electrode Material for Lithium/Sodium-Ion Batteries. ChemistrySelect 2018, 3, 8973–8979. [Google Scholar] [CrossRef]
- Xiong, P.; Bai, P.; Li, A.; Li, B.; Cheng, M.; Chen, Y.; Huang, S.; Jiang, Q.; Bu, X.H.; Xu, Y. Bismuth Nanoparticle@Carbon Composite Anodes for Ultralong Cycle Life and High-Rate Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1904771. [Google Scholar] [CrossRef]
- Yang, H.; Chen, L.-W.; He, F.; Zhang, J.; Feng, Y.; Zhao, L.; Wang, B.; He, L.; Zhang, Q.; Yu, Y. Optimizing the Void Size of Yolk-Shell Bi@Void@C Nanospheres for High-Power-Density Sodium-Ion Batteries. Nano Lett. 2020, 20, 758–767. [Google Scholar] [CrossRef]
- Xue, P.; Wang, N.; Fang, Z.; Lu, Z.; Xu, X.; Wang, L.; Du, Y.; Ren, X.; Bai, Z.; Dou, S.; et al. Rayleigh-Instability-Induced Bismuth Nanorod@Nitrogen-Doped Carbon Nanotubes as A Long Cycling and High Rate Anode for Sodium-Ion Batteries. Nano Lett. 2019, 19, 1998–2004. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, X.; He, Y.; Liang, J.; Liang, K.; Tardy, B.L.; Richardson, J.J.; Hu, M.; Wu, H.; Zhang, Y.; et al. Superstructured Mesocrystals through Multiple Inherent Molecular Interactions for Highly Reversible Sodium Ion Batteries. Sci. Adv. 2021, 7, eabh3482. [Google Scholar] [CrossRef]
- Cheng, X.; Shao, R.; Li, D.; Yang, H.; Wu, Y.; Wang, B.; Sun, C.; Jiang, Y.; Zhang, Q.; Yu, Y. A Self-Healing Volume Variation Three-Dimensional Continuous Bulk Porous Bismuth for Ultrafast Sodium Storage. Adv. Funct. Mater. 2021, 31, 2011264. [Google Scholar] [CrossRef]
- Guo, S.; Wei, C.; Wang, L.; Mei, S.; Xiang, B.; Zheng, Y.; Zhang, X.; Javanbakht, M.; Gao, B.; Chu, P.K.; et al. Micro-Sized Porous Bulk Bismuth Caged by Carbon for Fast Charging and Ultralong Cycling in Sodium-Ion Batteries. Cell Rep. Phys. Sci. 2023, 4, 101463. [Google Scholar] [CrossRef]
- Wang, C.; Du, D.; Song, M.; Wang, Y.; Li, F. A High-Power Na3V2(PO4)3-Bi Sodium-Ion Full Battery in a Wide Temperature Range. Adv. Energy Mater. 2019, 9, 1900022. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, F.; Peng, L.; Qin, T.; Kang, F.; Yang, C. Inlaying Bismuth Nanoparticles on Graphene Nanosheets by Chemical Bond for Ultralong-Lifespan Aqueous Sodium Storage. Angew. Chem. Int. Ed. 2023, 62, 202212439. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yuan, H.; Lan, J.-L.; Yu, Y.; Lin, Y.-H.; Yang, X. Bio-Inspired Spider-Web-like Membranes with a Hierarchical Structure for High Performance Lithium/Sodium Ion Battery Electrodes: The Case of 3D Freestanding and Binder-Free Bismuth/CNF Anodes. Nanoscale 2017, 9, 13298–13304. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, Z.; Guo, J.; Pan, A.; Cai, Z.; Liang, S. Bismuth Nanosheets Grown on Carbon Fiber Cloth as Advanced Binder-Free Anode for Sodium-Ion Batteries. Electrochem. Commun. 2017, 81, 10–13. [Google Scholar] [CrossRef]
- Qiu, J.; Li, S.; Su, X.; Wang, Y.; Xu, L.; Yuan, S.; Li, H.; Zhang, S. Bismuth Nano-Spheres Encapsulated in Porous Carbon Network for Robust and Fast Sodium Storage. Chem. Eng. J. 2017, 320, 300–307. [Google Scholar] [CrossRef]
- Zhang, R.; Bao, J.; Wang, Y.; Sun, C.-F. Concentrated Electrolytes Stabilize Bismuth-Potassium Batteries. Chem. Sci. 2018, 9, 6193–6198. [Google Scholar] [CrossRef]
- Shen, C.; Cheng, T.L.; Liu, C.Y.; Huang, L.; Cao, M.Y.; Song, G.Q.; Wang, D.; Lu, B.A.; Wang, J.W.; Qin, C.C.; et al. Bismuthene from Sonoelectrochemistry as a Superior Anode for Potassium-Ion Batteries. J. Mater. Chem. A 2020, 8, 453–460. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, Y.; Li, D.; Yang, H.; Chen, F.; Huang, F.; Jiang, Y.; Wu, Y.; An, X.; Yu, Y. From 0D to 3D: Dimensional Control of Bismuth for Potassium Storage with Superb Kinetics and Cycling Stability. Adv. Energy Mater. 2021, 11, 2102263. [Google Scholar] [CrossRef]
- Liu, M.; Xing, Y.; Wang, J.; Wang, D.; Huang, L.; Wu, X.; Liu, Z.; Wu, Y. Besides the Capacitive and Diffusion Control: Inner-Surface Controlled Bismuth Based Electrode Facilitating Potassium-Ion Energy Storage. Adv. Funct. Mater. 2021, 31, 2101868. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, H.; Wei, C.; Huang, F.; Yao, Y.; Bai, R.; Jiang, Y.; Li, S. Synergistic Effect of 1D Bismuth Nanowires/2D Graphene Composites for High Performance Flexible Anodes in Sodium-Ion Batteries. J. Mater. Chem. A 2023, 11, 8081–8090. [Google Scholar] [CrossRef]
- Sottmann, J.; Herrmann, M.; Vajeeston, P.; Hu, Y.; Ruud, A.; Drathen, C.; Emerich, H.; Fjellvaag, H.; Wragg, D.S. How Crystallite Size Controls the Reaction Path in Nonaqueous Metal Ion Batteries: The Example of Sodium Bismuth Alloying. Chem. Mater. 2016, 28, 2750–2756. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Zhang, S.; Hu, X.; Zhang, K.; Zhou, W.; Guo, S.; Xu, F.; Zeng, H. Ultrathin Bismuth Nanosheets for Stable Na-Ion Batteries: Clarification of Structure and Phase Transition by in Situ Observation. Nano Lett. 2019, 19, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, J.; Chen, M.; Wang, J.; Liu, X.; Wei, B.; Wang, Z.; Li, J.; Gu, L.; Zhang, Q.; et al. Few-Layer Bismuthene with Anisotropic Expansion for High-Areal-Capacity Sodium-Ion Batteries. Adv. Mater. 2019, 31, 1807874. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Wu, S.; Cheng, J.; Chen, D.; Shen, D.; Wang, H.; Tong, Z.; Li, H.; Liu, B.; Kai, J.-J.; et al. Bismuth Nanorod Networks Confined in a Robust Carbon Matrix as Long-Cycling and High-Rate Potassium-Ion Battery Anodes. J. Mater. Chem. A 2020, 8, 8440–8446. [Google Scholar] [CrossRef]
- Cui, R.C.; Zhou, H.Y.; Li, J.C.; Yang, C.C.; Jiang, Q. Ball-Cactus-Like Bi Embedded in N-Riched Carbon Nanonetworks Enables the Best Potassium Storage Performance. Adv. Funct. Mater. 2021, 31, 2103067. [Google Scholar] [CrossRef]
- Qin, T.; Chu, X.; Deng, T.; Wang, B.; Zhang, X.; Dong, T.; Li, Z.; Fan, X.; Ge, X.; Wang, Z.; et al. Reinventing the Mechanism of High-Performance Bi Anode in Aqueous K+ Rechargeable Batteries. J. Energy Chem. 2020, 48, 21–28. [Google Scholar] [CrossRef]
- Guo, M.; Yuan, C.; Zhang, T.; Yu, X. Solid-State Electrolytes for Rechargeable Magnesium-Ion Batteries: From Structure to Mechanism. Small 2022, 18, 2106981. [Google Scholar] [CrossRef]
- Asif, M.; Kilian, S.; Rashad, M. Uncovering Electrochemistries of Rechargeable Magnesium-Ion Batteries at Low and High Temperatures. Energy Storage Mater. 2021, 42, 129–144. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Lv, R.; Wang, D.; Liu, P.; Luo, J. Rechargeable Mg Metal Batteries Enabled by a Protection Layer Formed in Vivo. Energy Storage Mater. 2020, 26, 408–413. [Google Scholar] [CrossRef]
- Park, H.; Lim, H.-K.; Oh, S.H.; Park, J.; Lim, H.-D.; Kang, K. Tailoring Ion-Conducting Interphases on Magnesium Metals for High-Efficiency Rechargeable Magnesium Metal Batteries. ACS Energy Lett. 2020, 5, 3733–3740. [Google Scholar] [CrossRef]
- Shao, Y.; Gu, M.; Li, X.; Nie, Z.; Zuo, P.; Li, G.; Liu, T.; Xiao, J.; Cheng, Y.; Wang, C.; et al. Highly Reversible Mg Insertion in Nanostructured Bi for Mg Ion Batteries. Nano Lett. 2014, 14, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Cen, Y.; Dong, J.; Zhu, T.; Cai, X.; Wang, X.; Hu, B.; Xu, C.; Yu, D.; Liu, Y.; Chen, C. Bi Nanorods Anchored in N-Doped Carbon Shell as Anode for High-Performance Magnesium Ion Batteries. Electrochim. Acta 2021, 397, 139260. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, J.; Wang, X.; Li, Y.; Xia, W.; Liu, Q.; Hu, J.; Wei, T.; Ling, Y.; Liu, B.; et al. In-Situ Synthesis of Bi Nanospheres Anchored in 3D Interconnected Cellulose Nanocrystal Derived Carbon Aerogel as Anode for High-Performance Mg-Ion Batteries. Chem. Eng. J. 2023, 451, 138824. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, Y.; Xu, H.; Zhao, X. Bismuth Nanoparticle-Embedded Carbon Microrod for High-Rate Electrochemical Magnesium Storage. ACS Appl. Mater. Interfaces 2023, 15, 23353–23360. [Google Scholar] [CrossRef] [PubMed]
- Penki, T.R.; Valurouthu, G.; Shivakumara, S.; Sethuraman, V.A.; Munichandraiah, N. In Situ Synthesis of Bismuth (Bi)/Reduced Graphene Oxide (RGO) Nanocomposites as High-Capacity Anode Materials for a Mg-Ion Battery. New J. Chem. 2018, 42, 5996–6004. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Yang, F.; Zeng, Y.; Liu, X.; Lu, X. Bismuth Nanoparticles@carbon Composite as a Stable and High Capacity Anode for High-Voltage Bismuth-Manganese Batteries. Energy Storage Mater. 2021, 41, 623–630. [Google Scholar] [CrossRef]
- Kravchyk, K.V.; Piveteau, L.; Caputo, R.; He, M.; Stadie, N.P.; Bodnarchuk, M.I.; Lechner, R.T.; Kovalenko, M.V. Colloidal Bismuth Nanocrystals as a Model Anode Material for Rechargeable Mg-Ion Batteries: Atomistic and Mesoscale Insights. ACS Nano 2018, 12, 8297–8307. [Google Scholar] [CrossRef]
- Xu, X.; Chao, D.; Chen, B.; Liang, P.; Li, H.; Xie, F.; Davey, K.; Qiao, S.-Z. Revealing the Magnesium-Storage Mechanism in Mesoporous Bismuth via Spectroscopy and Ab-Initio Simulations. Angew. Chem. Int. Ed. 2020, 59, 21728–21735. [Google Scholar] [CrossRef]
- Feng, X.; Fang, H.; Wu, N.; Liu, P.; Jena, P.; Nanda, J.; Mitlin, D. Review of Modification Strategies in Emerging Inorganic Solid-State Electrolytes for Lithium, Sodium, and Potassium Batteries. Joule 2022, 6, 543–587. [Google Scholar] [CrossRef]
- Teng, W.; Wu, J.; Liang, Q.; Deng, J.; Xu, Y.; Liu, Q.; Wang, B.; Ma, T.; Nan, D.; Liu, J.; et al. Designing Advanced Liquid Electrolytes for Alkali Metal Batteries: Principles, Progress, and Perspectives. Energy Environ. Mater. 2023, 6, e12355. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.; Ren, L.; Guan, X.; Wang, D.; Dong, A.; Zhang, C.; Li, G.; Luo, J. Controlling Li Ion Flux through Materials Innovation for Dendrite-Free Lithium Metal Anodes. Adv. Funct. Mater. 2019, 29, 1905940. [Google Scholar] [CrossRef]
- Chen, X.-R.; Zhao, B.-C.; Yan, C.; Zhang, Q. Review on Li Deposition in Working Batteries: From Nucleation to Early Growth. Adv. Mater. 2021, 33, 2004128. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Mitlin, D. Tutorial Review on Structure—Dendrite Growth Relations in Metal Battery Anode Supports. Chem. Soc. Rev. 2020, 49, 7284–7300. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liu, F.; Feng, Y.; Zhou, J.; Rui, X.; Yu, Y. Lithium Metal Batteries: The Synergetic Effect of Lithium Bisoxalatodifluorophosphate and Fluoroethylene Carbonate on Dendrite Suppression for Fast Charging Lithium Metal Batteries (Small 30/2020). Small 2020, 16, 2070164. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, S.; Lu, G.; Wang, L.; Jiang, Y.; Liu, F.; Yao, Y.; Yang, H.; Ma, M.; Ye, S.; et al. Red Phosphorous-Derived Protective Layers with High Ionic Conductivity and Mechanical Strength on Dendrite-Free Sodium and Potassium Metal Anodes. Adv. Energy Mater. 2021, 11, 2003381. [Google Scholar] [CrossRef]
- Cheng, X.; Li, D.; Peng, S.; Shi, P.; Yu, H.; Jiang, Y.; Li, S. In-Situ Alloy-Modified Sodiophilic Current Collectors for Anode-Less Sodium Metal Batteries. Batteries 2023, 9, 408. [Google Scholar] [CrossRef]
- Li, D.; Sun, Y.; Li, M.; Cheng, X.; Yao, Y.; Huang, F.; Jiao, S.; Gu, M.; Rui, X.; Ali, Z.; et al. Rational Design of an Artificial SEI: Alloy/Solid Electrolyte Hybrid Layer for a Highly Reversible Na and K Metal Anode. ACS Nano 2022, 16, 16966–16975. [Google Scholar] [CrossRef]
- Huang, G.; Guo, P.; Wang, J.; Chen, S.; Liang, J.; Tao, R.; Tang, S.; Zhang, X.; Cheng, S.; Cao, Y.-C.; et al. Lithiophilic V2O5 Nanobelt Arrays Decorated 3D Framework Hosts for Highly Stable Composite Lithium Metal Anodes. Chem. Eng. J. 2020, 384, 123313. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Wang, C.; Dou, H.; Zhang, X. Targeted Deposition in a Lithiophilic Silver-Modified 3D Cu Host for Lithium-Metal Anodes. Energy Environ. Mater. 2023, 6, e12412. [Google Scholar] [CrossRef]
- Liu, X.; Xu, P.; Zhang, J.; Hu, X.; Hou, Q.; Lin, X.; Zheng, M.; Dong, Q. A Highly Reversible Lithium Metal Anode by Constructing Lithiophilic Bi-Nanosheets. Small 2021, 17, 2102016. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, H.; Yang, H.; Yu, Y.; Luo, J.; Li, T.; Li, W.; Zhang, Y.-N.; Kang, Y. Thermodynamic Regulation of Dendrite-Free Li Plating on Li3Bi for Stable Lithium Metal Batteries. Nano Lett. 2021, 21, 8664–8670. [Google Scholar] [CrossRef] [PubMed]
- An, M.; Wang, H.; Cheng, W.; Li, G.; Liu, S.; Gao, X. Li-Bi and Li-In Alloys-Based Composite Anode for Lithium Metal Batteries. J. Alloys Compd. 2023, 966, 171619. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wang, H.-M.; Liu, X.; Zhou, C.; Li, G.-R.; Liu, S.; Gao, X.-P. A Dimensionally Stable Lithium Alloy Based Composite Electrode for Lithium Metal Batteries. Chem. Eng. J. 2022, 450, 138074. [Google Scholar] [CrossRef]
- Xu, J.; Xie, Y.; Zheng, J.; Liu, C.; Lai, Y.; Zhang, Z. A Sodiophilic Carbon Cloth Decorated with Bi-MOF Derived Porous Bi@C Nanosheets for Stable Na Metal Anode. J. Electroanal. Chem. 2021, 903, 115853. [Google Scholar] [CrossRef]
- Yang, G.; Li, N.; Sun, C. High-Performance Sodium Metal Batteries with Sodium–Bismuth Alloy Anode. ACS Appl. Energy Mater. 2020, 3, 12607–12612. [Google Scholar] [CrossRef]
- Ye, S.; Wang, L.; Liu, F.; Shi, P.; Yu, Y. Integration of Homogeneous and Heterogeneous Nucleation Growth via 3D Alloy Framework for Stable Na/K Metal Anode. eScience 2021, 1, 75–82. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Y.; Cui, Z.; Tao, Y.; Li, B.; Wang, W. Recent Progress of Functional Separators in Dendrite Inhibition for Lithium Metal Batteries. Energy Storage Mater. 2021, 35, 157–168. [Google Scholar] [CrossRef]
- Sun, X.; Yang, S.; Zhang, T.; Shi, Y.; Dong, L.; Ai, G.; Li, D.; Mao, W. Regulating Li-Ion Flux with a High-Dielectric Hybrid Artificial SEI for Stable Li Metal Anodes. Nanoscale 2022, 14, 5033–5043. [Google Scholar] [CrossRef]
- Li, F.; He, J.; Liu, J.; Wu, M.; Hou, Y.; Wang, H.; Qi, S.; Liu, Q.; Hu, J.; Ma, J. Gradient Solid Electrolyte Interphase and Lithium-Ion Solvation Regulated by Bisfluoroacetamide for Stable Lithium Metal Batteries. Angew. Chem. Int. Ed. 2021, 60, 6600–6608. [Google Scholar] [CrossRef]
- Qi, S.; He, J.; Liu, J.; Wang, H.; Wu, M.; Li, F.; Wu, D.; Li, X.; Ma, J. Phosphonium Bromides Regulating Solid Electrolyte Interphase Components and Optimizing Solvation Sheath Structure for Suppressing Lithium Dendrite Growth. Adv. Funct. Mater. 2021, 31, 2009013. [Google Scholar] [CrossRef]
- Shi, Y.; Wan, J.; Liu, G.-X.; Zuo, T.-T.; Song, Y.-X.; Liu, B.; Guo, Y.-G.; Wen, R.; Wan, L.-J. Interfacial Evolution of Lithium Dendrites and Their Solid Electrolyte Interphase Shells of Quasi-Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 18120–18125. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xi, Q.; Li, Y.; Fu, H.; Hua, Y.; Shankar, E.G.; Kakarla, A.K.; Yu, J.S. Regulating Dendrite-Free Zinc Deposition by Red Phosphorous-Derived Artificial Protective Layer for Zinc Metal Batteries. Adv. Sci. 2022, 9, 2200155. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Meng, F.; Zhang, Z.; Liang, J.; Hu, Y.; Kong, W.; Zhang, X.L.; Jin, Z. Stabilizing Lithium Metal Anode by Molecular Beam Epitaxy Grown Uniform and Ultrathin Bismuth Film. Nano Energy 2020, 76, 105068. [Google Scholar] [CrossRef]
- Shao, Y.; Qin, Y.; Song, Y.; Xu, K.; Wang, H.; Shen, C.; Chen, R.; Lyu, Y.; Liu, Y.; Guo, B. Bismuth-Contained Lithiophilic Protective Layer on Lithium Metal Anode In Situ Generated via Electrochemical Method. ACS Sustain. Chem. Eng. 2022, 10, 11493–11500. [Google Scholar] [CrossRef]
- Tu, H.; Li, S.; Luo, Z.; Xu, L.; Zhang, H.; Xiang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Bi-Doped Carbon Dots for a Stable Lithium Metal Anode. Chem. Commun. 2022, 58, 6449–6452. [Google Scholar] [CrossRef]
- Hu, F.; Li, Y.; Wei, Y.; Yang, J.; Hu, P.; Rao, Z.; Chen, X.; Yuan, L.; Li, Z. Construct an Ultrathin Bismuth Buffer for Stable Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2020, 12, 12793–12800. [Google Scholar] [CrossRef]
- Ma, M.; Lu, Y.; Yan, Z.; Chen, J. In Situ Synthesis of a Bismuth Layer on a Sodium Metal Anode for Fast Interfacial Transport in Sodium-Oxygen Batteries. Batter. Supercaps 2019, 2, 663–667. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Wang, W.; Li, B. Stabilizing Sodium Metal Anode through Facile Construction of Organic-Metal Interface. J. Energy Chem. 2022, 66, 133–139. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Wang, G.; Xu, G.; Wu, M.; Liu, H.-K.; Dou, S.-X.; Wu, C. Bi Nanoparticles Embedded in 2D Carbon Nanosheets as an Interfacial Layer for Advanced Sodium Metal Anodes. Small 2021, 17, 2007578. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, A.; Dong, S.; Jiang, F.; Guo, Z.; Ge, X.; Qu, X.; Zhou, X.; Cui, G. A Bismuth-Based Protective Layer for Magnesium Metal Anode in Noncorrosive Electrolytes. ACS Energy Lett. 2021, 6, 2594–2601. [Google Scholar] [CrossRef]
- Zhou, L.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Host Materials Anchoring Polysulfides in Li–S Batteries Reviewed. Adv. Energy Mater. 2021, 11, 2001304. [Google Scholar] [CrossRef]
- Lee, B.-J.; Kang, T.-H.; Lee, H.-Y.; Samdani, J.S.; Jung, Y.; Zhang, C.; Yu, Z.; Xu, G.-L.; Cheng, L.; Byun, S.; et al. Revisiting the Role of Conductivity and Polarity of Host Materials for Long-Life Lithium–Sulfur Battery. Adv. Energy Mater. 2020, 10, 1903934. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Luo, D.; Zhang, Y.; Zhao, Y.; Zhou, G.; Shui, L.; Wang, X.; Chen, Z. Engineering the Conductive Network of Metal Oxide-Based Sulfur Cathode toward Efficient and Longevous Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2002076. [Google Scholar] [CrossRef]
- Zheng, Y.; Yi, Y.; Fan, M.; Liu, H.; Li, X.; Zhang, R.; Li, M.; Qiao, Z.-A. A High-Entropy Metal Oxide as Chemical Anchor of Polysulfide for Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 23, 678–683. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Wu, D.; Wei, H.; Guo, Y.; Chen, H.; Li, Z.; Wang, L.; Xiong, C.; Meng, Q.; et al. High-Density Oxygen Doping of Conductive Metal Sulfides for Better Polysulfide Trapping and Li2S-S8 Redox Kinetics in High Areal Capacity Lithium–Sulfur Batteries. Adv. Sci. 2022, 9, 2200840. [Google Scholar] [CrossRef]
- Ma, L.; Qian, J.; Li, Y.; Cheng, Y.; Wang, S.; Wang, Z.; Peng, C.; Wu, K.; Xu, J.; Manke, I.; et al. Binary Metal Single Atom Electrocatalysts with Synergistic Catalytic Activity toward High-Rate and High Areal-Capacity Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2208666. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, J.; Yuan, M.; Dai, K.; Zhang, J.; Li, S.; He, H.; Liu, Z.; Zhang, G. Local Charge Rearrangement to Boost the Chemical Adsorption and Catalytic Conversion of Polysulfides for High-Performance Lithium-Sulfur Batteries. J. Mater. Chem. A 2021, 9, 7566–7574. [Google Scholar] [CrossRef]

| Application | Products | Mass Specific Capacity (mAh g−1) | Volumetric Specific Capacity (mAh cm−3) | Operation Voltage (V) | Volume Expansion Ratio (%) |
|---|---|---|---|---|---|
| LIBs | Li3Bi | 385 | 3800 | 0.8/0.7 | 208 |
| SIBs | Na3Bi | 385 | 3800 | 0.77/0.67 | 352 |
| PIBs | K3Bi | 385 | 3800 | 1.0/0.4/0.3 | 509 |
| MIBs | Mg3Bi2 | 385 | 3800 | 0.25 | 196 |
| Materials | Synthesis Methods | Electrolytes | ICE (%) | Cycling Performance (a) | Rate Capability (b) | Refs. |
|---|---|---|---|---|---|---|
| Bi@C nanowires | pyrolysis | 1 M LiPF6 in EC/DEC/DMC | 63.1 | 408/100th/0.1 | 240 (58.8)/1 | [27] |
| Bi@C microspheres | aerosol spray pyrolysis | 1 M LiPF6 in EC/DEC/DMC | 36.6 | 280/100th/0.1 | 90 (30.1%)/2 | [69] |
| Bi/C nanofibers | pyrolysis | 1 M LiPF6 in EC/DEC | 61.5 | 316.7/500th/0.1 | 159.3 (49.7%)/3.2 | [75] |
| Bi/C nanowires | mechanical pressure injection method | 1 M LiPF6 in EC/DEC | 46.7 | 307.3/50th/0.2 | - | [67] |
| Bi@C-TiOx | solvothermal | 1 M LiPF6 in EC/DMC/EMC | - | 119.5/5000th/10 | 225 (67.5%)/10 | [71] |
| Bi/C | solvothermal | 1 M LiPF6 in EC/DMC | 52 | 248/100th/1 | 208 (26.1%)/2 | [66] |
| yolk−shell Bi@C−N | thermal reduction and carbonization | 1 M LiPF6 in EC/DEC/DMC | 73 | 1700 mAh cm−3/500th/1 | 1635 mAh cm−3 (41.8%)/2 | [76] |
| Bi@C | carbothermal reduction | 1 M LiPF6 in EC/DEC with 10% FEC | 64 | 256/1400th/1 | 131 (23.9%)/5 | [70] |
| Bi@PC | carbothermal reduction | 1 M LiPF6 in EC/DEC/DMC | 61.8 | 380/500th/0.5 | 215 (30.1%)/2 | [72] |
| C-Bi/PMC | annealing | 1 M LiPF6 in EC/DMC with 1% VC and 5% FEC | 68 | 400/500th/0.25 | 90 (21.3%)/5 | [73] |
| Bi powder | commercial | 2 M LiBH4 in THF | - | 381/1000th/8 C | 230 (56.8%)/64 C | [74] |
| Bi@C/C NL | pyrolysis | 1 M LiPF6 in EC/DMC (3:7) with 5% FEC | 62.3 | 373/1500th/3 | 278 (48.3%)/3 | [77] |
| Materials | Synthesis Methods | Electrolytes | ICE (%) | Cycling Performance (a) | Rate Capability (b) | Refs. |
|---|---|---|---|---|---|---|
| Bi@graphene | hydrothermal | 1 M NaClO4 in EC/PC | 55.6 | 358/50th/0.04 | 250 (69.8%)/1.28 | [23] |
| Arrayed Bi | dealloying | 1 M NaClO4 in PC with 5%FEC | 55 | 301.9/150th/0.05 | 102.3 (29.2%)/2 | [84] |
| Bi@C | aerosol spray pyrolysis | 1 M NaClO4 in EC/PC | 36.6 | 123.5/100th/0.1 | 83.4 (32.1%)/2 | [69] |
| Bi/CNF | electrospinning | 1 M NaPF6 in EC/DMC | 61.6 | 483.8/200th/0.1 | 170.7 (32.0%)/2 | [94] |
| Bi/CFC | hydrothermal | 1 M NaPF6 in EC/DMC/EMC with 5%FEC | 61.2 | 350/300th/0.05 | ~100 (28.5%)/2 | [95] |
| Bi-NS@C | molten salt calcination | 1 M NaClO4 in EC/PC | - | 106/1000th/0.2 | 110 (64.7%)/2 | [96] |
| Bulk Bi | commercial | 1 M NaPF6 in G2 | 94.8 | 389/2000th/0.4 | 356.0 (90.2%)/2 | [28] |
| Bi/C nanofibers | electrospinning | 1 M NaPF6 in EC/DMC with 5%FEC | 55.8 | 273.2/500th/0.1 | 69.0 (22.8%)/3.2 | [75] |
| Bi@Graphite | intercalation | 1 M NaPF6 in DME | 74.5 | ~140/10,000/3.2 | 113 (70%)/48 | [18] |
| Bi@C Nanoplates | thermal treatment | 1 M NaPF6 in EC/DMC with 5%FEC | 69.1 | 200/200th/0.15 | 74 (24%)/2 | [85] |
| Bi@3DGF | thermal treatment | 1 M NaPF6 in DME | 36 | 185.2/2000th/10 | 180 (78.3%)/50 | [26] |
| Bi@C | annealing | 1 M NaPF6 in DME | 50.3 | 265/30,000th/8 | 232 (71%)/60 | [86] |
| Bi@N−C | annealing | 1 M NaPF6 in DME | 85.7 | 302/1000th/1 | 368 (89.7%)/2 | [88] |
| Bi/C | annealing | 1 M NaPF6 in DME | 36.5 | 203/1000th/10 | 178 (60%)/100 | [25] |
| 3DPBi | liquid phase reduction | 1 M NaPF6 in DME | 65.9 | 374/3000th/10 | 354 (95.6%)/60 | [90] |
| HBiC | annealing | 1 M NaPF6 in DME | 79.9 | 264/15,000th/5 | 72.5 (22.9%)/200 | [89] |
| P-Bi/C | annealing | 1 M NaPF6 in DME | 95.2 | 178/20,000th/50 | 101 (27.3%)/72 | [91] |
| Bi/rGO | solution synthesis method | 1 M KFSI in EC/DEC | 63 | 290/50th/0.05 | 235 (76.1%)/0.5 | [24] |
| bulk Bi | commercial | 1 M KPF6 in DME | 87.2 | 322.7/300th/0.8 | - | [47] |
| Bi@C | carbothermal reduction | 5 M KTFSI in DME | 46.3 | 151/35th/0.1 | - | [97] |
| FBNs | electrochemical cathodic exfoliation | 1 M KPF6 in DME | - | 201/2500th/20 | 182 (43.0%)/20 | [98] |
| 2D-Bi | solution synthesis method | 1 M KPF6 in DME | 89.2 | 344/750th/10 | 345 (87.3%)/30 | [99] |
| CBN | solution synthesis method | 1 M KPF6 in DME | - | 200/5000th/30 | 254.5 (58.6%)/30 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Li, D.; Jiang, Y.; Huang, F.; Li, S. Advances in Electrochemical Energy Storage over Metallic Bismuth-Based Materials. Materials 2024, 17, 21. https://doi.org/10.3390/ma17010021
Cheng X, Li D, Jiang Y, Huang F, Li S. Advances in Electrochemical Energy Storage over Metallic Bismuth-Based Materials. Materials. 2024; 17(1):21. https://doi.org/10.3390/ma17010021
Chicago/Turabian StyleCheng, Xiaolong, Dongjun Li, Yu Jiang, Fangzhi Huang, and Shikuo Li. 2024. "Advances in Electrochemical Energy Storage over Metallic Bismuth-Based Materials" Materials 17, no. 1: 21. https://doi.org/10.3390/ma17010021
APA StyleCheng, X., Li, D., Jiang, Y., Huang, F., & Li, S. (2024). Advances in Electrochemical Energy Storage over Metallic Bismuth-Based Materials. Materials, 17(1), 21. https://doi.org/10.3390/ma17010021









