Investigation of Ionic Polymers’ Stabilizing and Flocculating Properties in Dispersed Activated Carbons Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Charge Density, Zeta Potential and Aggregate Sizes Determination
2.3. Adsorption Studies
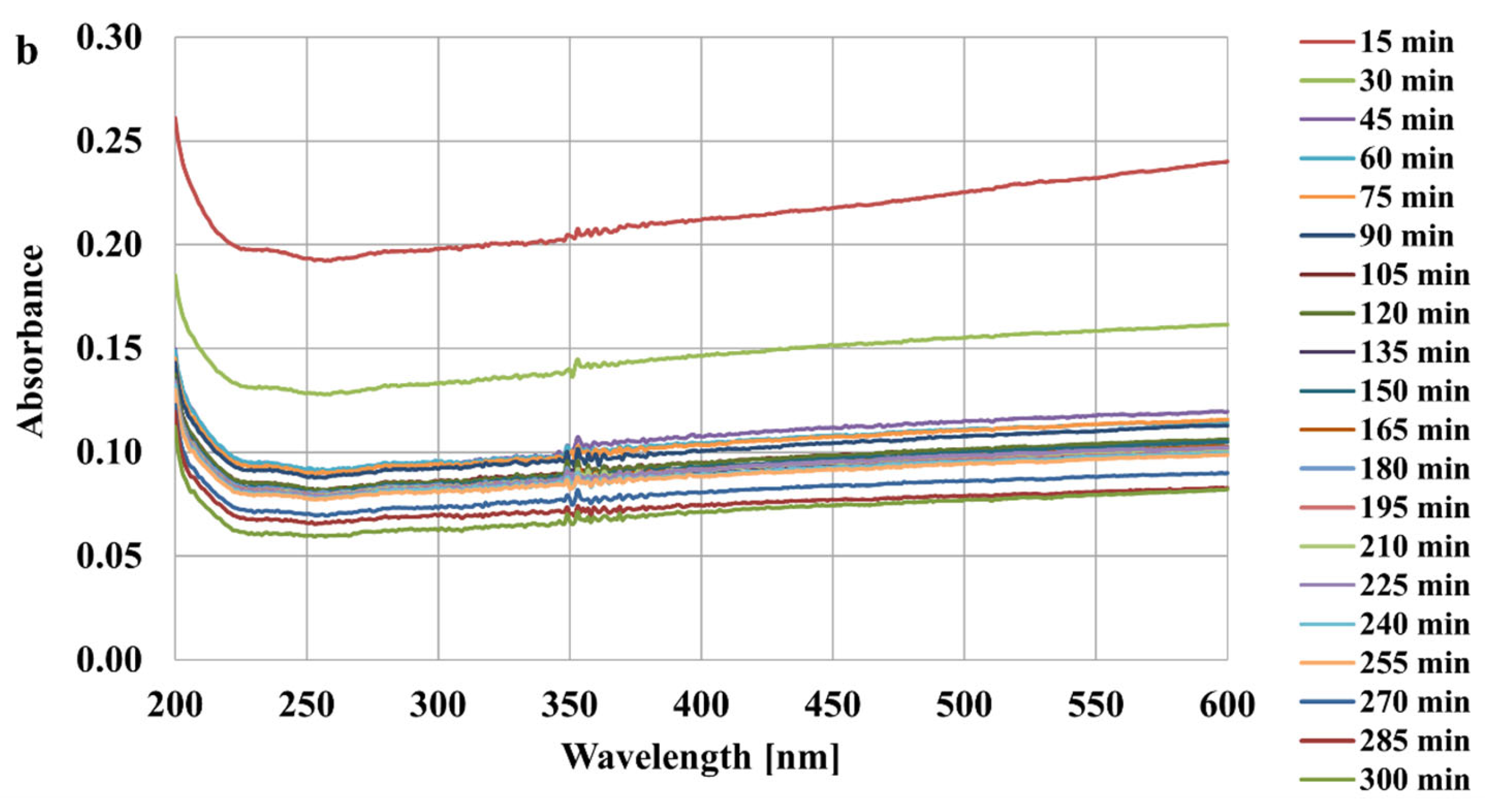
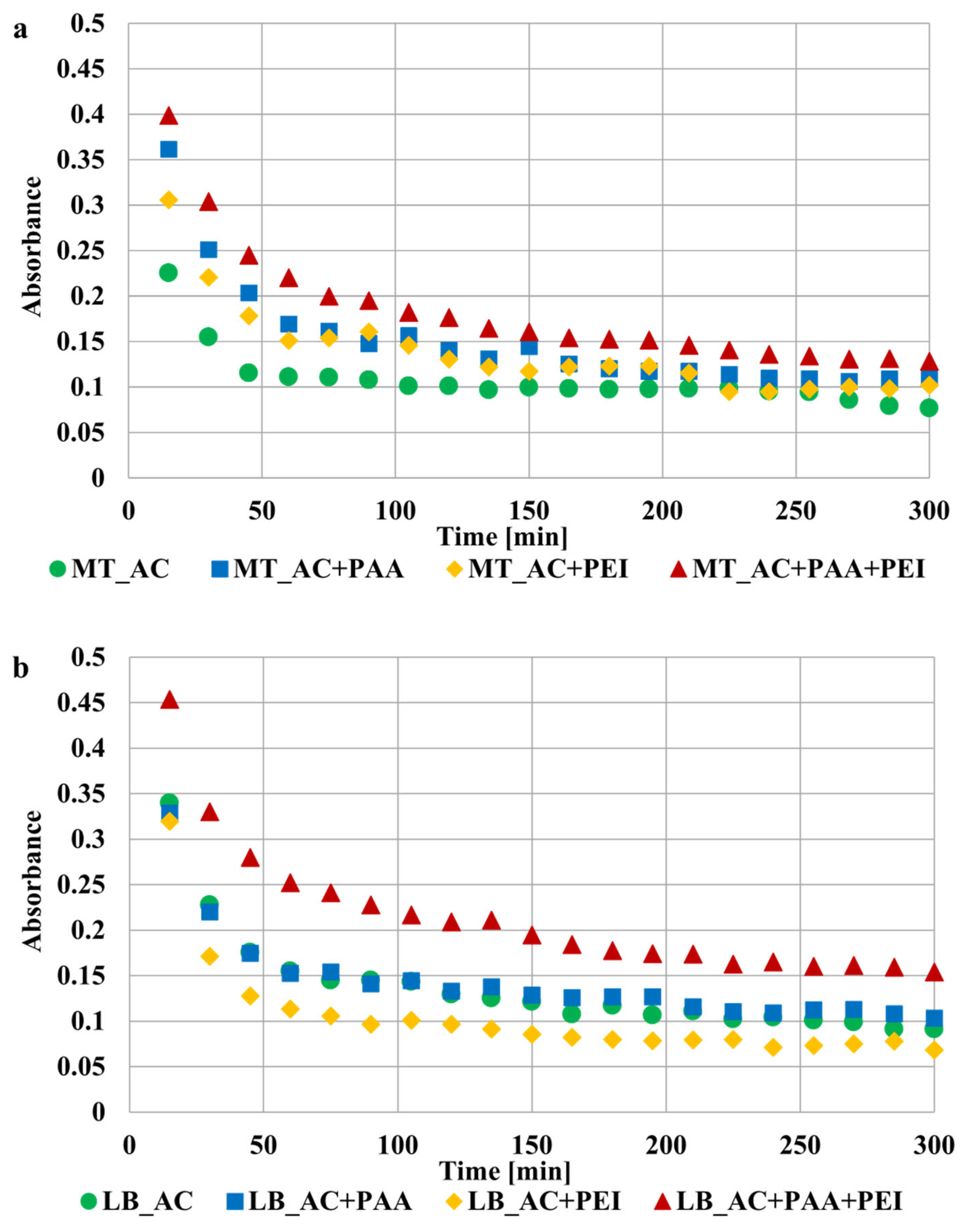
2.4. Stability Tests
3. Results and Discussion
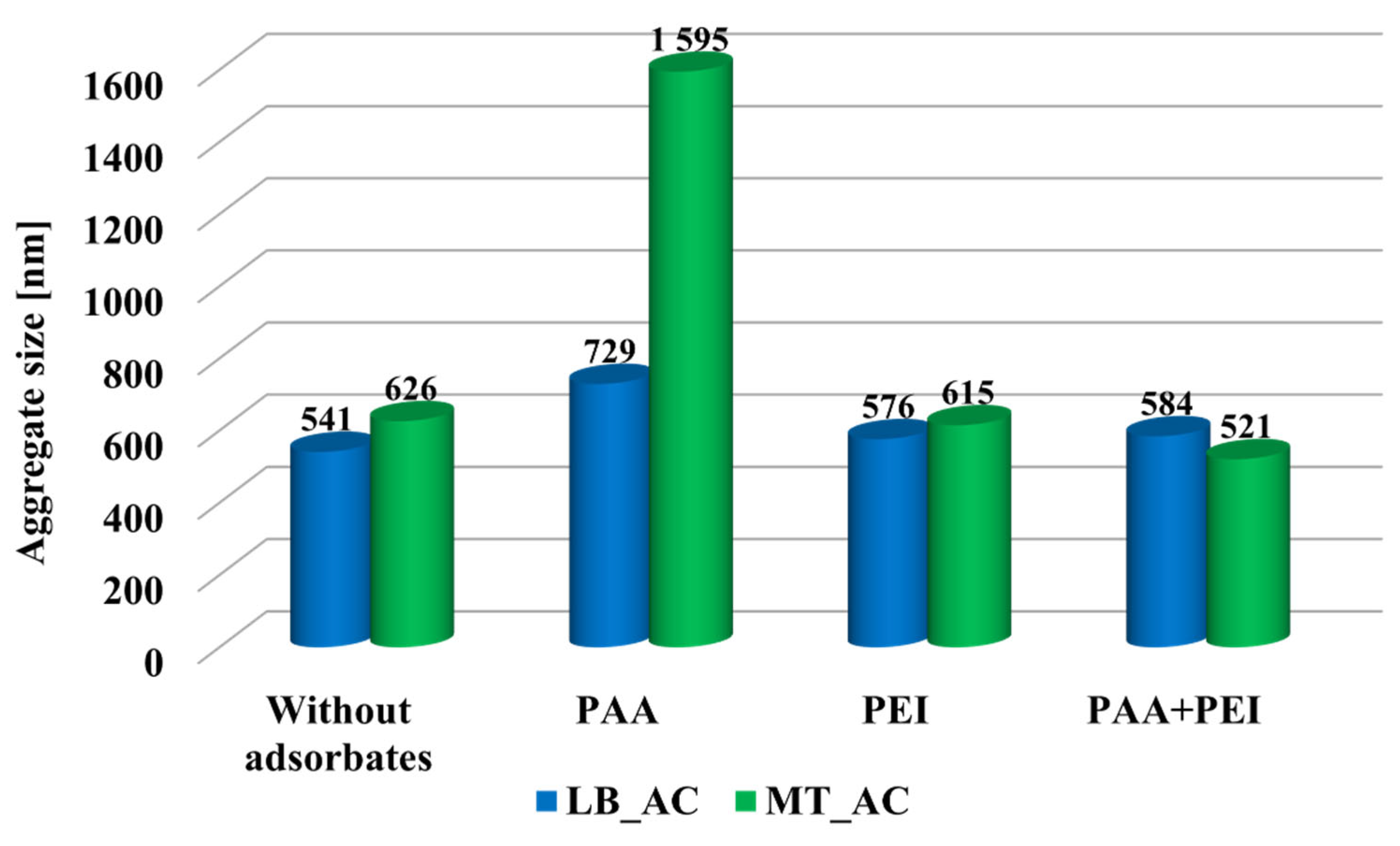
3.1. Electrical Double-Layer Structure and Aggregation Properties of Activated Carbons in the Presence of Ionic Polymers
3.2. Adsorption Layer Structure of Ionic Polymers on the Activated Carbons Surface
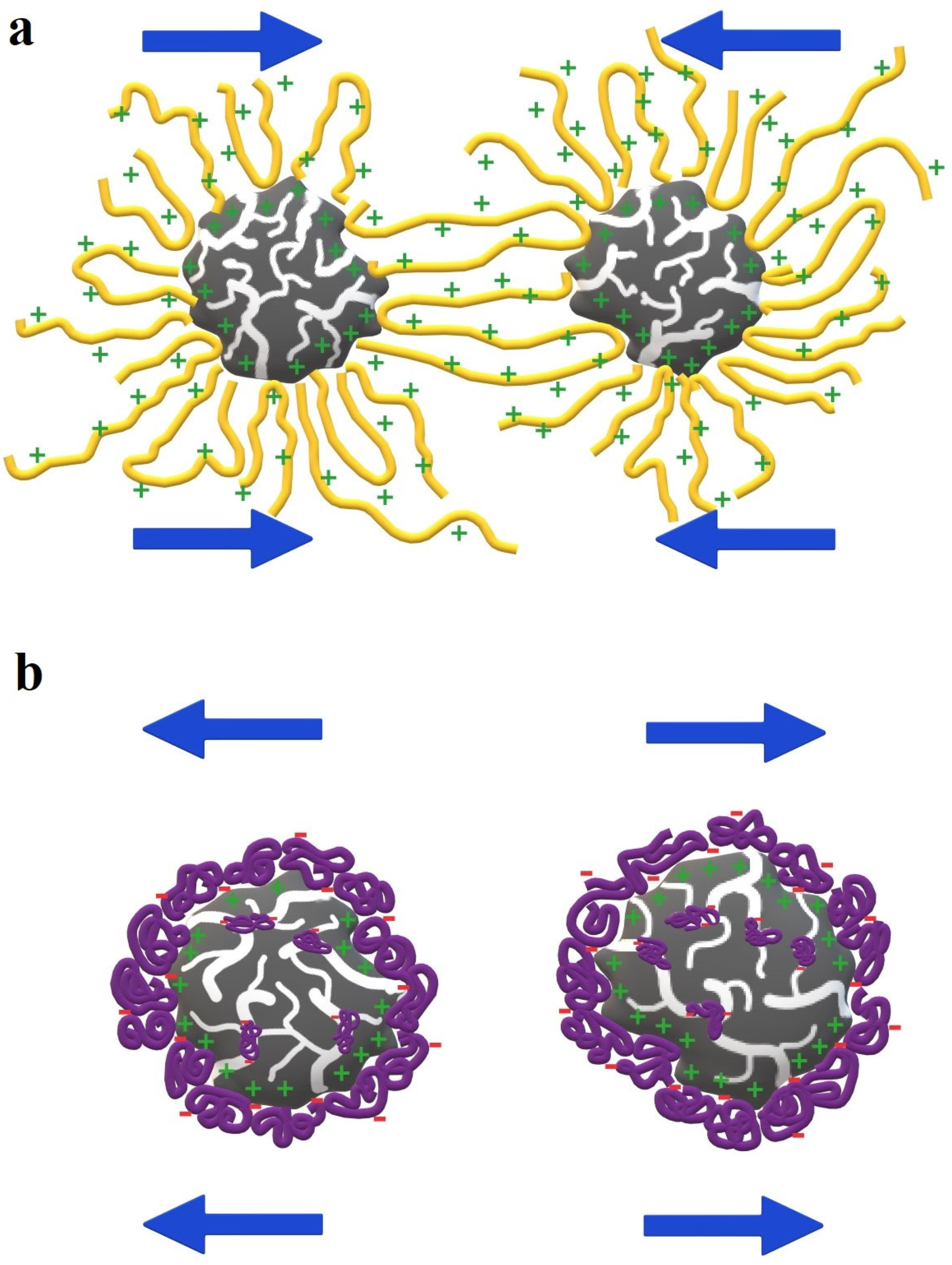
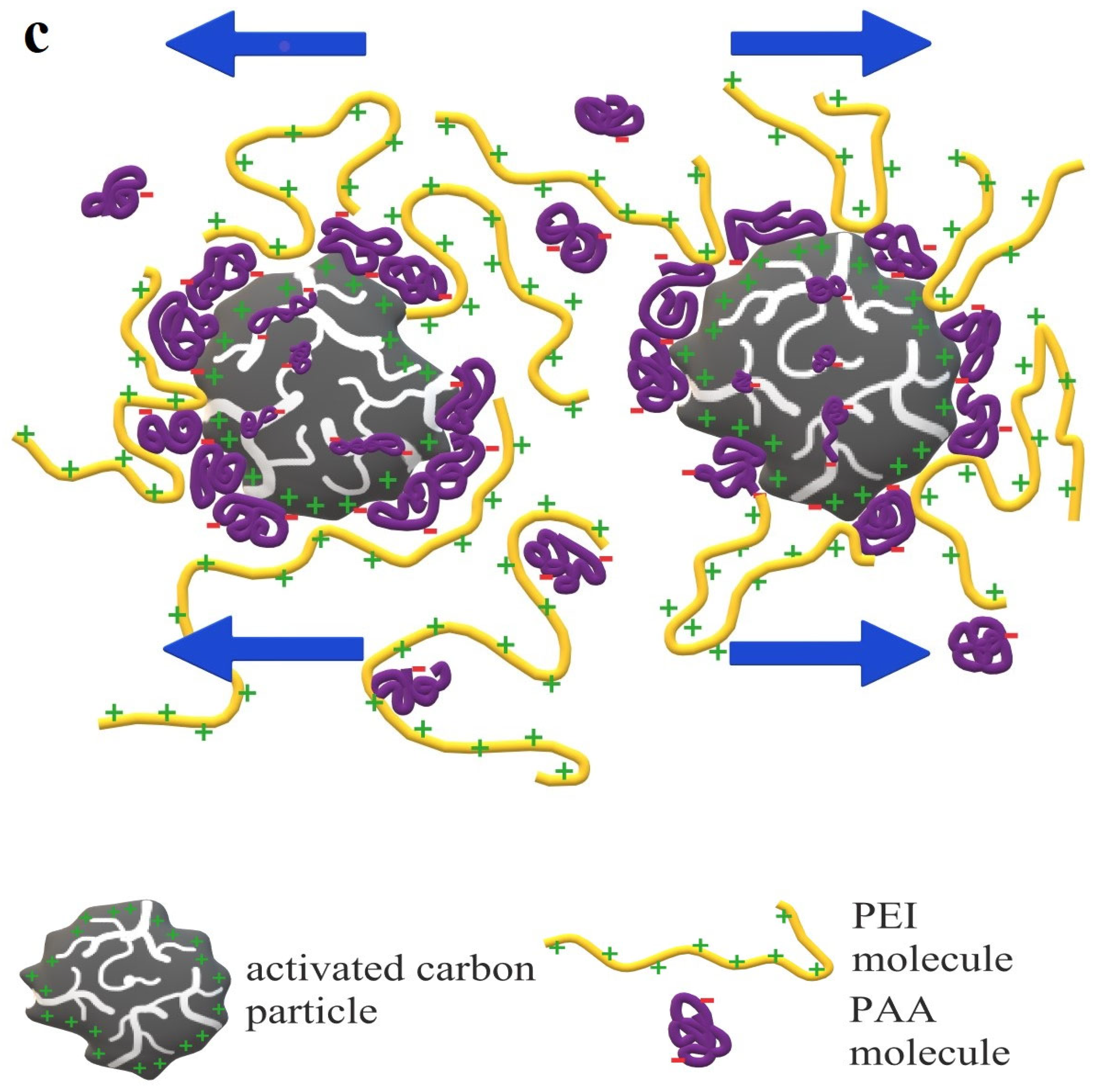
3.3. Stability Mechanisms of Activated Carbons Suspensions in the Presence of Ionic Polymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Biochars and activated carbons as adsorbents of inorganic and organic compounds from multicomponent systems—A review. Adv. Colloid Interface Sci. 2022, 305, 102687. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Krahnstöver, T.; Wintgens, T. Optimizing the flocculation of powdered activated carbon in wastewater treatment by dosing iron salt in single-and two-stage processes. J. Water Proc. Eng. 2017, 20, 130–137. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Chibowski, S.; Urban, T. Effect of the presence of cationic polyacrylamide on the surface properties of aqueous alumina suspension-stability mechanism. Appl. Surf. Sci. 2014, 320, 843–851. [Google Scholar] [CrossRef]
- Ueno, K.; Watanabe, M. From colloidal stability in ionic liquids to advanced soft materials using unique media. Langmuir 2011, 27, 9105–9115. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Nowicki, P. Peat-based activated carbons as adsorbents for simultaneous separation of organic molecules from mixed solution of poly (acrylic acid) polymer and sodium dodecyl sulfate surfactant. Colloids Surf. A 2020, 585, 124179. [Google Scholar] [CrossRef]
- Kong, H.J.; Bike, S.G.; Li, V.C. Electrosteric stabilization of concentrated cement suspensions imparted by a strong anionic polyelectrolyte and a non-ionic polymer. Cem. Concr. Res. 2006, 36, 842–850. [Google Scholar] [CrossRef]
- Kim, S.; Hyun, K.; Moon, J.Y.; Clasen, C.; Ahn, K.H. Depletion stabilization in nanoparticle–polymer suspensions: Multi-length-scale analysis of microstructure. Langmuir 2015, 31, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.; Schädler, V.; Willenbacher, N.; Wagner, N.J. Electrosteric stabilization of colloidal dispersions. Langmuir 2002, 18, 6381–6390. [Google Scholar] [CrossRef]
- Kong, H.J.; Bike, S.G.; Li, V.C. Development of a self-consolidating engineered cementitious composite employing electrosteric dispersion/stabilization. Cem. Concr. Compos. 2003, 25, 301–309. [Google Scholar] [CrossRef]
- Semenov, A.N.; Shvets, A.A. Theory of colloid depletion stabilization by unattached and adsorbed polymers. Soft Matter 2015, 11, 8863–8878. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Farokh, A.; Rahmani, E.; Eshaghi, M.M.; Aslani, A.; Rahdar, A.; Ferreira, L.F.R. Polyacrylic acid mediated targeted drug delivery nano-systems: A review. J. Drug Deliv. Sci. Technol. 2023, 80, 104169. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly (acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B 2017, 152, 252–259. [Google Scholar] [CrossRef]
- Cozens, E.J.; Roohpour, N.; Gautrot, J.E. Comparative adhesion of chemically and physically crosslinked poly (acrylic acid)-based hydrogels to soft tissues. Eur. Polym. J. 2021, 146, 110250. [Google Scholar] [CrossRef]
- Jung, H.; Jeon, S.; Jo, D.H.; Huh, J.; Kim, S.H. Effect of crosslinking on the CO2 adsorption of polyethyleneimine-impregnated sorbents. Chem. Eng. J. 2017, 307, 836–844. [Google Scholar] [CrossRef]
- Wang, Z.; Park, H.N.; Won, S.W. Adsorption and Desorption Properties of Polyethylenimine/Polyvinyl Chloride Cross-Linked Fiber for the Treatment of Azo Dye Reactive Yellow 2. Molecules 2021, 26, 1519. [Google Scholar] [CrossRef] [PubMed]
- Gęca, M.; Wiśniewska, M.; Nowicki, P. Simultaneous Removal of Polymers with Different Ionic Character from Their Mixed Solutions Using Herb-Based Biochars and Activated Carbons. Molecules 2022, 27, 7557. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P.; Diehl, E.; Heck, W.; Sappok, R. Surface oxides of carbon. Angew. Chem. Int. Ed. Engl. 1964, 3, 669–677. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Scope of doped mesoporous (<10 nm) surfactant-modified alumina templated carbons for hydrogen storage applications. Int. J. Energy Res. 2019, 43, 4264–4280. [Google Scholar] [CrossRef]
- Singh, S.B.; Dastgheib, S.A. Physicochemical transformation of graphene oxide during heat treatment at 110–200 °C. Carbon Trends 2023, 10, 100251. [Google Scholar] [CrossRef]
- Girgis, B.S.; Yunis, S.S.; Soliman, A.M. Characteristics of activated carbon from peanut hulls in relation to conditions of preparation. Mater. Lett. 2002, 57, 164–172. [Google Scholar] [CrossRef]
- Gómez-Serrano, V.; Cuerda-Correa, E.M.; Fernandez-Gonzalez, M.C.; Alexandre-Franco, M.F.; Macias-Garcia, A. Preparation of activated carbons from chestnut wood by phosphoric acid-chemical activation. Study of microporosity and fractal dimension. Mater. Lett. 2005, 59, 846–853. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Porous structure and adsorptive properties of pineapple peel based activated carbons prepared via microwave assisted KOH and K2CO3 activation. Microporous Mesoporous Mater. 2012, 148, 191–195. [Google Scholar] [CrossRef]
- Sayğılı, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Ibrahim, T.; Moctar, B.L.; Tomkouani, K.; Gbandi, D.B.; Victor, D.K.; Phinthè, N. Kinetics of the adsorption of anionic and cationic dyes in aqueous solution by low-cost activated carbons prepared from sea cake and cotton cake. Am. Chem. Sci. J. 2013, 4, 38–57. [Google Scholar] [CrossRef]
- Chibowski, S.; Wiśniewska, M.; Marczewski, A.W.; Pikus, S. Application of the SAXS method and viscometry for determination of the thickness of adsorbed polymer layers at the ZrO2 –polymer solution interface. J. Colloid Interface Sci. 2003, 267, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Janusz, W. Electrical double layer in the system TiO2 (anathase)/aqueous solution of NaCl. Pol. J. Chem. 1994, 68, 1871–1880. [Google Scholar]
- Ohshima, H. A Simple Expression for Henry’s Function for the Retardation Effect in Electrophoresis of Spherical Colloidal Particles. J. Colloid Interface Sci. 1994, 168, 269–271. [Google Scholar] [CrossRef]
- Crummett, W.B.; Hummel, R.A. The determination of traces of polyacrylamides in water. J. Am. Water Works Assoc. 1963, 55, 209–219. [Google Scholar] [CrossRef]
- Patkowski, J.; Myśliwiec, D.; Chibowski, S. Validation of a new method for spectrophotometric determination of polyethylenimine. Int. J. Polym. Anal. 2016, 21, 486–494. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Nowicki, P.; Jędruchniewicz, K. Cd(II) and As(V) removal from the multicomponent solutions in the presence of ionic polymers using carbonaceous adsorbents obtained from herbs. Pure Appl. Chem. 2023, 95, 563–578. [Google Scholar] [CrossRef]
- Gęca, M.; Wiśniewska, M.; Urban, T.; Nowicki, P. Temperature Effect on Ionic Polymers Removal from Aqueous Solutions Using Activated Carbons Obtained from Biomass. Materials 2023, 16, 350. [Google Scholar] [CrossRef]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Márquez, C.O.; Garcia, V.J.; Cambardella, C.A.; Schultz, R.C.; Isenhart, T.M. Aggregate-Size Stability Distribution and Soil Stability. Soil Sci. Soc. Am. J. 2004, 68, 725. [Google Scholar]
- Bey, H.B.; Hot, J.; Baumann, R.; Roussel, N. Consequences of competitive adsorption between polymers on the rheological behaviour of cement pastes. Cem. Concr. Compos. 2014, 54, 17–20. [Google Scholar] [CrossRef]
- Pelssers EG, M.; Stuart MA, C.; Fleer, G.J. Kinetics of bridging flocculation. Role of relaxations in the polymer layer. J. Chem. Soc. Faraday Trans. 1990, 86, 1355. [Google Scholar] [CrossRef]
- Wiśniewska, M. A review of temperature influence on adsorption mechanism and conformation of water soluble polymers on the solid surface. J. Dispers. Sci. Technol. 2011, 32, 1605–1623. [Google Scholar] [CrossRef]


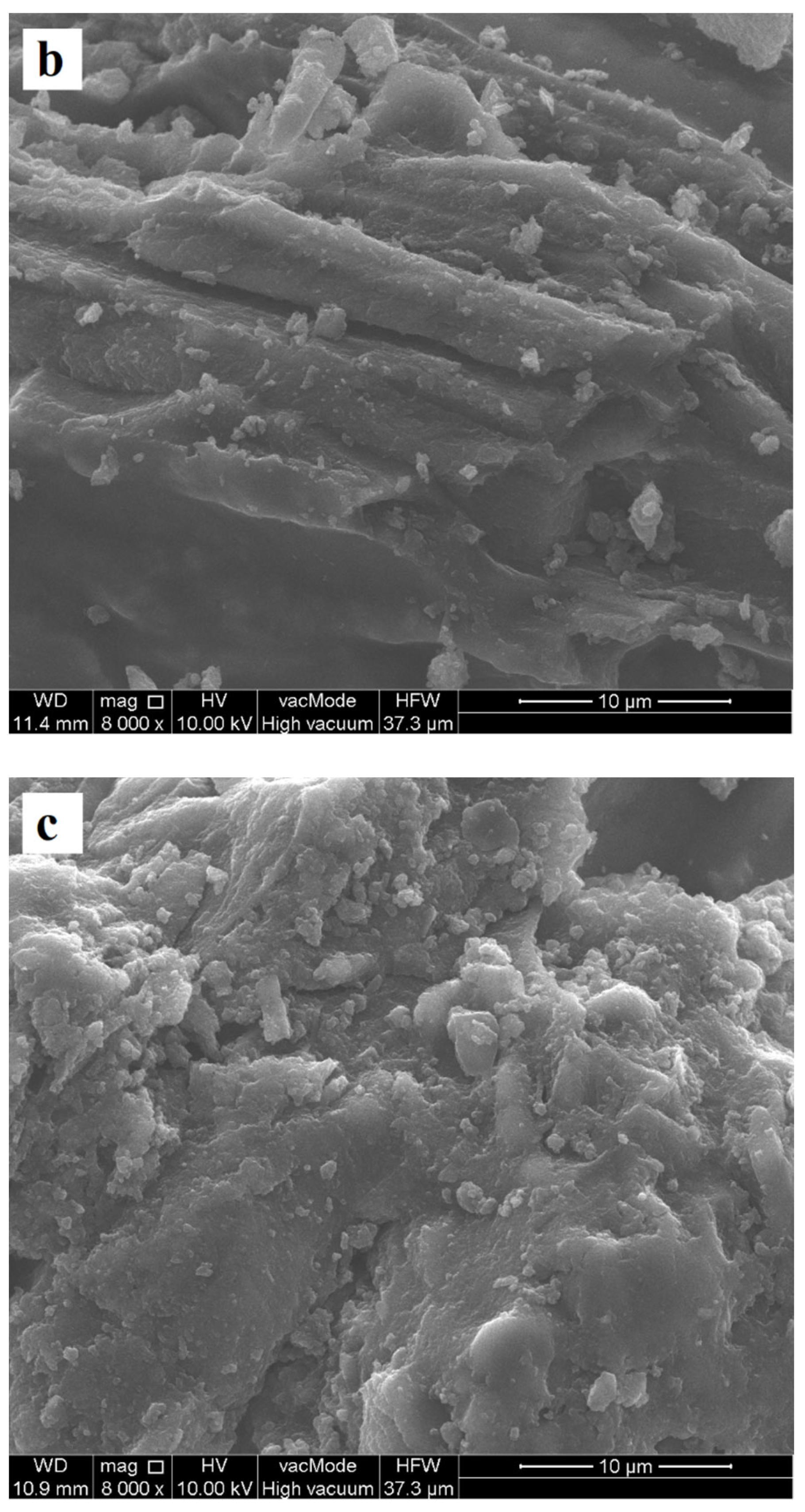



| Precursor | Preparation Conditions | Surface Area [m2/g] | Total Pore Volume [cm3/g] | Reference |
|---|---|---|---|---|
| Lemon balm herb | Impregnation with H3PO4, two stage heating program (200 °C, 500 °C) | 950 | 1.10 | Present study |
| Mint herb | Impregnation with H3PO4, two stage heating program (200 °C, 500 °C) | 1145 | 1.47 | Present study |
| Peanut hulls | Chemical activation with ZnCl2, heating for 6 h at temp. range 300–750 °C | 420 | 0.17 | [21] |
| Chestnut wood | Impregnation with H3PO4, heating at 500 °C for 4 h | 783 | 0.44 | [22] |
| Pineapple peels | Carbonized at 700 °C, activation with KOH in microwave oven | 1006 | 0.59 | [23] |
| Pineapple peels | Carbonized at 700 °C, activation with K2CO3 in microwave oven | 680 | 0.45 | [23] |
| Tomato processing solid waste | Impregnation with ZnCl2, carbonization at 600 °C, for 1 h | 1093 | 1.57 | [24] |
| Cotton cake | Impregnation with H3PO4, heating at 450 °C for 2 h | 584 | 0.30 | [25] |
| Shea cake | Impregnation with H3PO4, heating at 450 °C for 2 h | 1148 | 0.61 | [25] |
| System | Surface Charge Density [μC/cm2] | Zeta Potential [mV] | ||
|---|---|---|---|---|
| LB_AC | MT_AC | LB_AC | MT_AC | |
| Without adsorbates | 4.52 | 2.54 | 3.90 | −4.72 |
| PAA | 2.52 | 1.47 | −3.00 | −4.19 |
| PEI | 14.33 | 14.59 | 26.02 | 19.77 |
| PAA + PEI | 11.23 | 3.40 | 32.08 | 33.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gęca, M.; Wiśniewska, M.; Nowicki, P. Investigation of Ionic Polymers’ Stabilizing and Flocculating Properties in Dispersed Activated Carbons Systems. Materials 2024, 17, 693. https://doi.org/10.3390/ma17030693
Gęca M, Wiśniewska M, Nowicki P. Investigation of Ionic Polymers’ Stabilizing and Flocculating Properties in Dispersed Activated Carbons Systems. Materials. 2024; 17(3):693. https://doi.org/10.3390/ma17030693
Chicago/Turabian StyleGęca, Marlena, Małgorzata Wiśniewska, and Piotr Nowicki. 2024. "Investigation of Ionic Polymers’ Stabilizing and Flocculating Properties in Dispersed Activated Carbons Systems" Materials 17, no. 3: 693. https://doi.org/10.3390/ma17030693
APA StyleGęca, M., Wiśniewska, M., & Nowicki, P. (2024). Investigation of Ionic Polymers’ Stabilizing and Flocculating Properties in Dispersed Activated Carbons Systems. Materials, 17(3), 693. https://doi.org/10.3390/ma17030693








