Pore Structure and Deformation Correlation of an Aluminum Foam Sandwich Subject to Three-Point Bending
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
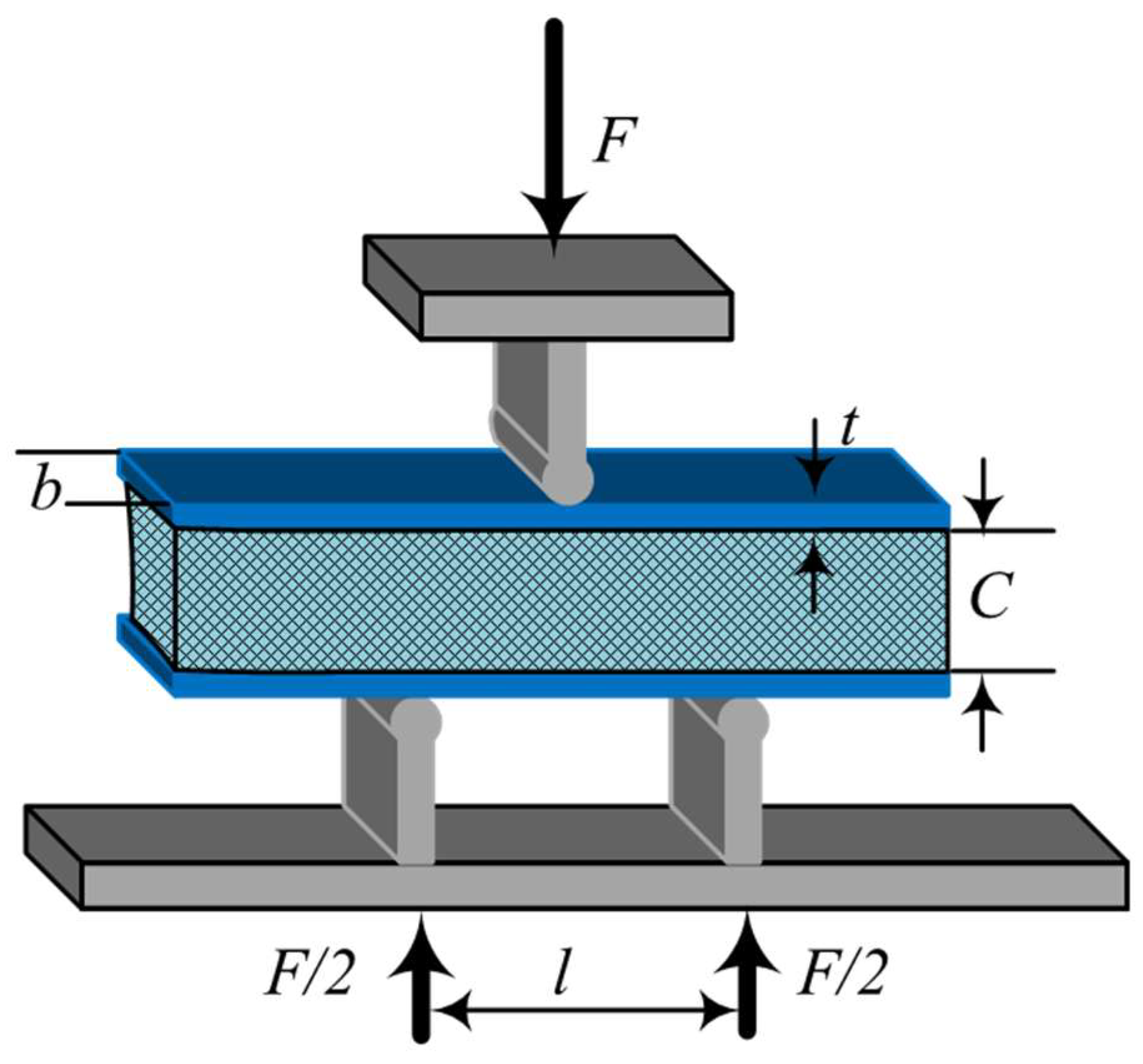
2.3. Three-Point Bending Test
3. Results and Discussion
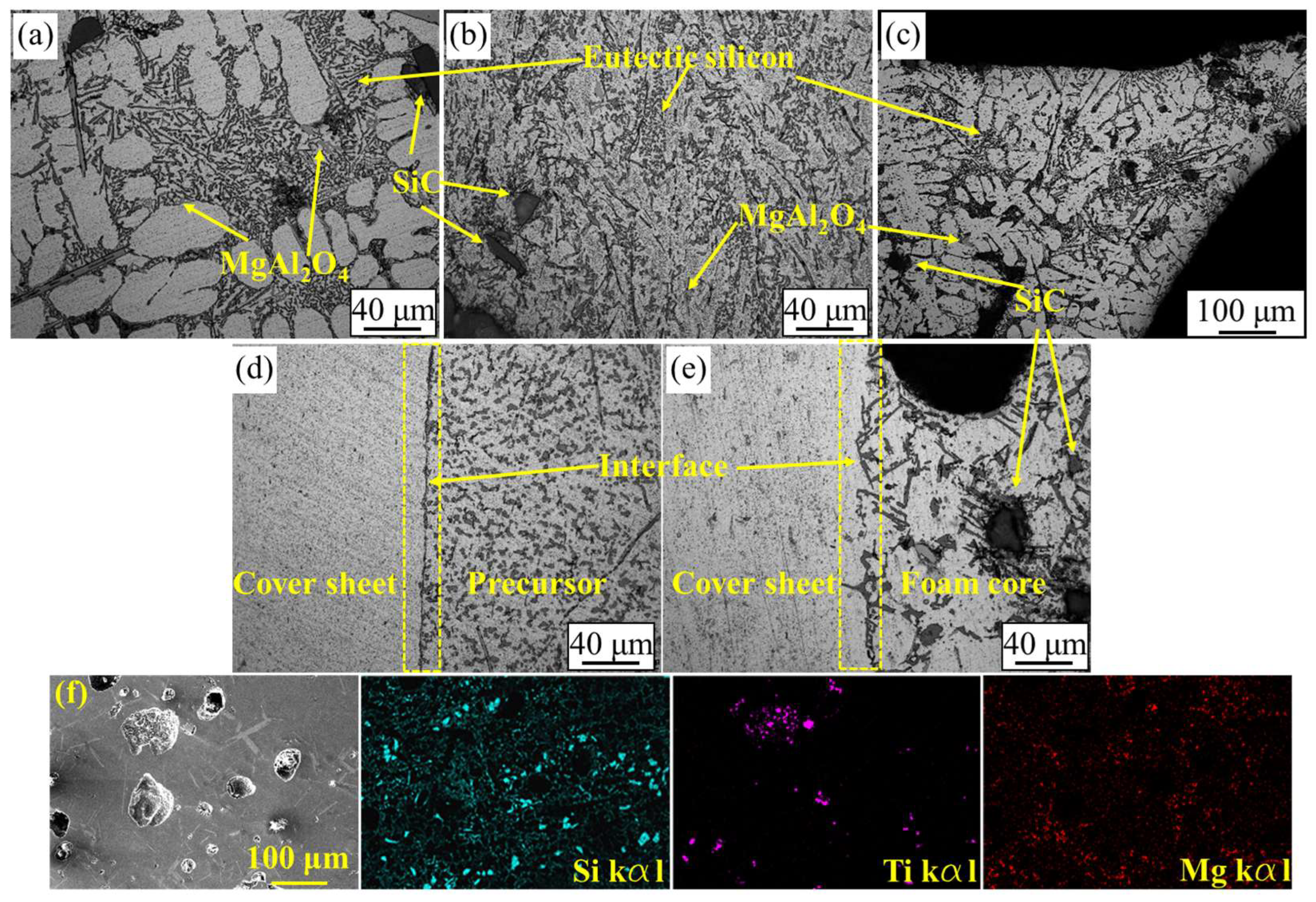
3.1. Microstructure and Morphology
3.2. Foaming Process
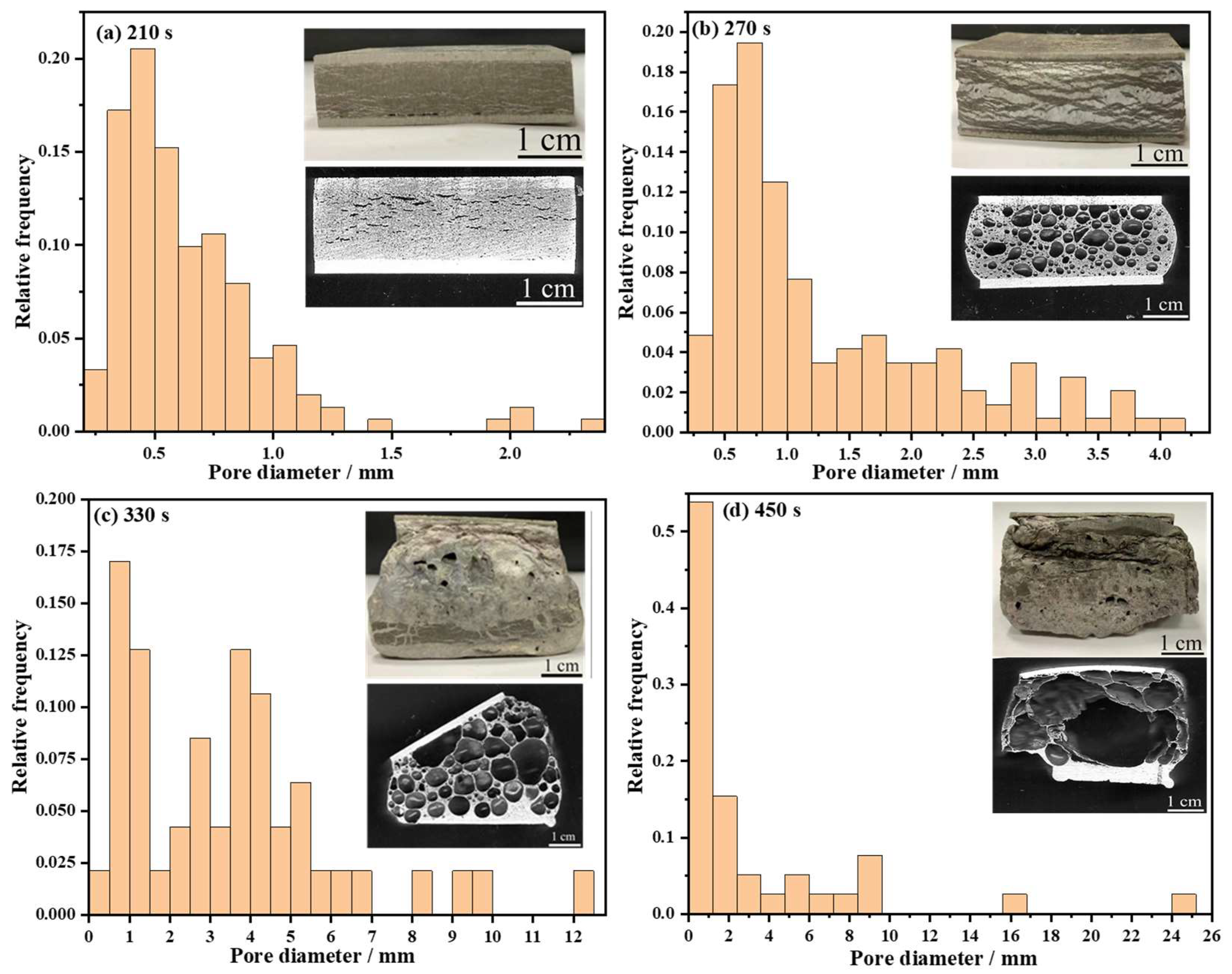
3.2.1. Effect of Foaming Temperature on Pore Structure
3.2.2. Effect of Foaming Time on Pore Structure
3.2.3. Foaming Mechanism of AFS
3.3. Three-Bending Test
3.3.1. Pore Structure of AFS Samples
3.3.2. Three-Bending Deformation Process
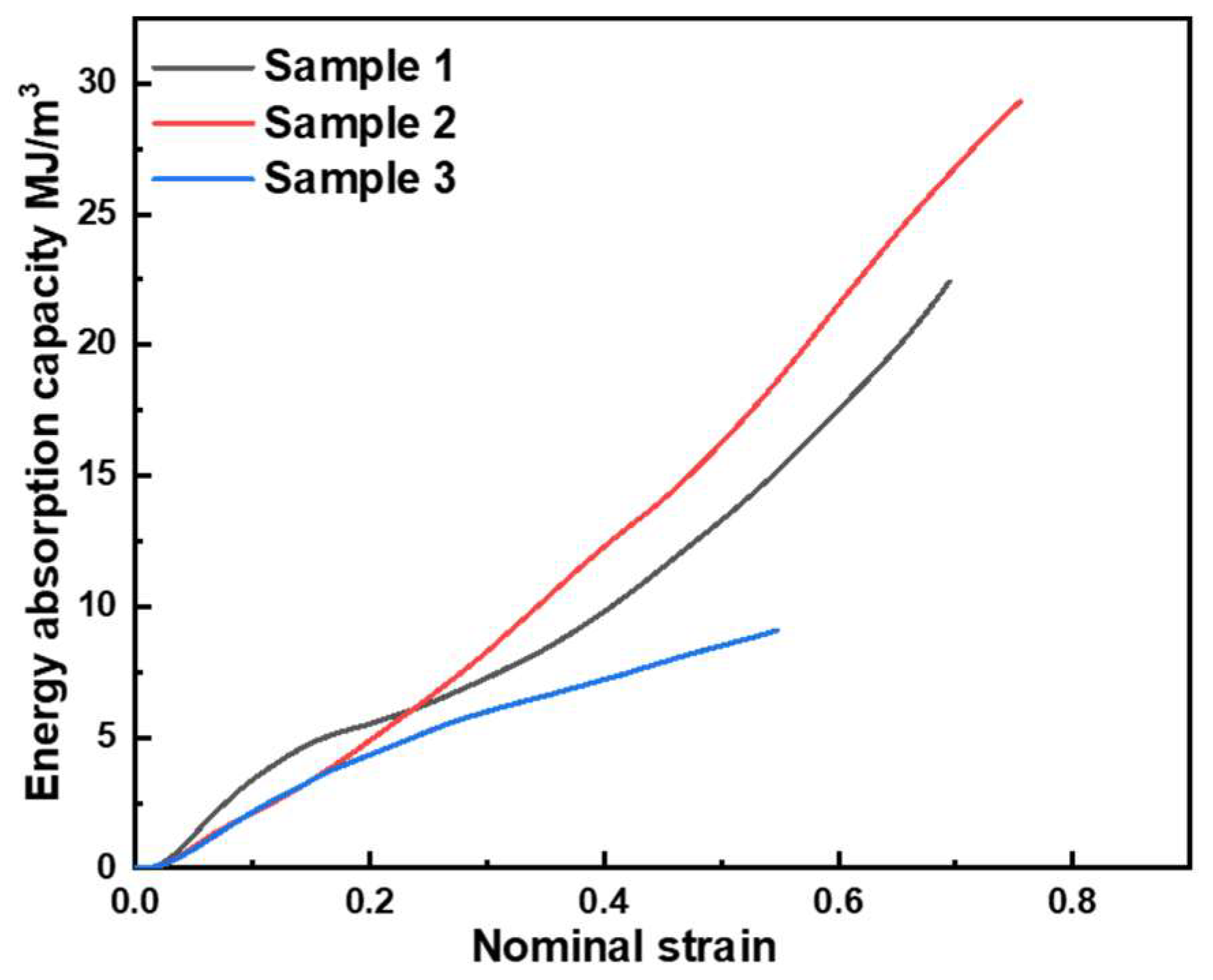
3.3.3. Energy Absorption Capacity
3.3.4. Fracture Morphology Analysis
4. Conclusions
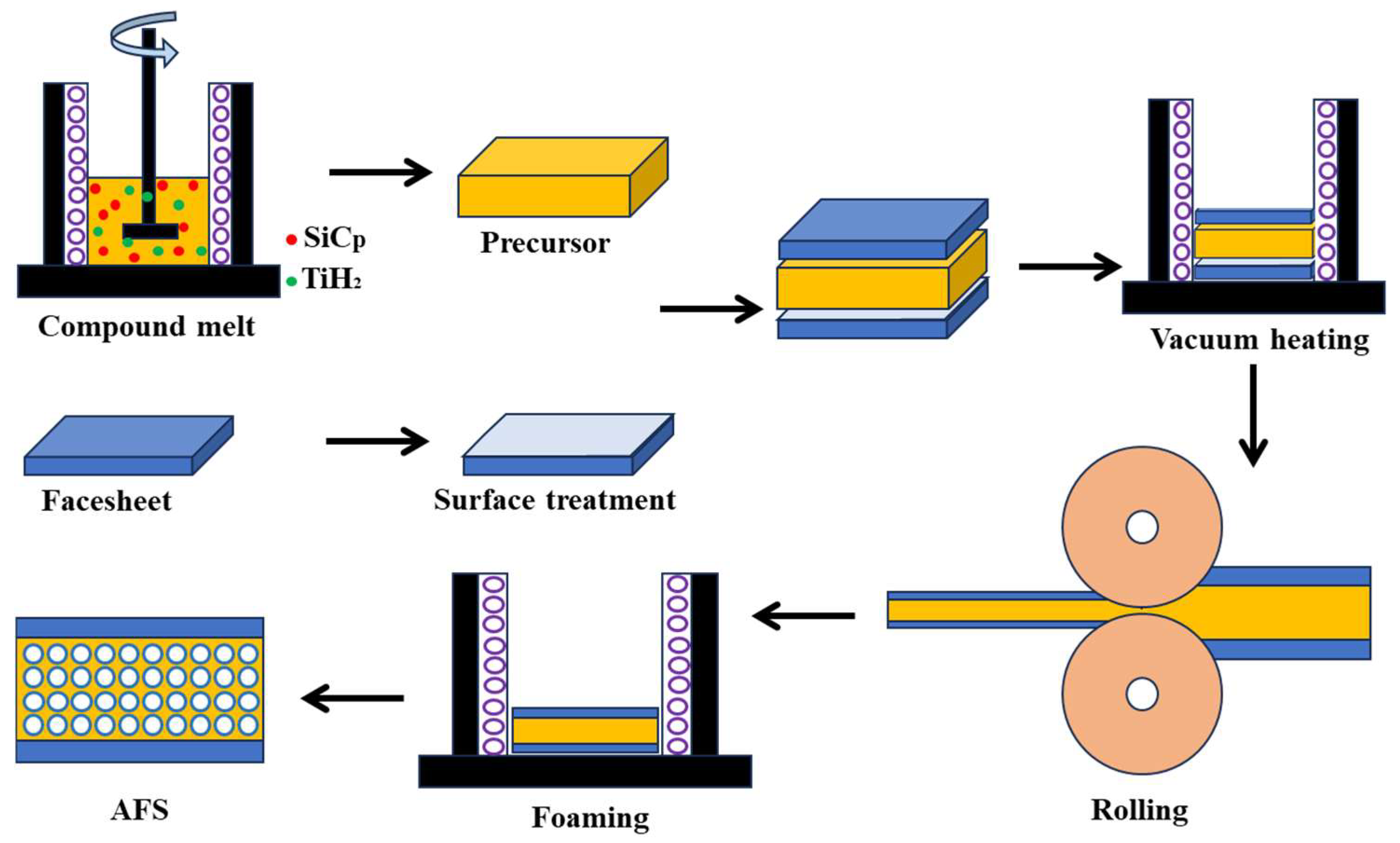
- The foamable precursor was prepared by the melting route. Rapid cooling followed immediately after the addition of TiH2 to minimize the release thereof;
- In the hot-rolling process, vacuum heating to 480 °C was applied over 3 h to prevent interface oxidation and obtain good bonding. The interface was mainly fixed by mechanical bonding with a little metallic bonding in some areas after hot rolling;
- In the foaming process, two kinds of foaming mechanism were concluded, i.e., a crack-like CDM and pore AEM. These two foaming mechanisms are all based on the pressure difference between the inside and outside of the pores. In addition, the metallic bonding of the interface was obtained after this foaming process. The microstructure of the foam matrix, if changed by hot-rolling, can be recovered in the foaming process;
- The pore structure and pore size affect the bending properties of the AFS. Fewer pore layers in the AFS result in a significant stress drop and then the bending properties become more akin to those of a denser material. However, the pore diameter of the AFS distributed mainly in the range of 0~1 mm presents a typical sandwich shear failure mode. The AFS with a uniform pore structure has a polygonal pore shape and the pore diameters are concentrated in the range of 0.5~3 mm, giving rise to a good energy absorption capacity, and the bending stress–strain curve fluctuates greatly after the first stress drop;
- The SEM images of the AFS bending fracture morphology show multiple brittle fracture characteristics in such samples, proving that the AlSi12 matrix foam core fails in brittle fracture under bending.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibson, J.; Ashby, L. Cellular Solids: Structure and Properties; Cambridge University Press & Assessment: Cambridge, UK, 1988; Volume 9. [Google Scholar]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Banhart, J.; García-Moreno, F.; Heim, K.; Seeliger, H.W. Light-weighting in transportation and defence using aluminium foam sandwich structures. In Light Weighting for Defense, Aerospace, and Transportation; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Schuler, P.; Frank, R.; Uebel, D.; Fischer, S.F.; Buhrig-Polaczek, A.; Fleck, C. Influence of heat treatments on the microstructure and mechanical behaviour of open cell AlSi7Mg0.3 foams on different lengthscales. Acta Mater. 2016, 109, 32–45. [Google Scholar] [CrossRef]
- Lu, X.; Luo, H.; Yang, S.; Wei, Y.; Xu, J.; Yao, Z. Two-step foaming process combined with hot-rolling in fabrication of an aluminium foam sandwich panel. Mater. Lett. 2020, 265, 127427. [Google Scholar] [CrossRef]
- Wan, L.; Huang, Y.; Lv, S.; Feng, J. Fabrication and interfacial characterization of aluminum foam sandwich via fluxless soldering with surface abrasion. Compos. Struct. 2015, 123, 366–373. [Google Scholar] [CrossRef]
- Lin, H.; Luo, H.J.; Zhang, Z.G.; Ma, J.F.; Yao, G.C. Study on improvement of aluminum foams by rapid foaming. Mater. Lett. 2017, 188, 288–290. [Google Scholar] [CrossRef]
- Hangai, Y.; Kamada, H.; Utsunomiya, T.; Kitahara, S.; Kuwazuru, O.; Yoshikawa, N. Aluminum alloy foam core sandwich panels fabricated from die casting aluminum alloy by friction stir welding route. J. Mater. Process. Tech. 2014, 214, 1928–1934. [Google Scholar] [CrossRef]
- Phongphisutthinan, C.; Wattanapornphan, P.; Suga, T.; Mizutani, M.; Katayama, S. Laser welding for joining of open-cell aluminum foam to solid shell. Weld. World 2019, 63, 825–839. [Google Scholar] [CrossRef]
- Neugebauer, R.; Lies, C.; Hohlfeld, J.; Hipke, T. Adhesion in sandwiches with aluminum foam core. Prod. Eng. 2007, 1, 271–278. [Google Scholar] [CrossRef]
- Mu, Y.; Yao, G.; Liang, L.; Luo, H.; Zu, G. Deformation mechanisms of closed-cell aluminum foam in compression. Scr. Mater. 2010, 63, 629–632. [Google Scholar] [CrossRef]
- Kenesei, P. The influence of cell-size distribution on the plastic deformation in metal foams. Scr. Mater. 2004, 50, 295–300. [Google Scholar] [CrossRef]
- Crupi, V.; Montanini, R. Aluminium foam sandwiches collapse modes under static and dynamic three-point bending. Int. J. Impact Eng. 2007, 34, 509–521. [Google Scholar] [CrossRef]
- Ding, X.; Liu, Y.; Wan, T. A novel hot-pressing method to prepare foamable precursor of aluminum foam sandwich (AFS). Mater. Lett. 2020, 259, 126895. [Google Scholar] [CrossRef]
- Durante, M.; Formisano, A.; Viscusi, A.; Carrino, L. An innovative manufacturing method of aluminum foam sandwiches using a mesh-grid reinforcement as mold. Int. J. Adv. Manuf. Technol. 2020, 107, 3039–3048. [Google Scholar] [CrossRef]
- Lin, H.; Luo, H.; Huang, W.; Zhang, X.; Yao, G. Diffusion bonding in fabrication of aluminum foam sandwich panels. J. Mater. Process. Tech. 2016, 230, 35–41. [Google Scholar] [CrossRef]
- Nabavi, A.; Khaki, J.V. Manufacturing of aluminum foam sandwich panels: Comparison of a novel method with two different conventional methods. J. Sandw. Struct. Mater. 2011, 13, 177–187. [Google Scholar] [CrossRef]
- Shunmugasamy, V.C.; Mansoor, B. Flexural Response of an Aluminum Foam Core/Stainless Steel Facesheet Sandwich Composite. JOM 2019, 71, 4024–4033. [Google Scholar] [CrossRef]
- Sun, X.; Huang, P.; Zhang, X.; Han, N.; Lei, J.; Yao, Y.; Zu, G. Densification Mechanism for the Precursor of AFS under Different Rolling Temperatures. Materials 2019, 12, 3933. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, I.; Kume, Y.; Kobashi, M.; Kanetake, N. Deformation Behavior in Die Forging of Aluminum Foam Sandwich. Procedia Eng. 2014, 81, 564–567. [Google Scholar] [CrossRef]
- Sha, J.B.; Yip, T.H. In situ surface displacement analysis on sandwich and multilayer beams composed of aluminum foam core and metallic face sheets under bending loading. Mater. Sci. Eng. A 2004, 386, 91–103. [Google Scholar] [CrossRef]
- Yan, C.; Wang, J.; Song, X. Fatigue behavior and damage mechanism of aluminum foam sandwich with carbon-fiber face-sheets. J. Mech. Sci. Technol. 2020, 34, 1119–1127. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, A.; Zhu, Z.; Zhang, H.; Qu, X.; Hu, Y. Local compression properties of the aluminium foam sandwich panels on the elevated temperatures. Mater. Res. Express 2019, 6, 096555. [Google Scholar] [CrossRef]
- Ren, C.-X.; Hu, Z.-F.; Yao, C.; Mo, F. Experimental study on the quasi-static compression behavior of multilayer aluminum foam sandwich structure. J. Alloys Compd. 2019, 810, 151860. [Google Scholar] [CrossRef]
- Liang, X.; Luo, H.; Mu, Y.; Wu, L.; Lin, H. Experimental study on stress attenuation in aluminum foam core sandwich panels in high-velocity impact. Mater. Lett. 2017, 203, 100–102. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Jiang, B.; Lu, F. Local indentation of aluminum foam core sandwich beams at elevated temperatures. Compos. Struct. 2016, 145, 142–148. [Google Scholar] [CrossRef]
- Lamanna, E.; Gupta, N.; Cappa, P.; Strbik, O.M.; Cho, K. Evaluation of the dynamic properties of an aluminum syntactic foam core sandwich. J. Alloys Compd. 2017, 695, 2987–2994. [Google Scholar] [CrossRef]
- Vodenitcharova, T.; Kabir, K.; Hoffman, M. Indentation of metallic foam core sandwich panels with soft aluminium face sheets. Mater. Sci. Eng. A 2012, 558, 175–185. [Google Scholar] [CrossRef]
- Kabir, K.; Vodenitcharova, T.; Hoffman, M. Response of aluminium foam-cored sandwich panels to bending load. Compos. Part B Eng. 2014, 64, 24–32. [Google Scholar] [CrossRef]
- Malachevsky, M.T.; D’Ovidio, C.A. Thermal evolution of titanium hydride optimized for aluminium foam fabrication. Scr. Mater. 2009, 61, 1–4. [Google Scholar] [CrossRef]
- You, X.H.; Wang, F.; Wang, L.C. The Structure Control of Aluminum Foams Produced by Powder Compacted Foaming Process. Acta Metall. Sin. (Engl. Lett.) 2004, 17, 279–282. [Google Scholar]
- Wang, L.; Yao, G.; Liu, Y.; Zu, G. Study on Crack-like Pores of Al Foams Made via the Powder-Metallurgy Route. Adv. Eng. Mater. 2010, 12, 50–53. [Google Scholar] [CrossRef]
- Aguirre-Perales, L.Y.; Jung, I.-H.; Drew, R.A.L. Foaming behavior of powder metallurgical Al–Sn foams. Acta Mater. 2012, 60, 759–769. [Google Scholar] [CrossRef]
- Degischer, H.-P.; Kriszt, B. Handbook of Cellular Metals Production, Processing, Applications; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Xia, X.C.; Feng, J.L.; Ding, J.; Song, K.H.; Chen, X.W.; Zhao, W.M.; Liao, B.; Hur, B.Y. Fabrication and characterization of closed-cell magnesium-based composite foams. Mater. Des. 2015, 74, 36–43. [Google Scholar] [CrossRef]
- Homma, S.; Ogata, S.; Koga, J.; Matsumoto, S. Gas–solid reaction model for a shrinking spherical particle with unreacted shrinking core. Chem. Eng. Sci. 2005, 60, 4971–4980. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Yu, J.; Qian, C.; Lu, F. Deformation and failure mechanisms of sandwich beams under three-point bending at elevated temperatures. Compos. Struct. 2014, 111, 285–290. [Google Scholar] [CrossRef]
- Zu, G.; Song, B.; Zhong, Z.; Li, X.; Mu, Y.; Yao, G. Compounds, Static three-point bending behavior of aluminum foam sandwich. J. Alloys Compd. 2012, 540, 275–278. [Google Scholar] [CrossRef]
- Asavavisithchai, S.; Kennedy, A.R. The effect of Mg addition on the stability of Al-Al2O3 foams made by a powder metallurgy route. Scr. Mater. 2006, 7, 1331–1334. [Google Scholar] [CrossRef]
- Lumley, R.N.; Sercombe, T.B.; Schaffer, G.M. Surface oxide and the role of magnesium during the sintering of aluminum. Met. Mater. Trans. A 1999, 30, 457–463. [Google Scholar] [CrossRef]
- McLeod, A.D.; Gabryel, C.M. Kinetics of the growth of spinel, MgAl2O4, on alumina particulate in aluminum alloys containing magnesium. Met. Trans. A 1992, 4, 1279–1283. [Google Scholar] [CrossRef]
- Takamatsu, S.; Arai, T.; Sayama, A.; Suzuki, S. Characteristics of Pore Morphology in Aluminum Alloy Foams Fabricated by Semi-Solid Route among Multiple Experimental Runs. Metals 2023, 13, 1654. [Google Scholar] [CrossRef]
- Thulasikanth, V.; Padmanabhan, R. Fabrication of sustainable closed-cell aluminium foams using recycled fly ash and eggshell powder. Mater. Today Commun. 2023, 37, 107302. [Google Scholar] [CrossRef]
- Vidwans, A.; Trovalusci, P.; Fantuzzi, N.; Correia, J. Application of column buckling theory to steel aluminium foam sandwich panels. Structures 2023, 54, 607–617. [Google Scholar] [CrossRef]
- Hangai, Y.; Takagi, T.; Koyama, S.; Suzuki, R.; Kamakoshi, Y. Refoaming of deformed aluminum foam fabricated by precursor foaming process using remaining foaming agent and densification using friction stir welding. Mater. Lett. 2023, 351, 135008. [Google Scholar] [CrossRef]
- Kováčik, J.; Jerz, J.; Gopinathan, A.; Simančík, F.; Marsavina, L.; Linul, E. Effect of sample shape on compression behavior of aluminum foams. Mater. Today Proc. 2023, 78, 308–313. [Google Scholar] [CrossRef]
- Hangai, Y.; Yamamoto, Y.; Goto, Y.; Okada, K.; Yoshikawa, N. Friction Welding of Polycarbonate Plate and Aluminum Foam Fabricated by Precursor Foaming Process. Metals 2023, 13, 1366. [Google Scholar] [CrossRef]
- Byakova, A.; Gnyloskurenko, S.; Vlasov, A.; Yevych, Y.; Semenov, N. The Mechanical Performance of Aluminum Foam Fabricated by Melt Processing with Different Foaming Agents: A Comparative Analysis. Metals 2022, 12, 1384. [Google Scholar] [CrossRef]







| Bonding Type | Fabrication Method | Foam Core Structure | Cover Sheet | References |
|---|---|---|---|---|
| Metallic joints | Extrusion | C-AlSi7 foam | AlMn1 | [13] |
| Hot pressing | C-AlMg4Si8 foam | Al | [14] | |
| Direct foaming to bond coversheet | C-AlSi10 foam | Stainless steel wire mesh-grid | [15] | |
| Friction stir welding | C-ADC12 foam | ADC6 plate | [8] | |
| Hot pressing | C-AlSi12 foam | Q235 steel | [16] | |
| Self-propagating high-temperature synthesis | O-Al foam | Al | [17] | |
| Direct foaming to bond coversheet | C-Al foam | Steel sheet | [10] | |
| Laser welding | O-AC2A foam | A6063 | [9] | |
| Soldering | O-Al foam | 316 Steel sheet | [18] | |
| Hot rolling | C-AlSi12 foam | A6063 | [19] | |
| Hot rolling | C-AlSi7 foam | Al | [20] | |
| Soldering | C-Al foam | 5056 Al | [6] | |
| Mechanical joints | Adhesive bonding | Alporas foam | Stainless steel | [21] |
| Adhesive bonding | C-7075Al foam | Carbon fiber | [22] | |
| Adhesive bonding | C-Al foam | Al-1060 | [23] | |
| Adhesive bonding | C-Al foam | 5052 Al | [24] | |
| No joining | C-Al foam | Steel sheet | [25] | |
| Inorganic adhesive bonding | C-Al foam | Al (99.2%) | [26] | |
| In injection process | O-A356 alloy foam | Carbon fabric | [27] | |
| Adhesive bonding | C-Al foam | Al (99.5%) | [13] | |
| Adhesive bonding | Alporas foam | AA 1100-O | [28] | |
| Adhesive bonding | Alporas foam | AA 1100-O/AA 3104-H19 | [29] |
| Temperature (°C) | 680 | 700 | 720 | 740 | 750 | |
|---|---|---|---|---|---|---|
| Time (s) | ||||||
| 150 | ✕ | ✕ | √ | √ | √ | |
| 210 | ✕ | √ | √ | √ | √ | |
| 240 | ✕ | ✕ | ✕ | ✕ | √ | |
| 270 | √ | √ | √ | √ | √ | |
| 330 | √ | √ | √ | √ | √ | |
| 390 | √ | √ | √ | √ | ✕ | |
| 450 | √ | √ | ✕ | ✕ | ✕ | |
| 510 | √ | ✕ | ✕ | ✕ | ✕ | |
| Density (g/cm3) | Bending Strength (σby MPa) | Energy Absorption Capacity (Et MJ/m3) | Specific Energy Absorption (Es MJ/m3g−1) | Elasticity Modulus (E) | Foaming Temp. (°C) | Foaming Time (s) | |
|---|---|---|---|---|---|---|---|
| Sample 1 | 1.352 | 49.9 | 13.3 | 1.42 | 857.46 | 700 | 330 |
| Sample 2 | 1.023 | 31.3 | 16.3 | 1.87 | 637.91 | 720 | 270 |
| Sample 3 | 1.201 | 29.6 | 8.5 | 0.79 | 484.33 | 680 | 450 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Jing, L.; Zhou, W.; Yang, H.; Yuan, P.; Li, X. Pore Structure and Deformation Correlation of an Aluminum Foam Sandwich Subject to Three-Point Bending. Materials 2024, 17, 567. https://doi.org/10.3390/ma17030567
Lu X, Jing L, Zhou W, Yang H, Yuan P, Li X. Pore Structure and Deformation Correlation of an Aluminum Foam Sandwich Subject to Three-Point Bending. Materials. 2024; 17(3):567. https://doi.org/10.3390/ma17030567
Chicago/Turabian StyleLu, Xiaotong, Lei Jing, Wenhao Zhou, Hui Yang, Pingyun Yuan, and Xiaocheng Li. 2024. "Pore Structure and Deformation Correlation of an Aluminum Foam Sandwich Subject to Three-Point Bending" Materials 17, no. 3: 567. https://doi.org/10.3390/ma17030567
APA StyleLu, X., Jing, L., Zhou, W., Yang, H., Yuan, P., & Li, X. (2024). Pore Structure and Deformation Correlation of an Aluminum Foam Sandwich Subject to Three-Point Bending. Materials, 17(3), 567. https://doi.org/10.3390/ma17030567





