Numerical Analysis of the Influence of Air Flow Rate on the Development of the Porous Structure of Activated Carbons Prepared from Macadamia Nut Shells
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, Y. Analysis of the impacts of carbon dioxide emission on climate change. Theor. Nat. Sci. 2023, 7, 120–127. [Google Scholar] [CrossRef]
- Song, J.; Tong, G.; Chao, J.; Chung, J.; Zhang, M.; Lin, W.; Zhang, T.; Bentler, P.M.; Zhu, W. Data driven pathway analysis and forecast of global warming and sea level rise. Sci. Rep. 2023, 13, 5536. [Google Scholar] [CrossRef]
- Pisciotta, M.; Pilorgé, H.; Feldmann, J.; Jacobson, R.; Davids, J.; Swett, S.; Sasso, Z.; Wilcox, J. Current state of industrial heating and opportunities for decarbonisation. Prog. Energ. Combust. Sci. 2022, 91, 100982. [Google Scholar] [CrossRef]
- Keller, D.P.; Lenton, A.; Littleton, E.W.; Oschlies, A.; Scott, V.; Vaughan, N.E. The effects of carbon dioxide removal on the carbon cycle. Curr. Clim. Change Rep. 2018, 4, 250–265. [Google Scholar] [CrossRef]
- Kim, S.; Ko, Y.; Lee, G.J.; Lee, J.W.; Xu, R.; Ahn, H.; Kang, Y.T. Sustainable energy harvesting from post-combustion CO2 capture using amine-functionalized solvents. Energy 2023, 267, 126532. [Google Scholar] [CrossRef]
- Khan, U.; Ogbaga, C.C.; Abiodun, O.-A.O.; Adeleke, A.A.; Ikubanni, P.P.; Okoye, P.U.; Okolie, J.A. Assessing absorption-based CO2 capture: Research progress and techno-economic assessment overview. Carbon Capture Sci. Technol. 2023, 8, 100125. [Google Scholar] [CrossRef]
- Underschultz, J.; Dodds, K.; Michael, K.; Sharma, S.; Wall, T.; Whittaker, S. Carbon capture and storage. In Sustainability in the Mineral and Energy Sectors; CRC Press: Boca Raton, FL, USA, 2016; pp. 437–452. [Google Scholar] [CrossRef]
- Cantador-Fernandez, D.; Suescum-Morales, D.; Esquivel, D.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 adsorption by ethane periodic mesoporous organosilica at low temperatures and high pressure. J. Environ. Chem. Eng. 2023, 11, 110582. [Google Scholar] [CrossRef]
- Maquíñez-Buitrago, D.F.; Ramos-Rincón, J.M.; Giraldo, L.; Moreno-Piraján, J.C. Carbon foams for CO2 adsorption: Synthesis, characterization and application. Hybrid Adv. 2024, 6, 100219. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.; Wei, X.; Gao, S.; Jiang, L.; Yi, Y. Study of CO2 adsorption on carbon aerogel fibers prepared by electrospinning. J. Environ. Manag. 2024, 349, 119432. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B. Activated carbons—Preparation, characterization and their application in CO2 capture: A review. Environ. Sci. Pollut. Res. 2024, 31, 40008–40062. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Zhang, H.; Zhou, T.; Xie, L.; Wang, B.; Jiang, X. Biomass-derived functional materials: Preparation, functionalization, and applications in adsorption and catalytic separation of carbon dioxide and other atmospheric pollutants. Sep. Purif. Technol. 2025, 354, 129099. [Google Scholar] [CrossRef]
- Siemak, J.; Mikołajczak, G.; Pol-Szyszko, M.; Michalkiewicz, B. Activated carbon for CO2 adsorption from avocado seeds activated with NaOH: The significance of the production method. Materials 2024, 17, 4157. [Google Scholar] [CrossRef]
- Liu, C.; Zhi, Y.; Yu, Q.; Tian, L.; Demir, M.; Colak, S.G.; Farghaly, A.A.; Wang, L.; Hu, X. Sulfur-enriched nanoporous carbon: A novel approach to CO2 adsorption. ACS Appl. Nano Mater. 2024, 7, 5434–5441. [Google Scholar] [CrossRef]
- Trinh, K.T.; Tsubota, T. Supercapacitors composed of Japanese cedar bark-based activated carbons with various activators. Mater. Chem. Phys. 2023, 307, 128148. [Google Scholar] [CrossRef]
- Tetteh, I.K.; Issahaku, I.; Tetteh, A.Y. Recent advances in synthesis, characterization, and environmental applications of activated carbons and other carbon derivatives. Carbon Trends 2024, 14, 100328. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Conv. Bioref. 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Castro, P.; Ferrarini, S.; Rimoli, M.; Merlo, A.; Nogueira, R.; Pires, E. Preparation and characterization of steam and CO2 activated carbon from Brazil nut shell. Biosci. J. 2023, 39, e39054. [Google Scholar] [CrossRef]
- Kumar, N.S.; Grekov, D.; Pré, P.; Alappat, B.J. Statistical approach to describe the properties of nanoporous carbons from lignin by chemical activation. Sustain. Mater. Technol. 2024, 40, e00939. [Google Scholar] [CrossRef]
- Ying, W.; Tian, S.; Liu, H.; Zhou, Z.; Kapeso, G.; Zhong, J.; Zhang, W. In situ dry chemical synthesis of nitrogen-doped activated carbon from bamboo charcoal for carbon dioxide adsorption. Materials 2022, 15, 763. [Google Scholar] [CrossRef]
- Yahia, E.H.; Serafin, J.; Román-Martínez, M.C.; Saidi, M.; Gallego, A.R.; Atlas, S.; Ouzzine, M. Valorization of argan paste cake waste: Enhanced CO2 adsorption on chemically activated carbon. J. Anal. Appl. Pyrol. 2024, 181, 106637. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kamińska, A.; Serafin, J.; Michalkiewicz, B. Chemical activation of banana peel waste-derived biochar using KOH and urea for CO2 capture. Materials 2024, 17, 872. [Google Scholar] [CrossRef]
- Nandi, R.; Jha, M.K.; Guchhait, S.K.; Sutradhar, D.; Yadav, S. Impact of KOH activation on rice husk derived porous activated carbon for carbon capture at flue gas alike temperatures with high CO2/N2 selectivity. ACS Omega 2023, 8, 4802–4812. [Google Scholar] [CrossRef]
- Saputro, E.; Wulan, V.; Winata, B.; Yogaswara, R.; Erliyanti, N. Process of activated carbon form coconut shells through chemical activation. Natur. Sci. J. Sci. Technol. 2020, 9, 23–28. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Wu, J.; Li, X.; Ma, H.; Zhou, J. Sequential H3PO4–CO2 assisted synthesis of lignin-derived porous carbon: CO2 activation kinetics investigation and textural properties regulation. Renew. Energy 2022, 191, 639–648. [Google Scholar] [CrossRef]
- Bai, J.; Huang, J.; Yu, Q.; Demir, M.; Kilic, M.; Altay, B.N.; Hu, X.; Wang, L. N-doped porous carbon derived from macadamia nut shell for CO2 adsorption. Fuel Process. Technol. 2023, 249, 107854. [Google Scholar] [CrossRef]
- Raffah, B.M.; Knani, S.; Bouzid, M.; Alruqi, A.B.; Vieira, Y.; Dotto, G.L.; Lefi, N.; Lamine, A.B. Morphological, sterical, and localized thermodynamics in the adsorption of CO2 by activated biocarbon from the white rot fungi Trametes gibbosa. Sci. Total Environ. 2024, 939, 173326. [Google Scholar] [CrossRef]
- Aouay, F.; Attia, A.; Dammak, L.; Ben Amar, R.; Deratani, A. Activated carbon prepared from waste coffee grounds: Characterization and adsorption properties of dyes. Materials 2024, 17, 3078. [Google Scholar] [CrossRef]
- Zayed, A.M.; Metwally, B.S.; Masoud, M.A.; Mubarak, M.F.; Shendy, H.; Wahed, M.S.M.A. From non-conventional agricultural waste into sustainable and eco-friendly activated carbon through specified thermo-chemical protocol. Appl. Nanosci. 2024, 14, 21–32. [Google Scholar] [CrossRef]
- Koli, A.; Battu, A.K.; Motkuri, R.K.; Sabale, S. Hierarchical porous activated carbon derived from agro-waste for potential CO2 capture and efficient dye removal applications. Biomass Conv. Bioref. 2024, 14, 10177–10188. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Shi, E.; Liu, S.; Zhang, S.; Zou, Y. Molecular dynamics study on structures and adsorption capacity of activated carbon prepared from corn straw and sewage sludge by molten salt method. Appl. Phys. A 2024, 130, 117. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, G.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G. A green strategy to prepare nitrogen-oxygen co-doped porous carbons from macadamia nut shells for post-combustion CO2 capture and supercapacitors. J. Anal. Appl. Pyrol. 2023, 171, 105952. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press Inc.: Cambridge, MA, USA, 1982. [Google Scholar] [CrossRef]
- Zelenka, T.; Horikawa, T.; Do, D.D. Artifacts and misinterpretations in gas physisorption measurements and characterization of porous solids. Adv. Colloid Interf. Sci. 2023, 311, 102831. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, M. Computer analysis of microporous structure by employing the LBET class models with various variants of the adsorption energy distribution in comparison to the classical equations. Langmuir 2007, 23, 2569–2581. [Google Scholar] [CrossRef]
- Kwiatkowski, M. Computer analyses of new numerical methods for the description of adsorption process and the reliability of identification of microporous structure parameters. J. Mol. Model. 2008, 14, 183–200. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Broniek, E.; Fierro, V.; Celzard, A. An evaluation of the impact of the amount of potassium hydroxide on the porous structure development of activated carbons. Materials 2021, 14, 2045. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, M.; Kalderis, D.; Tono, W.; Tsubota, T. Numerical analysis of the micropore structure of activated carbons focusing on optimum CO2 adsorption. J. CO2 Utiliz. 2022, 60, 101996. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Cansado, I.P.d.P.; Mourão, P.M. numerical analysis of the porous structure of activated carbons derived from synthetic polymers. Materials 2024, 17, 3122. [Google Scholar] [CrossRef]
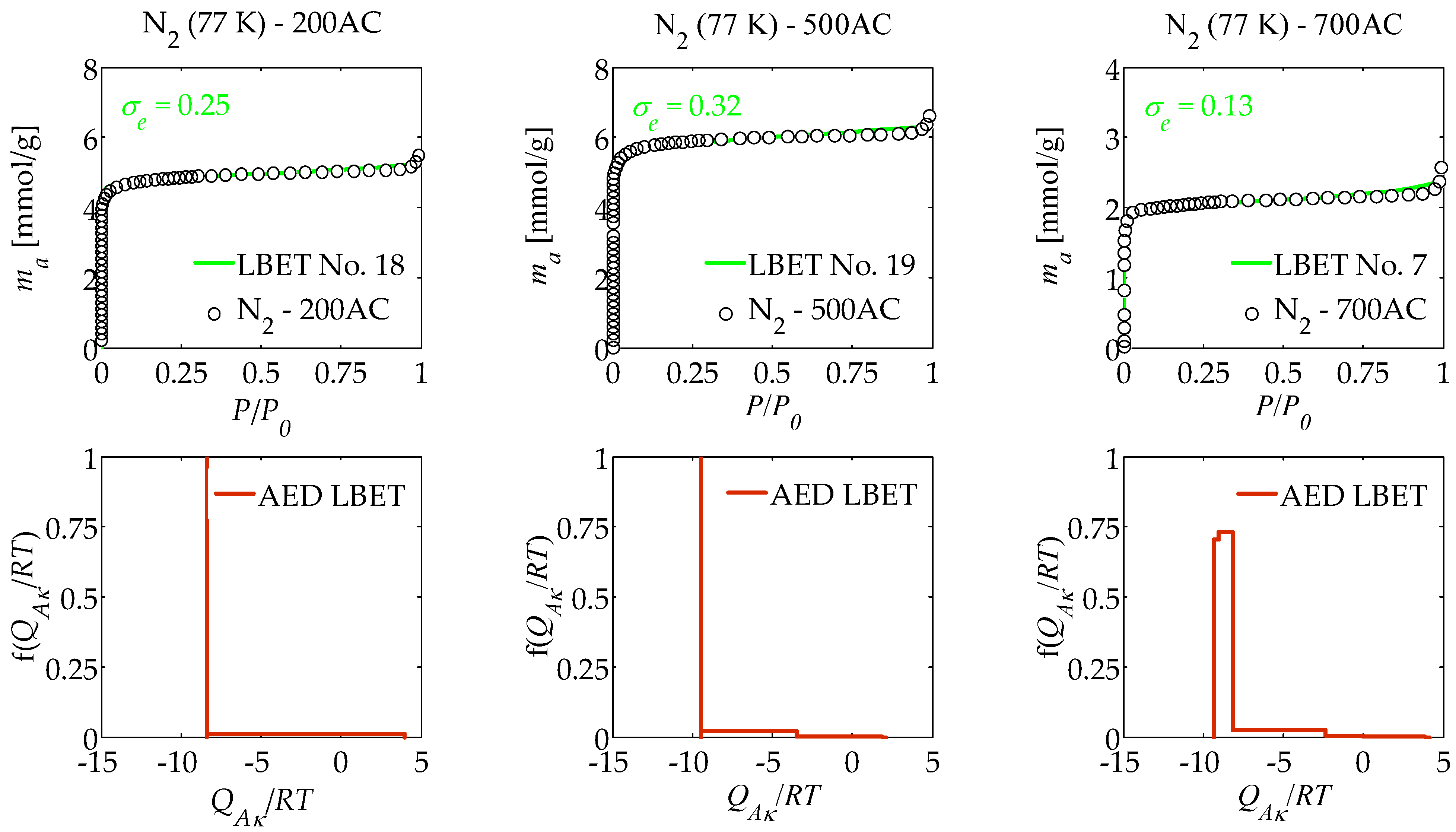
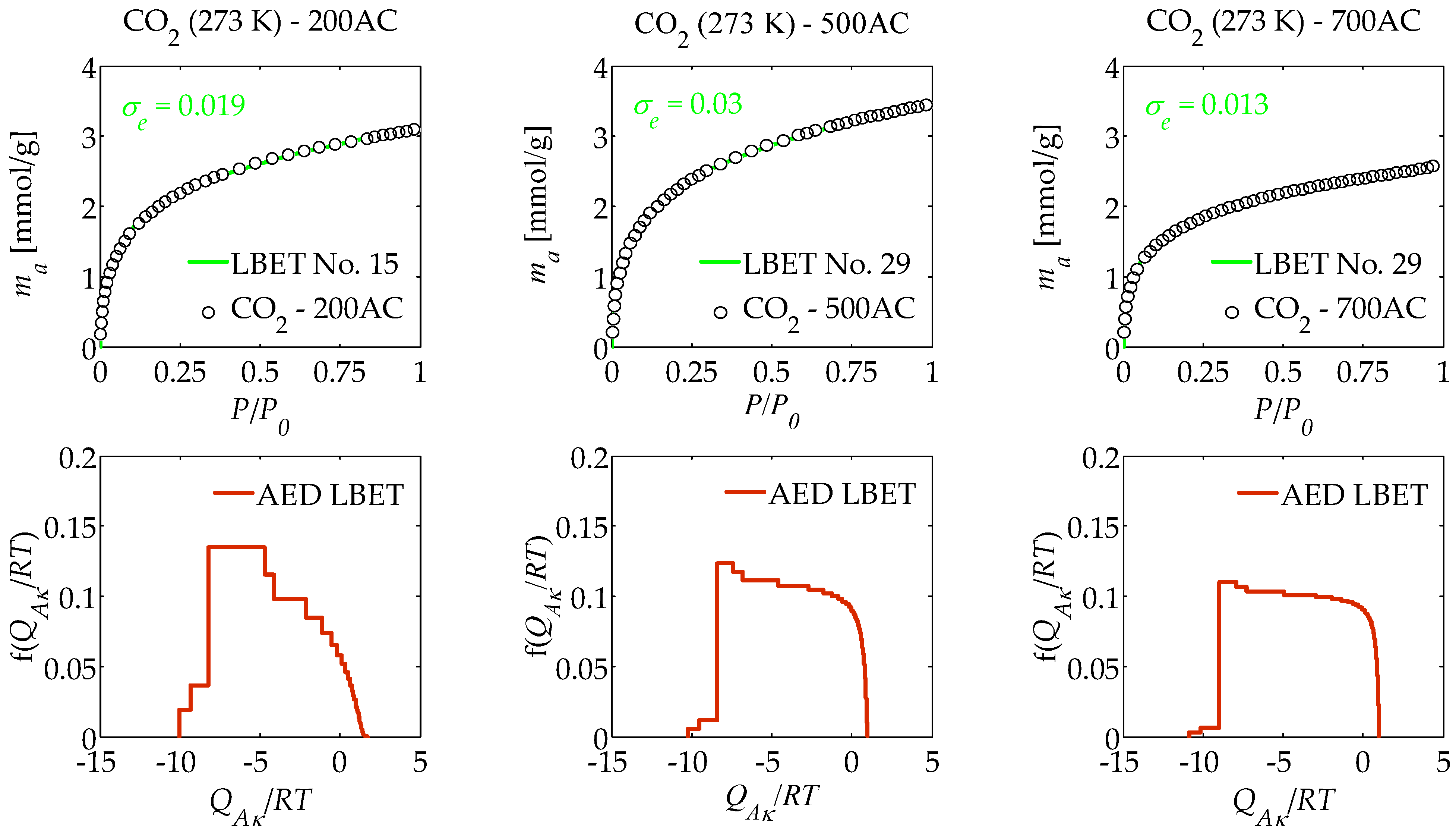
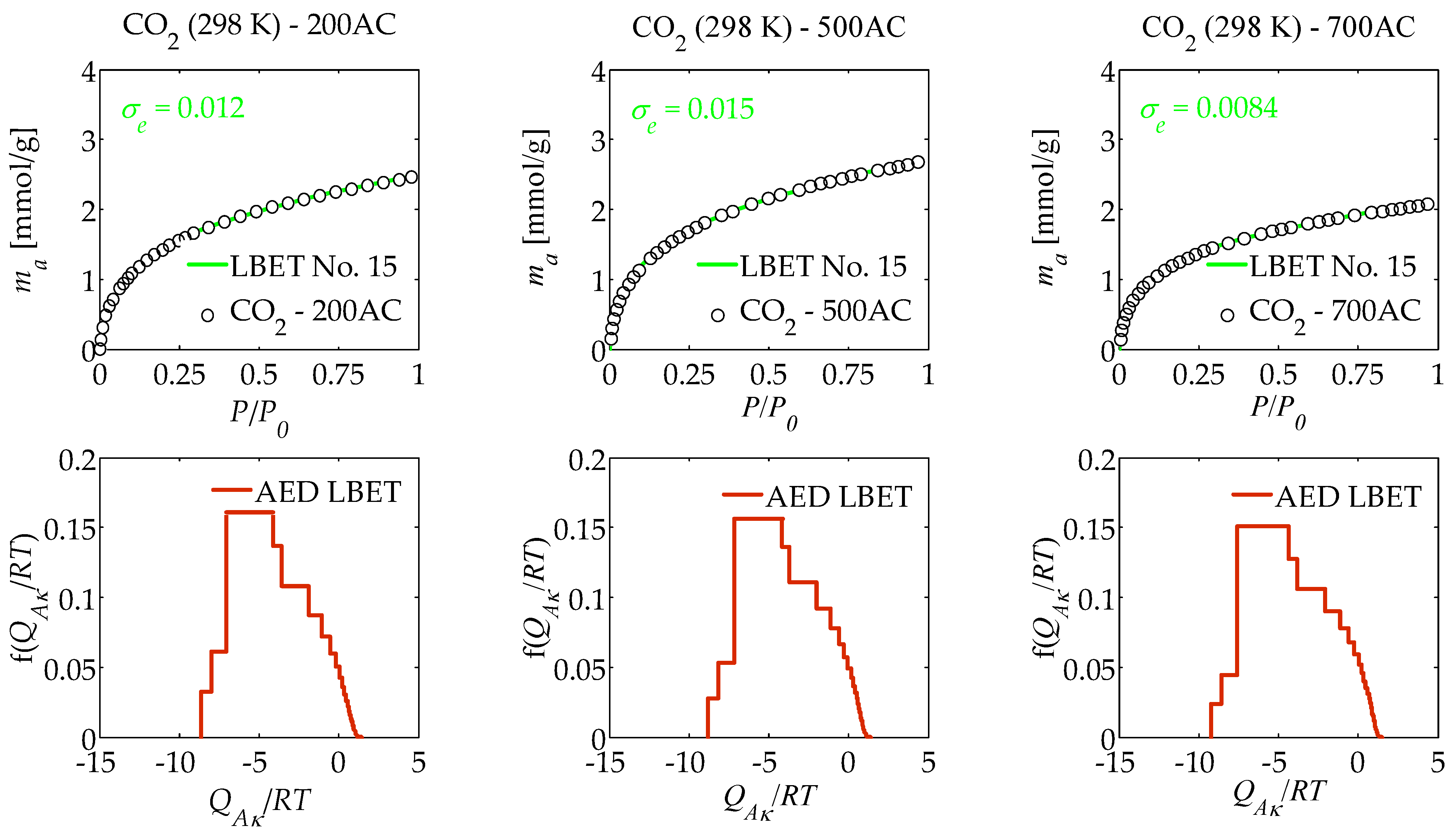
| AC | Model No. | VhA [cm3/g] | α | β | QA/RT | BC | h | σe | wid |
|---|---|---|---|---|---|---|---|---|---|
| 200AC | 18 | 0.161 | 0.14 | 2.37 | −8.42 | 3.94 | 2 | 0.25 | 0.81 |
| 500AC | 19 | 0.179 | 0.14 | 1.26 | −9.48 | 1.00 | 3 | 0.32 | 0.47 |
| 700AC | 7 | 0.642 | 0.18 | 3.33 | −9.33 | 7.73 | 5 | 0.13 | 0.52 |
| AC | Model No. | VhA [cm3/g] | α | β | QA/RT | BC | h | σe | wid |
|---|---|---|---|---|---|---|---|---|---|
| 200AC | 15 | 0.211 | 0.90 | 5.11 | −10.03 | 2.07 | 9 | 0.019 | 0.43 |
| 500AC | 29 | 0.246 | 0.98 | 2.65 | −10.22 | 1.00 | 9 | 0.03 | 0.19 |
| 700AC | 29 | 0.185 | 0.99 | 2.22 | −10.89 | 1.00 | 9 | 0.013 | 0.21 |
| AC | Model No. | VhA [cm3/g] | α | β | QA/RT | BC | h | σe | wid |
|---|---|---|---|---|---|---|---|---|---|
| 200AC | 15 | 0.170 | 0.85 | 4.89 | −8.66 | 1.51 | 9 | 0.012 | 0.81 |
| 500AC | 15 | 0.184 | 0.87 | 5.20 | −8.80 | 1.41 | 9 | 0.015 | 0.73 |
| 700AC | 15 | 0.143 | 0.89 | 4.74 | −9.25 | 1.63 | 9 | 0.0084 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowski, M.; Zhang, G. Numerical Analysis of the Influence of Air Flow Rate on the Development of the Porous Structure of Activated Carbons Prepared from Macadamia Nut Shells. Materials 2024, 17, 6264. https://doi.org/10.3390/ma17246264
Kwiatkowski M, Zhang G. Numerical Analysis of the Influence of Air Flow Rate on the Development of the Porous Structure of Activated Carbons Prepared from Macadamia Nut Shells. Materials. 2024; 17(24):6264. https://doi.org/10.3390/ma17246264
Chicago/Turabian StyleKwiatkowski, Mirosław, and Guojie Zhang. 2024. "Numerical Analysis of the Influence of Air Flow Rate on the Development of the Porous Structure of Activated Carbons Prepared from Macadamia Nut Shells" Materials 17, no. 24: 6264. https://doi.org/10.3390/ma17246264
APA StyleKwiatkowski, M., & Zhang, G. (2024). Numerical Analysis of the Influence of Air Flow Rate on the Development of the Porous Structure of Activated Carbons Prepared from Macadamia Nut Shells. Materials, 17(24), 6264. https://doi.org/10.3390/ma17246264







