On the Possibility of the Deformation of Mg and Alloys Without Preheating of Initial Billets: Understanding Their Corrosion Performance via Electrochemical Tests
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- KoBo extrusion enables the deformation of Mg and its alloys without preheating of the initial billet. The obtained materials are without cracking and reduce the diameter of the ingots significantly, resulting in the production of long elements, such as rods or wires. The next step of the KoBo improvement should be research on the possibility of thin-walled structure fabrication;
- KoBo extrusion improves the corrosion resistance of the initial ingots of Mg and its alloys; however, there is no clear relationship between grain refinement and corrosion resistance improvement, and smaller grain size does not always lead to higher corrosion resistance. The properties of the specific alloy must be treated as a separate case, since the intensity of the dynamic recrystallization is related to the extrusion ratio and strain induced by the reversible die;
- The corrosion resistance of the KoBo-extruded alloys depends mainly on the alloy chemistry and its microstructure; however, it is also dominated by the formation of stresses during extrusion with an oscillating die. Importantly, we do not have any clear information about the specific temperature inside the extruder. The effect of the internal heating resulting from the friction of the material and the extruder during processing should be further investigated;
- In the current research on magnesium alloys, the imperative issue of balancing the structural characteristics and mechanical properties of the alloys remains to be resolved. A reduction in the deformation temperature of Mg alloys will generate savings and reduce CO2 gas emissions, which is crucial to ensure the sustainable growth of industry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atrens, A.; Song, G.L.; Cao, F.; Shi, Z.; Bowen, P.K. Advances in Mg Corrosion and Research Suggestions. J. Magnes. Alloys 2013, 1, 177–200. [Google Scholar] [CrossRef]
- Atrens, A.; Song, G.L.; Liu, M.; Shi, Z.; Cao, F.; Dargusch, M.S. Review of Recent Developments in the Field of Magnesium Corrosion. Adv. Eng. Mater. 2015, 17, 400–453. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the Relationship between Grain Size and Corrosion Rate of Metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Bahmani, A.; Arthanari, S.; Shin, K.S. Formulation of Corrosion Rate of Magnesium Alloys Using Microstructural Parameters. J. Magnes. Alloys 2020, 8, 134–149. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S.; Cohen, S. The Relation between Microstructure and Corrosion Behavior of Mg-Y-RE-Zr Alloys. J. Alloys Compd. 2007, 431, 269–276. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B.P. Influence of Chloride, Sulfate and Bicarbonate Anions on the Corrosion Behavior of AZ31 Magnesium Alloy. J. Alloys Compd. 2010, 496, 500–507. [Google Scholar] [CrossRef]
- Miyamoto, H. Corrosion of Ultrafine Grained Materials by Severe Plastic Deformation, an Overview. Mater. Trans. 2016, 57, 559–572. [Google Scholar] [CrossRef]
- Hoog, C.O.; Birbilis, N.; Estrin, Y. Corrosion of Pure Mg as a Function of Grain Size and Processing Route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Jiang, B.; Xiang, Q.; Atrens, A.; Song, J.; Pan, F. Influence of Crystallographic Texture and Grain Size on the Corrosion Behaviour of As-Extruded Mg Alloy AZ31 Sheets. Corros. Sci. 2017, 126, 374–380. [Google Scholar] [CrossRef]
- Gollapudi, S. Grain Size Distribution Effects on the Corrosion Behaviour of Materials. Corros. Sci. 2012, 62, 90–94. [Google Scholar] [CrossRef]
- Shan, Z.; Yang, J.; Fan, J.; Zhang, H.; Zhang, Q.; Wu, Y.; Dong, H. Microstructure Evolution and Mechanical Properties of an AZ61 Alloy Processed with TS-ECAP and EPT. Mater. Sci. Eng. A 2020, 780, 139195. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Zainal Abidin, N.I. Corrosion Mechanism Applicable to Biodegradable Magnesium Implants. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Lunder, O.; Lein, J.E.; Aune, T.K.; Nisancioglu, K. Role of Mg17Al12phase in the Corrosion of Mg Alloy AZ91. Corrosion 1989, 45, 741–748. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Feliú, S. Influence of Microstructure and Composition on the Corrosion Behaviour of Mg/Al Alloys in Chloride Media. Electrochim. Acta 2008, 53, 7890–7902. [Google Scholar] [CrossRef]
- Ubeda, C.; Garces, G.; Adeva, P.; Llorente, I.; Frankel, G.S.; Fajardo, S. The Role of the Beta-Mg17Al12 Phase on the Anomalous Hydrogen Evolution and Anodic Dissolution of AZ Magnesium Alloys. Corros. Sci. 2020, 165, 108384. [Google Scholar] [CrossRef]
- Zeng, R.C.; Sun, L.; Zheng, Y.F.; Cui, H.Z.; Han, E.H. Corrosion and Characterisation of Dual Phase Mg-Li-Ca Alloy in Hank’s Solution: The Influence of Microstructural Features. Corros. Sci. 2014, 79, 69–82. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk-Cieślak, B.; Kubásek, J.; Vojtěch, D.; Kuc, D.; Hadasik, E.; Mizera, J. Microstructure and Corrosion Resistance of a Duplex Structured Mg–7.5Li–3Al–1Zn. J. Magnes. Alloys 2021, 9, 467–477. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Kalita, D.; Maziarz, W.; Tański, T.; Borek, W.; Ostachowski, P.; Faryna, M. Effect of KOBO Extrusion and Following Cyclic Forging on Grain Refinement of Mg–9Li–2Al–0.5Sc Alloy. Met. Mater. Int. Korean Inst. Met. Mater. 2020, 26, 1004–1014. [Google Scholar] [CrossRef]
- Bochniak, W.; Korbel, A. KOBO Type Forming: Forging of Metals under Complex Conditions of the Process. J. Mater. Process. Technol. 2003, 134, 120–134. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk-cie, B.; Aydee, M.; Garcia, G. Effect of High Deformation without Preheating on Microstructure and Corrosion of Pure Mg. Metals 2024, 14, 949. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk-Cieślak, B.; Chlewicka, M.; Towarek, A.; Zielińska, A.; Koralnik, M.; Kuc, D.; Mizera, J. Evolution of Microstructure Dependent Corrosion Properties of Ultrafine AZ31 under Conditions of Extrusion with a Forward Backward Oscillating Die. J. Mater. Res. Technol. 2022, 18, 4486–4496. [Google Scholar] [CrossRef]
- Dobkowska, A.; Zielińska, A.; Donik, Č.; Łojkowski, M.; Adamczyk-Cieślak, B. Microstructure and Properties of an AZ61 Alloy after Extrusion with a Forward-Backward Oscillating Die without Preheating of the Initial Billet. J. Alloys Compd. 2023, 952, 169843. [Google Scholar] [CrossRef]
- Dobkowska, A.; Koralnik, M.; Adamczyk-Cieślak, B.; Kuc, D.; Chromiński, W.; Kubasek, J.; Mizera, J. The Effect of Extrusion Ratio on the Corrosion Resistance of Ultrafine-Grained Mg-4Li-3Al-Zn Alloy Deformed Using Extrusion with a Forward-Backward Oscillating Die. J. Mater. Eng. Perform. 2022, 31, 8932–8939. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk-Cieślak, B.; Koralnik, M.; Chromiński, W.; Kubasek, J.; Ciftci, J.; Kuc, D.; Mizera, J. Corrosion Behavior of Fine-Grained Mg-7.5Li-3Al-1Zn Fabricated by Extrusion with a Forward-Backward Rotating Die (KoBo). J. Magnes. Alloys 2021, 10, 811–820. [Google Scholar] [CrossRef]
- Dobkowska, A.; Zielińska, A.; Paulin, I.; Donik, C.; Koralnik, M.; Adamczyk-Cieślak, B.; Wieczorek-Czarnocka, M.; Kuc, D.; Kubasek, J.; Mikuszewski, T.; et al. Microstructural, Corrosion and Mechanical Properties of a WE43 Alloy: Conventional Extrusion versus SPD. J. Alloys Compd. 2024, 976, 173090. [Google Scholar] [CrossRef]
- Kirkland, N.; Birbilis, N.; Staiger, M. Assessing the Corrosion of Biodegradable Magnesium Implants: A Critical Review of Current Methodologies and Their Limitations. Acta Biomater. 2012, 61, 65–69. [Google Scholar] [CrossRef]
- Birbilis, N.; King, A.D.; Thomas, S.; Frankel, G.S.; Scully, J.R. Evidence for Enhanced Catalytic Activity of Magnesium Arising from Anodic Dissolution. Electrochim. Acta 2014, 132, 277–283. [Google Scholar] [CrossRef]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. Testing of General and Localized Corrosion of Magnesium Alloys: A Critical Review. J. Mater. Eng. Perform. 2004, 13, 517–529. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of Mechanical Properties and Corrosion Resistance of Mg-Ca Alloys through Microstructural Refinement by Indirect Extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Esmailzadeh, S.; Aliofkhazraei, M.; Sarlak, H. Interpretation of Cyclic Potentiodynamic Polarization Test Results for Study of Corrosion Behavior of Metals: A Review. Prot. Met. Phys. Chem. Surfaces 2018, 54, 976–989. [Google Scholar] [CrossRef]
- Benedetti, A.; Cirisano, F.; Delucchi, M.; Faimali, M.; Ferrari, M. Potentiodynamic Study of Al-Mg Alloy with Superhydrophobic Coating in Photobiologically Active/Not Active Natural Seawater. Colloids Surfaces B Biointerfaces 2016, 137, 167–175. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; A Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Chen, X.B.; Wang, B.J.; Wu, R.Z.; Han, E.H.; Birbilis, N. Composition and Microstructure Dependent Corrosion Behaviour of Mg-Li Alloys. Electrochim. Acta 2018, 260, 55–64. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Electrochemical Corrosion Behavior of Biodegradable Mg-Y-RE and Mg-Zn-Zr Alloys in Ringer’s Solution and Simulated Body Fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]
- Xu, D.K.; Han, E.H. Effect of Quasicrystalline Phase on Improving the Corrosion Resistance of a Duplex Structured Mg-Li Alloy. Scr. Mater. 2014, 71, 21–24. [Google Scholar]
- Tian, G.; Wang, J.; Xue, C.; Wang, S.; Yang, X.; Su, H.; Li, Q.; Li, X.; Yan, C.; Yang, Z. Improving Corrosion Resistance of Mg–Li Alloys by Sn Microalloying. J. Mater. Res. Technol. 2023, 26, 199–217. [Google Scholar] [CrossRef]
- Ma, H.; Wu, L.; Liu, C.; Liu, M.; Wang, C.; Li, D.; Chen, X.Q.; Dong, J.; Ke, W. First-Principles Modeling of the Hydrogen Evolution Reaction and Its Application in Electrochemical Corrosion of Mg. Acta Mater. 2020, 183, 377–389. [Google Scholar] [CrossRef]
- Bahmani, A.; Lotfpour, M.; Taghizadeh, M.; Kim, W.J. Corrosion Behavior of Severely Plastically Deformed Mg and Mg Alloys. J. Magnes. Alloys 2022, 10, 2607–2648. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M. Corrosion of Magnesium (Mg) Alloys and Metallurgical Influence. Corros. Magnes. Alloys 2011, 117–165. [Google Scholar] [CrossRef]
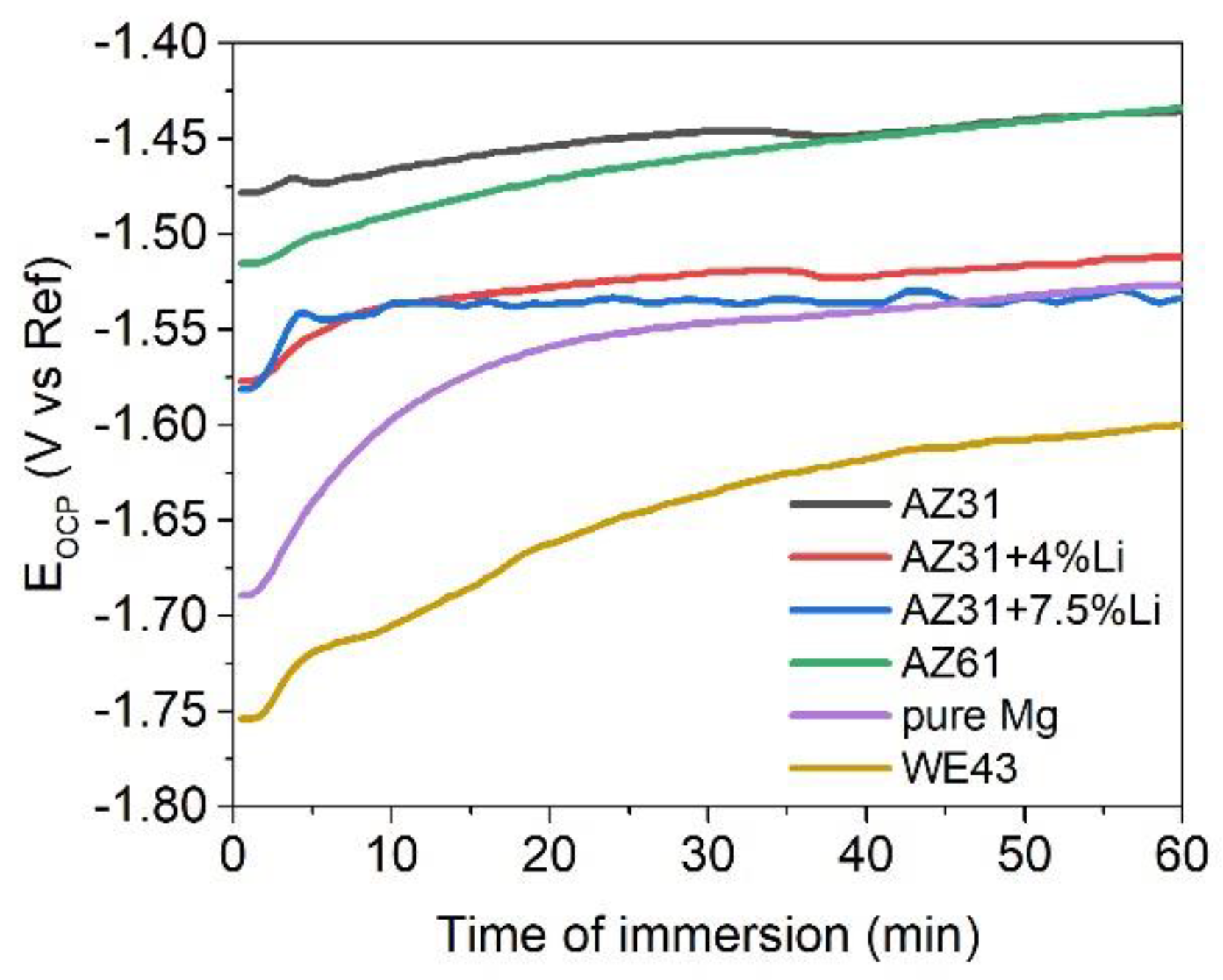

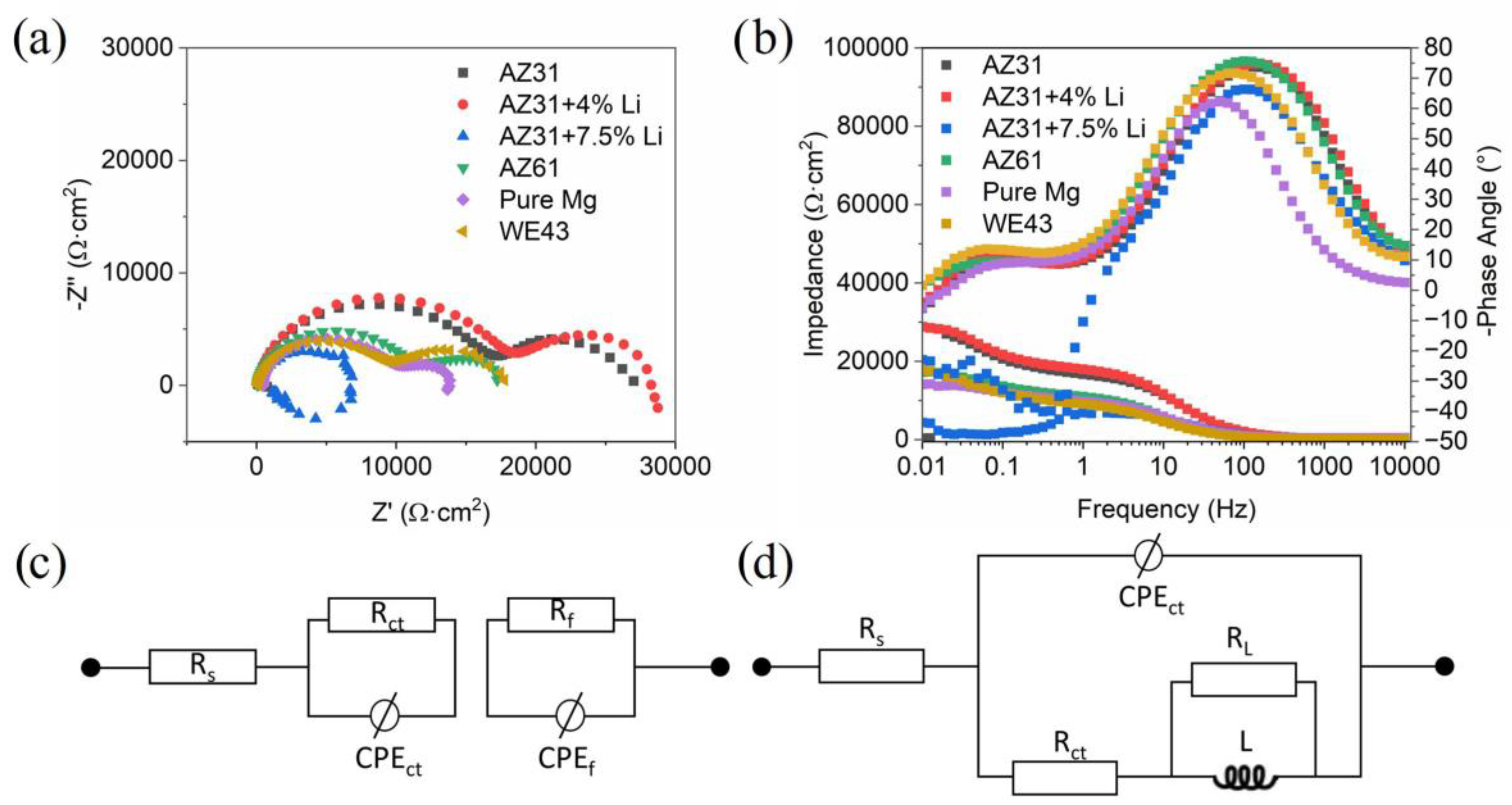
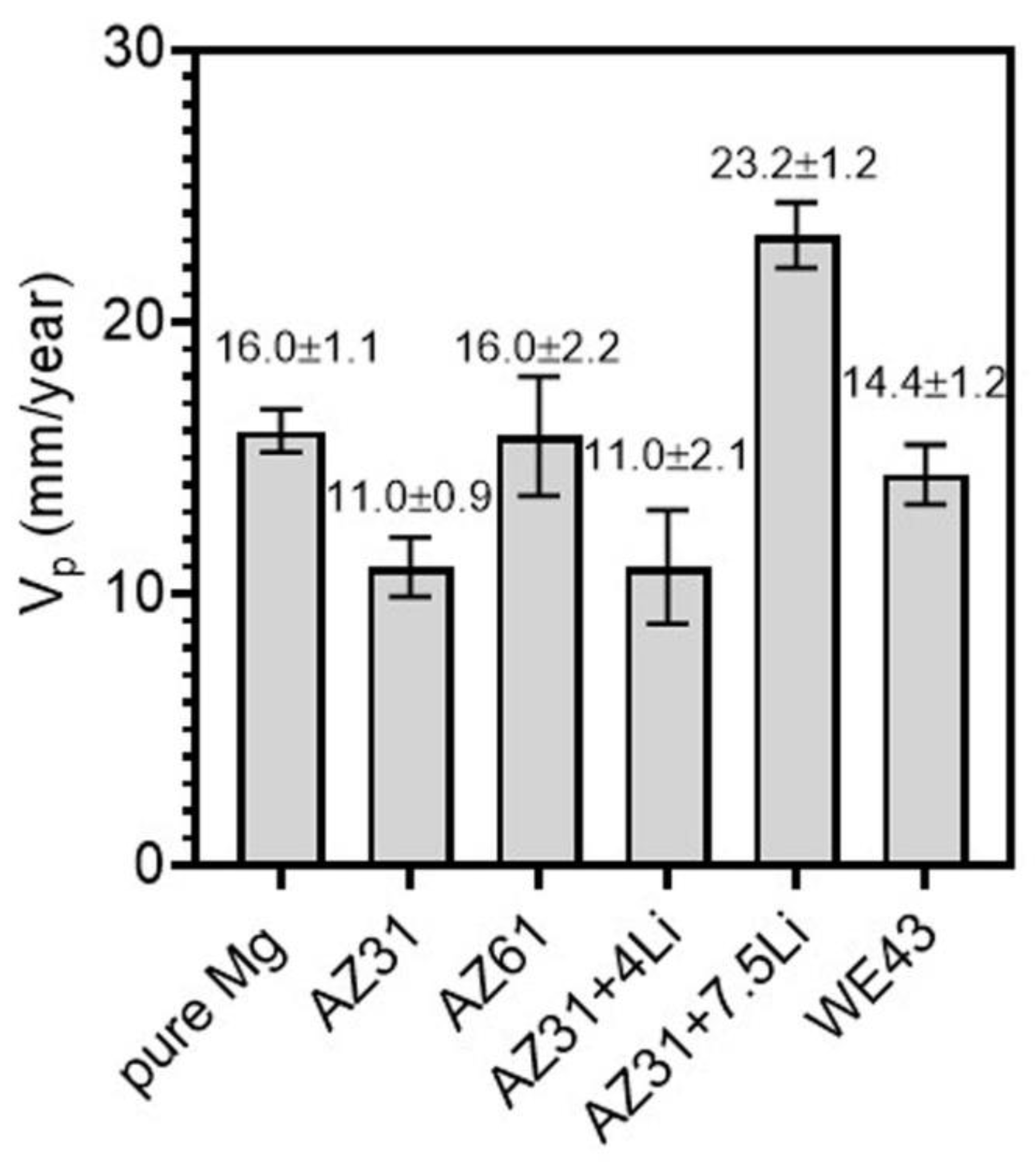
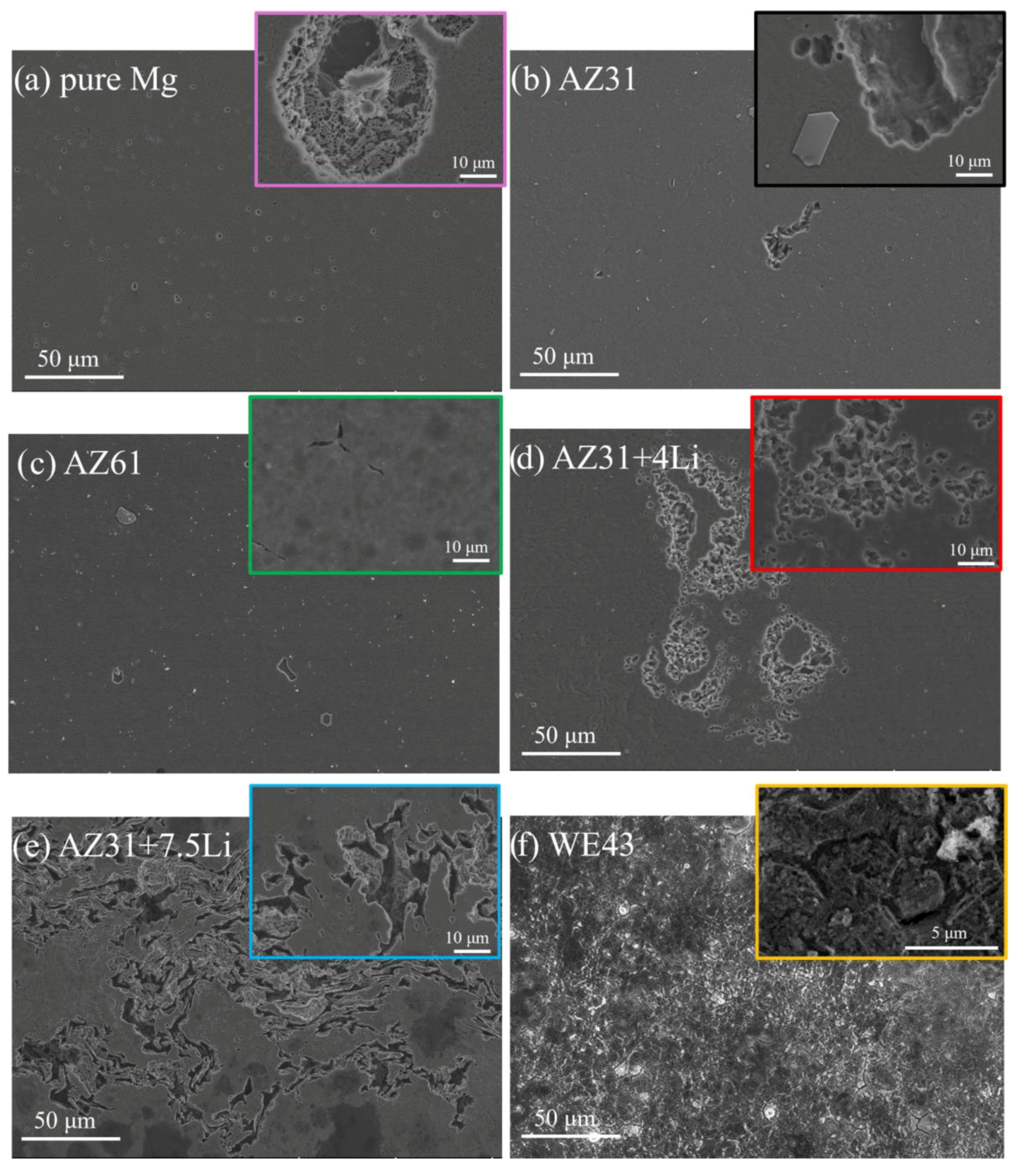
| Materials | Pure Mg | AZ31 | AZ61 | AZ31+4Li | AZ31+7.5Li | WE43 |
|---|---|---|---|---|---|---|
| Extrusion ratio | 7:1 | 10:1 | 7:1 | 10:1 | 10:1 | 5:1 |
| Parameters | Pure Mg | AZ31 | AZ61 | AZ31+4Li | AZ31+7.5Li | WE43 |
|---|---|---|---|---|---|---|
| Ecorr (V vs. Ref) | −1.54 | −1.39 | −1.44 | −1.51 | −1.58 | −1.57 |
| Eb (V vs. Ref) | −1.34 | −0.56 | - | −1.33 | −1.45 | −0.94 |
| ∆E | 0.20 | 0.83 | - | 0.18 | 0.13 | 0.63 |
| Materials | Rs (Ω∙cm2) | Rct (Ω∙cm2) | CPEct (µSsa/cm2) | a1 | RL (Ω∙cm2) | L (H∙cm2) | Rf (Ω∙cm2) | CPEf (µSsa/cm2) | a2 |
|---|---|---|---|---|---|---|---|---|---|
| Pure Mg | 61 | 1242 | 0.000018 | 0.94 | N/A | N/A | 634 | 0.000840 | 0.84 |
| AZ31 | 22 | 2266 | 0.000008 | 0.85 | N/A | N/A | 1420 | 0.000120 | 0.86 |
| AZ61 | 10 | 1454 | 0.000019 | 0.94 | N/A | N/A | 1016 | 0.001688 | 072 |
| AZ31+4Li | 17 | 2357 | 0.000009 | 0.93 | N/A | N/A | 1603 | 0.000155 | 0.71 |
| AZ31+7.5Li | 17 | 183 | 0.000025 | 0.88 | 691 | 182 | N/A | N/A | N/A |
| WE43 | 14 | 1201 | 0.000027 | 0.92 | N/A | N/A | 1294 | 0.001756 | 0.76 |
| Material | Phase Structure | Grain Size (µm) | Second Phases | Grain Orientation | Ref. |
|---|---|---|---|---|---|
| Pure Mg | α(Mg) phase | 3.88 µm; uniformly distributed | N/A | (10 and (2110) | [21] |
| AZ31 | α(Mg) phase | 4.38 µm; uniformly distributed | Coarse Al5Mn8 | (10) and (2110) with some randomly present (0001) | [21] |
| AZ61 | α(Mg) phase | 6.6 µm uniformly distributed | Coarse Al5Mn8 and nano β-Mg17Al12 located at grain boundaries | (10) and (2110) | [22] |
| AZ31+4Li | α(Mg) phase | 3.24 µm; uniformly distributed | Coarse AlLi and β-Mg17Al12 (nano) distributed in the grain interiors | (10) and (2110) | [23] |
| AZ31+7.5Li | α(Mg)+β(Li) | No EBSD data, based on SEM a few µm | Coarse AlLi and MgLi2Al with size around 1 µm, β-Mg17Al12 (nano) distributed in the grain interiors | No EBSD data | [24] |
| WE43 | α(Mg) phase | 3.3 µm; uniformly distributed | Mg41Nd5, Mg24Y5, Mg12Nd, and nano ternary Mg-Nd-Y | Random | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobkowska, A.; Kubasek, J. On the Possibility of the Deformation of Mg and Alloys Without Preheating of Initial Billets: Understanding Their Corrosion Performance via Electrochemical Tests. Materials 2024, 17, 6182. https://doi.org/10.3390/ma17246182
Dobkowska A, Kubasek J. On the Possibility of the Deformation of Mg and Alloys Without Preheating of Initial Billets: Understanding Their Corrosion Performance via Electrochemical Tests. Materials. 2024; 17(24):6182. https://doi.org/10.3390/ma17246182
Chicago/Turabian StyleDobkowska, Anna, and Jiri Kubasek. 2024. "On the Possibility of the Deformation of Mg and Alloys Without Preheating of Initial Billets: Understanding Their Corrosion Performance via Electrochemical Tests" Materials 17, no. 24: 6182. https://doi.org/10.3390/ma17246182
APA StyleDobkowska, A., & Kubasek, J. (2024). On the Possibility of the Deformation of Mg and Alloys Without Preheating of Initial Billets: Understanding Their Corrosion Performance via Electrochemical Tests. Materials, 17(24), 6182. https://doi.org/10.3390/ma17246182







