Magnetic and Biomedical Properties of Iron Nanoparticles Synthesized Using Vitex agnus-castus Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Vitex agnus-castus Plant
2.2. Green Synthesis of FeNPs
2.3. Characterization of FeNPs
2.4. Assessment of Cytotoxicity and Anticancer Potential of Green-Synthesized FeNPs
3. Results and Discussions
3.1. UV-Vis Spectrophotometry Analysis
3.2. Fourier-Transform Infrared Spectroscopy Analysis
3.3. Scanning Electron Microscopy Analysis
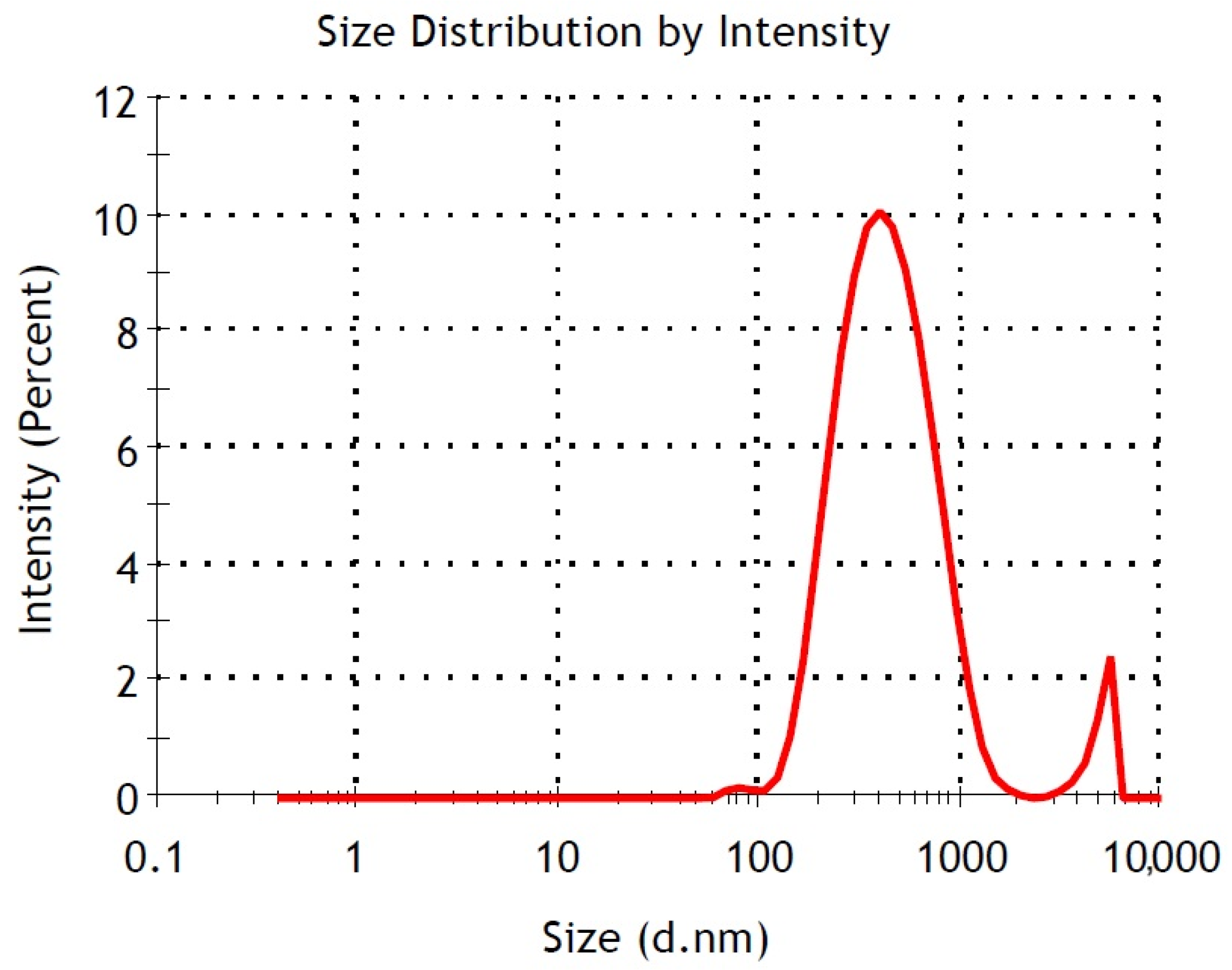
3.4. Particle Size (Zetasizer) Analysis
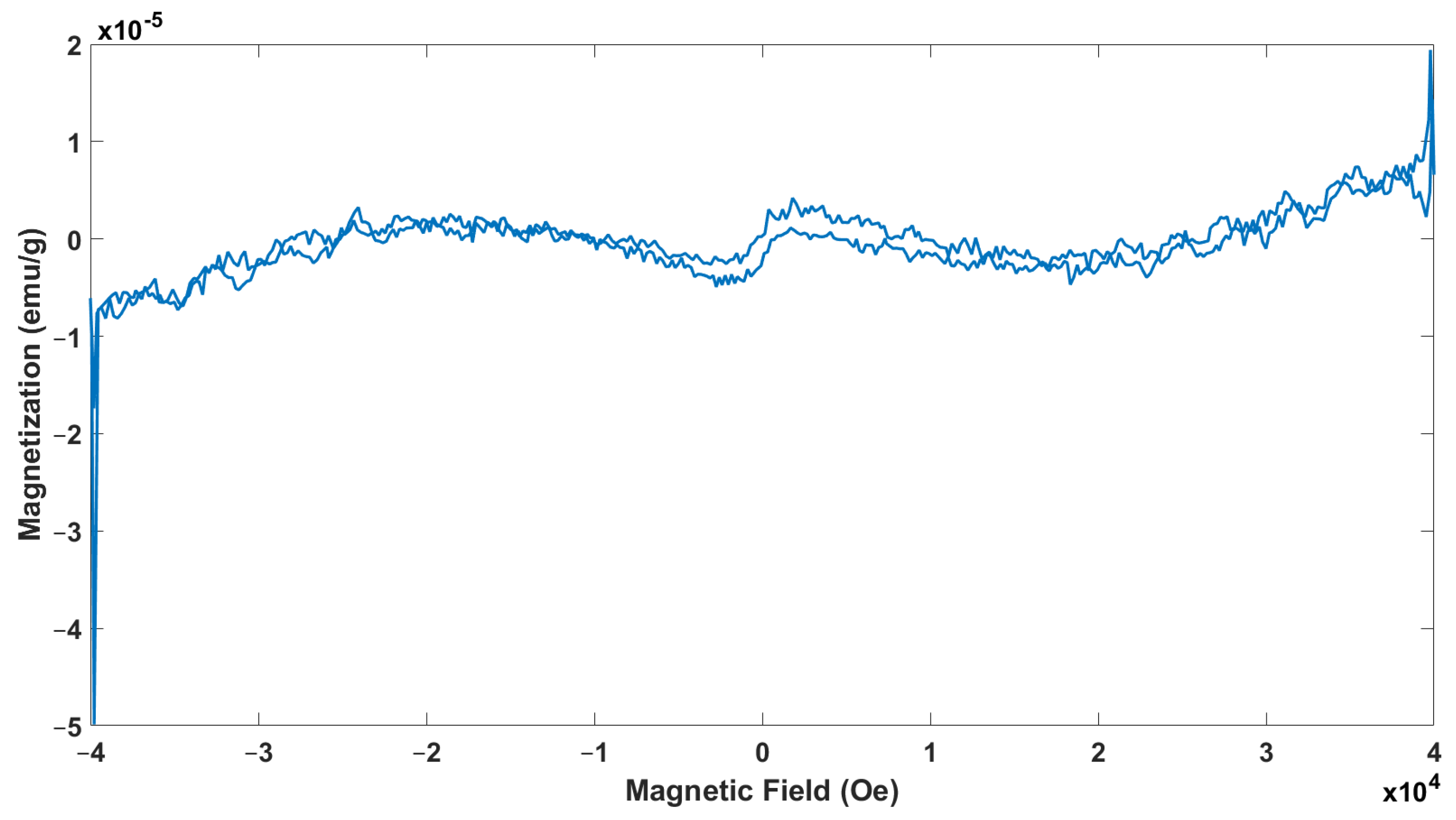
3.5. Physical Property Measurement System Analysis
3.6. Electron Spin Resonance Spectroscopy Analysis
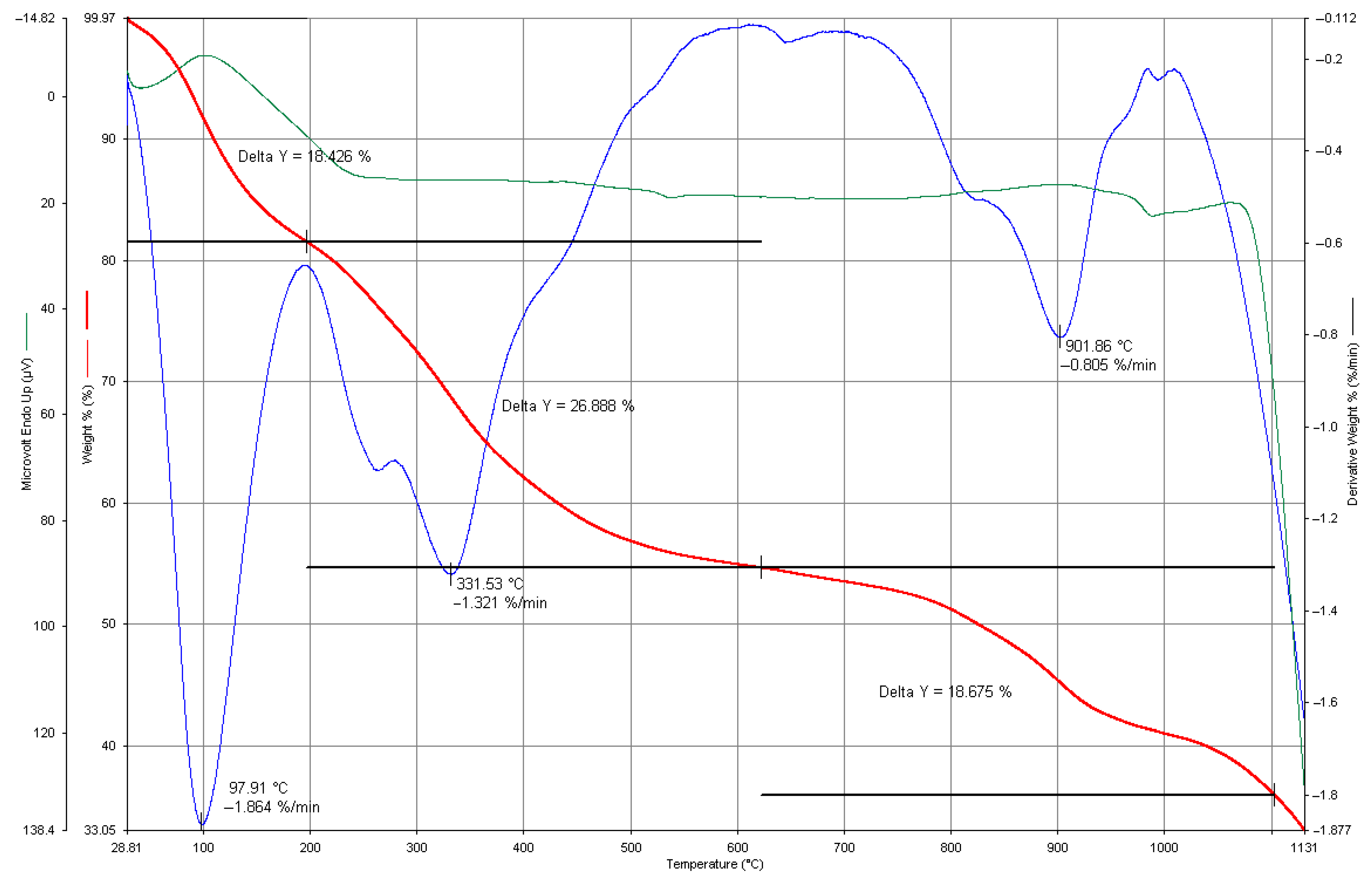
3.7. Thermogravimetric Analysis
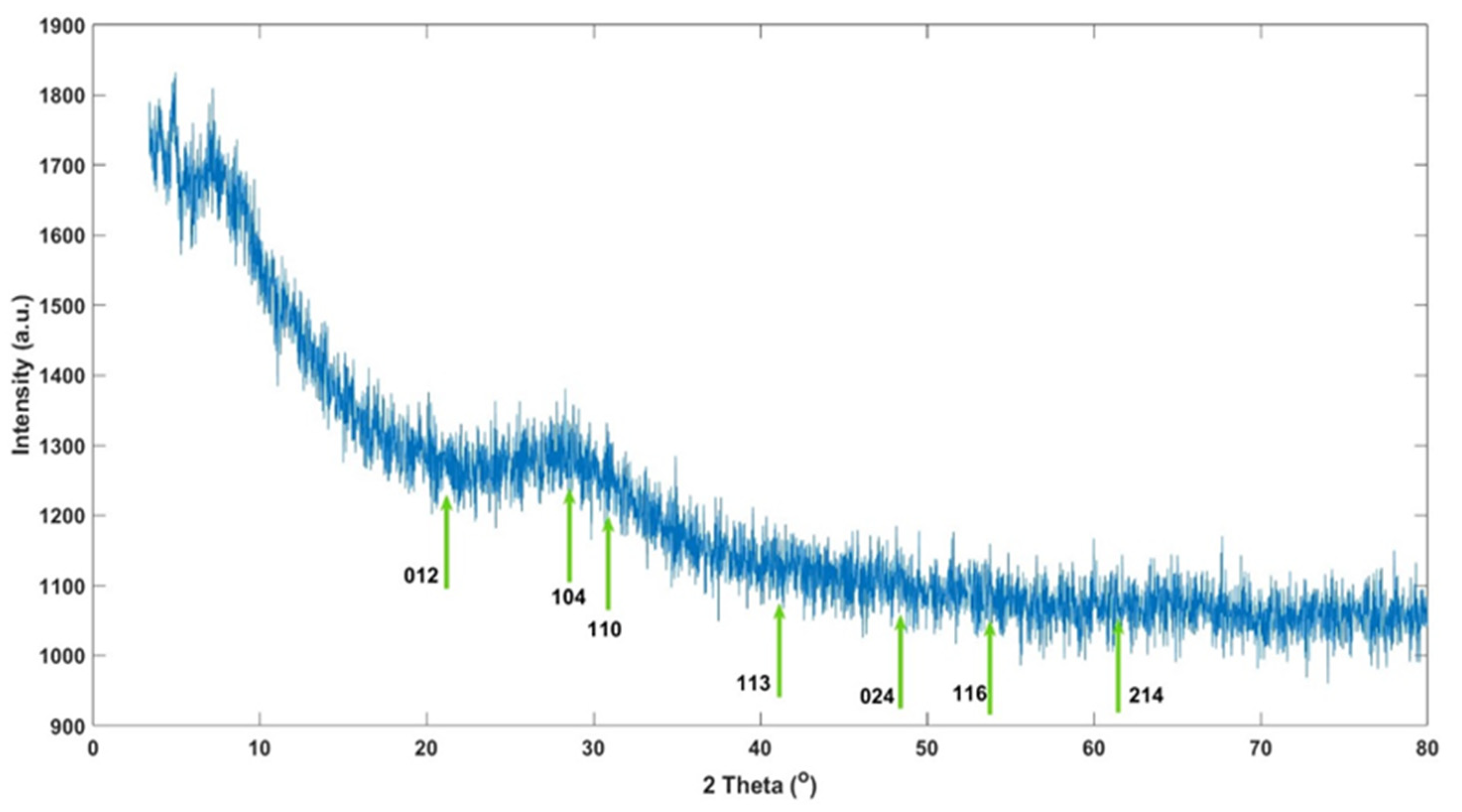
3.8. X-Ray Diffraction Analysis
3.9. Assessment of Cytotoxicity and Anticancer Activity in Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.; Kim, D.I.; Kim, J.Y.; Pané, S.; Nelson, B.J.; Chang, Y.T.; Choi, H. Magnetically Enhanced Intracellular Uptake of Superparamagnetic Iron Oxide Nanoparticles for Antitumor Therapy. ACS Nano 2023, 17, 15857–15870. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Niu, J.; Deng, L.; Yu, Y.; Zhang, L.; Chen, Q.; Zhao, J.; Wang, B.; Gao, H. Amphiphilic polymeric nanodrug integrated with superparamagnetic iron oxide nanoparticles for synergistic antibacterial and antitumor therapy of colorectal cancer. Acta Biomater. 2024, 173, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Morishita, S.; Higashinaka, R.; Matsuda, T.D.; Aoki, Y.; Kuzmann, E.; Homonnay, Z.; Katalin, S.; Pavić, L.; Kubuki, S. Synthesis, characterization and magnetic properties of ε-Fe2O3 nanoparticles prepared by sol-gel method. J. Magn. Magn. Mater. 2021, 538, 168264. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Cruz, M.M.; Thanh, N.T.; et al. Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: New insights and continuous production via flow chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Kong, H.; Kim, H.; Hwang, S.; Mun, J.; Yeo, J. Laser-Induced Hydrothermal Growth of Iron Oxide Nanoparticles on Diverse Substrates for Flexible Micro-Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 4102–4111. [Google Scholar] [CrossRef]
- Struchalin, P.G.; Thon, H.; Kuzmenkov, D.M.; Kutsenko, K.V.; Kosinski, P.; Balakin, B.V. Solar steam generation enabled by iron oxide nanoparticles: Prototype experiments and theoretical model. Int. J. Heat Mass Transf. 2020, 158, 119987. [Google Scholar] [CrossRef]
- Fatima, G.; Bibi, I.; Majid, F.; Kamal, S.; Nouren, S.; Ghafoor, A.; Raza, Q.; Al-Mijalli, S.H.; Alnafisi, N.M.; Iqbal, M. Mn-doped BaFe12O19 nanoparticles synthesis via micro-emulsion route: Solar light-driven photo-catalytic degradation of CV, MG and RhB dyes and antibacterial activity. Mater. Res. Bull. 2023, 168, 112491. [Google Scholar] [CrossRef]
- Balamurugan, M.; Saravanan, S.; Soga, T. Erratum: Synthesis of Iron Oxide Nanoparticles by Using Eucalyptus Globulus Plant Extract [e-J. Surf. Sci. Nanotech. Vol. 12, pp. 363–367 (2014)]. e-J. Surf. Sci. Nanotechnol. 2014, 12, 395. [Google Scholar] [CrossRef][Green Version]
- Widatalla, H.A.; Yassin, L.F.; Alrasheid, A.A.; Ahmed, S.A.; Widdatallah, M.O.; Eltilib, S.H.; Mohamed, A.A. Green synthesis of silver nanoparticles using green tea leaf extract, characterization and evaluation of antimicrobial activity. Nanoscale Adv. 2022, 4, 911–915. [Google Scholar] [CrossRef]
- Venkataraman, S. Plant Molecular Pharming and Plant-Derived Compounds towards Generation of Vaccines and Therapeutics against Coronaviruses. Vaccines 2022, 10, 1805. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Wu, W.; Hossain, A.; Hafeez, R.; Islam Masum, M.M.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in Plant and Microbe-Based Synthesis of Metallic Nanoparticles and Their Antimicrobial Activity against Plant Pathogens. Nanomaterials 2020, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Herlekar, M.; Barve, S.; Kumar, R. Plant-Mediated Green Synthesis of Iron Nanoparticles. J. Nanoparticles 2014, 2014, 140614. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloevera Plant Extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Dugoua, J.-J.; Seely, D.; Perri, D.; Koren, G.; Mills, E. Safety and efficacy of chastetree (Vitex agnus-castus) during pregnancy and lactation. J. Popul. Ther. Clin. Pharmacol. 2008, 15, e74–e79. Available online: https://jptcp.com/index.php/jptcp/article/view/191 (accessed on 21 November 2024).
- Habbab, A.; Sekkoum, K.; Belboukhari, N.; Cheriti, A.; Aboul-Enein, H.Y. Analgesic and Anti-Inflammatory Effects of Essential Oils of Vitex agnuscastus L. from South-West of Algeria. Curr. Bioact. Compd. 2017, 13, 165–169. [Google Scholar] [CrossRef]
- Niroumand, M.C.; Heydarpour, F.; Farzaei, M.H. Pharmacological and therapeutic effects of Vitex agnus-castus L.: A review. Pharmacogn. Rev. 2018, 12, 103–114. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Casticin from Vitex species: A short review on its anticancer and anti-inflammatory properties. J. Integr. Med. 2018, 16, 147–152. [Google Scholar] [CrossRef]
- Adamov, G.V.; Rendyuk, T.D.; Saybel, O.L.; Dargaeva, T.D.; Tsitsilin, A.N.; Bokov, D.O. Vitex agnus-castus: Botanical features and area, chemical composition of fruit, pharmacological properties, and medicinal uses. J. Appl. Pharm. Sci. 2022, 12, 34–44. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Al-Suede, F.S. The Anti-proliferative Activity of Vitex agnus-castus Leaves Methanol Extract against Breast and Prostate Cancer Cell Line. Am. J. Phytomed. Clin. Ther. 2015, 3, 2321–2748. Available online: www.ajpct.org (accessed on 16 October 2024).
- Niraimathee, V.A.; Subha, V.; Ravindran, R.S.E.; Renganathan, S. Green synthesis of iron oxide nanoparticles from Mimosa pudica root extract. Int. J. Environ. Sustain. Dev. 2016, 15, 227–240. [Google Scholar] [CrossRef]
- Tong, A.; Tang, X.; Zhang, F.; Wang, B. Study on the shift of ultraviolet spectra in aqueous solution with variations of the solution concentration. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 234, 118259. [Google Scholar] [CrossRef] [PubMed]
- Bratovcic, A. Biosynthesis of Green Silver Nanoparticles and Its UV-Vis Characterization. 1 January 2020. Available online: https://www.academia.edu/68559162/Biosynthesis_of_Green_Silver_Nanoparticles_and_Its_UV_Vis_Characterization (accessed on 21 November 2024).
- Şahin, A.; Altınsoy, Ş.; Kızılbey, K. An approach for cationic dyes removal from wastewater: Green synthesis of iron nanoparticles using Prunus avium stems extracts. Kuwait J. Sci. 2024, 51, 100226. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Kumar, K.S.; Reddy, P.S.; Sreedhar, B. Biobased green method to synthesise palladium and iron nanoparticles using Terminalia chebula aqueous extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 102, 128–133. [Google Scholar] [CrossRef]
- Kaya, B.; Darendelioğlu, E.; Dervişoğlu, G.; Tartik, M. Determination of comparative biological activities of silver nanoparticles formed by biological synthesis using achillea vermicularis. Pak. J. Bot. 2018, 50, 1423–1432. [Google Scholar]
- Nahari, M.H.; Al Ali, A.; Asiri, A.; Mahnashi, M.H.; Shaikh, I.A.; Shettar, A.K.; Hoskeri, J. Green Synthesis and Characterization of Iron Nanoparticles Synthesized from Aqueous Leaf Extract of Vitex leucoxylon and Its Biomedical Applications. Nanomaterials 2022, 12, 2404. [Google Scholar] [CrossRef]
- Takeno, Y.; Murakami, Y.; Sato, T.; Tanigaki, T.; Park, H.S.; Shindo, D.; Ferguson, R.M.; Krishnan, K.M. Morphology and magnetic flux distribution in superparamagnetic, single-crystalline Fe3O4 nanoparticle rings. Appl. Phys. Lett. 2014, 105, 183102. [Google Scholar] [CrossRef]
- Sayed, F.N.; Polshettiwar, V. Facile and Sustainable Synthesis of Shaped Iron Oxide Nanoparticles: Effect of Iron Precursor Salts on the Shapes of Iron Oxides. Sci. Rep. 2015, 5, 9733. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Amjad, M. Euphorbia herita leaf extract as a reducing agent in a facile green synthesis of iron oxide nanoparticles and antimicrobial activity evaluation. Inorg. Nano-Met. Chem. 2021, 51, 1147–1154. [Google Scholar] [CrossRef]
- Smuleac, V.; Varma, R.; Sikdar, S.; Bhattacharyya, D. Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics. J. Membr. Sci. 2011, 379, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, S.; Wang, F.; Megharaj, M.; Naidu, R.; Chen, Z. Heterogeneous Fenton-like oxidation of malachite green by iron-based nanoparticles synthesized by tea extract as a catalyst. Sep. Purif. Technol. 2015, 154, 161–167. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Schwarzenberger, K.; Gatzemeier, J.; Lei, Z.; Eckert, K. Magnetically Induced Aggregation of Iron Oxide Nanoparticles for Carrier Flotation Strategies. ACS Appl. Mater. Interfaces 2021, 13, 20830–20844. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Kant, K.M. Effect of size and shape dependent anisotropy on superparamagnetic property of CoFe2O4 nanoparticles and nanoplatelets. Phys. B Condens. Matter. 2017, 520, 152–163. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idée, J.M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Kim, J.Y.; Seo, C.; Hong, S.; Lee, S.; Eo, S.H. Altitudinal range-size distribution of breeding birds and environmental factors for the determination of species richness: An empirical test of altitudinal Rapoport’s rule and non-directional rescue effect on a local scale. PLoS ONE 2019, 14, e0203511. [Google Scholar] [CrossRef]
- Ullah, N.; Amin, A.; Alamoudi, R.A.; Rasheed, S.A.; Alamoudi, R.A.; Nawaz, A.; Raza, M.; Nawaz, T.; Ishtiaq, S.; Abbas, S.S. Fabrication and Optimization of Essential-Oil-Loaded Nanoemulsion Using Box–Behnken Design against Staphylococos aureus and Staphylococos epidermidis Isolated from Oral Cavity. Pharmaceutics 2022, 14, 1640. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Bououdina, M.; Ramalingam, R.J.; Al-Lohedan, H.A. Okra extract-assisted green synthesis of CoFe2O4 nanoparticles and their optical, magnetic, and antimicrobial properties. Mater. Chem. Phys. 2018, 204, 410–419. [Google Scholar] [CrossRef]
- Abdullah, J.A.A.; Díaz-García, Á.; Law, J.Y.; Romero, A.; Franco, V.; Guerrero, A. Sustainable Nanomagnetism: Investigating the Influence of Green Synthesis and pH on Iron Oxide Nanoparticles for Enhanced Biomedical Applications. Polymers 2023, 15, 3850. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.; Vizhi, R.E. Temperature Effects on the Structural, Morphological, and Magnetic Properties of Iron Oxide Nanoclusters Using Solvothermal Method. J. Supercond. Nov. Magn. 2024, 37, 905–919. [Google Scholar] [CrossRef]
- Chiesa, M.; Giamello, E. On the Role and Applications of Electron Magnetic Resonance Techniques in Surface Chemistry and Heterogeneous Catalysis. Catal. Lett. 2021, 151, 3417–3436. [Google Scholar] [CrossRef]
- Fokina, N.P.; Khutsishvili, K.O.; Atsarkin, V.A. Applied Magnetic Resonance ESR and Longitudinal Response in Metals Containing Localized Paramagnetic Centers with Spin S > 1/2. Appl. Magn. Reason. 2003, 24, 197–213. [Google Scholar] [CrossRef]
- Xia, Q.; Jiang, Z.; Li, D.; Wang, J.; Yao, Z. Green synthesis of a dendritic Fe3O4 @Feo composite modified with polar C-groups for Fenton-like oxidation of phenol. J. Alloys Compd. 2018, 746, 453–461. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef] [PubMed]
- Bowles, J.F.W.; Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties Reactions Occurrence and Uses. Weinheim and New York (VCH Verlagsgeseiischaft mbH). 1996, xxxi + 573 pp. Price DM 328.00. ISBN 3-527-28576-8. Miner. Mag. 1997, 61, 740–741. [Google Scholar] [CrossRef]
- Jezierska, A.; Jerzykiewicz, L.B.; Kołodziejczak, J.; Sobczak, J.M. Synthesis, X-ray crystal structure and DFT study of potential ligands of (2Z)-3-[(2-hydroxyphenyl)amino]-1-phenyl”alk”-2-en-1-one type. J. Mol. Struct. 2007, 839, 33–40. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: Beginning a new era in cancer theranostics. Nanomedicine 2016, 11, 3157–3177. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, , 2016; Volume 1724. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen, Mathematisch-Physikalische Klasse 1918, 1918, 98–100. [Google Scholar]
- Saikia, I.; Hazarika, M.; Yunus, S.; Pal, M.; Das, M.R.; Borah, J.C.; Tamuly, C. Green synthesis of Au-Ag-In-rGO nanocomposites and its α-glucosidase inhibition and cytotoxicity effects. Mater. Lett. 2018, 211, 48–50. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Chen, L.; Thompson, D.M.; Montoya, L.D. Effect of particle size on in vitro cytotoxicity of titania and alumina nanoparticles. J. Exp. Nanosci. 2014, 9, 625–638. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef]
- Helmlinger, J.; Sengstock, C.; Groß-Heitfeld, C.; Mayer, C.; Schildhauer, T.A.; Köller, M.; Epple, M. Silver nanoparticles with different size and shape: Equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016, 6, 18490–18501. [Google Scholar] [CrossRef]
- Park, M.V.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 2011, 32, 9810–9817. [Google Scholar] [CrossRef]

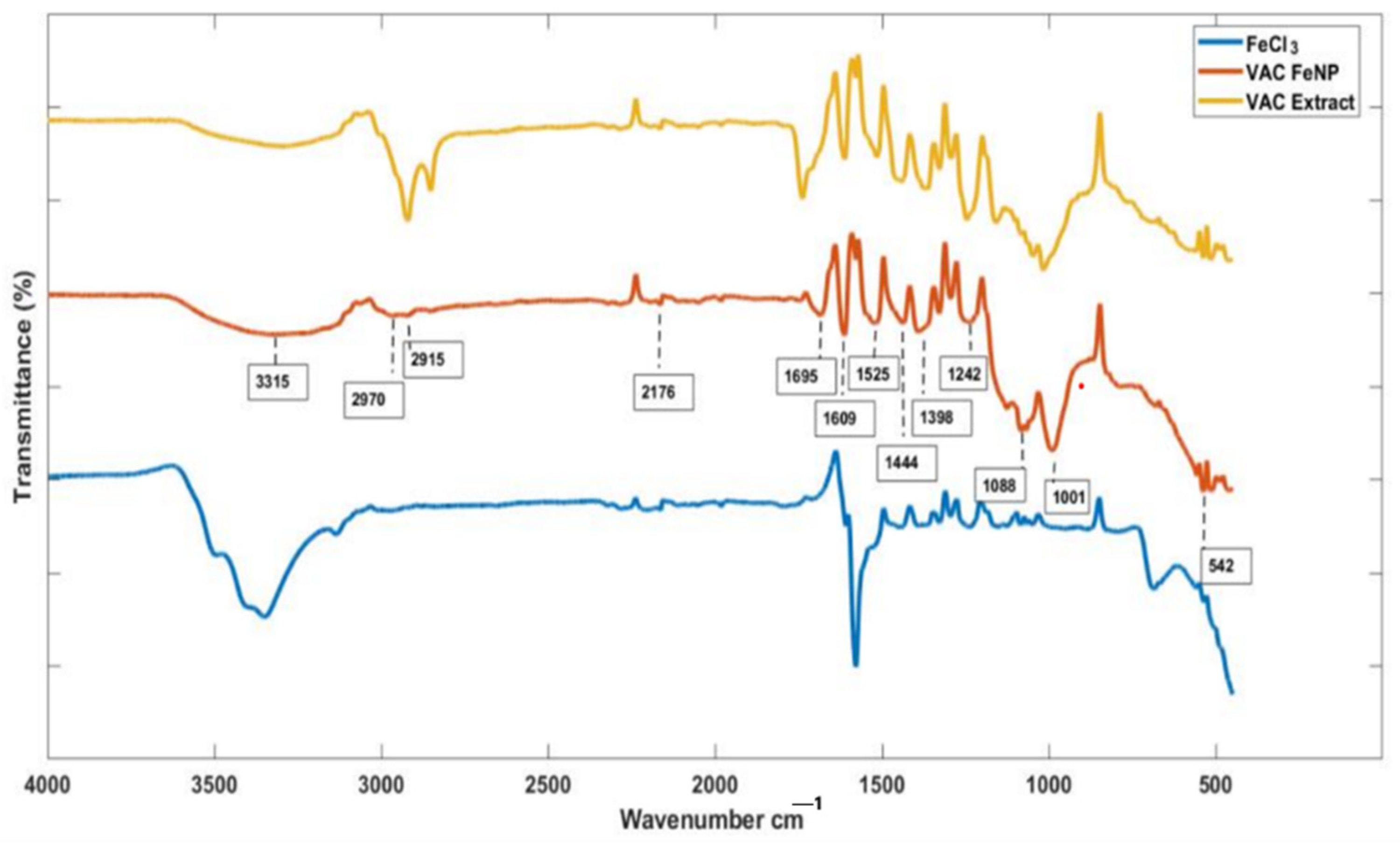



| Wavenumber (cm−1) | Functional Group | Role |
|---|---|---|
| 3000–3500 | O-H and N-H | Reducing agent, stabilizer |
| 1690–1760 | C=O (Carbonyl) | Interaction with nanoparticle surface |
| 1500–1600 | Aromatic C=C | Adsorption onto surface, stabilizer |
| 1340–1470, 2850–2970 | Alkanes | Stabilizing coating |
| 2200–2300 | C≡N | Presence of organic functional groups |
| 1040–1300 | Carboxylic acids, alcohols | Binding to nanoparticle surface |
| 400–600 | Fe-O | Chemical signature of nanoparticle formation |
| Element | Weight % | Atomic % |
|---|---|---|
| Mg | 23.1 | 37.1 |
| K | 16.6 | 16.5 |
| Ca | 16.4 | 16.0 |
| Fe | 43.8 | 30.4 |
| FeNPs | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Z-Ave (nm) | 442.3 | 398.9 | 390.5 | 390.4 | 393.3 | 421.4 | 421.8 | 428.5 |
| pDI | 0.360 | 0.337 | 0.342 | 0.351 | 0.343 | 0.331 | 0.427 | 0.373 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kızılbey, K.; Köprülü, E.N.; Temür, H.; Canım Ateş, S.; Özer, S. Magnetic and Biomedical Properties of Iron Nanoparticles Synthesized Using Vitex agnus-castus Extract. Materials 2024, 17, 6064. https://doi.org/10.3390/ma17246064
Kızılbey K, Köprülü EN, Temür H, Canım Ateş S, Özer S. Magnetic and Biomedical Properties of Iron Nanoparticles Synthesized Using Vitex agnus-castus Extract. Materials. 2024; 17(24):6064. https://doi.org/10.3390/ma17246064
Chicago/Turabian StyleKızılbey, Kadriye, Elif Nur Köprülü, Hatice Temür, Sezen Canım Ateş, and Sevil Özer. 2024. "Magnetic and Biomedical Properties of Iron Nanoparticles Synthesized Using Vitex agnus-castus Extract" Materials 17, no. 24: 6064. https://doi.org/10.3390/ma17246064
APA StyleKızılbey, K., Köprülü, E. N., Temür, H., Canım Ateş, S., & Özer, S. (2024). Magnetic and Biomedical Properties of Iron Nanoparticles Synthesized Using Vitex agnus-castus Extract. Materials, 17(24), 6064. https://doi.org/10.3390/ma17246064






