Synthesis and Phase Evolution of a Nanocrystalline FexCrNiAl (x = 1.0, 0.5, 0.25) High-Entropy Alloys by Mechanical Alloying
Highlights
- FexCrNiAl (x=1.0, 0.5, 0.25) high-entropy alloys were synthesized by mechanical alloying.
- The alloyed nanoscale FexCrNiAl powders presented non-equilibrium BCC supersaturated solid solution phase with the uniform elemental distribution and were refined to ~80 nm.
- The phase of alloyed powders transformed into the equilibrium phase of BCC and ordered BCC (B2) phases after high-temperature annealing.
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Alloying Behavior and Morphology of the Powders
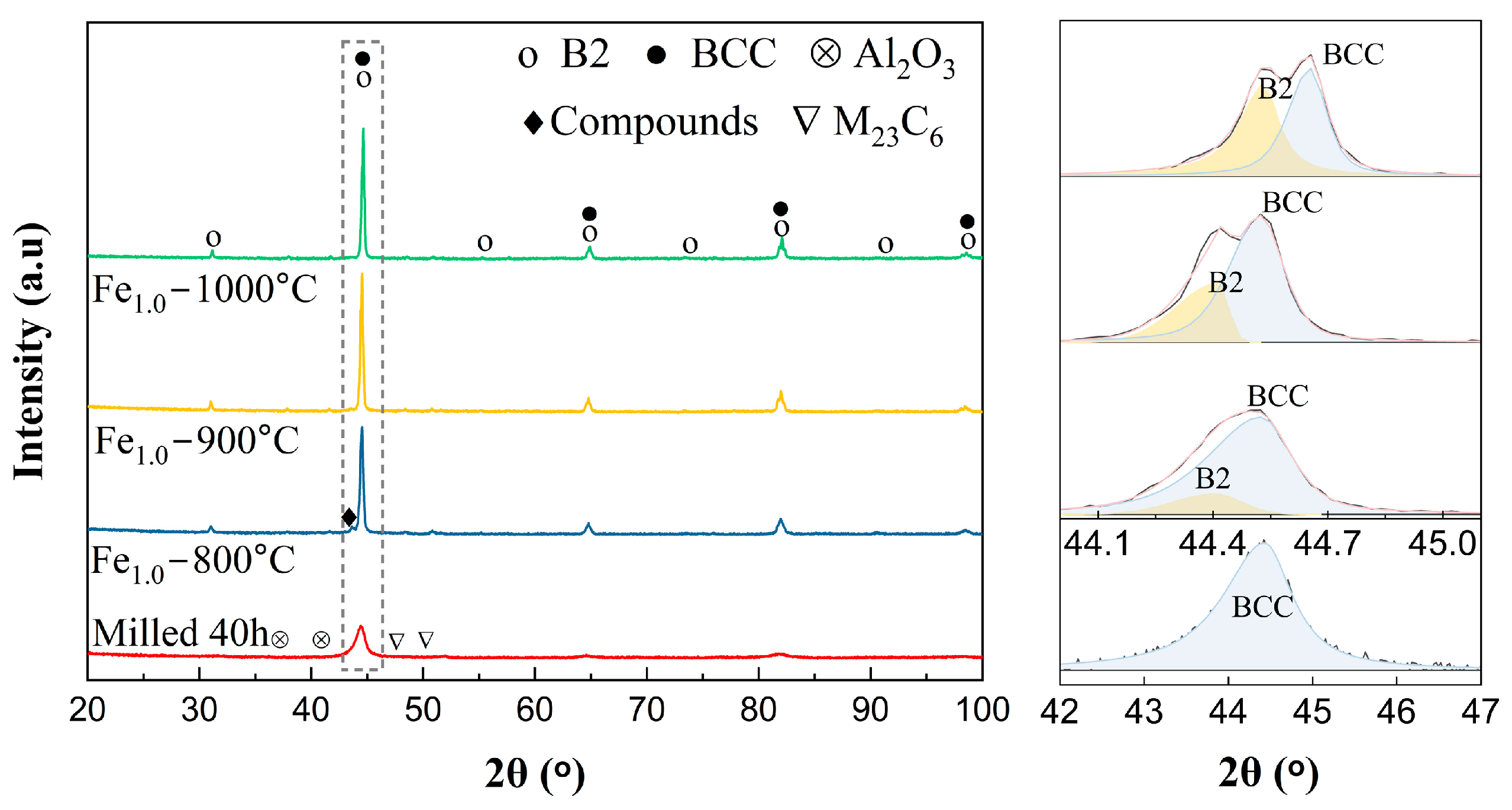
3.2. Phase Formation and High-Temperature Phase Evolution of the HEA Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys: Basic Concepts. In High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–30. ISBN 978-0-12-816067-1. [Google Scholar]
- Jeong, H.T.; Xing, Y.; Park, H.K.; Na, T.W.; Oh, S.H.; Kim, W.J. Achieving High Strength and Uniform Ductility in High-Entropy Alloys via Dynamic-Precipitation Accelerated Transformation-Induced Plasticity. Acta Mater. 2024, 272, 119945. [Google Scholar] [CrossRef]
- Asghari-Rad, P.; Sathiyamoorthi, P.; Nguyen, N.T.C.; Zargaran, A.; Kim, T.S.; Kim, H.S. A Powder-Metallurgy-Based Fabrication Route towards Achieving High Tensile Strength with Ultra-High Ductility in High-Entropy Alloy. Scr. Mater. 2021, 190, 69–74. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Zhang, H.; Chen, Y.; Luo, L.; Zhao, X.; Guo, F.; Xiao, P. Oxidation Behavior of Gas-Atomized AlCoCrFeNi High-Entropy Alloy Powder at 900–1100 °C. Corros. Sci. 2021, 181, 109257. [Google Scholar] [CrossRef]
- Yang, D.; Liu, Y.; Han, T.; Zhou, F.; Qu, N.; Liao, M.; Lai, Z.; Zhu, J. High Thermal Stability and Oxidation Behavior of FeCrNiAl-Based Medium-Entropy Alloys Prepared by Powder Metallurgy. J. Alloys Compd. 2022, 918, 165562. [Google Scholar] [CrossRef]
- Chen, B.; Li, X.; Chen, W.; Shang, L.; Jia, L. Microstructural Evolution, Mechanical and Wear Properties, and Corrosion Resistance of as-Cast CrFeNbTiMox Refractory High Entropy Alloys. Intermetallics 2023, 155, 107829. [Google Scholar] [CrossRef]
- Naseri, M.; Moghaddam, A.O.; Shaburova, N.; Mikhailov, D.; Gholami, D.; Mourad, A.-H.I.; Pellenen, A.; Trofimov, E. Upgrading the Strength-Ductility Trade-off and Wear Resistance of Al0.25CoCrFeNiCu and Al0.45CoCrFeNiSi0.45 High-Entropy Alloys through Severe Cold Rolling Process. Mater. Today Commun. 2024, 38, 108036. [Google Scholar] [CrossRef]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. Synthesis and Processing. In High-Entropy Alloys, 2nd ed.; Murty, B.S., Yeh, J.W., Ranganathan, S., Bhattacharjee, P.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–117. ISBN 978-0-12-816067-1. [Google Scholar]
- Zhang, Y. High-Entropy Materials, 1st ed.; Springer: Singapore, 2019; ISBN 978-981-13-8525-4. [Google Scholar]
- Ye, X.; Cheng, Z.; Liu, C.; Wu, X.; Yu, L.; Liu, M.; Fang, D.; Zhao, G.; Li, B. The Microstructure and Properties of Fe55Cr15Ni(30-x)Nbx Eutectic High-Entropy Alloys. Mater. Sci. Eng. A 2022, 841, 143026. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, D.; Wu, W.; Ni, S.; Song, M. Effect of Fe on Microstructure, Phase Evolution and Mechanical Properties of (AlCoCrFeNi)100-XFex High Entropy Alloys Processed by Spark Plasma Sintering. Intermetallics 2018, 103, 1–11. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Liu, Y.; Fang, Q.; Wu, Y.; Chen, S.; Liu, C.T. Microstructure and Mechanical Properties of Equimolar FeCoCrNi High Entropy Alloy Prepared via Powder Extrusion. Intermetallics 2016, 75, 25–30. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Wen, H.; Zhang, D.; Chen, Z.; Zheng, B.; Zhou, Y.; Lavernia, E.J. Microstructure and Strengthening Mechanisms in an FCC Structured Single-Phase Nanocrystalline Co25Ni25Fe25Al7.5Cu17.5 High-Entropy Alloy. Acta Mater. 2016, 107, 59–71. [Google Scholar] [CrossRef]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Comparative Study of the Microstructures and Mechanical Properties of Direct Laser Fabricated and Arc-Melted AlxCoCrFeNi High Entropy Alloys. Mater. Sci. Eng. A 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Han, T.; Liu, Y.; Yang, D.; Qu, N.; Liao, M.; Lai, Z.; Jiang, M.; Zhu, J. Effect of Annealing on Microstructure and Mechanical Properties of AlCrFe2Ni2 Medium Entropy Alloy Fabricated by Laser Powder Bed Fusion Additive Manufacturing. Mater. Sci. Eng. A 2022, 839, 142868. [Google Scholar] [CrossRef]
- Han, T.; Chen, J.; Wei, Z.; Qu, N.; Liu, Y.; Yang, D.; Zhao, S.; Lai, Z.; Jiang, M.; Zhu, J. Effect of Cooling Rate on Microstructure and Mechanical Properties of AlCrFe2Ni2 Medium Entropy Alloy Fabricated by Laser Powder Bed Fusion. J. Mater. Res. Technol. 2023, 25, 4063–4073. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, W.; Xia, Z.; Xiong, W.; Fu, Z. Influence of Synthesis Method on Microstructure and Mechanical Behavior of Co-Free AlCrFeNi Medium-Entropy Alloy. Intermetallics 2019, 108, 45–54. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical Alloying and Milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Varalakshmi, S.; Kamaraj, M.; Murty, B.S. Synthesis and Characterization of Nanocrystalline AlFeTiCrZnCu High Entropy Solid Solution by Mechanical Alloying. J. Alloys Compd. 2008, 460, 253–257. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, J.; Wang, Y.; Liu, Y.; Zhang, Y. Effects of Milling Process Parameters and PCAs on the Synthesis of Al0.8Co0.5Cr1.5CuFeNi High Entropy Alloy Powder by Mechanical Alloying. Mater. Des. 2022, 217, 110637. [Google Scholar] [CrossRef]
- Verma, P.K.; Singh, A.; Kumar, A. Microstructure Characterization and Phase Evolution of Equiatomic AlCoMoFeNi High Entropy Alloy Synthesized by Mechanical Alloying. Mater. Chem. Phys. 2024, 318, 129325. [Google Scholar] [CrossRef]
- Praveen, S.; Basu, J.; Kashyap, S.; Kottada, R.S. Exceptional Resistance to Grain Growth in Nanocrystalline CoCrFeNi High Entropy Alloy at High Homologous Temperatures. J. Alloys Compd. 2016, 662, 361–367. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-Ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Williamson, G.K.; Smallman, R.E., III. Dislocation Densities in Some Annealed and Cold-Worked Metals from Measurements on the X-Ray Debye-Scherrer Spectrum. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1956, 1, 34–46. [Google Scholar] [CrossRef]
- Wen, H.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J. Strengthening Mechanisms in a High-Strength Bulk Nanostructured Cu-Zn-Al Alloy Processed via Cryomilling and Spark Plasma Sintering. Acta Mater. 2013, 61, 2769–2782. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hu, Y.H.; Hsieh, C.A.; Yeh, J.W.; Chen, S.K. Competition between Elements during Mechanical Alloying in an Octonary Multi-Principal-Element Alloy System. J. Alloys Compd. 2009, 481, 768–775. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hu, Y.H.; Tsai, C.W.; Hsieh, C.A.; Kao, S.W.; Yeh, J.W.; Chin, T.S.; Chen, S.K. Alloying Behavior of Binary to Octonary Alloys Based on Cu-Ni-Al-Co-Cr-Fe-Ti-Mo during Mechanical Alloying. J. Alloys Compd. 2009, 477, 696–705. [Google Scholar] [CrossRef]
- Vaidya, M.; Prasad, A.; Parakh, A.; Murty, B.S. Influence of Sequence of Elemental Addition on Phase Evolution in Nanocrystalline AlCoCrFeNi: Novel Approach to Alloy Synthesis Using Mechanical Alloying. Mater. Des. 2017, 126, 37–46. [Google Scholar] [CrossRef]
- Chen, X.; Sui, Y.; Qi, J.; He, Y.; Wei, F.; Meng, Q.; Sun, Z. Microstructure of Al1.3CrFeNi Eutectic High Entropy Alloy and Oxidation Behavior at 1000 °C. J. Mater. Res. 2017, 32, 2109–2116. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-Entropy Alloys by Mechanical Alloying: A Review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Xiao, H.; Zhou, L.; Zhu, D.; Yang, S. Fabrication and Properties of Nanocrystalline Co0.5FeNiCrTi0.5 High Entropy Alloy by MA-SPS Technique. Mater. Des. 2013, 44, 535–539. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, W.; Fang, S.; Zhang, D.; Xiao, H.; Zhu, D. Alloying Behavior and Deformation Twinning in a CoNiFeCrAl0.6Ti0.4 High Entropy Alloy Processed by Spark Plasma Sintering. J. Alloys Compd. 2013, 553, 316–323. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Jiang, L.; Wang, T.; Li, T. Effects of Electro-Negativity on the Stability of Topologically Close-Packed Phase in High Entropy Alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Poletti, M.G.; Battezzati, L. Electronic and Thermodynamic Criteria for the Occurrence of High Entropy Alloys in Metallic Systems. Acta Mater. 2014, 75, 297–306. [Google Scholar] [CrossRef]
- Gao, M.C.; Zhang, C.; Gao, P.; Zhang, F.; Ouyang, L.Z.; Widom, M.; Hawk, J.A. Thermodynamics of Concentrated Solid Solution Alloys. Curr. Opin. Solid State Mater. Sci. 2017, 21, 238–251. [Google Scholar] [CrossRef]
- King, D.J.M.; Middleburgh, S.C.; McGregor, A.G.; Cortie, M.B. Predicting the Formation and Stability of Single Phase High-Entropy Alloys. Acta Mater. 2016, 104, 172–179. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Nanocrystalline CoCrFeNiCuAl High-Entropy Solid Solution Synthesized by Mechanical Alloying. J. Alloys Compd. 2009, 485, L31–L34. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Wang, W.M.; Lee, S.W.; Niihara, K. Characterization of Nanocrystalline CoCrFeNiTiAl High-Entropy Solid Solution Processed by Mechanical Alloying. J. Alloys Compd. 2010, 495, 33–38. [Google Scholar] [CrossRef]
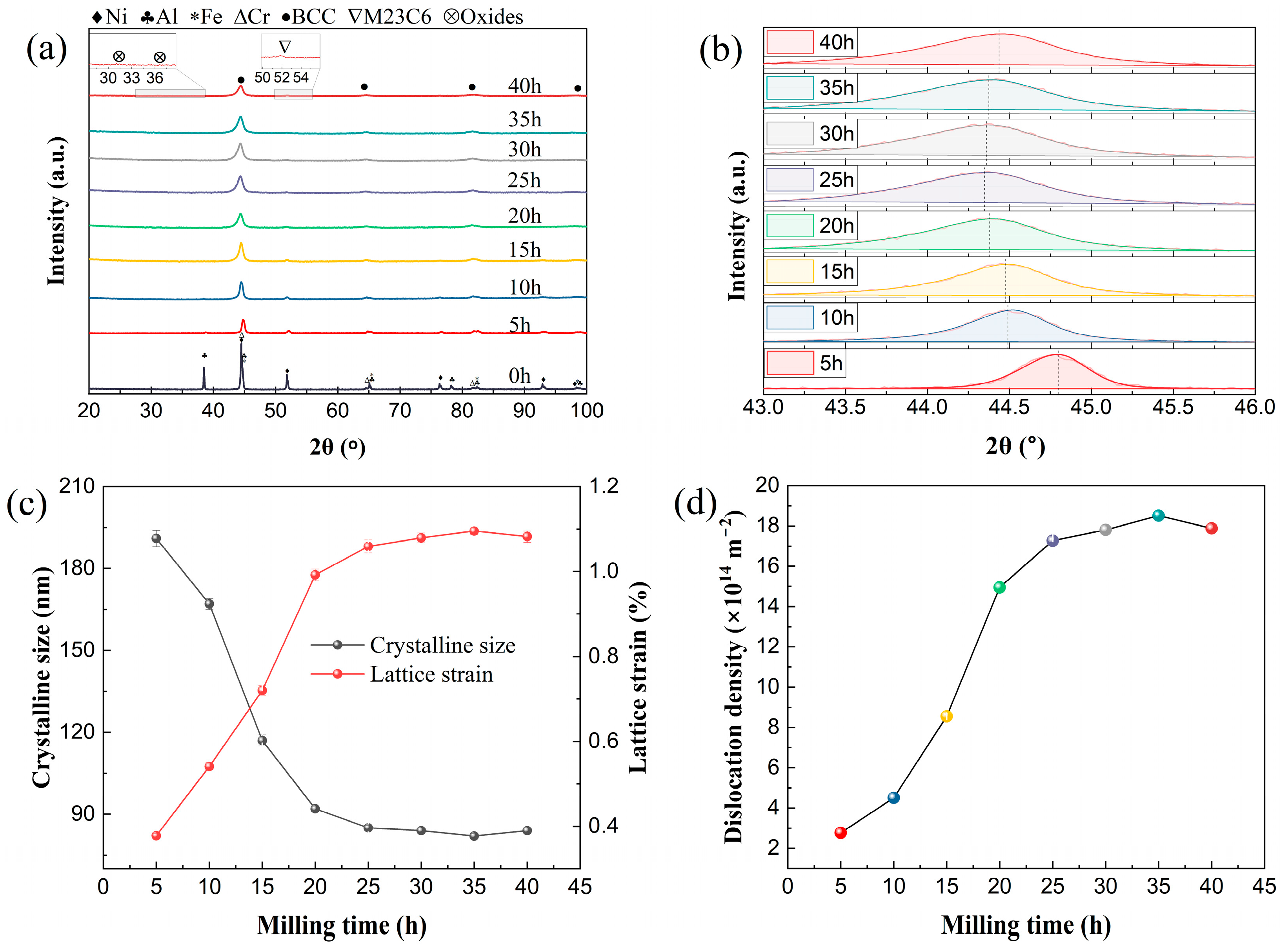
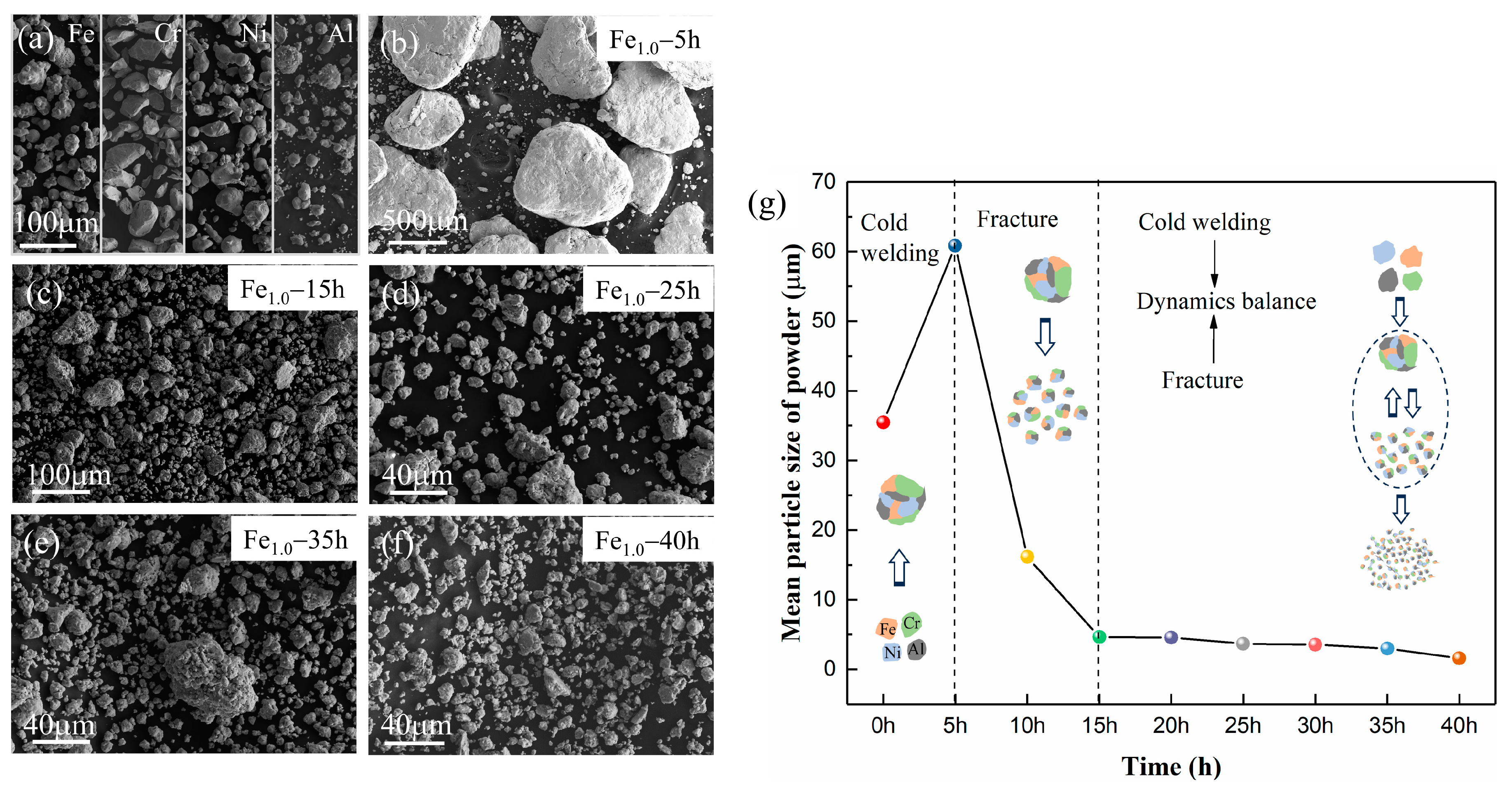
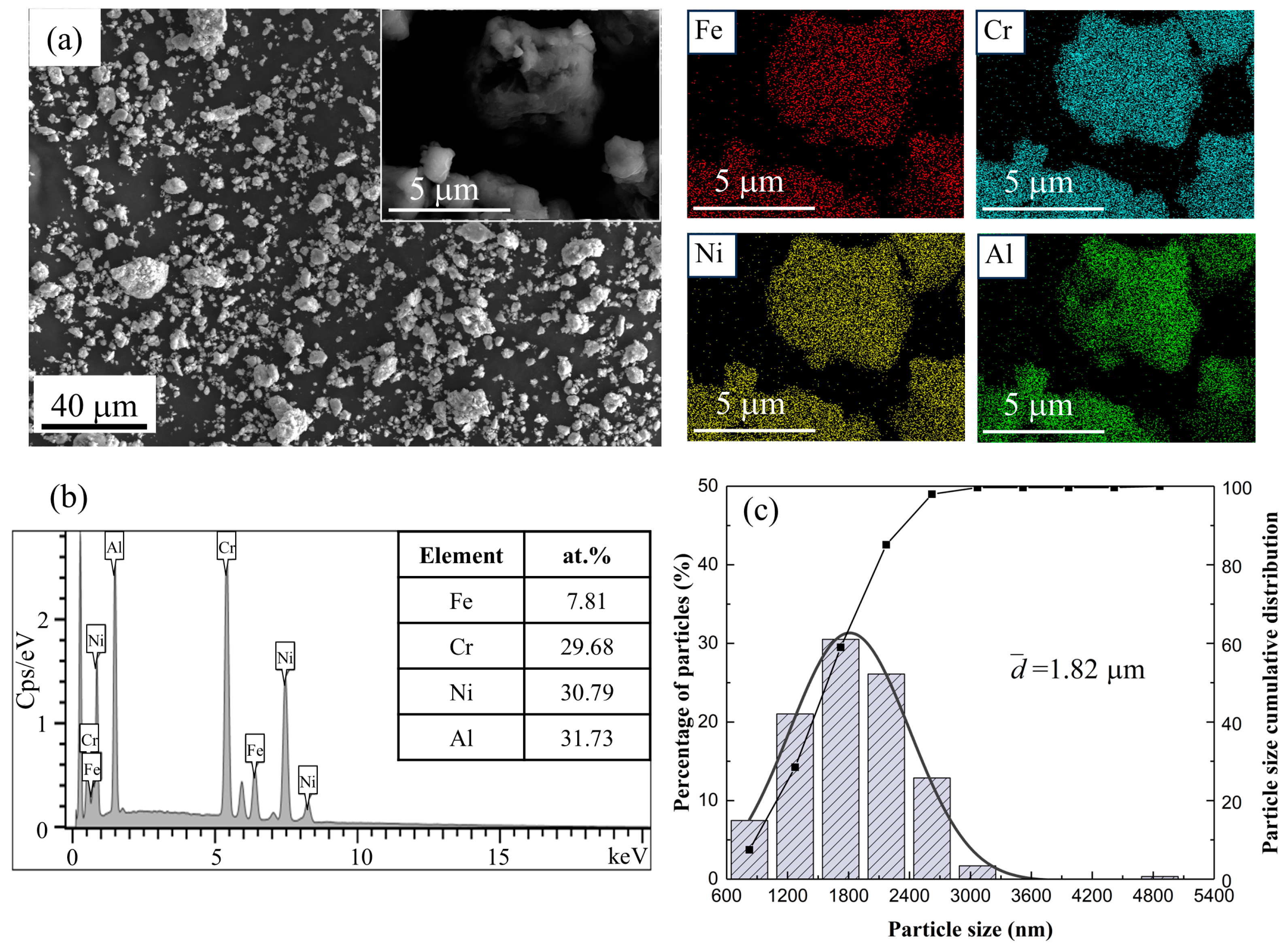
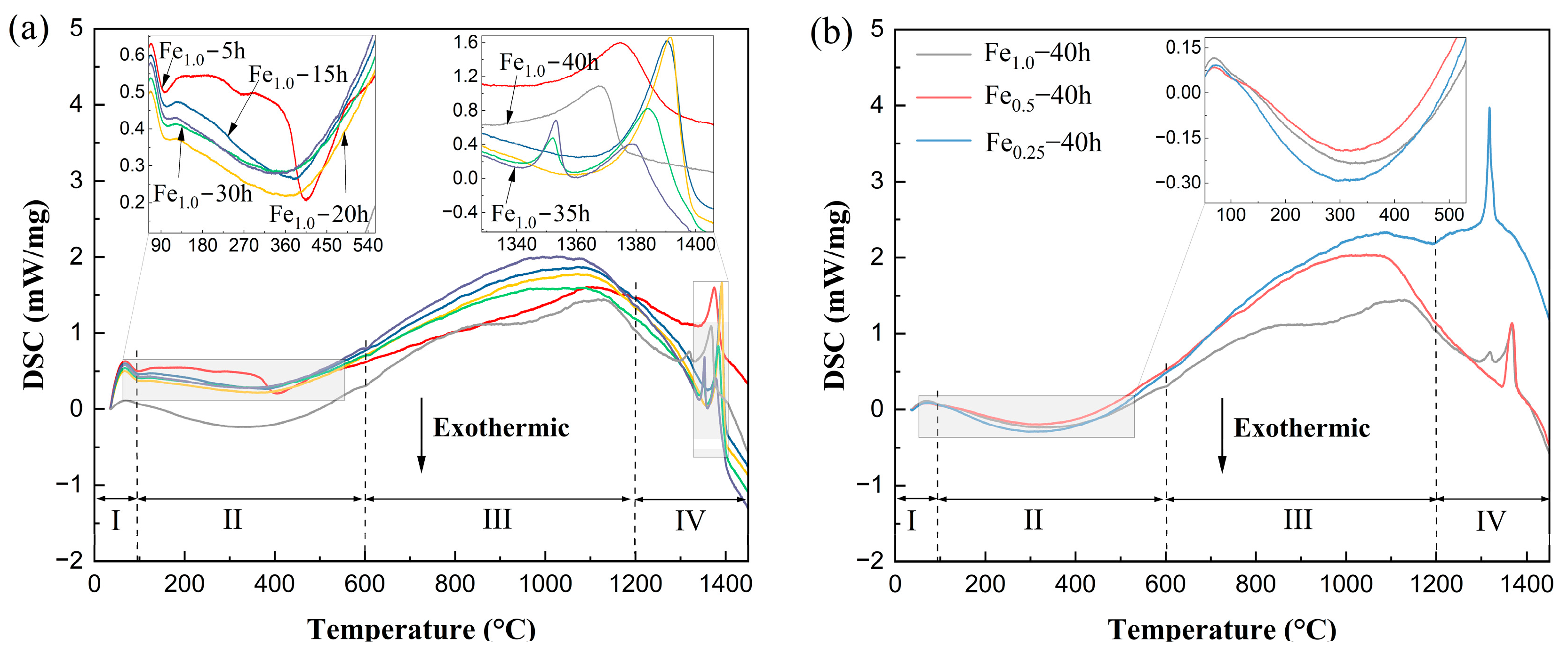
| Alloys | Milling Time (h) | Crystalline Size (nm) | Lattice Strain (%) | Dislocation Density (m−2) |
|---|---|---|---|---|
| Fe1.0 | 35 | 82 ± 1 | 1.0950 ± 0.0077 | 1.85 × 1015 |
| 40 | 84 ± 1 | 1.0820 ± 0.0133 | 1.79 × 1015 | |
| Fe0.5 | 35 | 83 ± 1 | 1.0860 ± 0.0163 | 1.82 × 1015 |
| 40 | 81 ± 1 | 1.0200 ± 0.0107 | 1.75 × 1015 | |
| Fe0.25 | 35 | 81 ± 1 | 1.1160 ± 0.0081 | 1.91 × 1015 |
| 40 | 80 ± 1 | 1.1100 ± 0.0128 | 1.92 × 1015 |
| Element | Tm (°C) | C.S. (400K) | r (Å) | D0 (400K) |
|---|---|---|---|---|
| Fe | 1538 | BCC | 1.27 | 10−31 |
| Cr | 1857 | BCC | 1.28 | 10−41 |
| Ni | 1453 | FCC | 1.25 | 10−37 |
| Al | 660 | FCC | 1.43 | 10−19 |
| Element | Fe | Cr | Ni | Al |
|---|---|---|---|---|
| Fe | — | −1 | −2 | −11 |
| Cr | −1 | — | −7 | −10 |
| Ni | −2 | −7 | — | −22 |
| Al | −11 | −10 | −22 | — |
| Alloys. | Mean Particle Size (μm) | Regions | Fe (at.%) | Cr (at.%) | Ni (at.%) | Al (at.%) |
|---|---|---|---|---|---|---|
| Fe1.0 | 1.59 | Nominal composition | 25.00 | 25.00 | 25.00 | 25.00 |
| EDS mapping | 26.22 | 23.56 | 24.10 | 26.12 | ||
| Fe0.5 | 1.80 | Nominal composition | 14.28 | 28.57 | 28.57 | 28.57 |
| EDS mapping | 15.39 | 29.26 | 29.84 | 25.51 | ||
| Fe0.25 | 1.82 | Nominal composition | 7.69 | 30.77 | 30.77 | 30.77 |
| EDS mapping | 7.81 | 29.68 | 30.79 | 31.73 |
| Alloy | ΔHmix (kJ/mol) | ΔSmix (J/K·mol) | Δχ | δ (%) | VEC | Ω |
|---|---|---|---|---|---|---|
| Fe1.0 | −13.2500 | 11.5256 | 0.1221 | 6.2600 | 6.7500 | 1.4353 |
| Fe0.5 | −15.0204 | 11.2387 | 0.1268 | 6.4630 | 6.5714 | 1.2174 |
| Fe0.25 | −16.0947 | 10.6859 | 0.1291 | 6.5587 | 6.4615 | 1.0709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Liao, M.; Huang, J.; Han, T.; Qu, N.; Wang, Y.; Zhu, J. Synthesis and Phase Evolution of a Nanocrystalline FexCrNiAl (x = 1.0, 0.5, 0.25) High-Entropy Alloys by Mechanical Alloying. Materials 2024, 17, 6061. https://doi.org/10.3390/ma17246061
Yang D, Liao M, Huang J, Han T, Qu N, Wang Y, Zhu J. Synthesis and Phase Evolution of a Nanocrystalline FexCrNiAl (x = 1.0, 0.5, 0.25) High-Entropy Alloys by Mechanical Alloying. Materials. 2024; 17(24):6061. https://doi.org/10.3390/ma17246061
Chicago/Turabian StyleYang, Danni, Mingqing Liao, Jingtao Huang, Tianyi Han, Nan Qu, Yalin Wang, and Jingchuan Zhu. 2024. "Synthesis and Phase Evolution of a Nanocrystalline FexCrNiAl (x = 1.0, 0.5, 0.25) High-Entropy Alloys by Mechanical Alloying" Materials 17, no. 24: 6061. https://doi.org/10.3390/ma17246061
APA StyleYang, D., Liao, M., Huang, J., Han, T., Qu, N., Wang, Y., & Zhu, J. (2024). Synthesis and Phase Evolution of a Nanocrystalline FexCrNiAl (x = 1.0, 0.5, 0.25) High-Entropy Alloys by Mechanical Alloying. Materials, 17(24), 6061. https://doi.org/10.3390/ma17246061







