Preparation of Alginate Hydrogel Beads on a Superhydrophobic Surface with Calcium Salt Powder to Enhance the Mechanical Strength and Encapsulation Efficiency of Ingredients
Highlights
- Alginate hydrogel beads prepared on a superhydrophobic surface with solid CaCl2 powder.
- High mechanical strength of the beads achieved through the use of a solid cross-linker.
- High encapsulation efficiency due to bead preparation in the gas phase.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
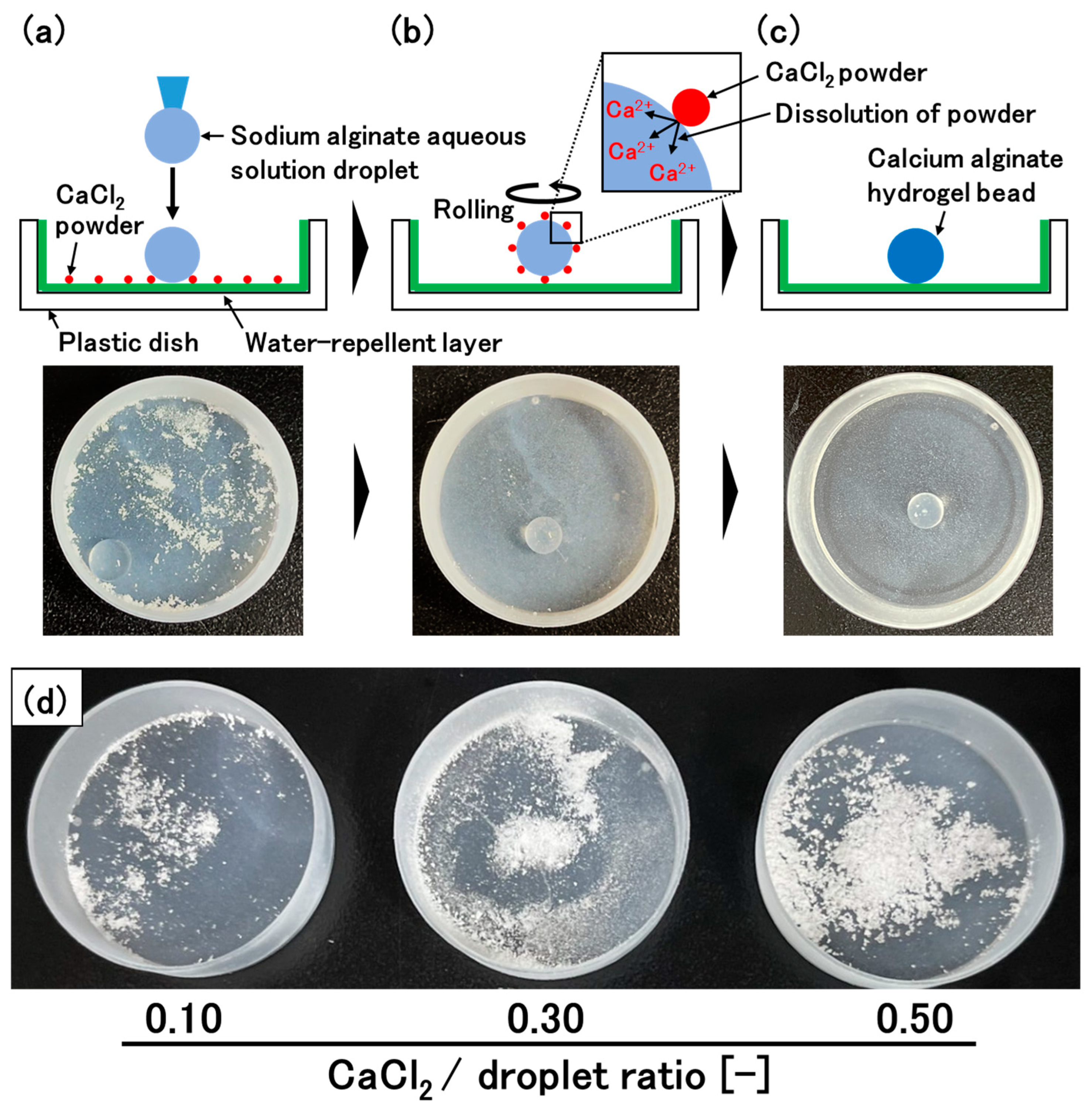
2.2. Preparation of the Calcium Alginate Hydrogel Beads
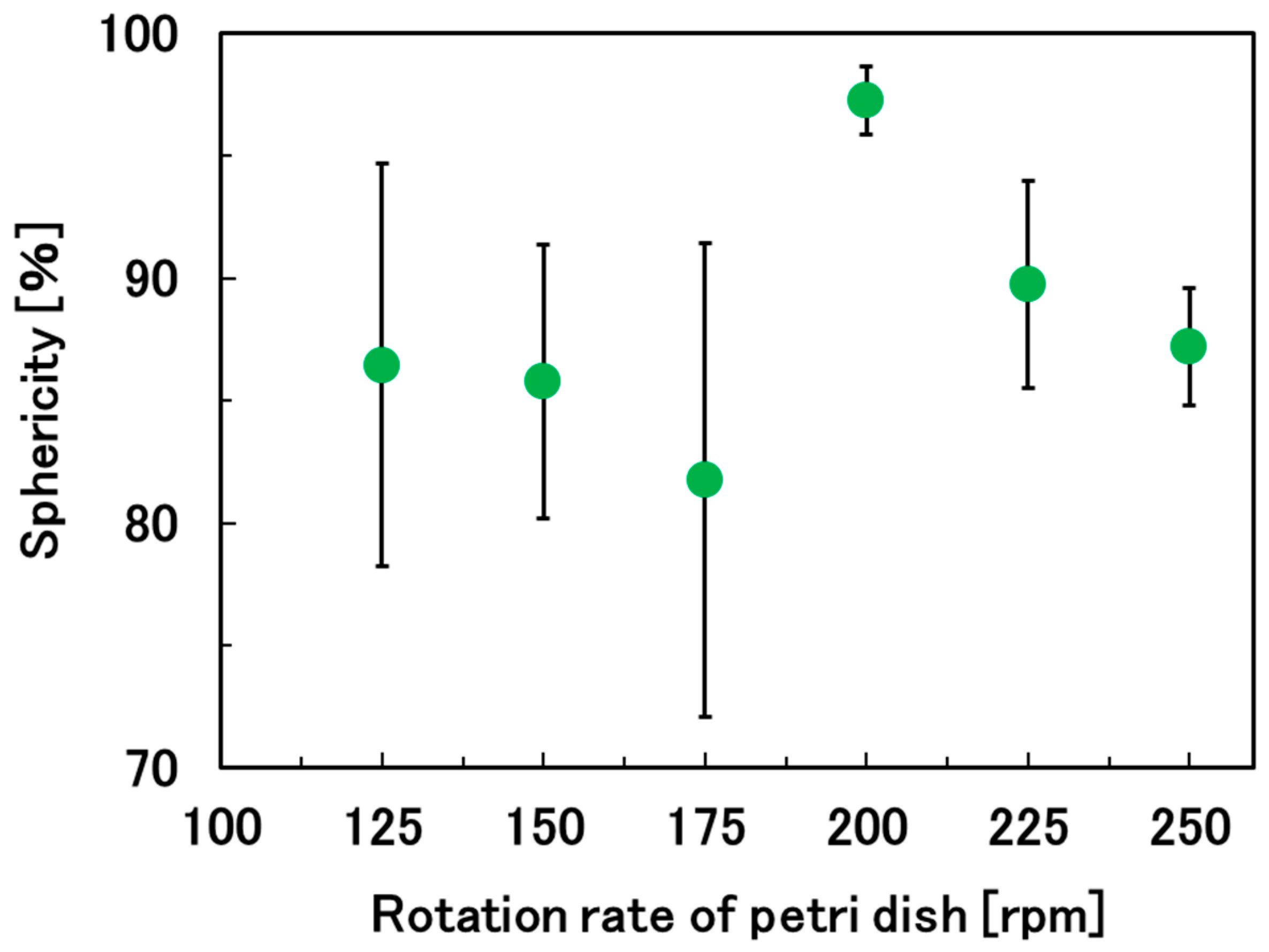
2.3. Optimization of the Rotation Rate of the Petri Dish to Prepare Spherical Hydrogel Beads
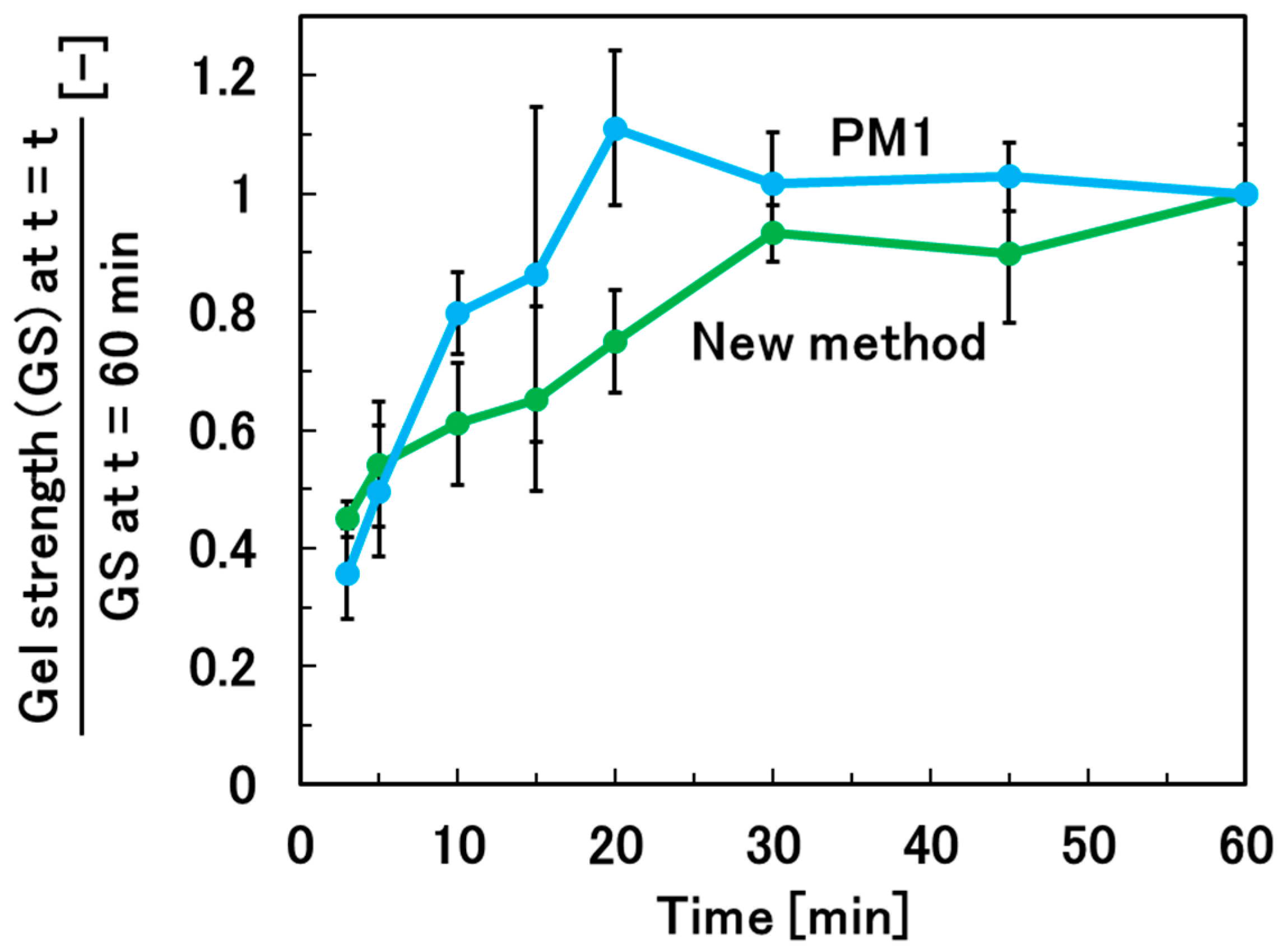
2.4. Determination of Gelation Time Required for Sufficient Gelation
2.5. Measurement of Mechanical Strength
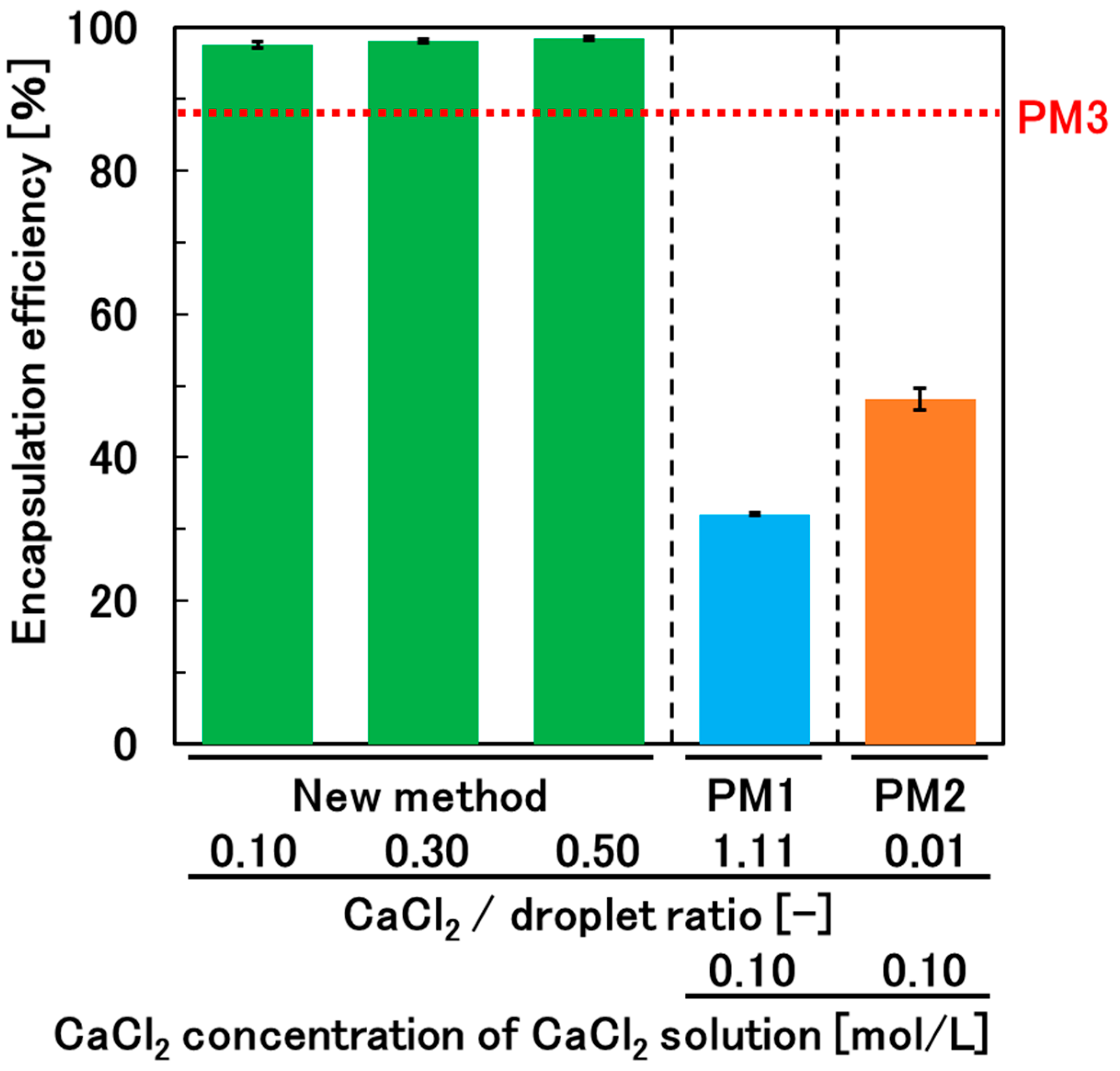
2.6. Determination of the Encapsulation Efficiency of Glucose
2.7. Permeability Evaluation
3. Results and Discussion
3.1. Optimization of the Rotation Rate of the Petri Dish to Prepare Spherical Hydrogel Beads
3.2. Optimization of the Gelation Time for Sufficient Gelation of the Hydrogel Beads
3.3. Mechanical Strength of the Hydrogel Beads
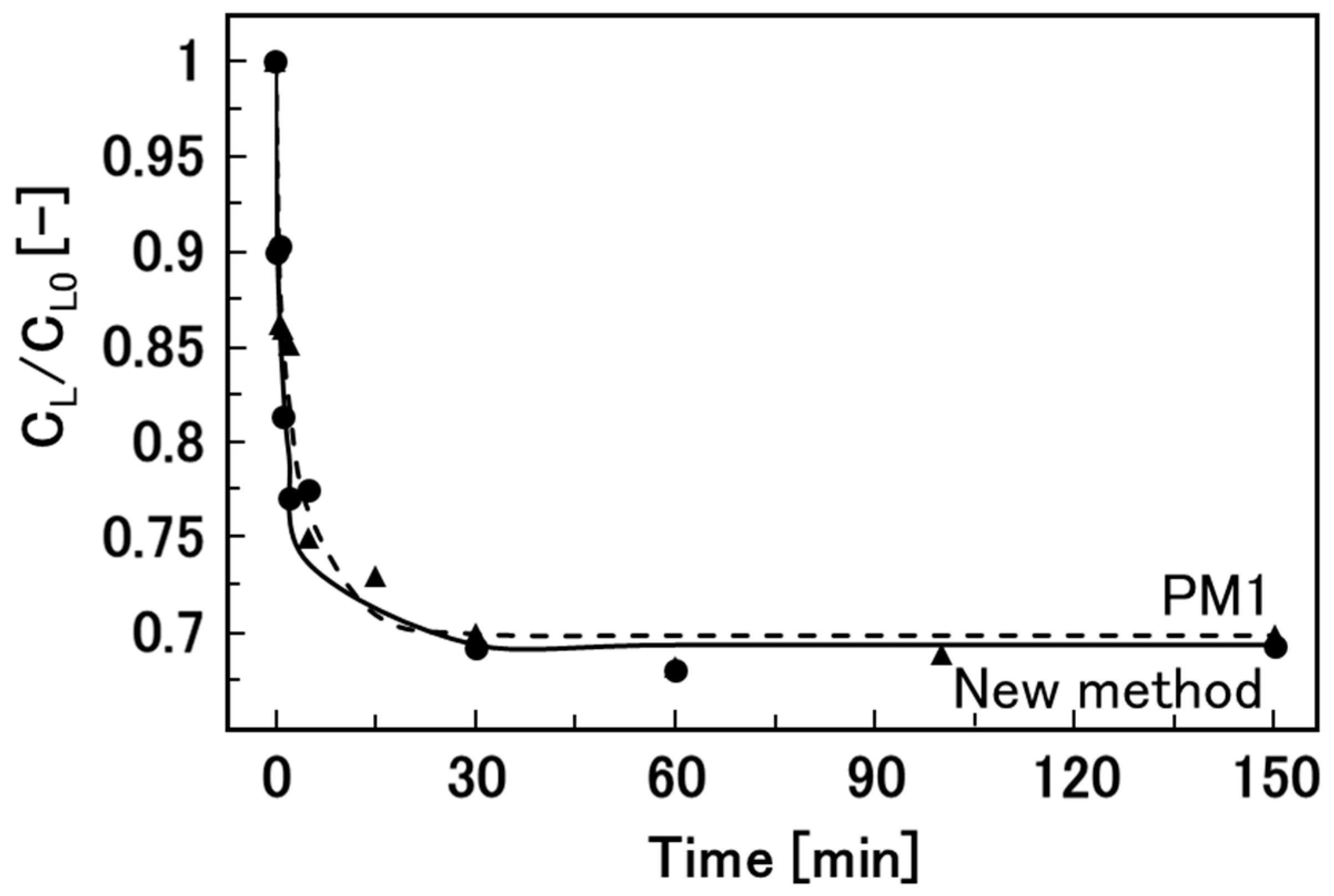
3.4. Encapsulation Efficiency and Permeability of the Hydrogel Beads
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, A.I.; Carneiro, J.; Campos, J.B.L.M.; Miranda, J.M. Production and characterization of a blood analogue based on alginate microparticles. Colloid Surf. A Physicochem. Eng. Asp. 2024, 693, 134022. [Google Scholar] [CrossRef]
- Ryu, J.; Rosenfeld, S.E.; McClements, D.J. Creation of plant-based meat analogs: Effects of calcium salt type on structure and texture of potato protein-alginate composite gels. Food Hydrocoll. 2024, 156, 110312. [Google Scholar] [CrossRef]
- Zhang, P.; Raza, S.; Cheng, Y.; Claudine, U.; Hayat, A.; Bashir, T.; Ali, T.; Ghasali, E.; Orooji, Y. Fabrication of maleic anhydride-acrylamide copolymer based sodium alginate hydrogel for elimination of metals ions and dyes contaminants from polluted water. Int. J. Biol. Macromol. 2024, 261, 129146. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Q.; Xu, W.; Yang, M.; Guo, W.; He, S.; Liu, W. Alginate-based hydrogels mediated biomedical applications: A review. Int. J. Biol. Macromol. 2024, 279, 135019. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and characterization of alginate hydrogels with high water-retaining capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef] [PubMed]
- Baishya, G.; Parasar, B.; Limboo, M.; Kumar, R.; Dutta, A.; Hussain, A.; Phukan, M.M.; Saikia, D. Advancements in nanocomposite hydrogels: A comprehensive review of biomedical applications. Discov. Mater. 2024, 4, 40. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Li, Y.; Li, H.; Ji, S.; Xia, Q. Pickering emulsions-chitosan hydrogel beads carrier system for loading of resveratrol: Formulation approach and characterization studies. React. Funct. Polym. 2021, 169, 105074. [Google Scholar] [CrossRef]
- Morales, E.; Quilaqueo, M.; Morales-Medina, R.; Drusch, S.; Navia, R.; Montillet, A.; Rubilar, M.; Poncelet, D.; Galvez-Jiron, F.; Acevedo, F. Pectin–chitosan hydrogel beads for delivery of functional food ingredients. Foods 2024, 13, 2885. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Phimolsiripol, Y.; Mousavi Khaneghah, A. A comprehensive review on the utilization of biopolymer hydrogels to encapsulate and protect probiotics in foods. Int. J. Biol. Macromol. 2024, 254, 127907. [Google Scholar] [CrossRef]
- Touzout, Z.; Abdellaoui, N.; Hadj-Hamou, A.S. Conception of pH-sensitive calcium alginate/poly vinyl alcohol hydrogel beads for controlled oral curcumin delivery systems. Antibacterial and antioxidant properties. Int. J. Biol. Macromol. 2024, 263, 130389. [Google Scholar] [CrossRef]
- Carroza, D.; Malavasi, G.; Ferrari, E.; Menziani, M.C. Alginate beads containing cerium-doped mesoporous glass and curcumin: Delivery and stabilization of therapeutics. Int. J. Biol. Macromol. 2023, 24, 880. [Google Scholar] [CrossRef] [PubMed]
- Raus, R.A.; Wan Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Q.; Xu, D.; Dong, Q.-W.; Song, X.-J.; Chen, Y.-B.; Cui, Y.-L. Biomedical potentials of alginate via physical, chemical, and biological modifications. Int. J. Biol. Macromol. 2024, 277, 134409. [Google Scholar] [CrossRef]
- Besiri, I.N.; Goudoulas, T.B.; Fattahi, E.; Becker, T. Experimental advances in the real-time recording of cross-linking alginate in situ gelation: A Review. Polymers 2023, 15, 2875. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Yu, X.; Kim, H. Diffusion characteristics of different molecular weight solutes in Ca–alginate gel beads. Colloids Surf. A Physicochem. Eng. Asp. 2015, 469, 158–165. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Ha, J.; Engler, C.R.; Wild, J.R. Biodegradation of coumaphos, chlorferon, and diethylthiophosphate using bacteria immobilized in Ca-alginate gel beads. Bioresour. Technol. 2009, 100, 1138–1142. [Google Scholar] [CrossRef]
- Khunawattanakul, W.; Jaipakdee, N.; Rongthong, T.; Chansri, N.; Srisuk, P.; Chitropas, P.; Pongjanyakul, T. Sodium Alginate-Quaternary Polymethacrylate Composites: Characterization of Dispersions and Calcium Ion Cross-Linked Gel Beads. Gels 2022, 8, 739. [Google Scholar] [CrossRef]
- Kim, C.-K.; Lee, E.-J. The controlled release of blue dextran from alginate beads. Int. J. Pharm. 1992, 79, 11–19. [Google Scholar] [CrossRef]
- Cheng, B.; Li, D.; Huo, Q.; Zhao, Q.; Lan, Q.; Cui, M.; Pan, W.; Yang, X. Two kinds of ketoprofen enteric gel beads (CA and CS-SA) using biopolymer alginate. Asian J. Pharm. Sci. 2018, 13, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Jia, Y.; Zhang, L.; Zhang, J.; Hu, W.; Wang, C. Swelling studies and in vitro release of acemetacin and BSA from alginate gel beads crosslinked with Ca 2+ or Ba 2+. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2012, 27, 669–674. [Google Scholar] [CrossRef]
- Elnashar, M.M.; Yassin, M.A.; El-Fetouh Abdel Moneim, A.; Bary, E.M.A. Surprising performance of alginate beads for the release of low-molecular-weight drugs. J. Appl. Polym. Sci. 2010, 116, 3021–3026. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Song, W.; Lima, A.C.; Mano, J.F. Bioinspired methodology to fabricate hydrogel spheres for multi-applications using superhydrophobic substrates. Soft Matter 2010, 6, 5868–5871. [Google Scholar] [CrossRef]
- Takei, T.; Terazono, K.; Araki, K.; Ozuno, Y.; Hayase, G.; Kanamori, K.; Nakanishi, K.; Yoshida, M. Encapsulation of hydrophobic ingredients in hard resin capsules with ultrahigh efficiency using a superoleophobic material. Polym. Bull. 2016, 73, 409–417. [Google Scholar] [CrossRef]
- Takei, T.; Hamada, S.; Terazono, K.; Yoshida, M. Air drying on superamphiphobic surfaces can reduce damage by organic solvents to microbial cells immobilized in synthetic resin capsules. Process Biochem. 2017, 54, 28–32. [Google Scholar] [CrossRef]
- Takei, T.; Yamasaki, Y.; Yuji, Y.; Sakoguchi, S.; Ohzuno, Y.; Hayase, G.; Yoshida, M. Millimeter-sized capsules prepared using liquid marbles: Encapsulation of ingredients with high efficiency and preparation of spherical core-shell capsules with highly uniform shell thickness using centrifugal force. J. Colloid Interface Sci. 2019, 536, 414–423. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Alatorre-Meda, M.; Alvarez-Lorenzo, C.; Mano, J.F. Superhydrophobic surfaces as a tool for the fabrication of hierarchical spherical polymeric carriers. Small 2015, 11, 3648–3652. [Google Scholar] [CrossRef]
- Song, B. Lotus leaf-inspired design of calcium alginate particles with superhigh drug encapsulation efficiency and pH responsive release. Colloids Surf. B Biointerfaces 2018, 172, 464–470. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975; pp. 92–97. [Google Scholar]
- Sakai, S.; Ono, T.; Ijima, H.; Kawakami, K. In vitro and in vivo evaluation of alginate/sol-gel synthesized aminopropyl-silicate/alginate membrane for bioartificial pancreas. Biomaterials 2002, 23, 4177–4183. [Google Scholar] [CrossRef] [PubMed]
- Hacimusalar, M.; Mehmetoglu, U. Determination of the effective diffusion coefficients of glucose and ethanol in calcium alginate gel by the moment analysis method. Chem. Eng. Sci. 1995, 50, 3001–3004. [Google Scholar] [CrossRef]


| Volume of the Alginate Droplet [g] | CaCl2/Droplet Ratio [-] | CaCl2 Concentration [mol/L] | Gel Bead Diameter [mm] | ||
|---|---|---|---|---|---|
| a | New method | 0.10 | 0.1 | - | 5.0 ± 0.3 |
| b | New method | 0.20 | 0.3 | - | 5.3 ± 0.1 |
| c | New method | 0.30 | 0.5 | - | 4.8 ± 0.2 |
| d | PM1 | 0.08 | 0.5 | 0.05 | 4.8 ± 0.4 |
| e | PM1 | 0.10 | 0.8 | 0.10 | 5.3 ± 0.2 |
| f | PM1 | 0.25 | 20.3 | 6.71 | 5.4 ± 0.0 |
| De (m2/s) | |
|---|---|
| New method | 1.92 × 10−9 |
| PM1 | 1.63 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosokawa, Y.; Goshima, T.; Kai, T.; Kobaru, S.; Ohzuno, Y.; Nii, S.; Kiyoyama, S.; Yoshida, M.; Takei, T. Preparation of Alginate Hydrogel Beads on a Superhydrophobic Surface with Calcium Salt Powder to Enhance the Mechanical Strength and Encapsulation Efficiency of Ingredients. Materials 2024, 17, 6027. https://doi.org/10.3390/ma17246027
Hosokawa Y, Goshima T, Kai T, Kobaru S, Ohzuno Y, Nii S, Kiyoyama S, Yoshida M, Takei T. Preparation of Alginate Hydrogel Beads on a Superhydrophobic Surface with Calcium Salt Powder to Enhance the Mechanical Strength and Encapsulation Efficiency of Ingredients. Materials. 2024; 17(24):6027. https://doi.org/10.3390/ma17246027
Chicago/Turabian StyleHosokawa, Yuhei, Takashi Goshima, Takami Kai, Saki Kobaru, Yoshihiro Ohzuno, Susumu Nii, Shiro Kiyoyama, Masahiro Yoshida, and Takayuki Takei. 2024. "Preparation of Alginate Hydrogel Beads on a Superhydrophobic Surface with Calcium Salt Powder to Enhance the Mechanical Strength and Encapsulation Efficiency of Ingredients" Materials 17, no. 24: 6027. https://doi.org/10.3390/ma17246027
APA StyleHosokawa, Y., Goshima, T., Kai, T., Kobaru, S., Ohzuno, Y., Nii, S., Kiyoyama, S., Yoshida, M., & Takei, T. (2024). Preparation of Alginate Hydrogel Beads on a Superhydrophobic Surface with Calcium Salt Powder to Enhance the Mechanical Strength and Encapsulation Efficiency of Ingredients. Materials, 17(24), 6027. https://doi.org/10.3390/ma17246027







