Modification of Light-Cured Composition for Permanent Dental Fillings; Mass Stability of New Composites Containing Quinoline and Quinoxaline Derivatives in Solutions Simulating the Oral Cavity Environment
Highlights
- Application of quinoline and quinoxaline derivatives as visible light initiators is described.
- Modification of light-cured composite for permanent dental fillings is discussed. Mass stability of the new composites in solutions simulating the oral cavity environment was investigated.
- Influence of storage conditions of the developed composites on sorption, solubility and mass changes was assessed.
- Results were analyzed considering the nature of the solution used, i.e., aqueous, hydrophobic, and acidic.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Methods
2.2.1. Preparation of Samples for Testing
2.2.2. Polymerization Kinetics Studies
2.2.3. Methodology for Testing Mass Stability
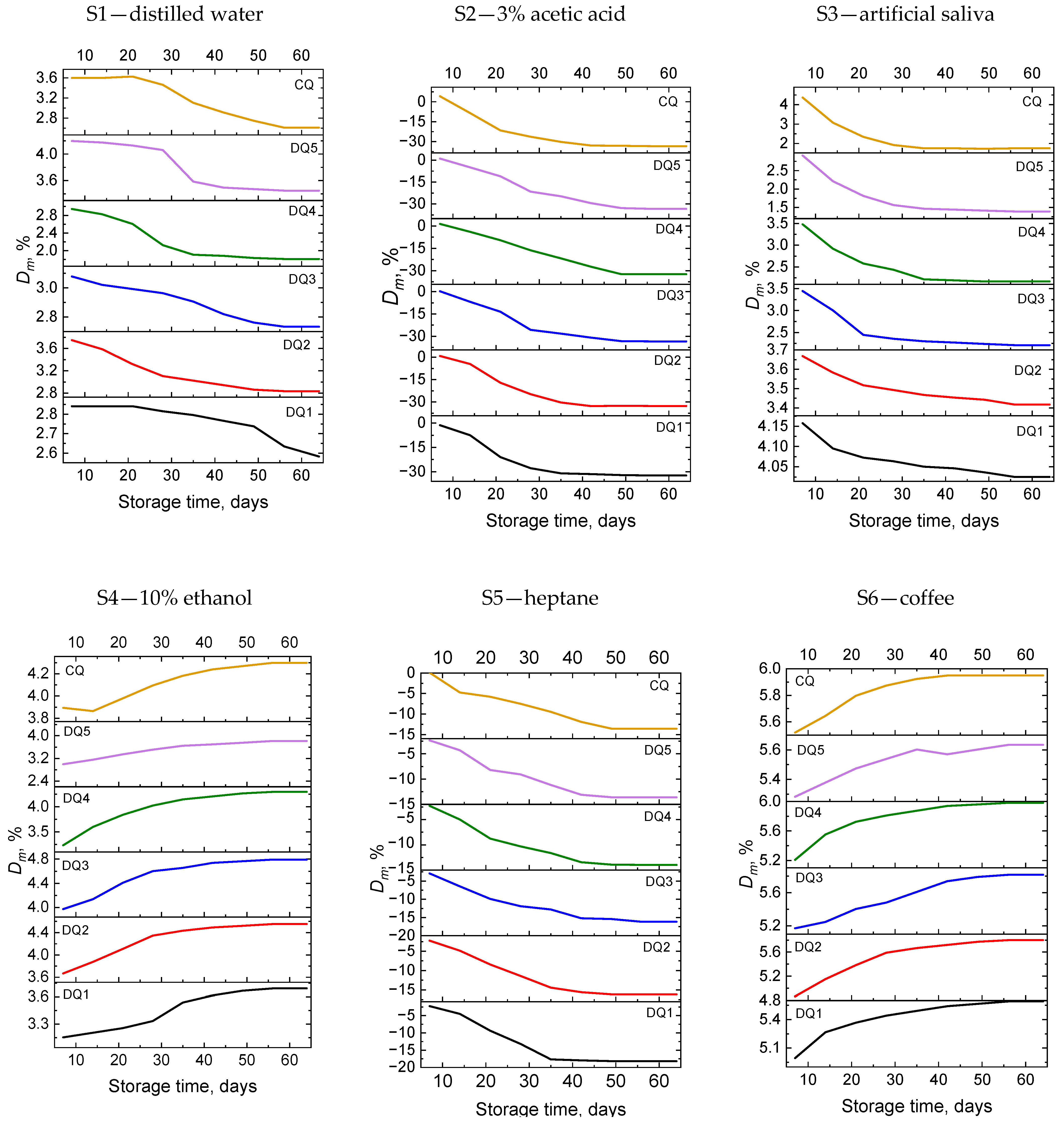
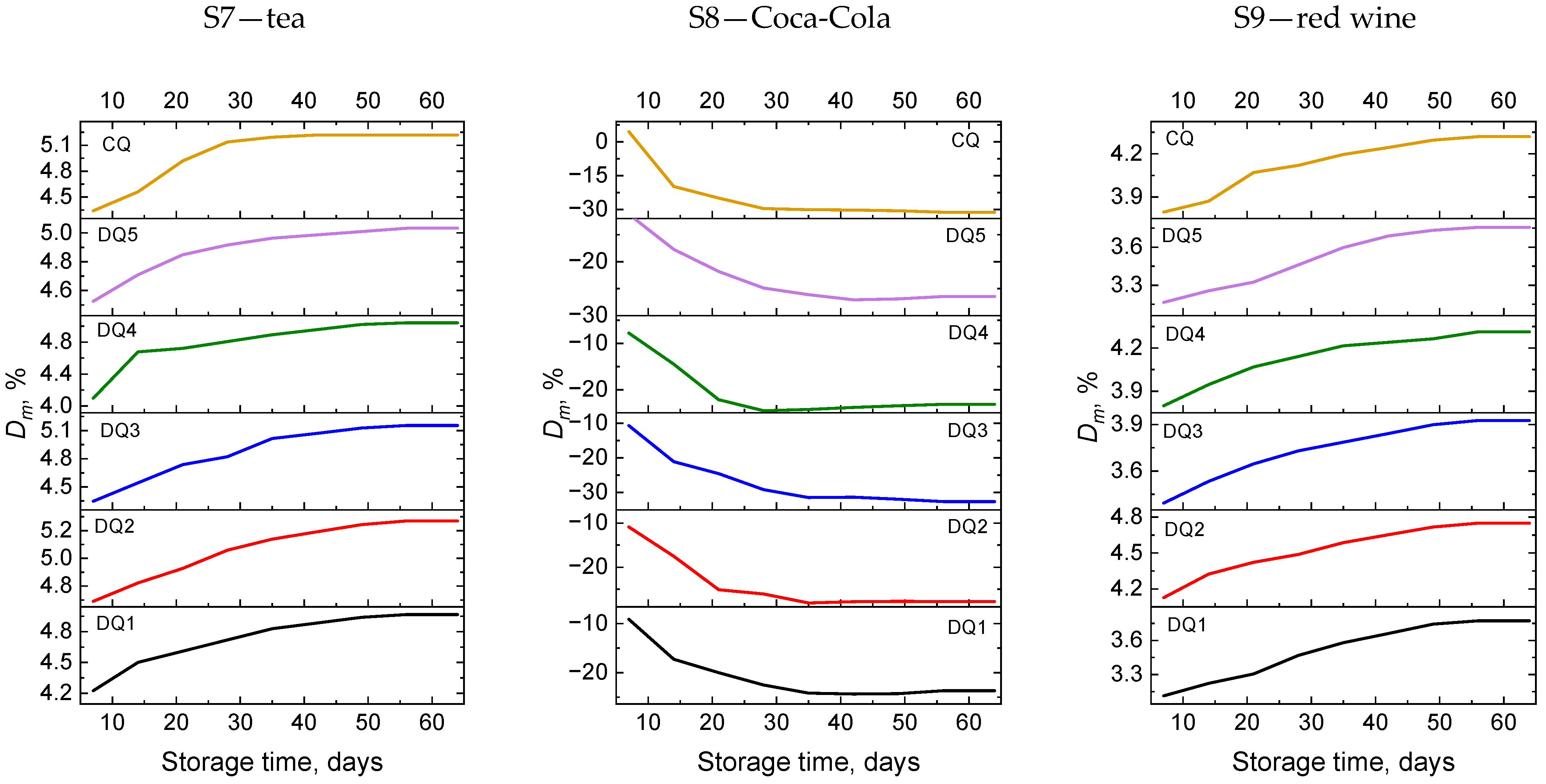
3. Results and Discussion
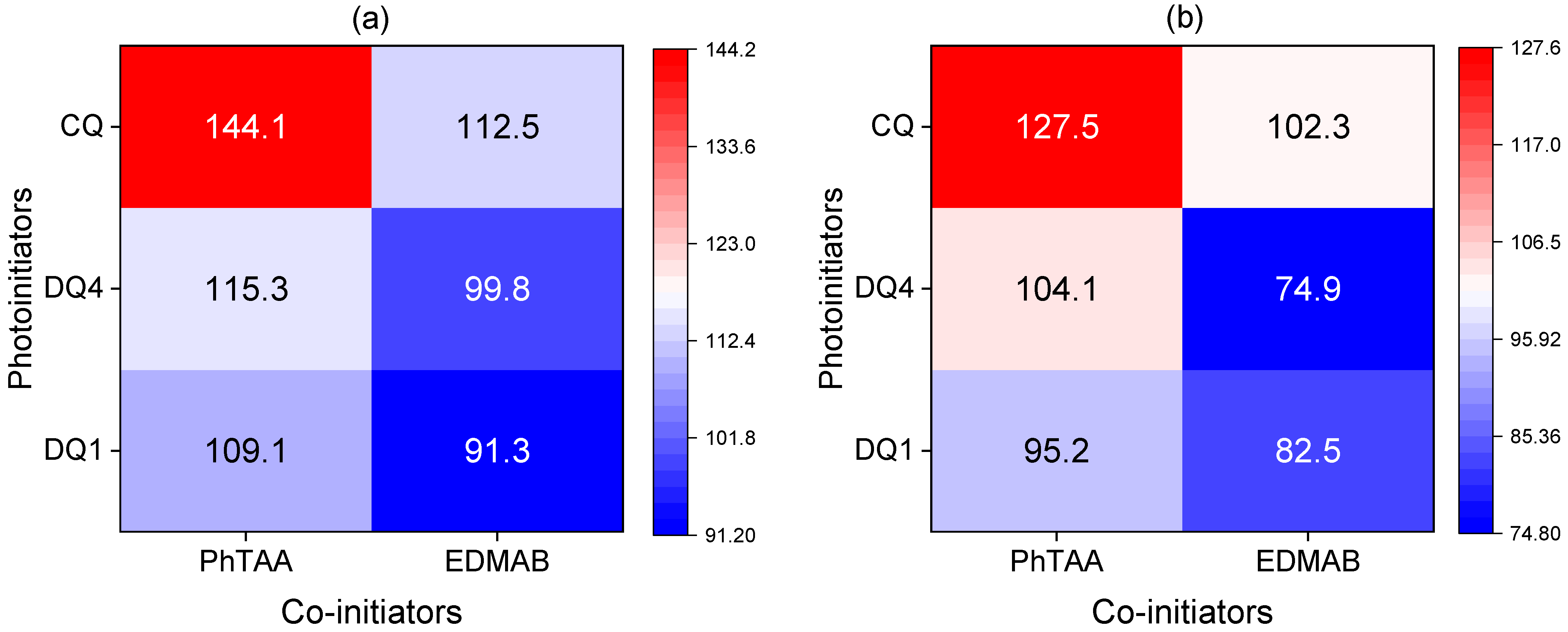
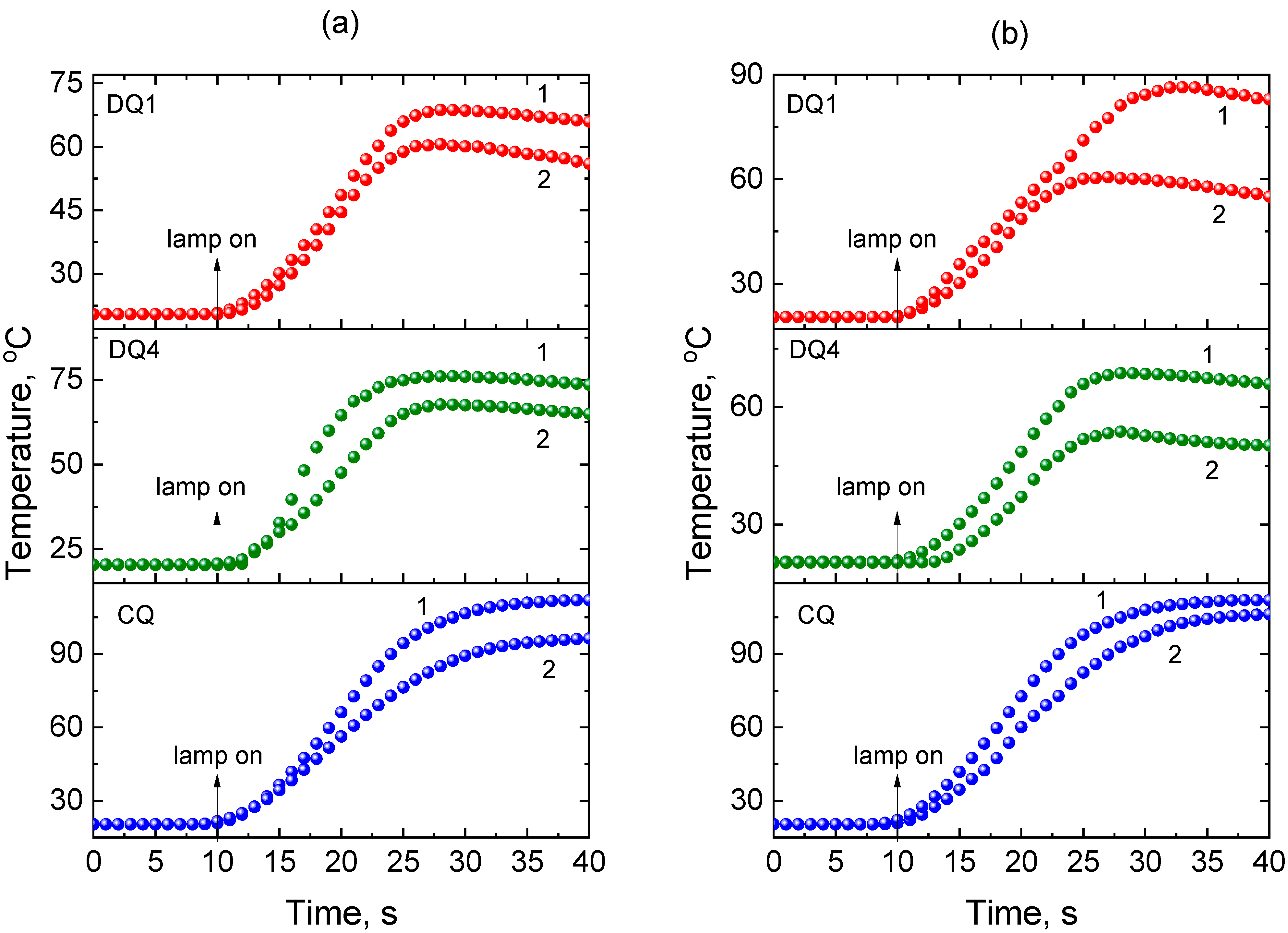
3.1. Photopolymerization
3.2. Weight Stability
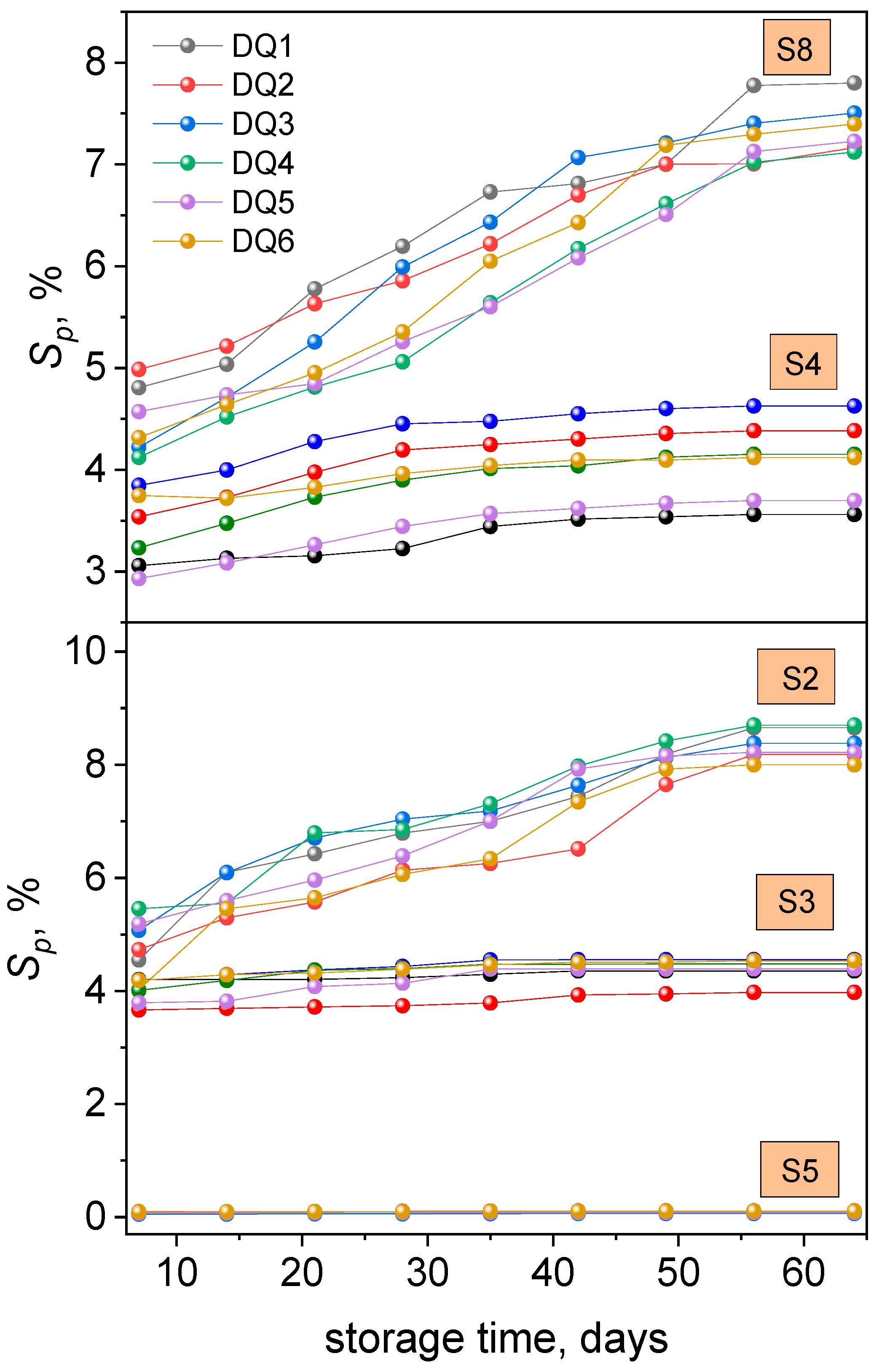
3.2.1. Sorption
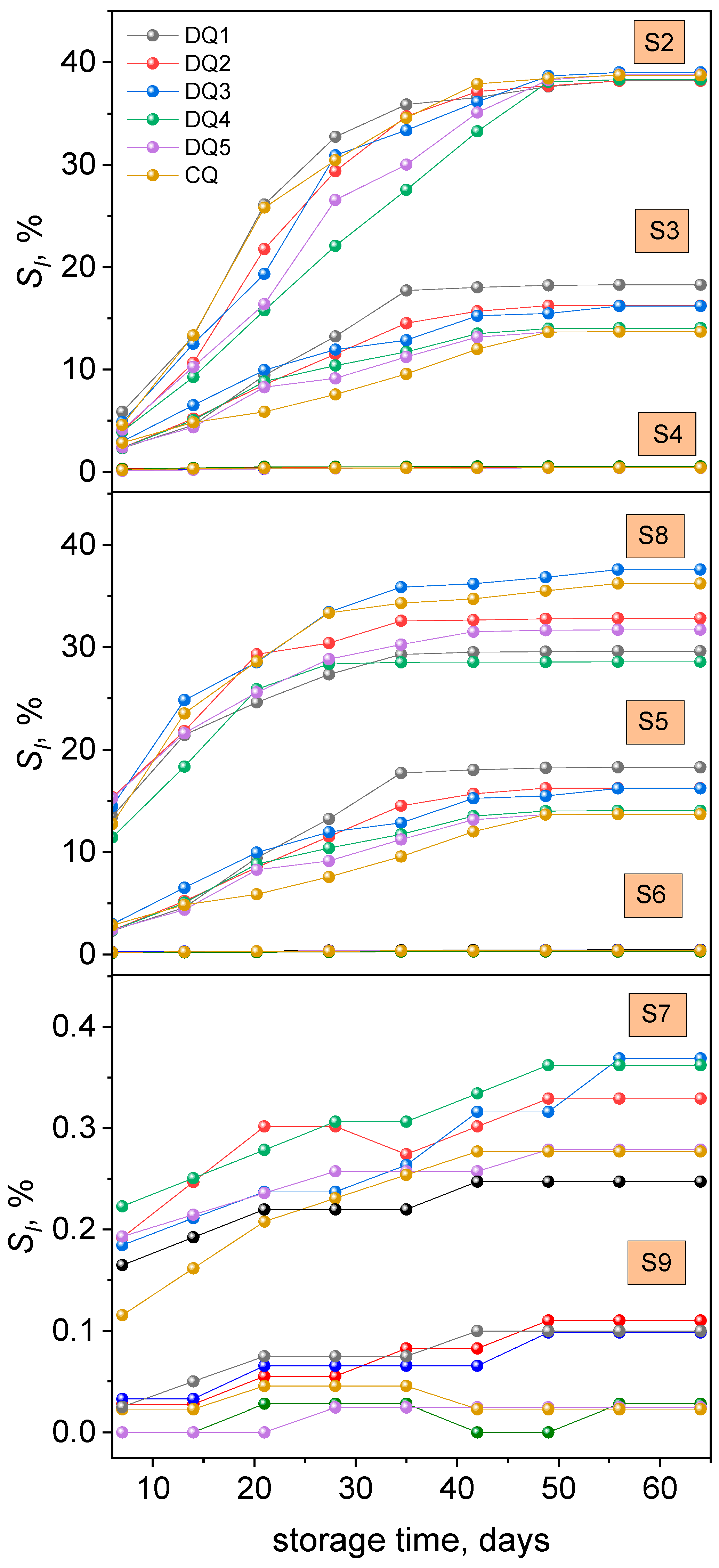
3.2.2. Solubility
3.2.3. Weight Change
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Abyu, G.Y.; Alsharif, U.; et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Syed-Picard, F.N.; Ray, H.L.; Kumta, P.N.; Sfeir, C. Scaffoldless Tissue-Engineered Dental Pulp Cell Constructs for Endodontic Therapy. J. Dent. Res. 2014, 93, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials, 13th ed.; In Elsevier eBook on VitalSource, Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chun, K.J.; Lee, J.Y. Comparative Study of Mechanical Properties of Dental Restorative Materials and Dental Hard Tissues in Compressive Loads. J. Dent. Biomech. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. On the Nature of Biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef]
- Schmalz, G. Determination of Biocompatibility. In Biocompatibility of Dental Materials; Schmalz, G., Arenholt-Bindslev, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 13–43. ISBN 978-3-540-77782-3. [Google Scholar]
- Dionysopoulos, D.; Gerasimidou, O.; Papadopoulos, C. Current Modifications of Dental Adhesive Systems for Composite Resin Restorations: A Review in Literature. J. Adhes. Sci. Technol. 2022, 36, 453–468. [Google Scholar] [CrossRef]
- Bowen, R.L.; Marjenhoff, W.A. Dental Composites/Glass Ionomers: The Materials. Adv. Dent. Res. 1992, 6, 44–49. [Google Scholar] [CrossRef]
- Ferracane, J.L. Current Trends in Dental Composites. Crit. Rev. Oral Biol. Med. 1995, 6, 302–318. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. New Developments of Polymeric Dental Composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin Composite—State of the Art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef]
- Jandt, K.D.; Sigusch, B.W. Future Perspectives of Resin-Based Dental Materials. Dent. Mater. 2009, 25, 1001–1006. [Google Scholar] [CrossRef]
- Chan, K.H.S.; Mai, Y.; Kim, H.; Tong, K.C.T.; Ng, D.; Hsiao, J.C.M. Review: Resin Composite Filling. Materials 2010, 3, 1228–1243. [Google Scholar] [CrossRef]
- Chen, M.-H. Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A. Resin Composites in Dentistry: The Monomer Systems. Eur. J. Oral Sci. 1997, 105, 97–116. [Google Scholar] [CrossRef] [PubMed]
- STANSBURY, J.W. Curing Dental Resins and Composites by Photopolymerization. J. Esthet. Restor. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Pączkowski, J.; Neckers, D.C. Photoinduced Electron Transfer Initiating Systems for Free-Radical Polymerization. In Electron Transfer in Chemistry; Wiley: Hoboken, NJ, USA, 2008; Volume 5. [Google Scholar]
- Pyszka, I.; Kucybała, Z.; Pączkowski, J. Reinvestigation of the Mechanism of the Free Radical Polymerization Photoinitiation Process by Camphorquinone–Coinitiator Systems: New Results. Macromol. Chem. Phys. 2004, 205, 2371–2375. [Google Scholar] [CrossRef]
- Allen, N.S. Photochemistry and Photophysics of Polymer Materials; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Cook, W.D. Photopolymerization Kinetics of Dimethacrylates Using the Camphorquinone/Amine Initiator System. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Nie, J.; Lindén, L.Å.; Rabek, J.F.; Ekstrand, J. Photocuring Kinetic Studies of New Dental Restorative Resins Based on Poly(Ethylene Glycol) Diacrylate and Tris[2-(Acryloyloxy)-Ethyl]Isocyanurate. Angew. Makromol. Chem. 1998, 257, 47–52. [Google Scholar] [CrossRef]
- ASMUSSEN, E. Factors Affecting the Quantity of Remaining Double Bonds in Restorative Resin Polymers. Eur. J. Oral Sci. 1982, 90, 490–496. [Google Scholar] [CrossRef]
- Łagocka, R.; Rogocka, M.; Granat, M.; Jakubowska, K.; Pawlik, A.; Mazurek-Mochol, M. Bis-GMA Monomer Elution from Modern Resin-Based Composites—Preliminary in Vitro Study. Farm. Pol. 2022, 78, 317–325. [Google Scholar] [CrossRef]
- Richter, C.A.; Birnbaum, L.S.; Farabollini, F.; Newbold, R.R.; Rubin, B.S.; Talsness, C.E.; Vandenbergh, J.G.; Walser-Kuntz, D.R.; vom Saal, F.S. In Vivo Effects of Bisphenol A in Laboratory Rodent Studies. Reprod. Toxicol. 2007, 24, 199–224. [Google Scholar] [CrossRef]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of Resin-Based Composites and Sealants Used in Dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kostoryz, E.L.; Wetmore, L.A.; Brockmann, W.G.; Yourtee, D.M.; Eick, J.D. Genotoxicity Assessment of Oxirane-Based Dental Monomers in Mammalian Cells. J. Biomed. Mater. Res. A 2004, 68A, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Issa, Y.; Watts, D.C.; Brunton, P.A.; Waters, C.M.; Duxbury, A.J. Resin Composite Monomers Alter MTT and LDH Activity of Human Gingival Fibroblasts In Vitro. Dent. Mater. 2004, 20, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Styllou, M.; Reichl, F.-X.; Styllou, P.; Urcan, E.; Rothmund, L.; Hickel, R.; Högg, C.; Scherthan, H. Dental Composite Components Induce DNA-Damage and Altered Nuclear Morphology in Gingiva Fibroblasts. Dent. Mater. 2015, 31, 1335–1344. [Google Scholar] [CrossRef]
- Nakamura, M.; Oshima, H.; Hashimoto, Y. Monomer Permeability of Disposable Dental Gloves. J. Prosthet. Dent. 2003, 90, 81–85. [Google Scholar] [CrossRef]
- Borjigin, T.; Schmitt, M.; Giacoletto, N.; Rico, A.; Bidotti, H.; Nechab, M.; Zhang, Y.; Graff, B.; Morlet-Savary, F.; Xiao, P.; et al. The Blue-LED-Sensitive Naphthoquinone-Imidazolyl Derivatives as Type II Photoinitiators of Free Radical Photopolymerization. Adv. Mater. Interfaces 2023, 10, 2202352. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Vallo, C.I. Effect of Different Photoinitiator Systems on Conversion Profiles of a Model Unfilled Light-Cured Resin. Dent. Mater. 2007, 23, 1313–1321. [Google Scholar] [CrossRef]
- de Oliveira, D.C.R.S.; Rocha, M.G.; Gatti, A.; Correr, A.B.; Ferracane, J.L.; Sinhoret, M.A.C. Effect of Different Photoinitiators and Reducing Agents on Cure Efficiency and Color Stability of Resin-Based Composites Using Different LED Wavelengths. J. Dent. 2015, 43, 1565–1572. [Google Scholar] [CrossRef]
- Rogalewicz, R.; Batko, K.; Voelkel, A. Identification of Organic Extractables from Commercial Resin-Modified Glass-Ionomers Using HPLC-MS. J. Environ. Monit. 2006, 8, 750–758. [Google Scholar] [CrossRef]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A Release from Short-Term Degraded Resin-Based Dental Materials. J. Dent. 2022, 116, 103894. [Google Scholar] [CrossRef]
- Durner, J.; Obermaier, J.; Draenert, M.; Ilie, N. Correlation of the Degree of Conversion with the Amount of Elutable Substances in Nano-Hybrid Dental Composites. Dent. Mater. 2012, 28, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and Hydrolytic Effects in Dental Polymer Networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Karabela, M.M. Sorption of Water, Ethanol or Ethanol/Water Solutions by Light-Cured Dental Dimethacrylate Resins. Dent. Mater. 2011, 27, 1003–1010. [Google Scholar] [CrossRef]
- Rahim, T.N.A.T.; Mohamad, D.; Md Akil, H.; Ab Rahman, I. Water Sorption Characteristics of Restorative Dental Composites Immersed in Acidic Drinks. Dent. Mater. 2012, 28, e63–e70. [Google Scholar] [CrossRef]
- Örtengren, U.; Wellendorf, H.; Karlsson, S.; Ruyter, I.E. Water Sorption and Solubility of Dental Composites and Identification of Monomers Released in an Aqueous Environment. J. Oral Rehabil. 2001, 28, 1106–1115. [Google Scholar] [CrossRef]
- Miettinen, V.M.; Narva, K.K.; Vallittu, P.K. Water Sorption, Solubility and Effect of Post-Curing of Glass Fibre Reinforced Polymers. Biomaterials 1999, 20, 1187–1194. [Google Scholar] [CrossRef]
- Yiu, C.K.Y.; King, N.M.; Carrilho, M.R.O.; Sauro, S.; Rueggeberg, F.A.; Prati, C.; Carvalho, R.M.; Pashley, D.H.; Tay, F.R. Effect of Resin Hydrophilicity and Temperature on Water Sorption of Dental Adhesive Resins. Biomaterials 2006, 27, 1695–1703. [Google Scholar] [CrossRef]
- Pyszka, I.; Jędrzejewska, B. Design of Dyes Based on the Quinoline or Quinoxaline Skeleton towards Visible Light Photoinitiators. Int. J. Mol. Sci. 2024, 25, 4289. [Google Scholar] [CrossRef]
- Kucková, L.; Jomová, K.; Švorcová, A.; Valko, M.; Segľa, P.; Moncoľ, J.; Kožíšek, J. Synthesis, Crystal Structure, Spectroscopic Properties and Potential Biological Activities of Salicylate–Neocuproine Ternary Copper(II) Complexes. Molecules 2015, 20, 2115–2137. [Google Scholar] [CrossRef]
- Abu Ali, H.; Abu Shamma, A.; Kamel, S. New Mixed Ligand Cobalt(II/III) Complexes Based on the Drug Sodium Valproate and Bioactive Nitrogen-Donor Ligands. Synthesis, Structure and Biological Properties. J. Mol. Struct. 2017, 1142, 40–47. [Google Scholar] [CrossRef]
- Dhumwad, S.D. Synthesis, Characterization and Biological Studies of Co(II), Ni(II), Cu(II) and Zn(II) Complexes of Schiff Bases Derived from 3-Formyl-2-Mercaptoquinolines. J. Chem. Pharm. Res. 2011, 3, 504–517. [Google Scholar]
- Jaman, Z.; Karim, M.R.; Siddiquee, T.A.; Mirza, A.H.; Ali, M.A. Synthesis of 5-Substituted 2, 9-Dimethyl-1,10-Phenanthroline Dialdehydes and Their Schiff Bases with Sulfur-Containing Amines. Int. J. Org. Chem. 2013, 3, 214–219. [Google Scholar] [CrossRef]
- Montana, M.; Mathias, F.; Terme, T.; Vanelle, P. Antitumoral Activity of Quinoxaline Derivatives: A Systematic Review. Eur. J. Med. Chem. 2019, 163, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Jabali, B.; Abu Ali, H. New Zinc(II) Complexes of the Non-Steroidal Anti-Inflammatory Drug (Indomethacin) and Various Nitrogen Donor Ligands. Synthesis, Characterization and Biological Activity. Polyhedron 2016, 117, 249–258. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pessoa, A.M.; Cordeiro, M.N.D.S.; Fernandes, R.; Prudêncio, C.; Noronha, J.P.; Vieira, M. Quinoxaline, Its Derivatives and Applications: A State of the Art Review. Eur. J. Med. Chem. 2015, 97, 664–672. [Google Scholar] [CrossRef]
- Paczkowski, J.; Kucybala, Z. Generalization of the Kinetic Scheme for a Dye-Photosensitized Free-Radical Polymerization Initiating System via an Intermolecular Electron-Transfer Process. Application of Marcus Theory. Macromolecules 1995, 28, 269–273. [Google Scholar] [CrossRef]
- Gonulol, N.; Ozer, S.; Sen Tunc, E. Water Sorption, Solubility, and Color Stability of Giomer Restoratives. J. Esthet. Restor. Dent. 2015, 27, 300–306. [Google Scholar] [CrossRef]
- KOKSAL, T.; DIKBAS, I. Color Stability of Different Denture Teeth Materials against Various Staining Agents. Dent. Mater. J. 2008, 27, 139–144. [Google Scholar] [CrossRef]
- ERTAS, E.; GÜLER, A.U.; YÜCEL, A.Ç.; KÖPRÜLÜ, H.; GÜLER, E. Color Stability of Resin Composites after Immersion in Different Drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef]
- Uchimura, J.Y.T.; Sato, F.; Bianchi, G.; Baesso, M.L.; Santana, R.G.; Pascotto, R.C. Color Stability Over Time of Three Resin-Based Restorative Materials Stored Dry and in Artificial Saliva. J. Esthet. Restor. Dent. 2014, 26, 279–287. [Google Scholar] [CrossRef]
- Haselton, D.R.; Diaz-Arnold, A.M.; Dawson, D.V. Color Stability of Provisional Crown and Fixed Partial Denture Resins. J. Prosthet. Dent. 2005, 93, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Yannikakis, S.A.; Zissis, A.J.; Polyzois, G.L.; Caroni, C. Color Stability of Provisional Resin Restorative Materials. J. Prosthet. Dent. 1998, 80, 533–539. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 4049:2019; Dentistry—Polymer-Based Restorative Materials, Ed. 5. International Organization for Standardization: Geneva, Switzerland, 2019.
- Atai, M.; Nekoomanesh, M.; Hashemi, S.A.; Amani, S. Physical and Mechanical Properties of an Experimental Dental Composite Based on a New Monomer. Dent. Mater. 2004, 20, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Silikas, N.; Zhang, Z.; Watts, D.C. Diffusion and Concurrent Solubility of Self-Adhering and New Resin–Matrix Composites during Water Sorption/Desorption Cycles. Dent. Mater. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Okulus, Z.; Héberger, K.; Voelkel, A. Sorption, Solubility, and Mass Changes of Hydroxyapatite-Containing Composites in Artificial Saliva, Food Simulating Solutions, Tea, and Coffee. J. Appl. Polym. Sci. 2014, 131, 39856. [Google Scholar] [CrossRef]
- Domingo, C.; Arcís, R.W.; Osorio, E.; Osorio, R.; Fanovich, M.A.; Rodríguez-Clemente, R.; Toledano, M. Hydrolytic Stability of Experimental Hydroxyapatite-Filled Dental Composite Materials. Dent. Mater. 2003, 19, 478–486. [Google Scholar] [CrossRef]
- Kanchanavasita, W.; Anstice, H.M.; Pearson, G.J. Water Sorption Characteristics of Resin-Modified Glass-Ionomer Cements. Biomaterials 1997, 18, 343–349. [Google Scholar] [CrossRef]
- Ratna, D.; Simon, G.P. Mechanical Characterization and Morphology of Carboxyl Randomized Poly(2-Ethyl Hexyl Acrylate) Liquid Rubber Toughened Epoxy Resins. Polymer 2001, 42, 7739–7747. [Google Scholar] [CrossRef]
- Vining, K.H.; Scherba, J.C.; Bever, A.M.; Alexander, M.R.; Celiz, A.D.; Mooney, D.J. Synthetic Light-Curable Polymeric Materials Provide a Supportive Niche for Dental Pulp Stem Cells. Adv. Mater. 2018, 30, 1704486. [Google Scholar] [CrossRef]
- Nason, C.; Roper, T.; Hoyle, C.; Pojman, J.A. UV-Induced Frontal Polymerization of Multifunctional (Meth)Acrylates. Macromolecules 2005, 38, 5506–5512. [Google Scholar] [CrossRef]
- Bjørndal, L.; Fransson, H.; Bruun, G.; Markvart, M.; Kjældgaard, M.; Näsman, P.; Hedenbjörk-Lager, A.; Dige, I.; Thordrup, M. Randomized Clinical Trials on Deep Carious Lesions: 5-Year Follow-Up. J. Dent. Res. 2017, 96, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hook, A.L.; Chang, C.-Y.; Yang, J.; Atkinson, S.; Langer, R.; Anderson, D.G.; Davies, M.C.; Williams, P.; Alexander, M.R. Discovery of Novel Materials with Broad Resistance to Bacterial Attachment Using Combinatorial Polymer Microarrays. Adv. Mater. 2013, 25, 2542–2547. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, M. Organotin (IV) Derivative of Piperic Acid and Phenylthioacetic Acid: Synthesis, Crystal Structure, Spectroscopic Characterizations and Biological Activities. Moroc. J. Chem. 2020, 8, 244–263. [Google Scholar] [CrossRef]
- Velmurugan, V.; Nandini Asha, R.; Ravindran Durai Nayagam, B.; Kumaresan, S.; Bhuvanesh, N. Synthesis, Characterization and Biological Activity of (Phenylthio)Acetic Acid:Theophylline Cocrystal. J. Chem. Crystallogr. 2021, 51, 225–234. [Google Scholar] [CrossRef]
- Sideridou, I.; Achilias, D.S.; Spyroudi, C.; Karabela, M. Water Sorption Characteristics of Light-Cured Dental Resins and Composites Based on Bis-EMA/PCDMA. Biomaterials 2004, 25, 367–376. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M. Dental Composites Based on Amorphous Calcium Phosphate—Resin Composition/Physicochemical Properties Study. J. Biomater. Appl. 2007, 21, 375–393. [Google Scholar] [CrossRef]
- Musanje, L.; Shu, M.; Darvell, B.W. Water Sorption and Mechanical Behaviour of Cosmetic Direct Restorative Materials in Artificial Saliva. Dent. Mater. 2001, 17, 394–401. [Google Scholar] [CrossRef]
| Photoinitiators | |
|---|---|
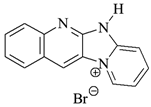 DQ1: quinoline [2,3-b]-1H-imidazo [1,2-a]pyridinium bromide | 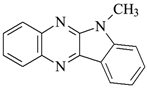 DQ4: 6-methyl-6H-indolo [2,3-b]quinoxaline |
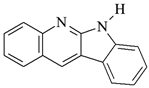 DQ2: 6H-indolo [2,3-b]quinoline | 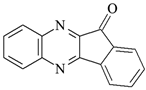 DQ5: 11H-indeno [1,2-b]qunioxalin-11-on |
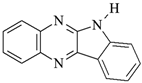 DQ3: 6H-indolo [2,3-b]quinoxaline | 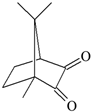 CQ: camphorquinone |
| Co-initiators | Solvent |
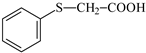 PhTAA: (phenylthio)acetic acid | 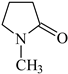 MP: 1-methyl-2-pyrolidinone |
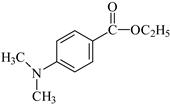 EDMAB: ethyl 4-dimethylaminobenzoate | |
| Monomers | |
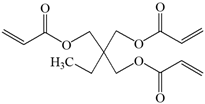 TMPTA: trimethylolpropane triacrylate | 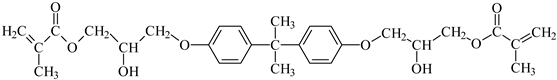 Bis-GMA: Bisphenol A glycerolate dimethacrylate |
| Developed Compositions | ||||||
|---|---|---|---|---|---|---|
| No. | Organic Matrix (A), % w/w | Inorganic Filler (B), g | Organic Matrix to Filler Ratio (A:B), % w/w | |||
| Photoinitiator | Co-Initiator | Monomer | Solvent | |||
| 1. | DQ1 0.05% | PhTAA 1.65% | TMPTA 88.20% | MP 10.10% | IDG 1.50 | 40:60 |
| 2. | DQ2 0.03% | PhTAA 1.65% | TMPTA 88.21% | MP 10.11% | IDG 1.50 | 40:60 |
| 3. | DQ3 0.03% | PhTAA 1.65% | TMPTA 88.21% | MP 10.11% | IDG 1.50 | 40:60 |
| 4. | DQ4 0.04% | PhTAA 1.65% | TMPTA 88.20% | MP 10.11% | IDG 1.50 | 40:60 |
| 5. | DQ5 0.04% | PhTAA 1.65% | TMPTA 88.20% | MP 10.11% | IDG 1.50 | 40:60 |
| 6. | CQ 9.91% | PhTAA 1.48% | TMPTA 79.50% | MP 9.11% | IDG 1.50 | 40:60 |
| Commercial composition | ||||||
| 1. | CQ 9.88% | EDMAB 1.70% | Bis-GMA 79.40% | MP 9.02% | IDG 1.50 | 40:60 |
| Sample | Component | Simulated Environment |
|---|---|---|
| S1 | distilled water | hydrated food with pH > 4.5 |
| S2 | 3% acetic acid solution | hydrated food with pH < 4.5 |
| S3 | artificial saliva | saliva |
| S4 | 10% aqueous solution of ethyl alcohol | food containing alcohol |
| S5 | heptane | fatty foods |
| S6 | coffee | coffee |
| S7 | tea | tea |
| S8 | Coca-Cola | Coca-Cola drink |
| S9 | red wine | wine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyszka, I.; Jędrzejewska, B. Modification of Light-Cured Composition for Permanent Dental Fillings; Mass Stability of New Composites Containing Quinoline and Quinoxaline Derivatives in Solutions Simulating the Oral Cavity Environment. Materials 2024, 17, 6003. https://doi.org/10.3390/ma17236003
Pyszka I, Jędrzejewska B. Modification of Light-Cured Composition for Permanent Dental Fillings; Mass Stability of New Composites Containing Quinoline and Quinoxaline Derivatives in Solutions Simulating the Oral Cavity Environment. Materials. 2024; 17(23):6003. https://doi.org/10.3390/ma17236003
Chicago/Turabian StylePyszka, Ilona, and Beata Jędrzejewska. 2024. "Modification of Light-Cured Composition for Permanent Dental Fillings; Mass Stability of New Composites Containing Quinoline and Quinoxaline Derivatives in Solutions Simulating the Oral Cavity Environment" Materials 17, no. 23: 6003. https://doi.org/10.3390/ma17236003
APA StylePyszka, I., & Jędrzejewska, B. (2024). Modification of Light-Cured Composition for Permanent Dental Fillings; Mass Stability of New Composites Containing Quinoline and Quinoxaline Derivatives in Solutions Simulating the Oral Cavity Environment. Materials, 17(23), 6003. https://doi.org/10.3390/ma17236003








