Superhydrophobic Surfaces as a Potential Skin Coating to Prevent Jellyfish Stings: Inhibition and Anti-Tentacle Adhesion in Nematocysts of Jellyfish Nemopilema nomurai
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
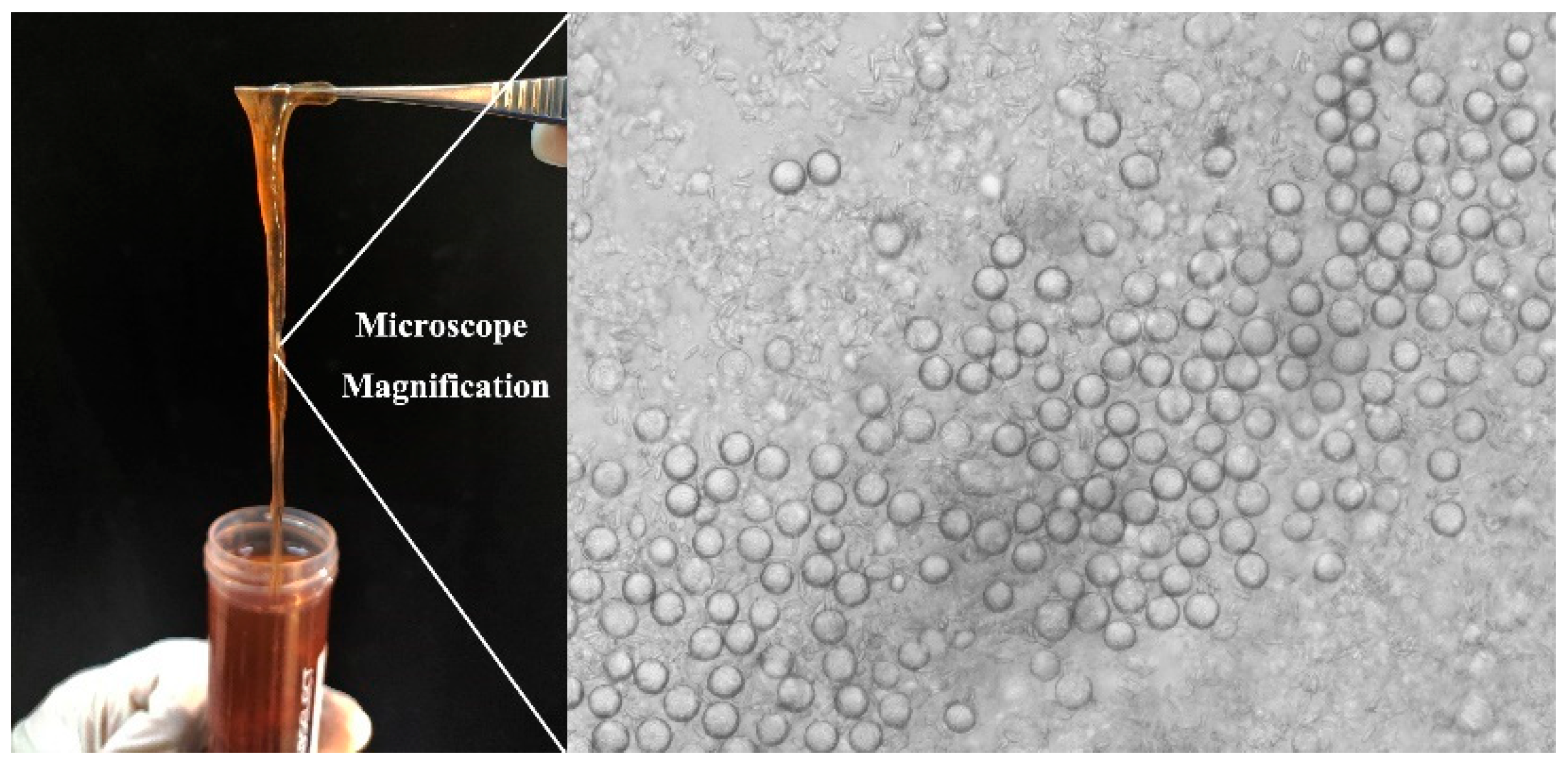
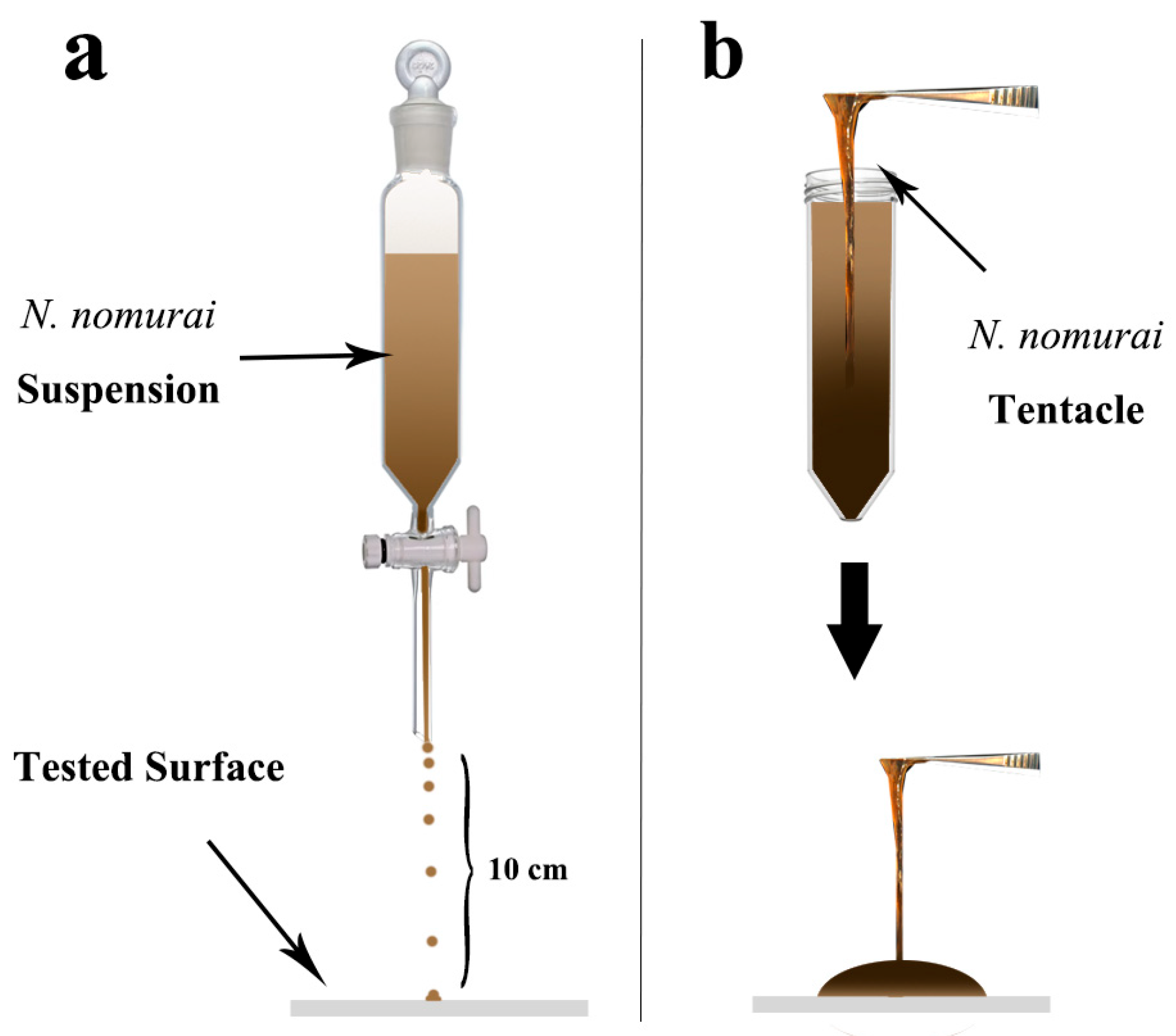
2.2. Nemopilema Nomurai Tentacles Collection
2.3. Surface Material Preparation
2.3.1. Gelatin and PDMS Surface Preparation
2.3.2. Preparation of Superhydrophobic SiO2 and TiO2
2.3.3. Preparation of CNF and ChNCs Composite Materials
2.3.4. Mechanical Strength Test of Surfaces
2.4. Contact-Angle Measurement
2.5. Scanning Electron Microscope Characterization
2.6. X-Ray Photoelectron Spectroscopy Characterization
2.7. Statistical Analysis
3. Results and Discussion
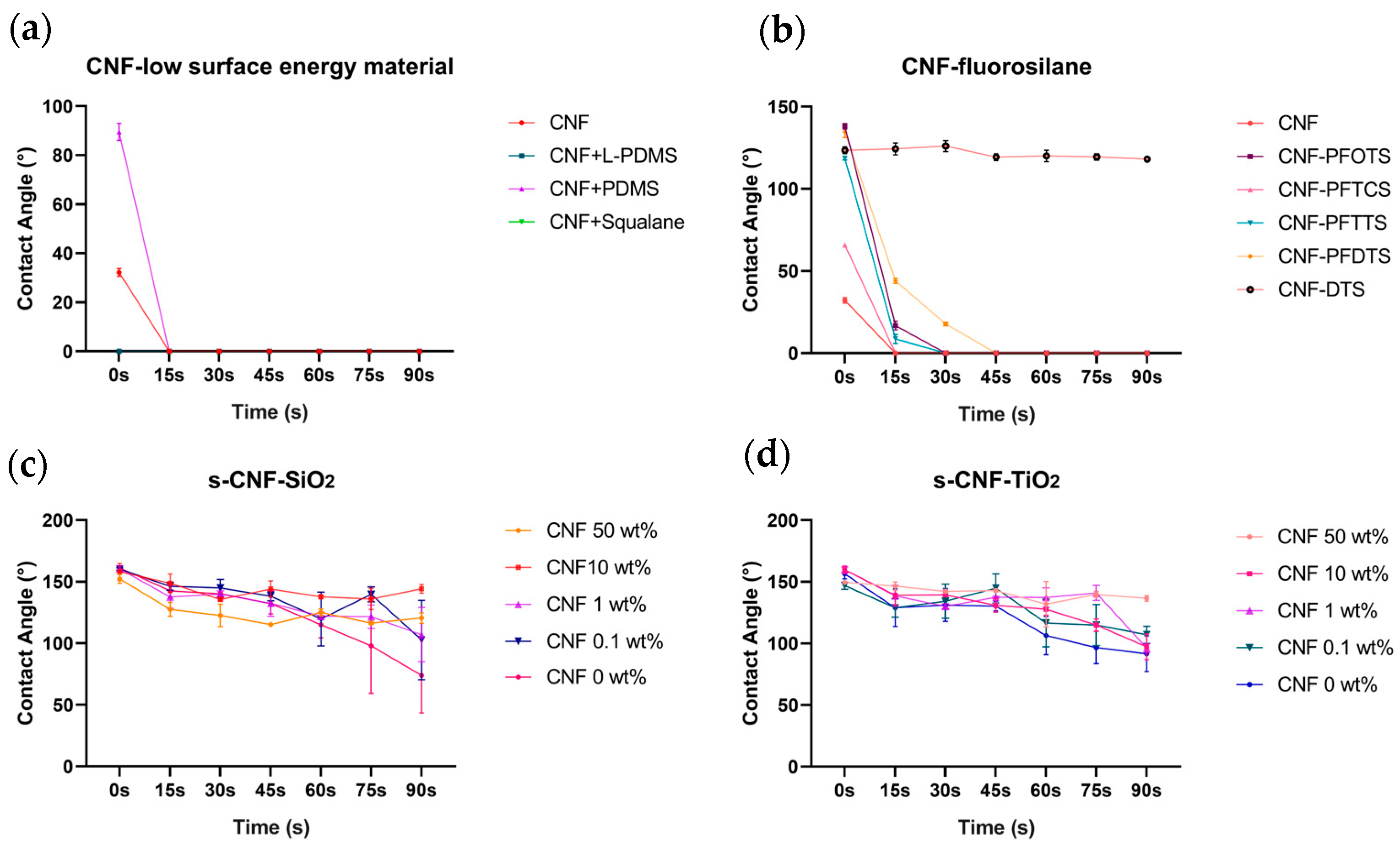
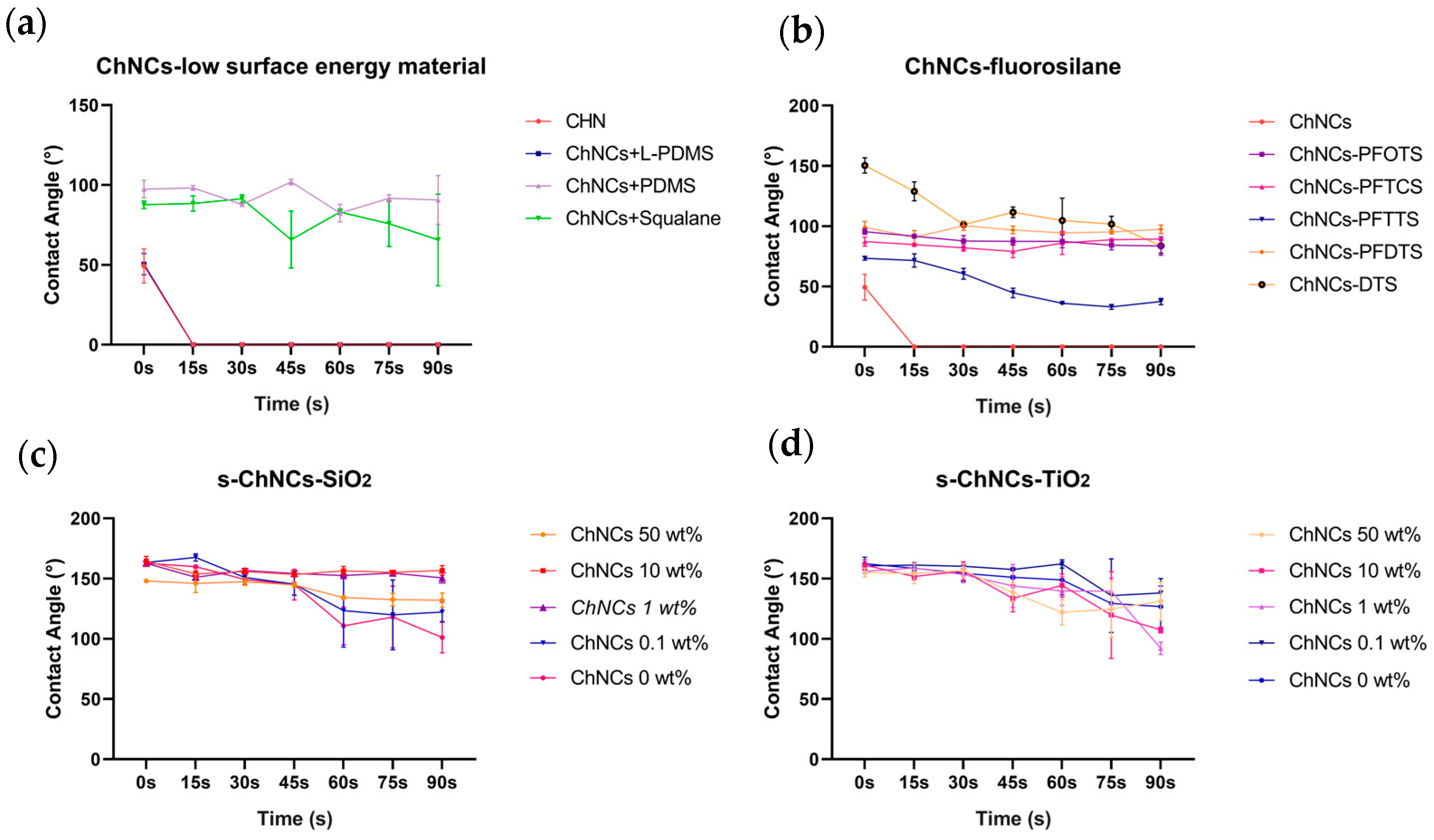
3.1. Superhydrophobic Surface Tentacles Adhesion and Characterization
3.2. Impact Test of Jellyfish Suspension on the Composite Material of CNF and ChNCs
3.3. Anti-Tentacle Abrasion of Superhydrophobic Composite Materials on Skin Surface
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amato, G.; Vita, F.; Gemelli, F.; Tigano, V.; Minciullo, P.L.; Gangemi, S. Jellyfish anaphylaxis: A wide spectrum of sensitization routes. Allergy Asthma Proc. 2020, 41, 158–166. [Google Scholar] [CrossRef]
- Rowley, O.C.; Courtney, R.; Northfield, T.; Seymour, J. Environmental drivers of the occurrence and abundance of the Irukandji jellyfish (Carukia barnesi). PLoS ONE 2022, 17, 21. [Google Scholar] [CrossRef]
- Park, S.; Lee, D.; Yang, Y.; Lee, H.; Lee, K. Analysis on underwater stability of the jellyfish sting protection net installed in the Haeundae beach. J. Korean Soc. Fish. Ocean. Technol. 2015, 51, 128–135. [Google Scholar] [CrossRef]
- Heeger, T.; Möller, H.; Mrowietz, U. Protection of human skin against jellyfish (Cyanea capillata) stings. Mar. Biol. 1992, 113, 669–678. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Biol. 2010, 60, 954–963. [Google Scholar] [CrossRef]
- Cannon, Q.; Wagner, E. Comparison of discharge mechanisms of Cnidarian cnidae and Myxozoan polar capsules. Rev. Fish. Sci. 2003, 11, 185–219. [Google Scholar] [CrossRef]
- Choudhary, I.; Hwang, D.H.; Lee, H.; Yoon, W.D.; Chae, J.; Han, C.H.; Yum, S.; Kang, C.; Kim, E. Proteomic Analysis of Novel Components of Nemopilema nomurai Jellyfish Venom: Deciphering the Mode of Action. Toxins 2019, 11, 153. [Google Scholar] [CrossRef]
- Rather, A.M.; Jana, N.; Hazarika, P.; Manna, U. Sustainable polymeric material for the facile and repetitive removal of oil-spills through the complementary use of both selective-absorption and active-filtration processes. J. Mater. Chem. A 2017, 5, 23339–23348. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, H.Z.; Wang, P.; Zhang, G.J. Rapidly one-step fabrication of durable superhydrophobic graphene surface with high temperature resistance and self-clean. J. Colloid Interface Sci. 2025, 679, 476–486. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Ma, X.; Zhu, H.; Qiao, Y.; Liu, X. “Petal effect”-inspired superhydrophobic and highly adhesive coating on magnesium with enhanced corrosion resistance and biocompatibility. Sci. China Mater. 2018, 61, 629–642. [Google Scholar] [CrossRef]
- Wan, B.B.; Ou, J.F.; Lv, D.M.; Xue, M.S.; Wang, F.J.; Wu, H.M. Superhydrophobic ceria on aluminum and its corrosion resistance. Surf. Interface Anal. 2016, 48, 173–178. [Google Scholar] [CrossRef]
- Wang, L.J.; Guo, X.D.; Zhang, H.M.; Liu, Y.X.; Wang, Y.X.; Liu, K.; Liang, H.F.; Ming, W.Y. Recent Advances in Superhydrophobic and Antibacterial Coatings for Biomedical Materials. Coatings 2022, 12, 32. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Lazarov, Y.; Stamenov, G.S.; Chaushev, T.A. When condensed matter physics meets biology: Does superhydrophobicity benefiting the cryopreservation of human spermatozoa? Cryobiology 2020, 92, 263–266. [Google Scholar] [CrossRef]
- Celik, N.; Sahin, F.; Ruzi, M.; Yay, M.; Unal, E.; Onses, M.S. Blood repellent superhydrophobic surfaces constructed from nanoparticle-free and biocompatible materials. Colloids Surf. B Biointerfaces 2021, 205, 111864. [Google Scholar] [CrossRef]
- Yousefi, S.Z.; Tabatabaei-Panah, P.S.; Seyfi, J.J.C.; Biointerfaces, S.B. Emphasizing the role of surface chemistry on hydrophobicity and cell adhesion behavior of polydimethylsiloxane/TiO2 nanocomposite films. Colloids Surf. B Biointerfaces 2018, 167, 492. [Google Scholar] [CrossRef]
- Aronov, D.; Rosen, R.; Ron, E.Z.; Rosenman, G. Tunable hydroxyapatite wettability: Effect on adhesion of biological molecules. Process Biochem. 2006, 41, 2367–2372. [Google Scholar] [CrossRef]
- Hwang, G.B.; Patir, A.; Allan, E.; Nair, S.P.; Parkin, I.P.; Nair, S.P. Interfaces. superhydrophobic and white light-activated bactericidal surface through a simple coating superhydrophobic and white light-activated bactericidal surface through a simple coating. ACS Appl. Mater. Interfaces 2017, 30, 29002–29009. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W.J.B. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Cheng, J.B.; Xue, L.; Zhang, B.S.; Zhang, P.P.; Cui, X.; Hong, S.; Wu, Y.P.; Zhang, X.C.; Liang, X.B. Durability and corrosion behaviors of superhydrophobic amorphous coatings: A contrastive investigation. RSC Adv. 2022, 12, 32813–32824. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Gao, M.; Guo, Y.C.; Xu, T.; Jiang, H.; Zhang, Z.J.; Ji, X.X.; Si, C.L. Nanocellulose-based functional materials for physical, chemical, and biological sensing: A review of materials, properties, and perspectives. Ind. Crop. Prod. 2024, 212, 21. [Google Scholar] [CrossRef]
- Quero, F.; Rosenkranz, A. Mechanical Performance of Binary and Ternary Hybrid MXene/Nanocellulose Hydro- and Aerogels—A Critical Review. Adv. Mater. Interfaces 2021, 8, 10. [Google Scholar] [CrossRef]
- Sobhan, A.; Muthukumarappan, K.; Cen, Z.S.; Wei, L. Characterization of nanocellulose and activated carbon nanocomposite films’ biosensing properties for smart packaging. Carbohydr. Polym. 2019, 225, 10. [Google Scholar] [CrossRef]
- Fang, Z.Q.; Hou, G.Y.; Chen, C.J.; Hu, L.B. Nanocellulose-based films and their emerging applications. Curr. Opin. Solid State Mat. Sci. 2019, 23, 15. [Google Scholar] [CrossRef]
- Huang, J.C.; Zhong, Y.; Zhang, L.N.; Cai, J. Extremely Strong and Transparent Chitin Films: A High-Efficiency, Energy-Saving, and “Green” Route Using an Aqueous KOH/Urea Solution. Adv. Funct. Mater. 2017, 27, 10. [Google Scholar] [CrossRef]
- King, C.; Shamshina, J.L.; Gurau, G.; Berton, P.; Khan, N.; Rogers, R.D. A platform for more sustainable chitin films from an ionic liquid process. Green Chem. 2017, 19, 117–126. [Google Scholar] [CrossRef]
- Hashiguchi, T.; Yamamoto, K.; Kadokawa, J.I. Fabrication of highly flexible nanochitin film and its composite film with anionic polysaccharide. Carbohydr. Polym. 2021, 270, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Deng, X.; Dong, Y.; Zhou, Z.; Zhang, Y.J.C.P. High-strength, transparent and superhydrophobic nanocellulose/nanochitin membranes fabricated via crosslinking of nanofibers and coating F-SiO2 suspensions. 2020, 247, 116694. Carbohydr. Polym. 247, 116694.
- Li, M.Y.; Gao, G.H.; Sheng, J.L.; Gao, X.; Wang, X.Q.; Chen, S.J.; Yu, J.Y. Renewable superhydrophobic PVB/SiO2 composite membranes with self-repairing for high-efficiency emulsion separation. Surf. Interfaces 2023, 36, 11. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Parkin, I.P. Repellent materials. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Li, Y.D.; Zhang, W.B.; Bai, S.W.; Zheng, X.L.; Huan, J.M.; Cao, G.L.; Yang, T.H.; Wang, M.; et al. Development of a facile and bi-functional superhydrophobic suspension and its applications in superhydrophobic coatings and aerogels in high-efficiency oil-water separation. Green Chemistry 2020, 22, 7424–7434. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.Y.; Wu, X.Y.; Gan, J.; Zhang, H.Q.; Cai, Y.J. A Superhydrophobic Moso Bamboo Cellulose Nano-Fibril Film Modified by Dopamine Hydrochloride. Front. Bioeng. Biotechnol. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.Y.; Shang, H.T.; Liu, C.E.; Wei, H. Light microscopic, electron microscopic, and immunohistochemical comparison of Bama minipig (Sus scrofa domestica) and human skin. Comp. Med. 2010, 60, 142–148. [Google Scholar] [PubMed]
- Zhao, C.X.; Liu, J.N.; Li, B.Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc-Air Batteries. Adv. Funct. Mater. 2020, 30, 9. [Google Scholar] [CrossRef]
- Fabre, H.; Mercier, D.; Galtayries, A.; Portet, D.; Delorme, N.; Bardeau, J.F. Impact of hydrophilic and hydrophobic functionalization of flat TiO2/Ti surfaces on proteins adsorption. Appl. Surf. Sci. 2018, 432, 15–21. [Google Scholar] [CrossRef]
- Bakshani, C.R.; Morales-Garcia, A.L.; Mike, A.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary conservation of the antimicrobial function of mucus: A first defence against infection. Biofilms Microbiomes 2018, 4, 14. [Google Scholar] [CrossRef]
- Shanks, A.L.; Graham, W.M. Chemical defense in a scyphomedusa. Mar. Ecol.-Prog. Ser. 1988, 45, 81–86. [Google Scholar] [CrossRef]
- Stabili, L.; Rizzo, L.; Caprioli, R.; Leone, A.; Piraino, S. Jellyfish Bioprospecting in the Mediterranean Sea: Antioxidant and Lysozyme-Like Activities from Aurelia coerulea (Cnidaria, Scyphozoa) Extracts. Mar. Drugs 2021, 19, 619. [Google Scholar] [CrossRef] [PubMed]
- Shpirer, E.; Chang, E.; Diamant, A.; Rubinstein, N.; Cartwright, P.; Huchon, D. Diversity and evolution of myxozoan minicollagens and nematogalectins. BMC Evol. Biol. 2014, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Mahadik, S.A.; Pedraza, F.; Mahadik, S.S.; Relekar, B.P.; Thorat, S.S. Biocompatible superhydrophobic coating material for biomedical applications. J. Sol.-Gel. Sci. Technol. 2017, 81, 791–796. [Google Scholar] [CrossRef]
- Iqbal, R.; Majhy, B.; Sen, A.K. Facile Fabrication and Characterization of a PDMS-Derived Candle Soot Coated Stable Biocompatible Superhydrophobic and Superhemophobic Surface. ACS Appl. Mater. Interfaces 2017, 9, 31170–31180. [Google Scholar] [CrossRef]
- Larbi, F.; García, A.; del Valle, L.J.; Hamou, A.; Puiggalí, J.; Belgacem, N.; Bras, J. Comparison of nanocrystals and nanofibers produced from shrimp shell α-chitin: From energy production to material cytotoxicity and Pickering emulsion properties. Carbohydr. Polym. 2018, 196, 385–397. [Google Scholar] [CrossRef]
- Brzicova, T.; Sikorova, J.; Milcova, A.; Vrbova, K.; Klema, J.; Pikal, P.; Lubovska, Z.; Philimonenko, V.; Franco, F.; Topinka, J.; et al. Nano-TiO2 stability in medium and size as important factors of toxicity in macrophage-like cells. Toxicol. Vitro 2019, 54, 178–188. [Google Scholar] [CrossRef] [PubMed]

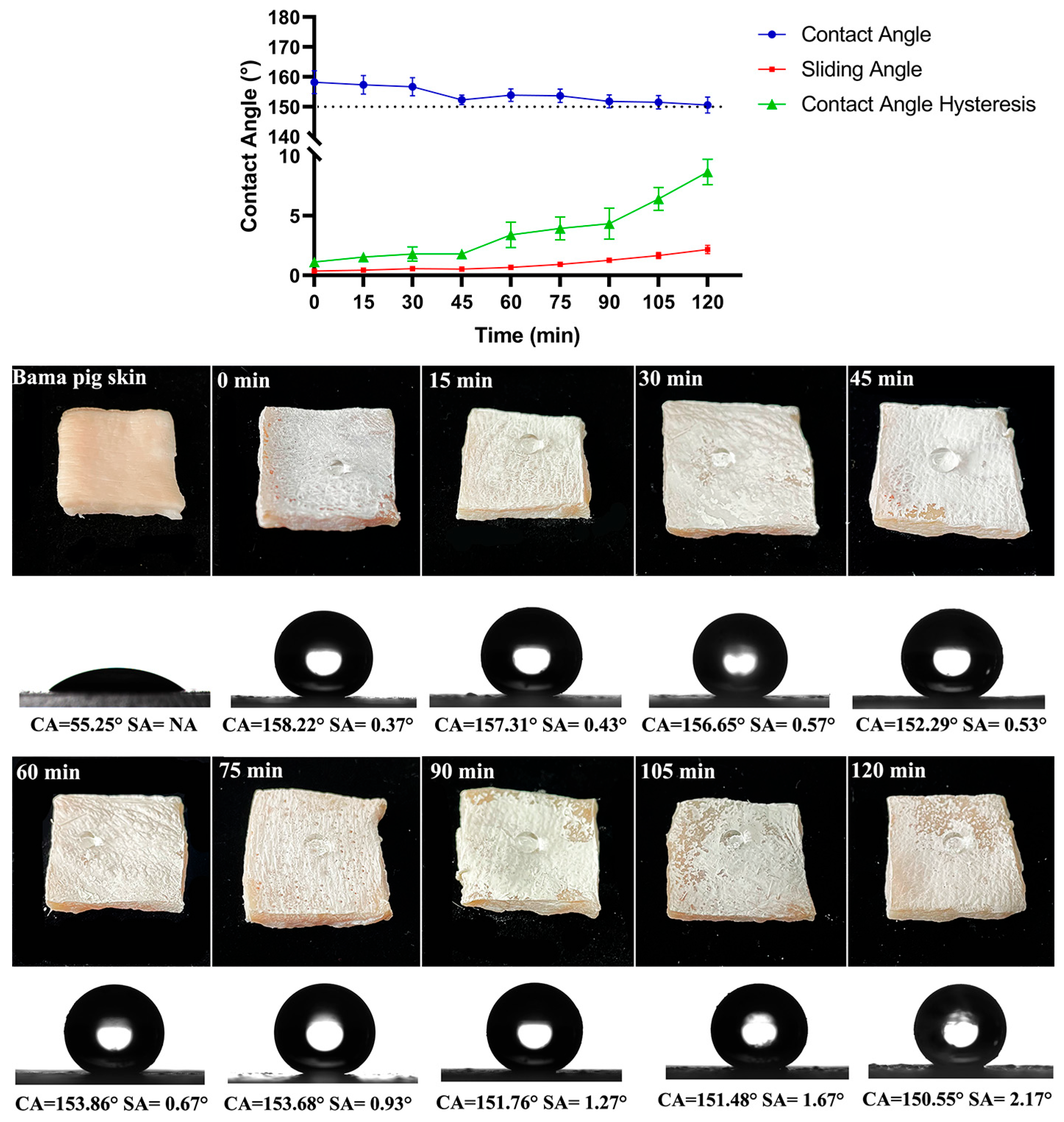

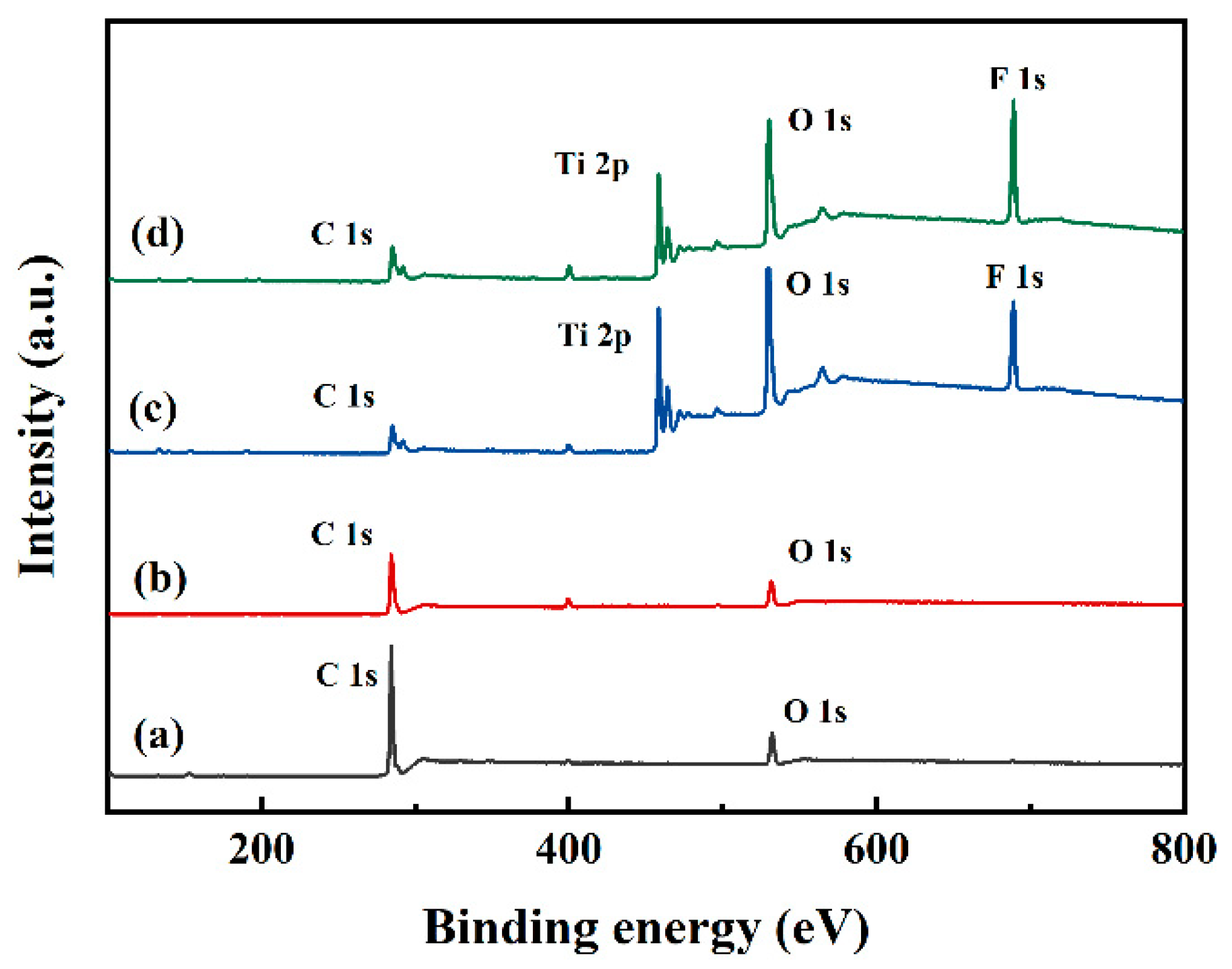
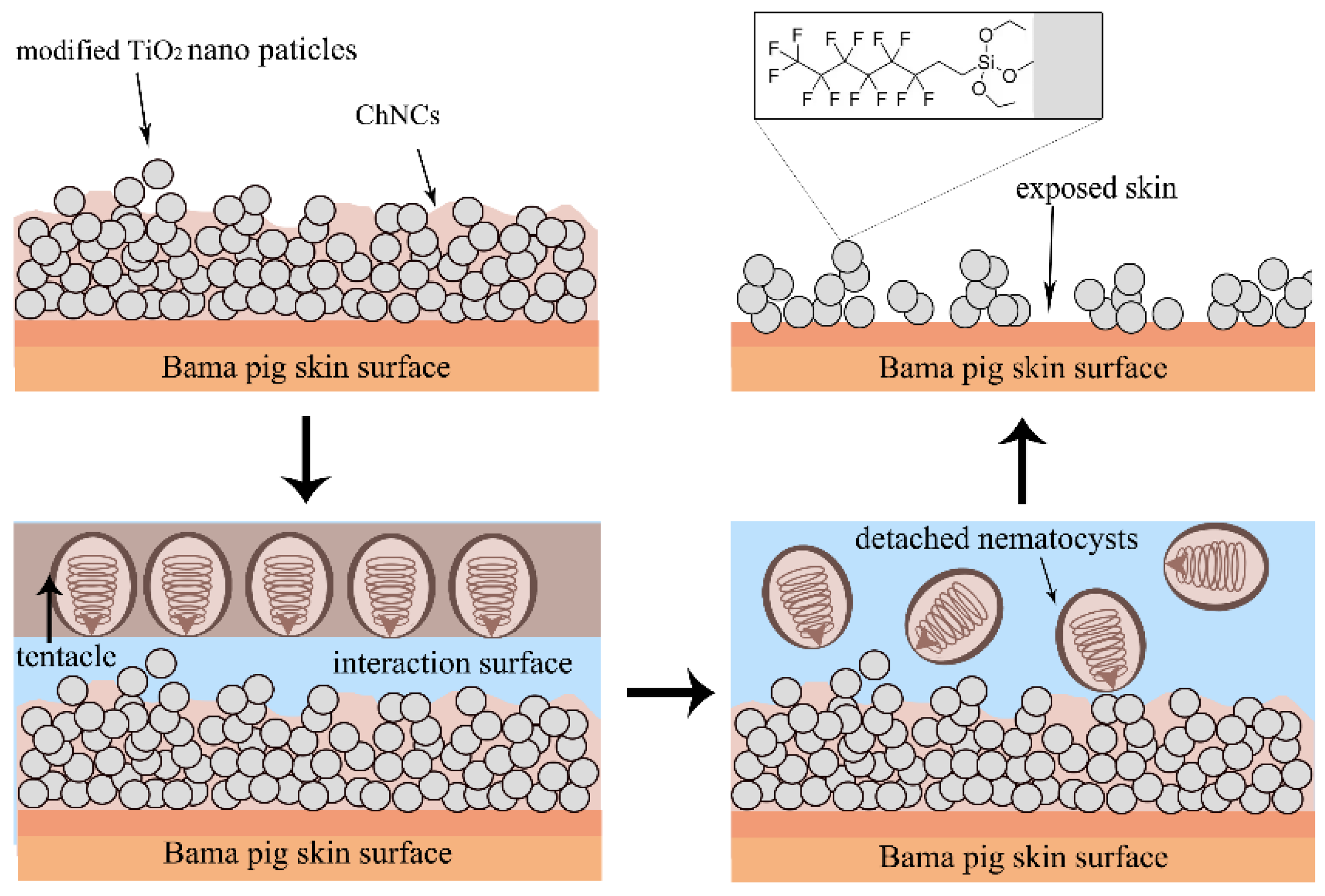
| Samples | Bama Pig Skin | s-SiO2-ChNCs (10 wt.%) | s-TiO2-ChNCs (0.1 wt.%) | s-SiO2-CNF (10 wt.%) | s-TiO2-CNF (50 wt.%) |
|---|---|---|---|---|---|
| Before-adhesion | 55.25° | 141.36° | 153.49° | 116.43° | 144.10° |
| Post-adhesion | 0° | 0° | 115.21° | 0° | 0° |
| Samples | [C] | [O] | [S] | [F] | [Ti] | [Si] |
|---|---|---|---|---|---|---|
| skin | 87.21 | 10.03 | 0.21 | 0 | 0 | 0 |
| skin-tentacles | 85.37 | 12.69 | 0.31 | 0 | 0 | 0 |
| s-TiO2-ChNCs | 27.42 | 42.76 | 0.53 | 13.31 | 14.47 | 1.78 |
| s-TiO2-ChNCs-tentacles | 32.88 | 36.10 | 0.63 | 17.27 | 11.34 | 1.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Sun, Y.; Li, R.; Liu, S.; Xing, R.; Li, P.; Yu, H. Superhydrophobic Surfaces as a Potential Skin Coating to Prevent Jellyfish Stings: Inhibition and Anti-Tentacle Adhesion in Nematocysts of Jellyfish Nemopilema nomurai. Materials 2024, 17, 5983. https://doi.org/10.3390/ma17235983
Xie Y, Sun Y, Li R, Liu S, Xing R, Li P, Yu H. Superhydrophobic Surfaces as a Potential Skin Coating to Prevent Jellyfish Stings: Inhibition and Anti-Tentacle Adhesion in Nematocysts of Jellyfish Nemopilema nomurai. Materials. 2024; 17(23):5983. https://doi.org/10.3390/ma17235983
Chicago/Turabian StyleXie, Yichen, Yuanyuan Sun, Rongfeng Li, Song Liu, Ronge Xing, Pengcheng Li, and Huahua Yu. 2024. "Superhydrophobic Surfaces as a Potential Skin Coating to Prevent Jellyfish Stings: Inhibition and Anti-Tentacle Adhesion in Nematocysts of Jellyfish Nemopilema nomurai" Materials 17, no. 23: 5983. https://doi.org/10.3390/ma17235983
APA StyleXie, Y., Sun, Y., Li, R., Liu, S., Xing, R., Li, P., & Yu, H. (2024). Superhydrophobic Surfaces as a Potential Skin Coating to Prevent Jellyfish Stings: Inhibition and Anti-Tentacle Adhesion in Nematocysts of Jellyfish Nemopilema nomurai. Materials, 17(23), 5983. https://doi.org/10.3390/ma17235983







