Photovoltaic Cells and Scintillators Towards Carbon Footprint Reduction: Advantages and Challenges for Ecological Safety
Abstract
1. Introduction
- Quartz mining and metallurgical silicon production;
- Mining and production of aluminium;
- Obtaining crystals and manufacturing wafers;
- Production of other components necessary for module assembly (glass, cables, EVA layers, aluminium frames, etc.);
- Assembly of cells and modules;
- Assembly of PV systems (on the roof).
- Where and how the metallic silicon and aluminium are produced;
- The type of silicon crystal: monocrystalline or multi-crystalline;
- The place and method of manufacturing the modules;
- The energy mix used;
- Panel construction: with aluminium frames or frameless glass–glass modules;
- Disposal method.
- The structure of the scintillation layer should contain (structural) inclusions in the form of (for example) microcapsules of a radioactive substance. This will increase the spherical luminescence efficiency and improve the photons’ light transmission coefficient to the iso-solar cell’s surface (i-PV). The outer scintillation layer should be surrounded by a mirror material reflecting light towards the photovoltaic panel.
- The possibility of both modulation of scintillation colours and observation with the naked eye is possible thanks to the doping of the materials with various elements. Initial observations of increased scintillation intensity and colour during X-ray exposure were observed after doping the organic structure with UO22+. An increase in luminescence in the X-ray beam (E > 20 keV) was observed. Increased radiation resistance and reduced hygroscopicity were also observed compared to commercially available CsI:Tl or NaI:Tl scintillators [34].
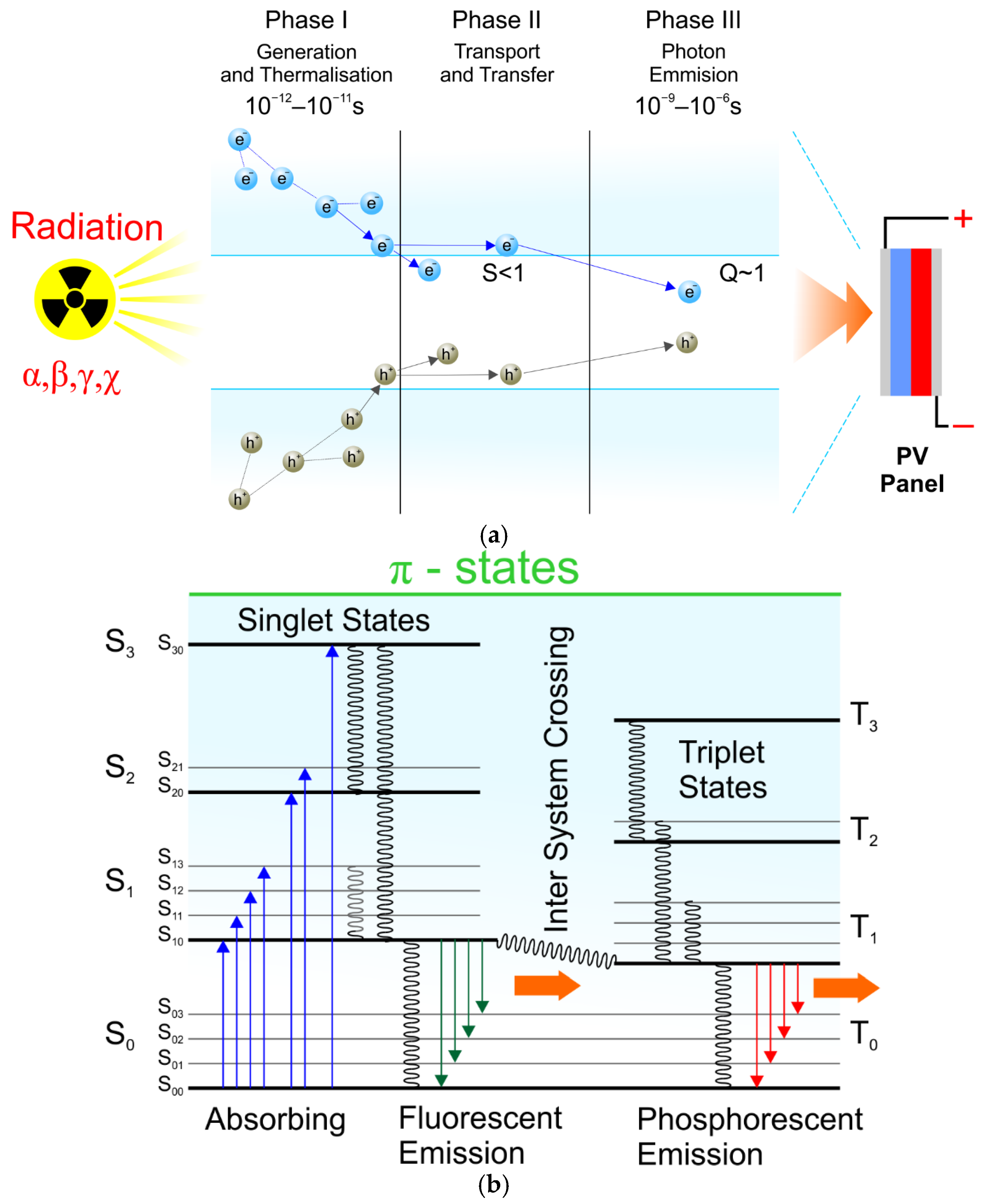
- A nuclear battery’s efficiency depends largely on matching the emitter (α, β, or ɣ source) to the scintillation material when converting radiation energy into photons of light. Nuclear batteries that use a single planar (layered) arrangement of components generally have low energy conversion efficiency. Spatial arrangements give much better results.
- According to Dujardin [35], the scintillator material’s high natural radioactivity makes dopant contents of K, Rb, and Lu highly desirable in applications related to radiation conversion.
- The perovskite single crystal can be a crucial scintillator in photovoltaic technology applications. It is characterised by high photoluminescence quantum efficiency and light efficiency conversion in a wide temperature range. According to literature reports [36,37,38], the emission lifetime is ~3.4 ns and the light efficiency is 1.5 ÷ 3 × 105 photons/MeV. It may be proportional to the energy gap of these materials, which is estimated to be below 2 eV. The latest technology, quantum dots and nanocrystals, can also improve light conversion in perovskite scintillators. Pellowski et al. [39] demonstrated that converting ionising radiation into photons of light generated in the scintillator structure can be—if the appropriate material is selected—a sufficient light source to generate an electric charge in photovoltaic cells. A new approach to obtaining a source of photons based on the volumetric arrangement of a radioactive isotope in the scintillator structure presents rational premises for increasing light intensity. The layered arrangement of the module “responsible” for the light source described in the literature so far has been ineffective, and the efficiency of isotope cells is low. The concept of a new type of iso-photovoltaic cell (i-PV) proposed in the article may constitute a breakthrough in unconventional energy. Zero-emission and practically unlimited lifetime of such energy sources may significantly supplement the global energy mix.
2. Results and Discussion
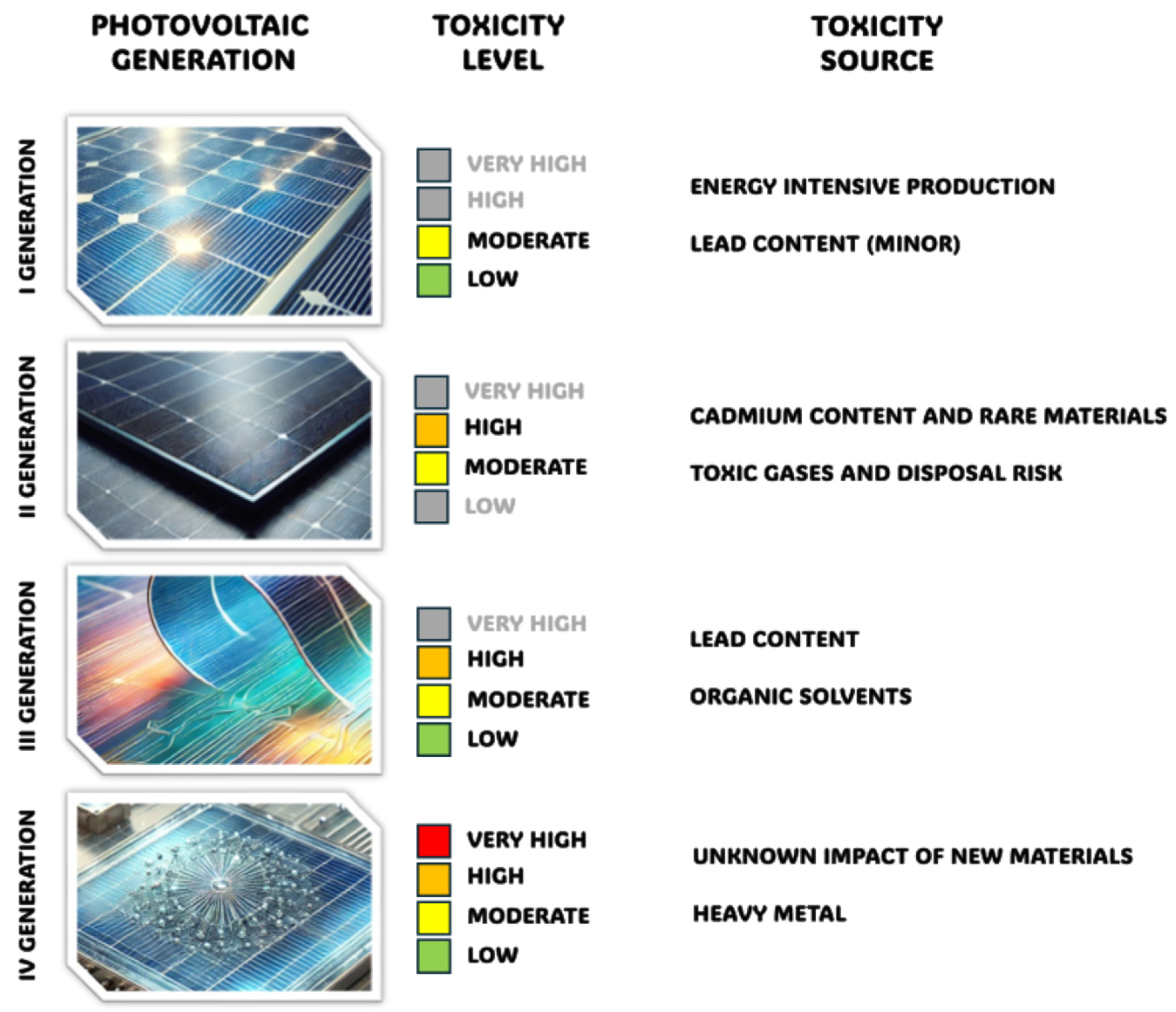
2.1. Toxicity and Effects of Scintillators on the Environment, Together with Risks Associated with Use and Disposal
2.1.1. Thallium-Doped Sodium Iodide
2.1.2. Thallium-Doped Caesium Iodide
2.1.3. Lead Tungstate
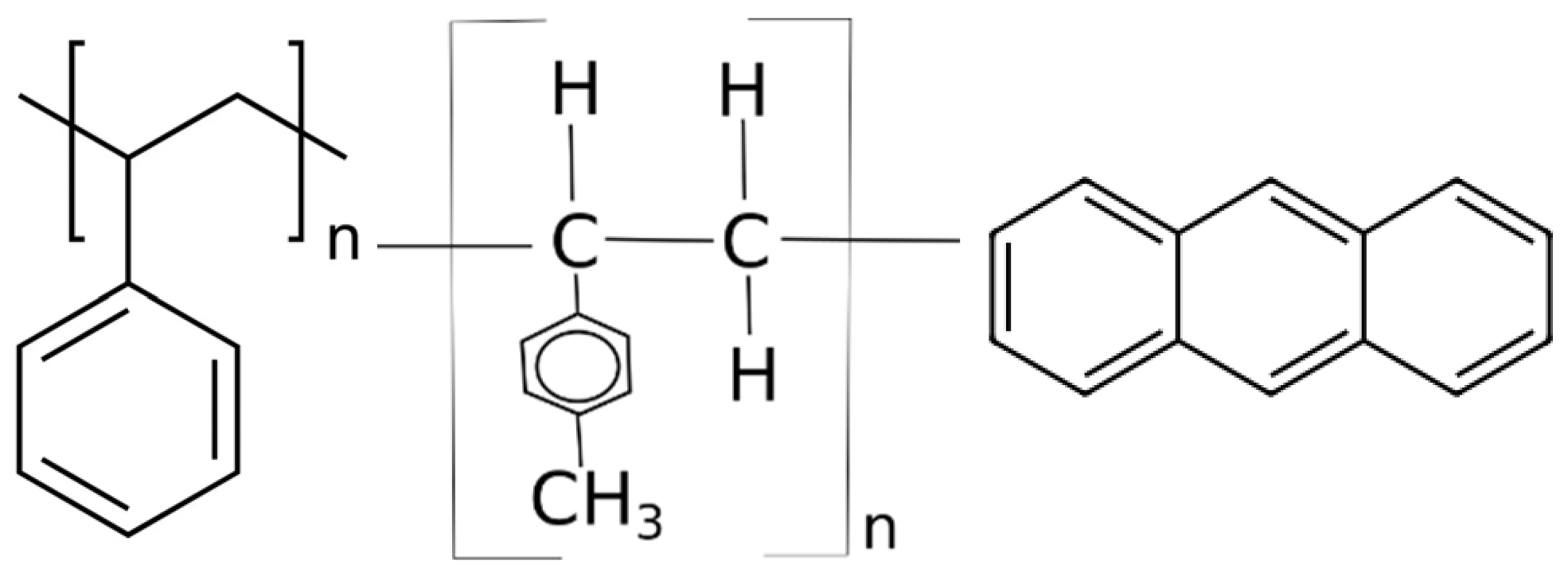
2.1.4. Organic Scintillators Based on Polystyrene
2.1.5. Organic Scintillators Based on Polyvinyl Toluene
2.1.6. Organic Scintillators Based on Anthracene
2.1.7. Nano-Scintillators
3. Factors Affecting the Durability and Lifespan of Scintillators
- Have high light efficiency (high efficiency—use of ionising radiation energy);
- Scintillations with long luminescence times;
- The wavelength emitted is consistent with the maximum sensitivity of the photocell;
- Non-hygroscopicity;
- Must be transparent to its light—minimal losses due to self-absorption;
- Effective collection of reflected internal light (high refractive index).
- Proper labelling: Scintillator materials should be labelled appropriately to inform operators of potential hazards;
- Personnel training: Personnel handling scintillators should be trained in radiation safety and proper disposal methods;
- Monitoring: Regular monitoring for radioactivity and contamination should be performed when handling scintillators.
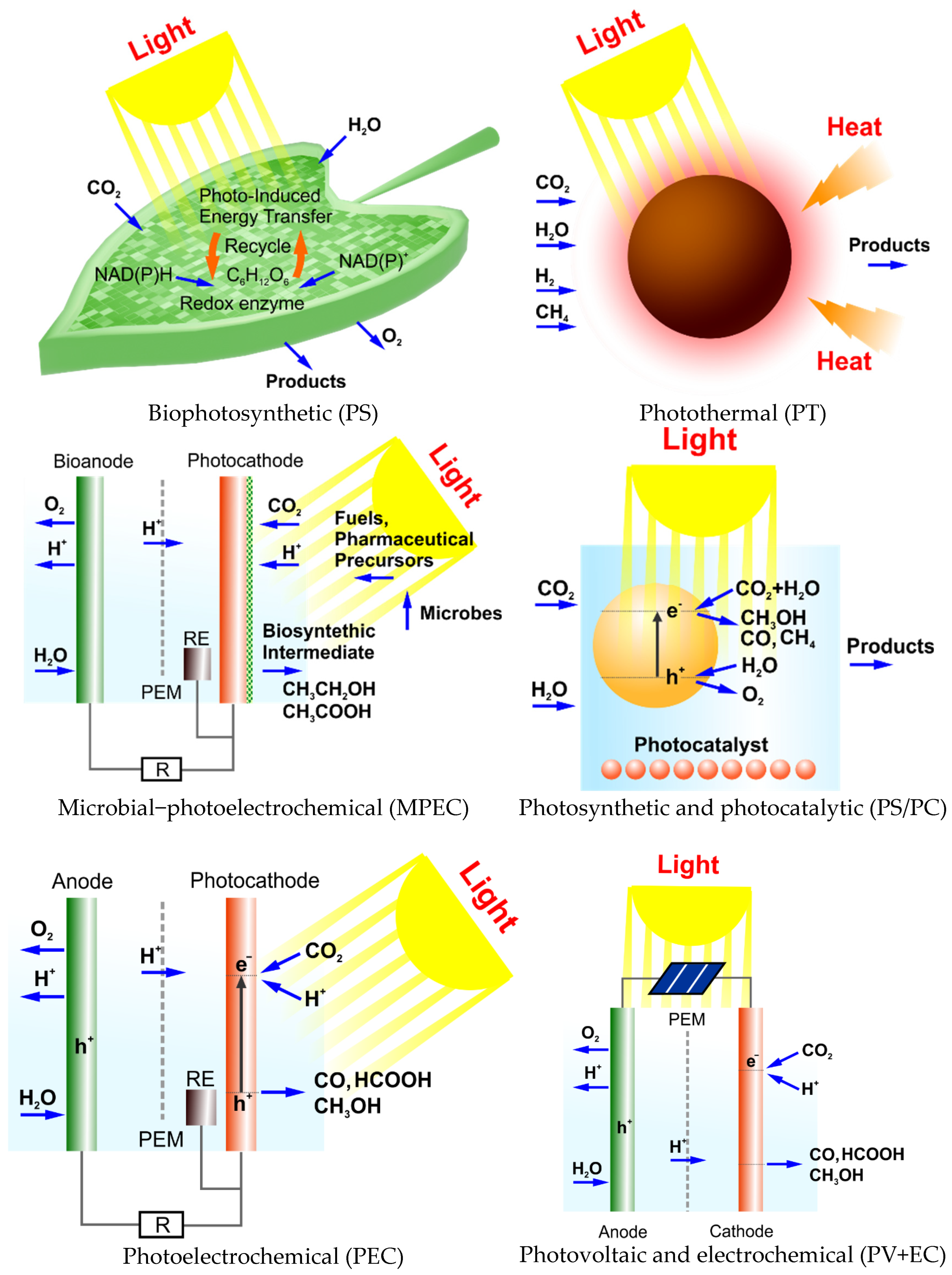
4. Types of Solar-Driven CO2 Conversion
5. The Carbon Footprint of Solar Cell Production
5.1. Silicon and Aluminium Production
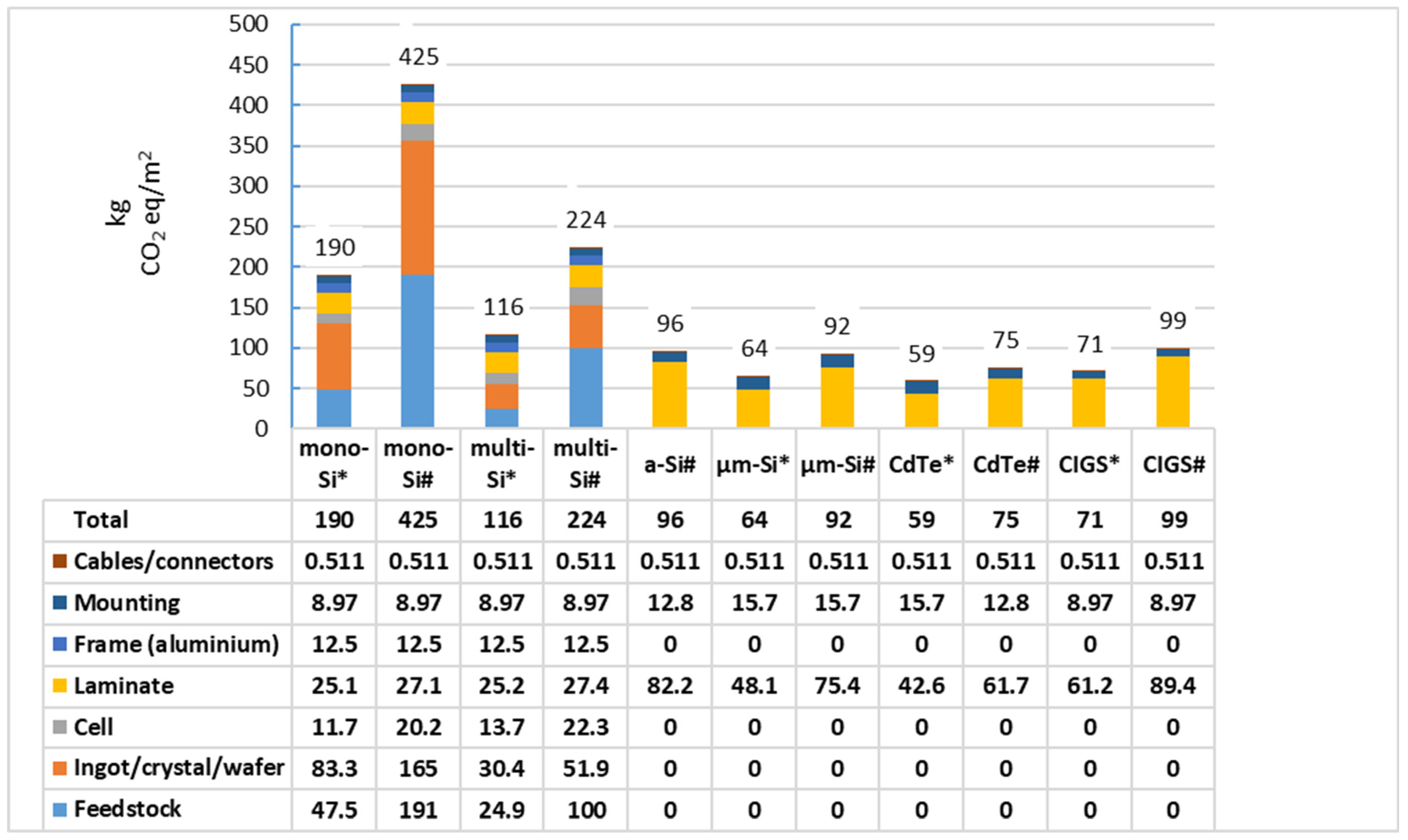
5.2. Production of Mono and Multi-Crystalline Silicon for Photovoltaic Applications
5.3. PV Panel Production Location and Energy Mix Used
5.4. Modules with Glass Instead of Backfilm
6. Recycling of Photovoltaic Modules
- ➢
- Removal of frame, junction boxes, and cables;
- ➢
- Separation of glass and silicon wafers;
- ➢
- Separation and purification of silicon cells and metals.
- ➢
- Module area—75%;
- ➢
- Polymer components—10%;
- ➢
- Aluminium—8%;
- ➢
- Silicon material—5%;
- ➢
- Copper—1%;
- ➢
- Other materials—1%, including traces of silver—0.1%.
7. SWOT Analysis of Scintillation Material Toxicity
7.1. Recycling of Scintillators
7.2. Impact of Scintillators on CO2 Emissions
- Inorganic crystals: The most used materials are sodium iodide (NaI), caesium iodide (CsI), gallium oxide, or gadolinium oxide. The extraction and processing of such raw materials are energy-intensive processes leading to CO2 emissions.
- Organic materials (e.g., plastic scintillators): The production of plastic scintillators requires crude oil-based raw materials, which are obtained in refinery processes that generate CO2.
- Synthesis of scintillator crystals: This is a highly energy-intensive process, especially for large single crystals, which requires high temperatures (even above 1000 °C) and long growth times. An example is the Czochralski method, which consumes a large amount of electrical energy, which is associated with CO2 emissions dependent on the energy source.
- Polymerization for plastic scintillators: The polymerization process (e.g., acrylic or styrene) also requires energy and leads to greenhouse gas emissions from the use of organic compounds and their processing.
- Compliance with regulations: Disposal must comply with local, state, and federal regulations regarding hazardous materials, especially if the scintillator contains any radioactive isotopes or hazardous chemicals;
- Hazardous waste: Scintillators contaminated with radioactive waste are classified as hazardous materials and must be disposed of through a licensed hazardous waste disposal service;
- Landfill: Non-radioactive scintillator waste can be disposed of in landfills, following strict guidelines to prevent environmental contamination;
- Incineration: In some cases, incineration can be a waste reduction method, but it must be handled carefully to avoid the release of harmful emissions;
- Specialty facilities: Some facilities specialize in the disposal of scientific instruments and materials and may have the capabilities to safely handle scintillators.
8. Internal and External Determinants in ESG Strategies
9. Conclusions
- Increasing the production of PV modules in the EU, which is estimated to save 40% of CO2 emissions compared to modules imported from China;
- Increasing the efficiency of photovoltaic modules;
- Increasing the lifetime of photovoltaic panels;
- Eliminating aluminium from production by developing frameless glass-to-glass module technology;
- Increasing the share of electricity from renewable energy sources in the production phase of PV systems;
- Conducting research on new PV technologies for applications in areas where conventional silicon PV cells are not cost-effective, such as low-light environments, greenhouse roofs, etc.;
- Conducting research and deployment of binary technologies such as combining PV panels with solar panels;
- Conducting research on technologies for recycling PV panel components and producing new PV panels from them.
- Using low-emission energy sources in production, such as solar or nuclear energy, especially for high-temperature synthesis;
- New synthesis methods: developing production methods that operate at lower temperatures or use alternative technologies, such as microwave crystal growth, which reduce the total energy required to produce scintillators and solar cells;
- Replacing inorganic materials with organic materials where possible—organic materials, although less efficient in detecting high-energy radiation, may offer lower CO2 emissions during production.
- Minimizing energy consumption in the synthesis and processing of materials;
- Using renewable or easier to recycle raw materials and materials;
- Improving production technologies with an emphasis on energy efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://climate.copernicus.eu/esotc/2023/temperature-and-thermal-stress (accessed on 25 October 2024).
- Schlömer, S.; Bruckner, T.; Fulton, L.; Hertwich, E.; McKinnon, A.; Perczyk, D.; Roy, J.; Schaeffer, R.; Sims, R.; Smith, P.; et al. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 2014. In Climate Change 2014: Mitigation of Climate Change; IPCC: Geneva, Switzerland, 2015. [Google Scholar]
- Available online: https://www.europarl.europa.eu/topics/en/article/20180305STO99003/reducing-carbon-emissions-eu-targets-and-policies (accessed on 25 October 2024).
- Pastuszak, J.; Wegierek, P. Photovoltaic cell generations and current research directions for their development. Materials 2022, 15, 5542. [Google Scholar] [CrossRef] [PubMed]
- Preet, S.; Smith, S.T. A comprehensive review on the recycling technology of silicon based photovoltaic solar panels: Challenges and future outlook. J. Clean. Prod. 2024, 448, 141661. [Google Scholar] [CrossRef]
- Aamir, I.M.; Malik, M.; Shahid, W.; Zaheer Ud Din, S.; Anwar, N.; Ikram, M. Materials for photovoltaics: Overview, generations, recent advancements and future prospects. In Thin Films Photovoltaics; IntechOpen: London, UK, 2022. [Google Scholar]
- Shah, N.; Shah, A.A.; Leung, P.K.; Khan, S.; Sun, K.; Zhu, X.; Liao, Q. A Review of third generation solar cells. Processes 2023, 11, 1852. [Google Scholar] [CrossRef]
- Maalouf, A.; Okoroafor, T.; Jehl, Z.; Babu, V.; Resalati, S. A comprehensive review on life cycle assessment of commercial and emerging thin-film solar cell systems. Renew. Sustain. Energy Rev. 2023, 186, 113652. [Google Scholar] [CrossRef]
- IEA. Renewable Electricity. In International Energy Agency Technical Report; IEA: Paris, France, 2022. [Google Scholar]
- IRENA. Global energy transformation: A roadmap to 2050. In International Renewable Energy Agency Technical Report; IRENA: Masdar City, United Arab Emirates, 2019. [Google Scholar]
- Spinelli, G.; Freitag, M.; Benesperi, I. What is necessary to fill the technological gap to design sustainable dye-sensitized solar cells? Sustain. Energy Fuels 2023, 7, 916. [Google Scholar] [CrossRef]
- Iwan, A.; Pellowski, W.; Bogdanowicz, K.A. Conversion of radiophotoluminescence irradiation into electricity in photovoltaic cells. A review of theoretical considerations and practical solutions. Energies 2021, 14, 6186. [Google Scholar] [CrossRef]
- Pellowski, W.; Iwan, A.; Bogdanowicz, K.A. New generation scintillators for the conversion of light photons generated by radiation-induced photoluminescence into electricity in iso-photovoltaic cells as an element of strengthening the energy security system. Przegląd Elektrotechniczny 2023, 10, 265. [Google Scholar]
- Singh, P.; Dosovitskiy, G.; Bekenstein, Y. Bright innovations: Review of next-generation advances in scintillator engineering. ACS Nano 2024, 18, 14029. [Google Scholar] [CrossRef]
- Kim, G.; Kim, H.; Kim, S. A study on absorbed dose measurement using a butyl acrylate-based plastic scintillator as a tissue-equivalent detector. J. Korean Phys. Soc. 2024, 85, 213. [Google Scholar] [CrossRef]
- Marie-Luce, R.; Mai, P.; Lerouge, F.; Cheref, Y.; Pierre, S.; Sabot, B.; Chaput, F.; Dujardin, C. Real-time detection and discrimination of radioactive gas mixtures using nanoporous inorganic scintillators. Nat. Photonics 2024, 18, 1037. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Abdoos, H.; Alamdari, S.; Menéndez, J.L. Flexible nanocomposite scintillator detectors for medical applications: A review. Sens. Actuators A Phys. 2024, 378, 115828. [Google Scholar] [CrossRef]
- Hawrami, R.; Ariesanti, E.; Farsoni, A. Growth and evaluation of improved CsI:Tl and NaI:Tl scintillators. Crystals 2022, 12, 1517. [Google Scholar] [CrossRef]
- Gao, T.J.; Wang, H.D.; Jing-Bin, L. The investigation of the luminescent structure of thallium-doped cesium iodide (CsI:Tl) basing on the first-principles coupled with spectral analysis. J. Phys. Condens. Matter 2022, 34, 5901. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Jia, X.; Jin, R.; Shi, B.; Chen, Y.; Xu, B. In situ reaction-based synthesis of a novel nanostructured Lu2SiO5-based environmental barrier coating with controllable Lu2O3 content. Ceram. Int. 2024, 50, 43345. [Google Scholar] [CrossRef]
- De Martinis, A.; Montalto, L.; Scalise, L. Luminescence and structural characterization of Gd2O2S scintillators doped with Tb3+, Ce3+, Pr3+ and F for imaging applications. Crystals 2022, 12, 854. [Google Scholar] [CrossRef]
- Viahin, O.; Sidletskiy, O.; Kurtsev, D.; Zorenko, Y. Luminescence properties of Lu2SiO5:Ce,Yb crystals under synchrotron radiation excitation. J. Lumin. 2023, 257, 119728. [Google Scholar] [CrossRef]
- Sykora, G.J.; Mann, S.E.; Mauri, G.; Schooneveld, E.M.; Rhodes, N.J. Review of thermal neutron scintillators: Evaluation metrics and future prospects for demanding applications. Opt. Mater. 2024, 24, 100373. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mao, Y.; Yuanbing, M. Recent advances, challenges, and opportunities of inorganic nanoscintillators. Front. Optoelectron. 2020, 13, 156. [Google Scholar] [CrossRef]
- Dormenev, V.; Brinkmann, K.-T.; Kazlou, D. Scintillation properties of garnets and oxyorthosilicates with different dopants. IEEE Trans. Nucl. Sci. 2023, 70, 1392. [Google Scholar] [CrossRef]
- Westphal, E.R.; Brown, A.D.; Quintana, E.C. Visible emission spectra of thermographic phosphors under X-ray excitation. Meas. Sci. Technol. 2021, 32, 094008. [Google Scholar] [CrossRef]
- Duan, M.L.; Kuang, X.Y.; Zhang, C.X.; Chai, R.P. Comparative analysis of energy-level splitting of Pr3+ doped in LiYF4 and LiBiF4 crystals: A complete energy matrix calculation. Chin. Phys. B 2011, 20, 013102. [Google Scholar] [CrossRef]
- Patel, D.N.; Sarkisov, S.S.; Darwish, A.M. Sensitive detection of short wavelength radiation with spectrum down-converting nanoparticles. In Optical Components and Materials XXI; SPIE—The International Society for Optical Engineering: Bellingham, WA, USA, 2024; Volume 12882. [Google Scholar]
- Delaey, M.; Van Bogaert, S.; Cosaert, E.; Mommen, W.; Poelman, D. Neodymium-doped gadolinium compounds as infrared emitters for multimodal imaging. Materials 2023, 16, 6471. [Google Scholar] [CrossRef]
- Markó, M.; Groitl, F.; Birk, J.O. Prototype of the novel CAMEA concept—A backend for neutron spectrometers. Rev. Sci. Instrum. 2018, 89, 015105. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, X.; Zhang, H.; Peng, H.; Ren, Q.; Xu, J.; Peng, Q.; Xie, S. Requirements of scintillation crystals with the development of PET scanners. Crystals 2022, 12, 1302. [Google Scholar] [CrossRef]
- Jagtap, S.; Chopade, P.; Tadepalli, S.; Bhalerao, A.; Gosavi, S. A review on the progress of ZnSe as inorganic scintillator. Opto-Electron. Rev. 2019, 27, 90. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Scintillation property of Tl-doped NaI transparent ceramics. Radiat. Phys. Chem. 2024, 215, 111367. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, X.; Liu, W. Emergence of uranium as a distinct metal center for building intrinsic X-ray scintillators. Angew. Chem. Int. Ed. 2018, 57, 7883. [Google Scholar] [CrossRef]
- Dujardin, C.; Auffray, E.; Bourret-Courchesne, E. Needs, trends and advances in inorganic scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 8. [Google Scholar] [CrossRef]
- Gandini, M.; Villa, I.; Beretta, M. Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators. Nat. Nanotechnol. 2020, 015, 462. [Google Scholar] [CrossRef]
- Budakovsky, S.V.; Galunov, N.Z.; Karavaeva, N.L. New effective organic scintillators for fast neutron and short-range radiation detection. IEEE Trans. Nucl. Sci. 2007, 54, 2734. [Google Scholar] [CrossRef]
- Witkiewicz-Lukaszek, S.; Gorbenko, V.; Zorenko, T. New types of composite scintillators based on the single crystalline films and crystals of Gd3(Al,Ga)5O12:Ce mixed garnets. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 264, 114909. [Google Scholar] [CrossRef]
- Pellowski, W.; Iwan, A.; Bogdanowicz, K.A. Conversion of light photons generated by radiatively excited photoluminescence into electrical energy in iso-photovoltaic (i-PV) cells—A new challenge for energy security. Przegląd Elektrotechniczny 2022, 9, 38. [Google Scholar]
- Wua, Y.; Maa, Y.; Zheng, H.; Ramakrishna, S. Piezoelectric materials for flexible and wearable electronics: A review. Mater. Des. 2021, 211, 110164. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. A comprehensive review on the state-of-the-art of piezoelectric energy harvesting. Nano Energy 2021, 80, 105567. [Google Scholar] [CrossRef]
- Irfan, S.; Yan, Z.; Khan, S.B. Advancements in thermoelectric materials: A comprehensive review. Mater. Sci. Energy Technol. 2024, 7, 349. [Google Scholar] [CrossRef]
- Brusa, E.; Carrera, A.; Delprete, C. A Review of piezoelectric energy harvesting: Materials, design, and readout circuits. Actuators 2023, 12, 457. [Google Scholar] [CrossRef]
- Ishibe, T.; Tomeda, A.; Komatsubara, Y.; Kitaura, R.; Uenuma, M.; Uraoka, Y.; Yamashita, Y.; Nakamura, Y. Carrier and phonon transport control by domain engineering for high-performance transparent thin film thermoelectric generator. Appl. Phys. Lett. 2021, 118, 151601. [Google Scholar] [CrossRef]
- Kima, K.B.; Jangb, W.; Choa, J.Y.; Wooa, S.B.; Jeona, D.H.; Ahna, J.H.; Honga, S.D.; Koob, H.Y.; Sunga, T.H. Transparent and flexible piezoelectric sensor for detecting human movement with a boron nitride nanosheet (BNNS). Nano Energy 2018, 54, 91. [Google Scholar] [CrossRef]
- Perera, C.S.; Vernon, K.C.; Mcleod, A. Simulations of the spontaneous emission of a quantum dot near a gap plasmon waveguide. J. Appl. Phys. 2014, 115, 054314. [Google Scholar] [CrossRef]
- Wong, V.K.; Ho, J.H.; Yap, E.H.; Chai, A.B. Dynamics of a piezoelectric energy harvester in a simulated rain environment. Adv. Mater. Res. 2014, 1043, 263. [Google Scholar] [CrossRef]
- Bhavanasi, V.; Kumar, V.; Parida, K.; Wang, J.; Lee, P.S. Enhanced piezoelectric energy harvesting performance of flexible PVDF-TrFE bilayer films with graphene oxide. ACS Appl. Mater. Interfaces 2016, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.K.; Ho, J.H.; Chai, A.B. Performance of a piezoelectric energy harvester in actual rain. Energy 2017, 124, 364. [Google Scholar] [CrossRef]
- Liang, Q.; Yan, X.; Gu, Y.; Zhang, K.; Liang, M.; Lu, S.; Zheng, X.; Zhang, Y. Highly transparent triboelectric nanogenerator for harvesting water-related energy reinforced by antireflection coating. Sci. Rep. 2015, 5, 9080. [Google Scholar] [CrossRef]
- Helseth, L. Electrical energy harvesting from water droplets passing a hydrophobic polymer with a metal film on its back side. J. Electrost. 2016, 81, 64. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, N.; Liu, J.; Wen, Z.; Sun, X.; Lee, S.T.; Sun, B. Integrating a silicon solar cell with a triboelectric nanogenerator via a mutual electrode for harvesting energy from sunlight and raindrops. ACS Nano 2018, 12, 2893. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.; He, B.; Yang, P. Graphene enabled all-weather solar cells for electricity harvest from sun and rain. Mater. Chem. A 2016, 4, 13235. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Kato, T.; Miyazaki, K.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Scintillation properties of In-doped NaI transparent ceramics. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2024, 546, 165155. [Google Scholar] [CrossRef]
- Loyd, M.; Pianassola, M.; Hurlbut, C.; Shipp, K.; Sideropoulos, L.; Weston, K.; Koschan, M.; Melcher, C.L.; Zhuravleva, M. Effects of temporary fogging and defogging in plastic scintillators. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2019, 922, 202. [Google Scholar] [CrossRef]
- Shevelev, V.S.; Ishchenko, A.V.; Vanetsev, A.S.; Nagirnyi, V.; Omelkov, S.I. Ultrafast hybrid nanocomposite scintillators: A review. J. Lumin. 2022, 242, 118534. [Google Scholar] [CrossRef]
- Lecoq, P. Development of new scintillators for medical applications. Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrometers Detect. Assoc. Equip. 2016, 809, 130. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Huet, C. Radiation damage in 20-cm long LYSO:Ce and BaF2:Y crystals. IEEE Trans. Nucl. Sci. 2024, 71, 2116. [Google Scholar] [CrossRef]
- Levin, D.S.; Friedman, P.S.; Ferretti, C. A prototype scintillator real-time beam monitor for ultra-high dose rate radiotherapy. Med. Phys. 2024, 51, 2905. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Wang, W.; Liu, F. High-quality CsI(Tl) single-crystal flake scintillators grown by the space-confined solution method. Opt. Mater. 2024, 151, 115333. [Google Scholar] [CrossRef]
- Ntoupis, V.; Michail, C.; Kalyvas, N.; Bakas, A.; Kandarakis, I.; Fountos, G.; Valais, I. Luminescence efficiency and spectral compatibility of cerium fluoride (CeF3) inorganic scintillator with various optical sensors in the diagnostic radiology X-ray energy range. Inorganics 2024, 12, 230. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, M.; Liu, Q. Reducing luminescence intensity and suppressing irradiation-induced darkening of Bi4Ge3O12 by Ce-doping for radiation detection. Adv. Opt. Mater. 2024, 12, 2301332. [Google Scholar] [CrossRef]
- Jayaprakash, S.S.; Tang, Y.; Wu, S.T.; Stand, L.; Tratsiak, Y.; Dong, Y. Metal halide perovskite polymer composites for indirect X-ray detection. Nanoscale 2024, 16, 17654. [Google Scholar] [CrossRef]
- Bhakta, S.; Raman, A.; Ajish, J.K. Development of plastic scintillator with better performance than the commercial counterpart (UPS-923A) and effect of fluorophore composition on light output. Appl. Radiat. Isot. 2024, 212, 111453. [Google Scholar] [CrossRef]
- Mao, J.; Chen, L.; Xia, W.; Gong, J.; Chen, J.; Liang, C. Measurement techniques for low-concentration tritium radiation in water: Review and prospects. Sensors 2024, 24, 5722. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Ning, Y.; Yang, X.; Huang, Y.; Zhang, Z.; Tang, J.; Zheng, P.; Yan, J.; Zhao, J. Preparation and application of nanostructured ZnO in radiation detection. Materials 2024, 17, 3549. [Google Scholar] [CrossRef]
- Lee, J.Y.; Adhikari, G.; Ha, C. A Study of NaI(Tl) crystal encapsulation using organic scintillators for the dark matter search. Nucl. Instrum. Methods Phys. Res. Sect. A-Accel. Spectrometers Detect. Assoc. Equip. 2020, 953, 163141. [Google Scholar] [CrossRef]
- Lee, H.; Yim, M.-S. Examination of scintillator-photovoltaic cell-based spent fuel radiation energy conversion for electricity generation. Prog. Nucl. Energy 2017, 94, 46. [Google Scholar] [CrossRef]
- Terranova, M.L. Nuclear batteries: Current context and near-term expectations. Int. J. Energy Res. 2022, 46, 19368. [Google Scholar] [CrossRef]
- Tavares, O.A.P.; Medeiros, E.L.; Terranova, M.L. 232U, 228Th, 227Ac, and 226Ra primary radioisotopes: High-power sources for nuclear batteries. Prog. Nucl. Energy 2024, 169, 105101. [Google Scholar] [CrossRef]
- Tavares, O.A.P.; Terranova, M.L. Physical viability for nuclear batteries. J. Radioanal. Nucl. Chem. 2023, 332, 3933. [Google Scholar] [CrossRef]
- He, J.; Janaký, C. Recent Advances in solar-driven carbon dioxide conversion: Expectations versus reality. ACS Energy Lett. 2020, 5, 1996. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, R.; Cheung, D.W.F.; Wu, L.; Luo, J. Solar driven CO2 reduction: From materials to devices. J. Mater. Chem. A 2023, 11, 12499. [Google Scholar] [CrossRef]
- Sævarsdottir, G.; Kvande, H.; Magnusson, T. Greenhouse gas emissions from silicon production: Development of carbon footprint with changing energy systems. In Proceedings of the 16th International Ferro-Alloys Congress (INFACON XVI) 2021, Virtual, 27–29 September 2021. [Google Scholar]
- Saevarsdottir, G.; Kvande, H.; Welch, B.J. Aluminum production in the times of climate change: The global challenge to reduce the carbon footprint and prevent carbon leakage. JOM 2020, 72, 296. [Google Scholar] [CrossRef]
- Bayliss, C. TMS light metals keynote presents realities of aluminum’s environmental sustainability. Light Met. Age 2019, 77, 38. [Google Scholar]
- Saevarsdottir, G.; Padamata, S.K.; Velasquez, B.N.; Kvande, H. The way towards zero carbon emissions in aluminum electrolysis. In Light Metals; Broek, S., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 637–645. [Google Scholar]
- Saevarsdottir, G.; Magnusson, T.; Kvande, H. Reducing the carbon footprint: Primary production of aluminum and silicon with changing energy systems. J. Sustain. Metall. 2021, 7, 848. [Google Scholar] [CrossRef]
- Available online: https://www.products.pcc.eu/pl/id/1249567/metal-krzemowy-pcc-bakkisilicon/ (accessed on 25 October 2024).
- Yuan, L.; Nain, P.; Kothari, M.; Anctil, A. Material intensity and carbon footprint of crystalline silicon module assembly over time. Sol. Energy 2024, 269, 112336. [Google Scholar] [CrossRef]
- de Wild-Scholten, M.J. Energy payback time and carbon footprint of commercial photovoltaic systems. Sol. Energy Mater. Sol. Cells. 2013, 119, 296. [Google Scholar] [CrossRef]
- Shaker, L.M.; Al-Amiery, A.A.; Hanoon, M.M.; Al-Azzawi, W.K.; Kadhum, A.A.H. Examining the influence of thermal effects on solar cells: A comprehensive review. Sustain. Energy Res. 2024, 11, 6. [Google Scholar] [CrossRef]
- Machín, A.; Márquez, F. Advancements in photovoltaic cell materials: Silicon, organic, and perovskite solar cells. Materials 2024, 17, 1165. [Google Scholar] [CrossRef]
- Solak, E.K.; Irmak, E. Advances in organic photovoltaic cells: A comprehensive review of materials, technologies and performance. RSC Adv. 2023, 13, 12244. [Google Scholar] [CrossRef]
- Ma, C.M.; Ge, Q.S. Method for calculating CO2 emissions from the power sector at the provincial level in China. Adv Clim. Change Res. 2014, 5, 92. [Google Scholar]
- Müller, A.; Friedrich, L.; Reichel, C.; Herceg, S.; Mittag, M.; Neuhaus, D.H. A comparative life cycle assessment of silicon PV modules: Impact of module design, manufacturing location and inventory. Sol. Energy Mater. Sol. Cells 2021, 230, 11127. [Google Scholar] [CrossRef]
- Reichel, C.; Müller, A.; Friedrich, L.; Herceg, S.; Mittag, M.; Protti, A.; Neuhaus, D.H. CO2 Emissions of silicon photovoltaic modules-impact of module design and production location. In Proceedings of the 8th World Conference on Photovoltaic Energy Conversion, Milan, Italy, 26–30 September 2022; EU PVSEC: Milano, Italy, 2022; Volume 1617. [Google Scholar]
- Bosnjakovic, M. Enviromental impact of PV power systems. Sustainability 2023, 15, 11888. [Google Scholar] [CrossRef]
- Gerold, E. Advancements and challenges in photovoltaic cell recycling: A comprehensive review. Sustainability 2024, 16, 2542. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Management of Radioactive Waste from the Use of Radioactive Material in Medicine, Industry, Agriculture, Research, and Education; IAEA Safety Standards Series No. GSG-2; IAEA: Vienna, Austria, 2013. [Google Scholar]
- IAEA. Disposal of Radioactive Waste; IAEA Safety Standards Series No. SSR-5; IAEA: Vienna, Austria, 2011. [Google Scholar]
- International Atomic Energy Agency. Handbook on Nuclear Law; IAEA: Vienna, Austria, 2003. [Google Scholar]
- Martiny, A.; Taglialatela, J.; Testa, F.; Iraldo, F. Determinants of environmental social and governance (ESG) performance: A systematic literature review. J. Clean. Prod. 2024, 456, 142213. [Google Scholar] [CrossRef]
- Kai, A.; Au, M.; Yang, Y.F.; Wang, H.; Chen, R.H.; Zheng, L.J. Mapping the landscape of ESG strategies: A bibliometric review and recommendations for future research. Sustainability 2023, 15, 16592. [Google Scholar] [CrossRef]
- Sierra, A.; Reinders, A. Designing innovative solutions for solar-powered electric mobility applications. Prog. Photovolt. Res. Appl. 2021, 29, 802. [Google Scholar] [CrossRef]


| Scintillator | Dopant/s | Function | Source |
|---|---|---|---|
| Caesium iodide (CsI(Tl)) | Thallium (Tl) | Improves the ability to detect gamma radiation and increases the efficiency of converting radiation energy into light. NaI(Tl) is one of the most commonly used scintillators in nuclear medicine and radiation detection. | [18,19] |
| Sodium iodide (NaI(Tl)) | |||
| Lutetium oxide (Lu2O3) | Cerium (Ce) | Improves the decay time (speed of light emission) and increases detection sensitivity, especially to gamma radiation. Cerium is used in scintillators used in positron emission tomography (PET) and high energy detection. | [20] |
| Gadolinite (Gd2O2S) | [21] | ||
| LSO (Lu2SiO5) | [22] | ||
| Zinc sulphide (ZnS) | Europium (Eu) | Increases emission brightness and is used in alpha and low-energy radiation detectors. Europium-doped scintillators are used in X-ray monitors and screens. | [23] |
| Samarium (Sm) | Dopant enables efficient detection of alpha radiation and neutrons. ZnS is used in the detection of alpha particles and thermal neutrons. | [24] | |
| Garnets (Y3Al5O12, YAG) | Yttrium (Y) | Dopant is used to increase sensitivity to X-rays and gamma rays and improve mechanical properties and thermal stability of scintillators. YAG is used in medical imaging systems. | [25] |
| Gadolinite (Gd2O2S) | [26] | ||
| Lithium fluoride (LiYF4) | Praseodymium (Pr) | Improves decay time and light efficiency, especially in scintillators used for detecting neutron radiation. | [27] |
| Gadolinite (Gd2O2S) | Terbium (Tb) | Dopant enables intense light emission due to absorption of X-rays. These materials are used in X-ray screens and beta radiation detection. | [28] |
| Garnets (Gd3Ga5O12) | Neodymium (Nd) | Improves decay time and is used in gamma and X-ray detection, especially in complex medical and imaging systems. | [29] |
| Fluorides (LiHoF4) | Holmium (Ho) | Is used to increase the sensitivity of scintillators to neutrons and is used in thermal neutron detection systems. | [30] |
| LSO (Lu2SiO5) | Lutetium (Lu) | Increases the density of the scintillator material, which improves the detection efficiency of high-energy radiation such as gamma radiation. Lutetium scintillators are mainly used in PET systems and ionizing radiation detection. | [31] |
| Factor | Effects | Source |
|---|---|---|
| Exposure to radiation | Radiation dose: Long-term exposure to radiation can lead to degradation of scintillators, especially in the case of organic materials. High doses of radiation can cause damage to the crystal structure of the scintillator, leading to a reduction in its light output. Radiation damage: Some scintillators, especially those based on organic materials (e.g., polystyrene or polyvinyltoluene), are susceptible to radiation damage. This damage can manifest itself in colour changes, reduced light output, and reduced operating life. | [59,60,61,62] |
| Moisture | Hygroscopicity: Some scintillators, such as sodium iodide (NaI), are hygroscopic, meaning they absorb moisture from the environment. Moisture can cause degradation of the material, leading to a loss of optical efficiency and a reduction in the transparency of the scintillator. Housing tightness: To prevent degradation caused by moisture, hygroscopic scintillators must be stored in tight, airtight housings, often filled with inert gases such as argon, or in moisture-proof shields. | [14,17,63] |
| Thermal factors | High temperature: High temperatures can damage scintillators, especially organic materials, resulting in degradation of their molecular structure. In extreme cases, this can cause cracking, colour changes, and loss of scintillation ability. Thermal phenomena: Sudden changes in temperature can lead to thermal stresses in scintillators, especially in crystalline materials such as bismuth germanate (BGO) or sodium iodide (NaI). These stresses can cause cracks or other mechanical damage. | [17] |
| Material aging | Chemical changes: Over time, some scintillators may undergo chemical aging processes that affect their performance. This can include oxidation, molecular degradation, or reactions with environmental contaminants. Mechanical changes: As a result of aging, the mechanical properties of a scintillator, such as brittleness or shock resistance, can deteriorate. This can affect their ability to operate for long periods in harsh environments. | [63,66] |
| Impurities and crystal defects | Contamination during manufacturing: The presence of impurities in the scintillator material can affect its service life. These impurities can reduce the efficiency of the conversion of radiation to light and accelerate the degradation of the material. Crystal defects: In the case of inorganic scintillators such as BGO, LSO (lutetium silicate), or NaI, defects in the crystal structure can affect the scintillator properties and lead to degradation of the optical performance over time. | [14] |
| Mechanical factors | Vibration and shock: Crystal scintillators such as NaI(Tl) or BGO are susceptible to mechanical damage. Vibrations, shocks, or drops can cause cracks, which in turn reduces their performance. Mechanical stress: Under conditions of strong mechanical stress, scintillators can deform, which negatively affects their performance. | [65] |
| Exposure to light | UV light exposure: Some scintillators, especially organic ones (e.g., PVT—polyvinyl toluene), are sensitive to UV radiation, which can lead to material degradation. Prolonged exposure to sunlight can reduce their ability to emit light after absorbing ionizing radiation. | [66,67] |
| Proper maintenance and storage | Dry storage: Hygroscopic scintillators, such as NaI(Tl), require proper storage in airtight containers in a dry environment. Temperature and humidity control: For many scintillators, especially those sensitive to moisture and temperature changes, it is crucial to control the environment in which they are stored and used. | [14,68] |
| Strengths | Weaknesses | Opportunities | Threats |
|---|---|---|---|
| Efficiency in radiation detection: | Toxicity of some components: | Development of alternative materials: | Technological competition is growing: |
| Scintillation materials, despite potential toxic effects, offer high efficiency in radiation detection, which is crucial in many fields such as medicine, industry, and science. | Some scintillators, such as NaI(Tl) containing thallium, are toxic and may pose a risk to human health and the environment. | There is potential to develop new, less toxic scintillators based on alternative chemical components. | Other radiation detection technologies, such as semiconductor detectors, may become more popular, which could limit the use of scintillators. |
| Possibility of chemical modification: | Chemical Exposure: | Increased demand for radiation detection: | Environmental Regulations: |
| Scintillators can be modified by adding dopants, which allows for improving their efficiency and reducing the toxicity of some components. | Scintillator manufacturing and use processes often involve exposure to toxic chemicals, which poses a health risk to workers and users. | Increased demand for radiation detection technologies in medicine and industry creates opportunities for innovation and improved toxicity practices. | Increased regulations regarding toxic chemicals may impact scintillator production and introduce additional restrictions. |
| Diversity of applications: | Recycling Issues: | Safety regulations and standards: | Negative health effects: |
| Due to their efficiency, scintillators are used in a wide range of applications, which can increase their value and importance in industry. | The high toxicity of some materials can limit their recycling capabilities, leading to waste management issues. | Introducing stricter regulations regarding toxic substances can lead to improved manufacturing practices and reduced risk. | Increasing public awareness of the toxicity of some materials may lead to a decrease in acceptance of scintillators containing hazardous substances. |
| Type of Process | Description |
|---|---|
| Identification and segregation | The used scintillators are assessed to identify their chemical composition and potential radioactive contamination. In the event that the scintillator is radioactive, it must be properly disposed of as radioactive waste. If it shows no contamination, the materials can be recycled. |
| Dismantling and material recovery | Scintillators consist of various components that can be recovered:
|
| Processing and reuse | Materials recovered from recycling can be reused to produce new scintillators, electronic components, or in other industrial processes. Recovered rare elements such as lutetium are particularly valuable in the technology industry and can be used not only in radiation detectors but also in other electronic devices. |
| Waste and disposal | Residues from the recycling process that cannot be reused must be properly disposed of. In the case of radioactive materials, strict regulations must be followed regarding the storage of radioactive waste. Chemical materials such as contaminated plastics are subject to special disposal procedures in accordance with environmental regulations. |
| Process safety | Scintillator recycling requires appropriate safety measures, both due to the possibility of exposure to radiation and contact with toxic chemicals such as thallium or heavy metals. Workers must be adequately protected and processes must be carried out in controlled conditions to minimise risks. |
| The Way to Proceed | Procedure Description |
|---|---|
| Hazardous waste disposal | For scintillators that contain toxic or radioactive materials, disposal is carried out in accordance with hazardous waste regulations. These materials must be collected, transported, and processed in accordance with stringent safety standards. Radioactive scintillators, which may contain radioactive elements, must be processed in special facilities adapted to the treatment of radioactive waste. This waste is typically stored in safe locations, such as underground radioactive waste storage facilities. |
| Recycling of materials | Many scintillators contain valuable elements such as lutetium (Lu), bismuth (Bi), and caesium (Cs) that can be recycled and reused. The recycling process involves the recovery of valuable materials that can be used to produce new scintillators. Sodium iodide (NaI) or caesium iodide (CsI) can be purified after use, where impurities are removed and the material is reused. |
| Mechanical and chemical processing | Some scintillators can be subjected to mechanical (e.g., grinding, separation) and chemical (e.g., dissolving in appropriate chemicals to separate components) processes that allow materials to be recovered for reuse or waste to be prepared for safe disposal. For example, bismuth germanate (Bi₄Ge₃O₁₂) can be processed in such a way as to recover bismuth or germanium for further industrial use. |
| Secure storage | If scintillators are not recyclable or reusable, they are properly disposed of in safe conditions. In the case of inorganic scintillators, which are not radioactive, they can be treated as industrial waste that is disposed of in landfills adapted to hazardous waste. Organic scintillators, such as polystyrene (PS) or polyvinyltoluene (PVT), can be incinerated in specialised installations, provided that the emission standards are met. |
| Disposal of radioactive materials | Scintillators containing radioactive materials, such as lutetite-177 or other isotopes used in certain types of scintillators, must be treated as radioactive waste. At the end of their useful life, they are stored in facilities intended for the storage of radioactive waste of low or medium activity. Radioactive waste can also undergo decontamination processes, which allow its radioactive activity to be reduced before further processing. |
| Recovery | In some cases, scintillator remanufacturing is possible, in which older or worn materials are refurbished and returned for reuse. This process may include repairing mechanical damage or cleaning the material to restore its original scintillation properties. |
| Regulatory Rules and Standards | The handling of used scintillators is strictly regulated by national and international regulations on environmental protection and hazardous waste management. Regulatory standards depend on the type of material and the country in which the scintillators are operated. Organizations such as the International Atomic Energy Agency (IAEA) or national environmental protection agencies (e.g., the National Atomic Energy Agency in Poland) regulate regulations on the safe handling of radioactive and hazardous waste. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwan, A.; Bogdanowicz, K.A.; Pich, R.; Gonciarz, A.; Pellowski, W.; Miedziak, J.; Przybyl, W. Photovoltaic Cells and Scintillators Towards Carbon Footprint Reduction: Advantages and Challenges for Ecological Safety. Materials 2024, 17, 5909. https://doi.org/10.3390/ma17235909
Iwan A, Bogdanowicz KA, Pich R, Gonciarz A, Pellowski W, Miedziak J, Przybyl W. Photovoltaic Cells and Scintillators Towards Carbon Footprint Reduction: Advantages and Challenges for Ecological Safety. Materials. 2024; 17(23):5909. https://doi.org/10.3390/ma17235909
Chicago/Turabian StyleIwan, Agnieszka, Krzysztof A. Bogdanowicz, Robert Pich, Agnieszka Gonciarz, Witalis Pellowski, Jacek Miedziak, and Wojciech Przybyl. 2024. "Photovoltaic Cells and Scintillators Towards Carbon Footprint Reduction: Advantages and Challenges for Ecological Safety" Materials 17, no. 23: 5909. https://doi.org/10.3390/ma17235909
APA StyleIwan, A., Bogdanowicz, K. A., Pich, R., Gonciarz, A., Pellowski, W., Miedziak, J., & Przybyl, W. (2024). Photovoltaic Cells and Scintillators Towards Carbon Footprint Reduction: Advantages and Challenges for Ecological Safety. Materials, 17(23), 5909. https://doi.org/10.3390/ma17235909










