Big Data Analysis, Design, Effect Fabrication, and Properties Analysis of SiO2/Cr/SiO2 Colored Solar Selective Absorbers with High PTCE and Chromaticity for Building Applications
Abstract
1. Introduction
- 1.
- Study on graphing the colored chromaticity coordinate distribution (CCD)-αs diagram in the CIE xy chromaticity diagram and analyzing the relationship between αs values and CCD;
- 2.1.
- Study on the design, fabrication, and analysis of seven SiO2/Cr/SiO2 CSSAs using colored CCD-αs diagrams;
- 2.2.
- Study on the impact of oxygen atom penetration on the crystallographic structure of CSSA during annealing;
- 3.
- Study on the effect of surface roughness on the properties of fabricated CSSAs;
- 4.
- Finally, provide a detailed discussion and explanation of the relationship between experimental results and surface roughness or simulations.
2. Experimental Details
2.1. Preparation of the SiO2/Cr/SiO2 Colored Solar Selective Absorbers
2.2. Characterization of the Thin Films
2.3. Optical Properties of Simulation for CSSAs Using the CUDA C Parallel Computation Technology
2.3.1. Thin Film Optical Simulation for the SiO2/Cr/SiO2 CSSA
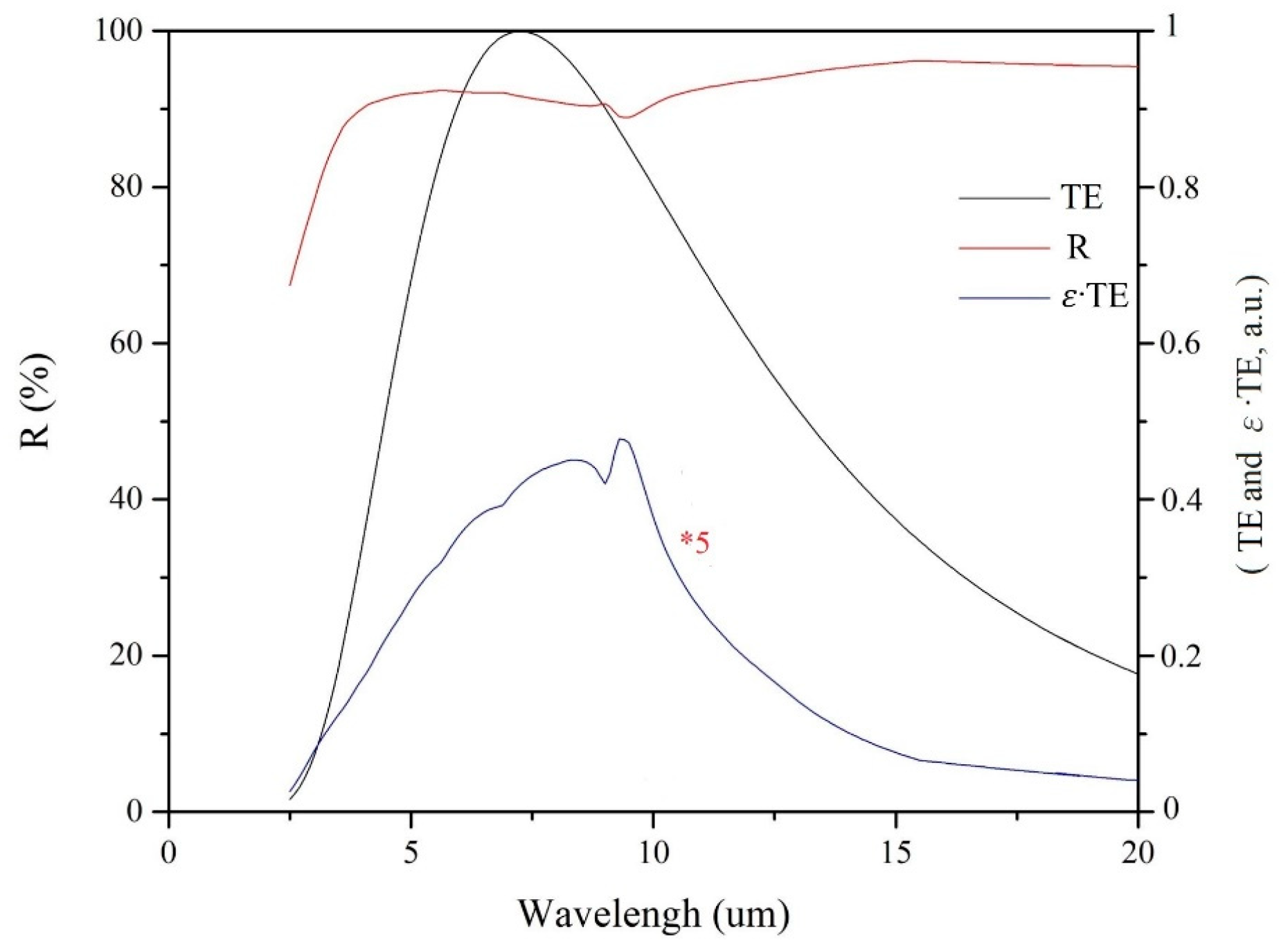
2.3.2. Analysis of Solar Absorption Efficiency, Emissivity, and αs for the SiO2/Cr/SiO2 CSSAs
2.3.3. Analysis of Chromaticity for the SiO2/Cr/SiO2 CSSA
3. Results and Discussion
3.1. Big Data Analysis of Optical Thin Film for the SiO2/Cr/SiO2 CSSAs
3.1.1. Simulation Settings and Optimal αs
3.1.2. Graphing Colored CCD-αs Diagram in the CIE xy Chromaticity Diagram and Analyzing the Relationship Between αs Value and CCD
3.2. Design and Fabrication of Seven CSSAs Utilizing Colored CCD-αs Diagrams: Comprehensive Property Analysis Including Investigation into Oxygen Atom Penetration and Cr Layer Oxidation
3.2.1. Design, Fabrication, and Analysis of Seven SiO2/Cr/SiO2 CSSAs Utilizing CCD-αs Diagrams
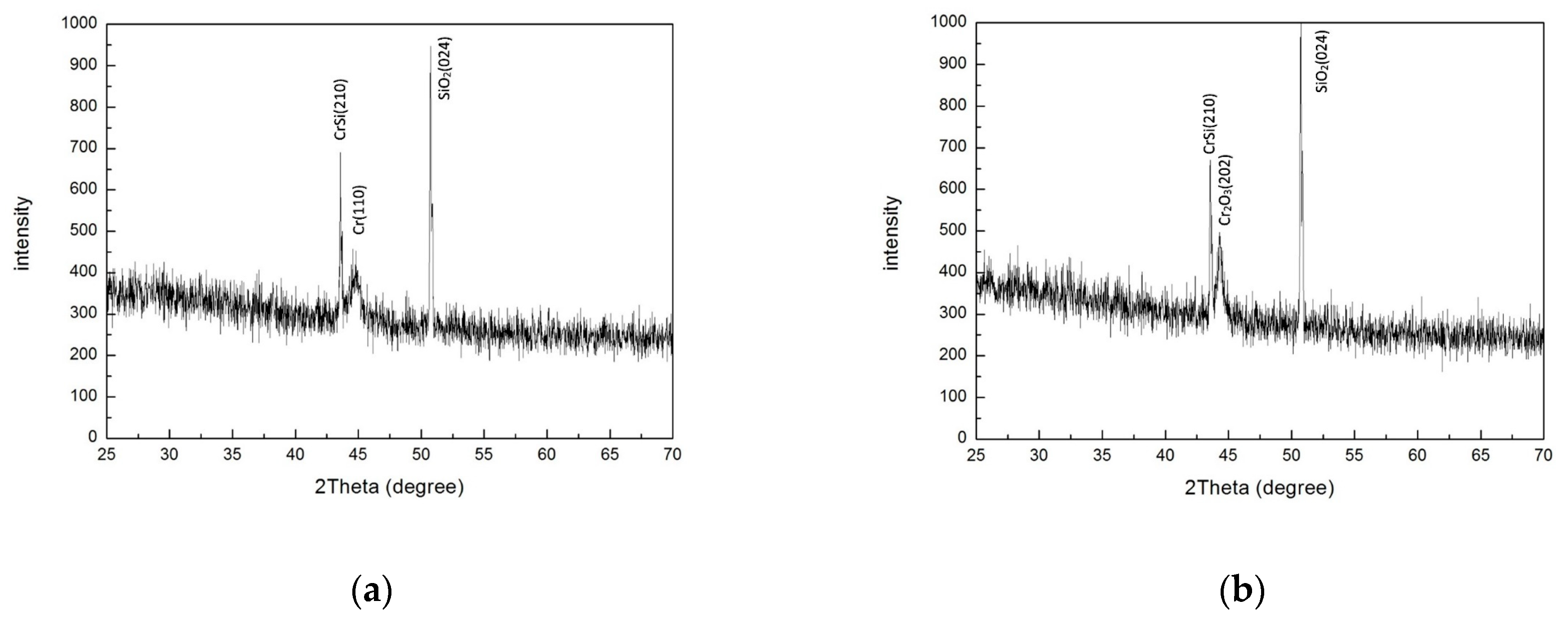
3.2.2. Exploring the Impact of Oxygen Atom Penetration on the Crystallographic Structure of CSSA During Annealing
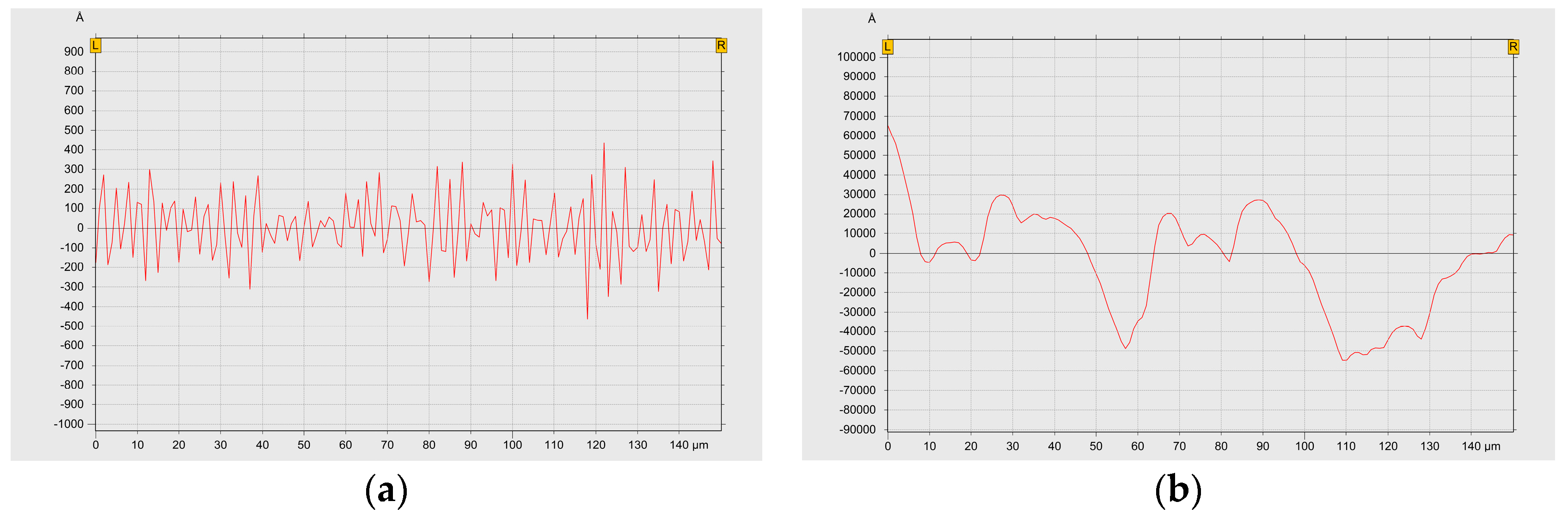
3.3. Effect of the Surface Roughness on Properties of the Fabricated CSSAs
3.4. Discussion and Explanation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lai, F.D. Effect of each layer thickness in Al2O3/Pt/Al2O3 solar absorber films on the photo-thermal conversion efficiency for rapid industrial production. Surf. Coat. Technol. 2020, 389, 125503. [Google Scholar] [CrossRef]
- Conserval Systems, Inc. Solar Wall. Available online: https://pdf.archiexpo.com/pdf/conserval-engineering/solarwall/60659-230117.html (accessed on 1 September 2024).
- Extended Campuses Building Expansion. Available online: https://in.nau.edu/facility-services/pdc/building-green/extended-campuses-building/ (accessed on 1 September 2024).
- Natural Ventilation System/Solar Chimney. Department of Environmental Engineering, Kitakyushu University, Japan. Available online: https://www.ibec.or.jp/jsbd/G/features.htm (accessed on 1 September 2024).
- Zeng, X.; Pelenovich, V.; Neena, D.; Xu, C.; Zhang, X.; Pogrebnjak, A.; Yang, B.; Liu, S. Thermal stability of high entropy graded solar selective absorbers deposited by non-equilibrium RF magnetron sputtering. J. Alloys Compd. 2023, 944, 169248. [Google Scholar] [CrossRef]
- Pelenovich, V.; Zeng, X.; Liu, Y.; Zhang, X.; Liu, H.; Pogrebnjak, A.; Vildanov, R.; Yang, B.; Liu, S. AlCrO protected textured stainless-steel surface for high temperature solar selective absorber applications. Surf. Coat. Technol. 2022, 436, 128282. [Google Scholar] [CrossRef]
- Liu, H.D.; Yang, B.; Mao, M.R.; Liu, Y.; Chen, Y.M.; Cai, Y.; Hu, X.J. Enhanced thermal stability of solar selective absorber based on nano-multilayered TiAlON films deposited by cathodic arc evaporation. Appl. Surf. Sci. 2020, 501, 501144025. [Google Scholar] [CrossRef]
- Qiu, X.-L.; Gao, X.-H.; He, C.-Y.; Chen, B.-H.; Liu, G. Structure, optical simulation and thermal stability of the HfB2-based high-temperature solar selective absorbing coatings. RSC Adv. 2019, 9, 29726–29733. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Yao, T.; Li, G.; Sun, Y.; Cao, X.; Ma, L.; Peng, S. Preparation of a novel TiN/TiNxOy/SiO2 composite ceramic films on aluminum substrate as a solar selective absorber by magnetron sputtering. J. Alloys Compd. 2020, 815, 152209. [Google Scholar] [CrossRef]
- Lai, F.D.; Yan, Y.S. Optical properties, contour map and fabrication of Al2O3/Pt/Al2O3/Ta multilayer films for solar selective absorptance layer. Sci. Adv. Mater. 2019, 11, 1106–1111. [Google Scholar] [CrossRef]
- Nuru, Z.Y.; Arendse, C.J.; Khamlich, S.; Kotsedi, L.; Maaza, M. Tantalum diffusion barrier layer to improve the thermal stability of AlxOy/Pt/AlxOy multilayer solar absorber. Sol. Energy 2014, 107, 89–96. [Google Scholar] [CrossRef]
- Nuru, Z.Y.; Arendse, C.J.; Khamlich, S.; Maaza, M. Optimization of AlxOy/Pt/AlxOy multilayer spectrally selective coatings for solar thermal applications. Vacuum 2012, 86, 2129–2135. [Google Scholar] [CrossRef]
- Wang, S.; Chen, F.; Lu, W. Advanced thin film photo-thermal materials and applications. Adv. Thermoelec. Mater. 2019, 8, 317–381. [Google Scholar]
- Lai, F.-D.; Li, W.-Y.; Chang, K.-C.; Wang, Y.-Z.; Chi, P.-L.; Su, J.-Y. Optical properties, optimized design and fabrication of SiO2/W/SiO2 films for solar selective absorber. Integr. Ferroelectr. 2012, 137, 77–84. [Google Scholar] [CrossRef]
- Lai, F.D.; Li, W.Y. SiO2/Ti/SiO2 Three-layer films used as solar selective absorber layer in heat energy collector. Integr. Ferroelec. 2013, 145, 158–164. [Google Scholar] [CrossRef]
- Shaffei, M.F.; Hussein, H.S.; Abouelata, A.M.A.; Osman, R.M.; Mohammed, M.S. Effect of sealing on characteristics of nano-porous aluminum oxide as black selective coatings. Clean. Eng. Technol. 2021, 4, 100156. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, Z.; Wang, J.; Hou, S.; Liu, Y.; Zhang, Q.; Mao, J.; Liu, X.; Cao, F. Realization of an efficient wide-angle solar selective absorber via the impedance matching. Sol. Energy Mater. Sol. Cells 2022, 238, 111582. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, X.; Gong, D.; Cheng, X.; Li, K. Optical properties and thermal stability of AlCrON based multilayer solar selective absorbing coating for high temperature applications. J. Mater. Res. Technol. 2021, 15, 6162–6174. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Ghanekar, A.; Zheng, Y. Scalable-manufactured metal–insulator–metal based selective solar absorbers with excellent high-temperature insensitivity. Appl. Energy 2021, 281, 116055. [Google Scholar] [CrossRef]
- Guo, H.-X.; Yu, D.-M.; He, C.-Y.; Qiu, X.-L.; Zhao, S.-S.; Liu, G.; Gao, X.-H. Double-layer solar absorber coating based on high entropy ceramic AlCrMoTaTiN: Structure, optical properties and failure mechanism. Surf. Interfaces 2021, 24, 101062. [Google Scholar] [CrossRef]
- He, C.-Y.; Gao, X.-H.; Yu, D.-M.; Guo, H.-X.; Zhao, S.-S.; Liu, G. Highly enhanced thermal robustness and photothermal conversion efficiency of solar-selective absorbers enabled by high-entropy alloy nitride MoTaTiCrN nanofilms. ACS Appl. Mater. Interfaces 2021, 13, 16987–16996. [Google Scholar] [CrossRef]
- Liu, H.; Xie, M.; Ai, Q.; Yu, Z. Ultra-broadband selective absorber for near-perfect harvesting of solar energy. J. Quant. Spectrosc. Radiat. Transfer. 2021, 266, 107575. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Bazri, S.; Badruddin, I.A.; Orooji, Y.; Saeidi, S.; Wongwises, S.; Mahian, O. Optical properties and thermal stability evaluation of solar absorbers enhanced by nanostructured selective coating films. Powder Technol. 2021, 377, 939–957. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Caratenuto, A.; Li, J.; Zhou, S.; Ran, R.; Chen, F.; Wang, Z.; Wan, K.-T.; Xiao, G.; et al. A new strategy towards spectral selectivity: Selective leaching alloy to achieve selective plasmonic solar absorption and infrared suppression. Nano Energy 2022, 92, 106717. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Shi, J.; Wei, D.; Zhao, H.; Ma, C. Solar selective absorber for emerging sustainable applications. Adv. Energy Sustain. Res. 2022, 3, 2100195. [Google Scholar] [CrossRef]
- Niranjan, K.; Kondaiah, P.; Biswas, A.; Kumar, V.; Srinivas, G.; Barshilia, H. Spectrally selective solar absorber coating of W/WAlSiN/SiON/SiO2 with enhanced absorption through gradation of optical constants: Validation by simulation. Coatings 2021, 11, 334. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Hu, E.-T.; Cai, Q.-Y.; Wang, J.; Tu, H.-T.; Yu, K.-H.; Chen, L.-Y.; Wei, W. Accurate design of solar selective absorber based on measured optical constants of nano-thin Cr Film. Coatings 2020, 10, 938. [Google Scholar] [CrossRef]
- Ji, S.-M.; Oh, S.-W.; Yoon, T.-H. Smart window based on angular-selective absorption of solar radiation with guest–host liquid crystals. Crystals 2021, 11, 131. [Google Scholar] [CrossRef]
- El-Fattah, H.A.A.; El-Mahallawi, I.S.; Shazly, M.H.; Khalifa, W.A. Optical properties and microstructure of TiNxOy and TiN thin films before and after annealing at different conditions. Coatings 2019, 9, 22. [Google Scholar] [CrossRef]
- Tyagi, P.K.; Kumar, R.; Said, Z. Recent advances on the role of nanomaterials for improving the performance of photovoltaic thermal systems: Trends, challenges and prospective. Nano Energy 2022, 93, 106834. [Google Scholar] [CrossRef]
- Said, Z.; Sundar, L.S.; Tiwari, A.K.; Ali, H.M.; Sheikholeslami, M.; Bellos, E.; Babar, H. Recent advances on the fundamental physical phenomena behind stability, dynamic motion, thermophysical properties, heat transport, applications, and challenges of nanofluids. Phys. Rep. 2022, 946, 1–94. [Google Scholar] [CrossRef]
- Anderson, T.; Duke, M.; Carson, J. The effect of colour on the thermal performance of building integrated solar collectors. Sol. Energy Mater. Sol. Cells 2010, 94, 350–354. [Google Scholar] [CrossRef]
- Selvakumar, N.; Karthik, G.; Jayaraj, S.; Barshilia, H.C. Sprayable PEDOT:PSS based spectrally selective coating for solar energy harvesting. Sol. Energy Mater. Sol. Cells 2021, 221, 110906. [Google Scholar] [CrossRef]
- Xuan, Z.; Li, J.; Liu, Q.; Yi, F.; Wang, S.; Lu, W. Artificial structural colors and applications. Innovation 2021, 2, 100081. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-X.; He, C.-Y.; Qiu, X.-L.; Shen, Y.-Q.; Liu, G.; Gao, X.-H. A novel multilayer high temperature colored solar absorber coating based on high-entropy alloy MoNbHfZrTi: Optimized preparation and chromaticity investigation. Sol. Energy Mater. Sol. Cells 2020, 209, 110444. [Google Scholar] [CrossRef]
- Lai, F.D.; Li, W.Y. Design, fabrication and analysis for Al2O3/Ti/Al2O3 colored solar selective absorbers for building applications. Coatings 2022, 12, 521. [Google Scholar] [CrossRef]
- Yang, R.; Liu, J.; Lin, L.; Qu, Y.; Zheng, W.; Lai, F. Optical properties and thermal stability of colored solar selective absorbing coatings with double-layer antireflection coatings. Sol. Energy 2016, 125, 453–459. [Google Scholar] [CrossRef]
- Chen, F.; Wang, S.-W.; Liu, X.; Ji, R.; Li, Z.; Chen, X.; Chen, Y.; Lu, W. Colorful solar selective absorber integrated with different colored units. Opt. Express 2017, 24, A92–A103. [Google Scholar] [CrossRef]
- Chen, F.; Wang, S.-W.; Liu, X.; Ji, R.; Yu, L.; Chen, X.; Lu, W. High performance colored selective absorbers for architecturally integrated solar applications. J. Mater. Chem. A 2015, 3, 7353–7360. [Google Scholar] [CrossRef]
- Wang, C.; Shi, J.; Geng, Z.; Ling, X. Polychromic Al–AlN cermet solar absorber coating with high absorption efficiency and excellent durability, Sol. Energy Mater. Sol. Cells 2016, 144, 14–22. [Google Scholar] [CrossRef]
- Dan, A.; Chattopadhyay, K.; Barshilia, H.C.; Basu, B. Colored selective absorber coating with excellent durability. Thin Solid Films 2016, 620, 17–22. [Google Scholar] [CrossRef]
- Schuler, A.; Boudaden, J.; Oelhafen, P.; De Chambrier, E.; Roecker, C.; Scartezzini, J.-L. Thin film multilayer design types for colored glazed thermal solar collectors. Sol. Energy Mater. Sol. Cells 2005, 89, 219–231. [Google Scholar] [CrossRef]
- Schüler, A.; Roecker, C.; Boudaden, J.; Oelhafen, P.; Scartezzini, J.-L. Potential of quarter-wave interference stacks for colored thermal solar collectors. Sol. Energy 2005, 79, 122–130. [Google Scholar] [CrossRef]
- No, L.; Šest, E.; Kapun, G.; Ruiz-Zepeda, F.; Binyamin, Y.; Merzel, F.; Jerman, I. High-solar-absorptance CSP coating characterization and reliability testing with isothermal and cyclic loads for service-life prediction. Energy Environ. Sci. 2019, 12, 1679–1694. [Google Scholar]
- Lai, F.D. Optical property analysis, contour map and fabrication of SiO2/Cr/SiO2/Cr multilayer films for high photo-thermal conversion efficiency. Integr. Ferroelectr. 2020, 210, 64–72. [Google Scholar] [CrossRef]
- Chang, S.-H.; Lin, S.-S.; Huang, K.-T. Effect of CrSi/CrSi2 content on the microstructure and properties of vacuum hot-pressed Cr-50 wt% Si alloys. Vacuum 2019, 162, 54–62. [Google Scholar] [CrossRef]
- Bhushan, T.; Chandrashekhar, A.; Prasat, S.V.; Reddy, I.R. Effect of substrate surface roughness on adhesion of titanium nitride coatings deposited by physical vapour deposition technique. Mater. Sci. Eng. 2020, 981, 042022. [Google Scholar] [CrossRef]
- Xiao, W.; Deng, H.; Zou, S.; Ren, Y.; Tang, D.; Lei, M.; Xiao, C.; Zhou, X.; Chen, Y. Effect of roughness of substrate and sputtering power on the properties of TiN coatings deposited by magnetron sputtering for ATF. J. Nucl. Mater. 2018, 509, 542–549. [Google Scholar] [CrossRef]
- Lee, L.C.; Ho, W.Y.; Lai, F.D. Effect of substrate surface roughness on the characteristics of CrN hard film. Mater. Chem. Phys. 1996, 43, 266–273. [Google Scholar] [CrossRef]
- Dan, A.; Barshili, H.C.; Chattopadhyay, K.; Basu, B. Solar energy absorption mediated by surface plasma polaritons in spectrally selective dielectric-metal-dielectric coatings: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1050–1077. [Google Scholar] [CrossRef]
- Cho, S.-H.; Seo, M.-K.; Kang, J.-H.; Yang, J.-K.; Kang, S.-Y.; Lee, Y.-H.; Hwang, K.H.; Lee, B.D.; Lee, J.-G.; Song, Y.-W.; et al. A Black Metal-dielectric Thin Film for High-contrast Displays. J. Korean Phys. Soc. 2009, 55, 501–507. [Google Scholar] [CrossRef]
- Caia, H.; Tonga, D.; Wanga, Y.; Songa, X.; Ding, B. Reactive synthesis of porous Cu3Si compound. J. Alloys Compd. 2011, 509, 1672–1676. [Google Scholar] [CrossRef]


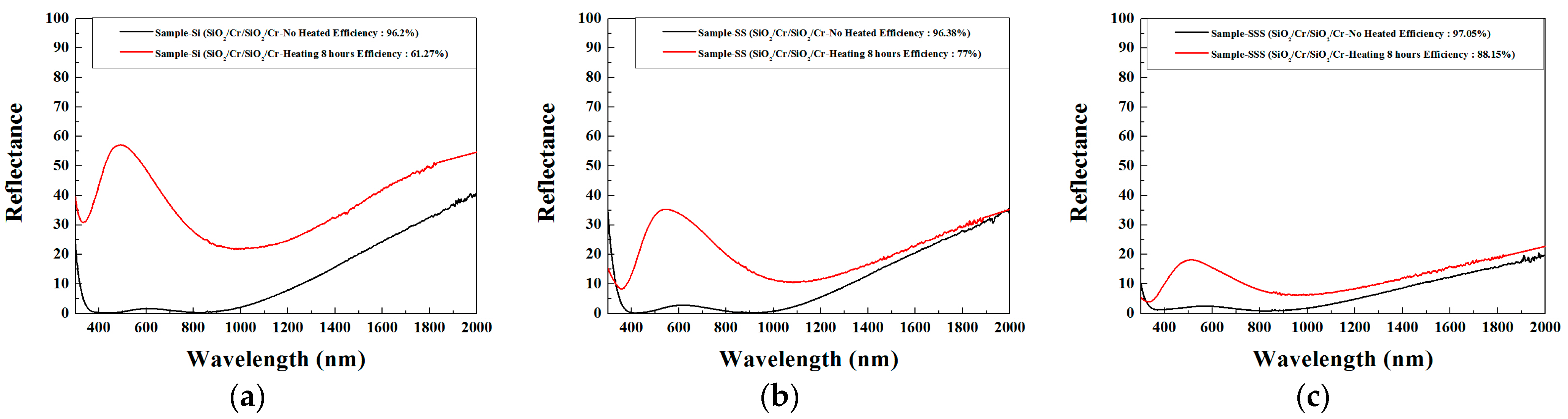

| Figure Number | Thickness Range (X) of a Cr Layer (nm) | Chromaticity Coordinate Distribution Region | αs Range (%) | Explanation of Chromaticity Coordinate Distribution and Preparation of Test Pieces |
|---|---|---|---|---|
| Figure 2b | 3 ≤ X < 4 | All Colors * | 90 to 95 | Its green CCD Area (CCA) is the smallest compared to other CCAs. Roughly speaking, αs within the same chromaticity area nearly remains the same. (Prepare a test piece of G). |
| Figure 2c | 4 ≤ X < 5 | All Colors | 90 to 97 | The CCA with αs exceeding 96% spans various hues including all colors. Notably, there is a broader region in the yellow-orange and orange regions. (Prepare a test piece of A). Across all diagrams from Figure 2b–g, the green CCA is most expansive in Figure 2c. (Prepare a test piece of B). |
| Figure 2d | 5 ≤ X < 6 | All Colors | 90 to 97 | With an increase in the αs, the chromaticity coordinate region shifts further from the white light region. The yellow and orange CCAs are notably broader. (Prepare two test pieces, C and D). |
| Figure 2e | 6 ≤ X < 7 | All Colors | 90 to 96 | The CCR shape in Figure 2e resembles that in Figure 2d but appears as a smaller version with a reduced CCA. (Prepare a test piece of E). |
| Figure 2f | 7 ≤ X < 8 | pink, orange, blue, and purple | 90 to 94 | Figure 2f also mirrors the reduced version of Figure 2c,d, with an even smaller CCA, and the highest αs is less than 94%. (Prepare a test piece of F). |
| Figure 2g | 8 ≤ X < 9 | pink, blue, and purple | 90 to 92 | There is only a small CCR when αs ≥ 90% and the highest αs is less than 92%. |
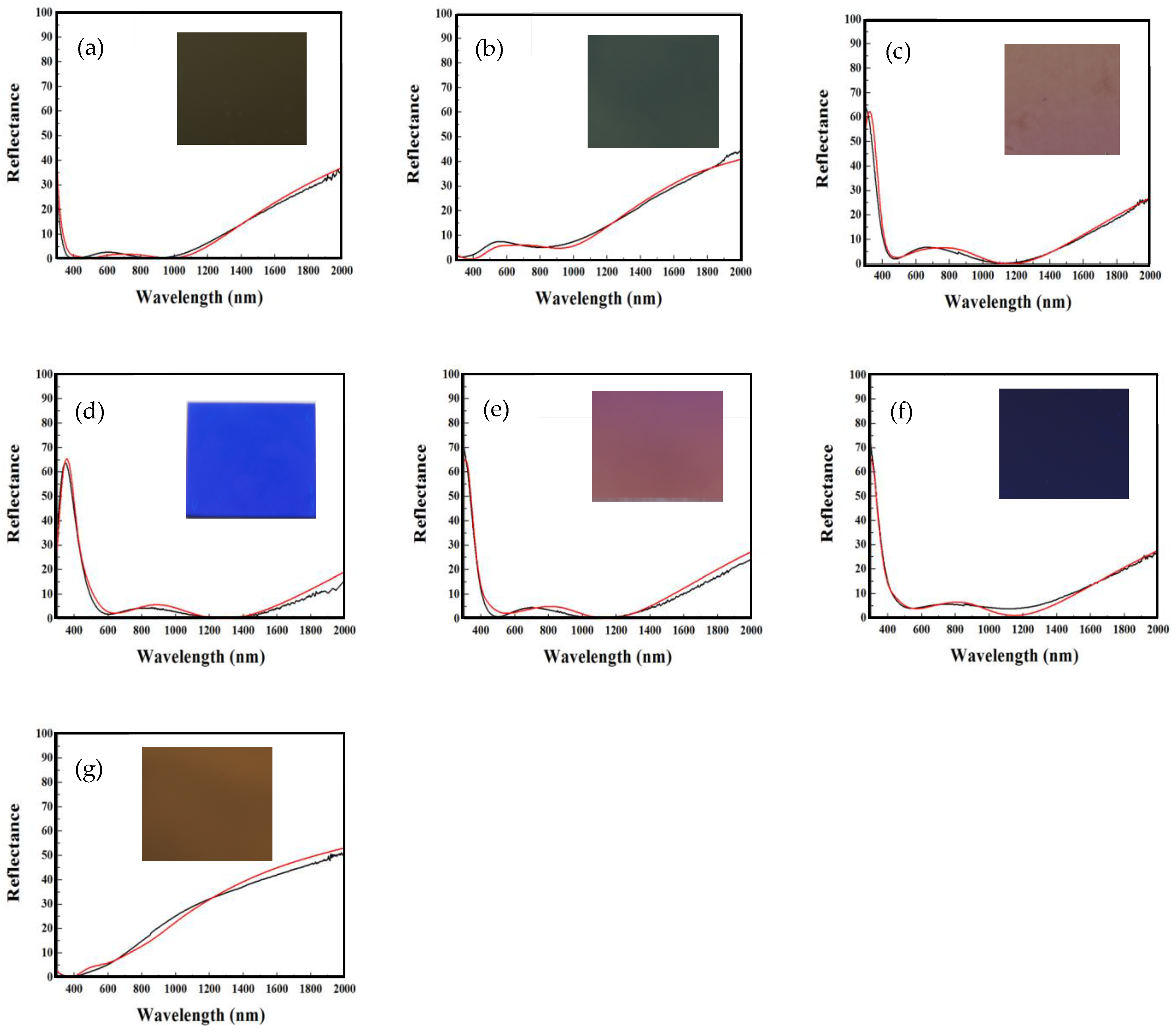
| Test Piece Encoding | In Figure | Designed Film Thickness (nm) | Reflectance Spectrum of Test Piece (Figure) | Calculated αs (%) | Measured αs (%) | Color |
|---|---|---|---|---|---|---|
| A | Figure 2b | 85/4.6/75 | Figure 3a | 96.3 | 96.2 | dark gold |
| B | Figure 2b | 62/4.8/61 | Figure 3b | 91.5 | 91.3 | grass-green |
| C | Figure 2c | 91/5.4/101 | Figure 3c | 94.4 | 94.5 | orange-pink |
| D | Figure 2c | 119/5.8/108 | Figure 3d | 92.2 | 92.1 | bright blue |
| E | Figure 2d | 96/6.4/86 | Figure 3e | 95.1 | 95.2 | purplish-pink |
| F | Figure 2e | 95/7.2/88 | Figure 3f | 93.3 | 93.1 | purple |
| G | Figure 2a | 46/3.8/43 | Figure 3g | 86.8 | 85.6 | earthy gold |
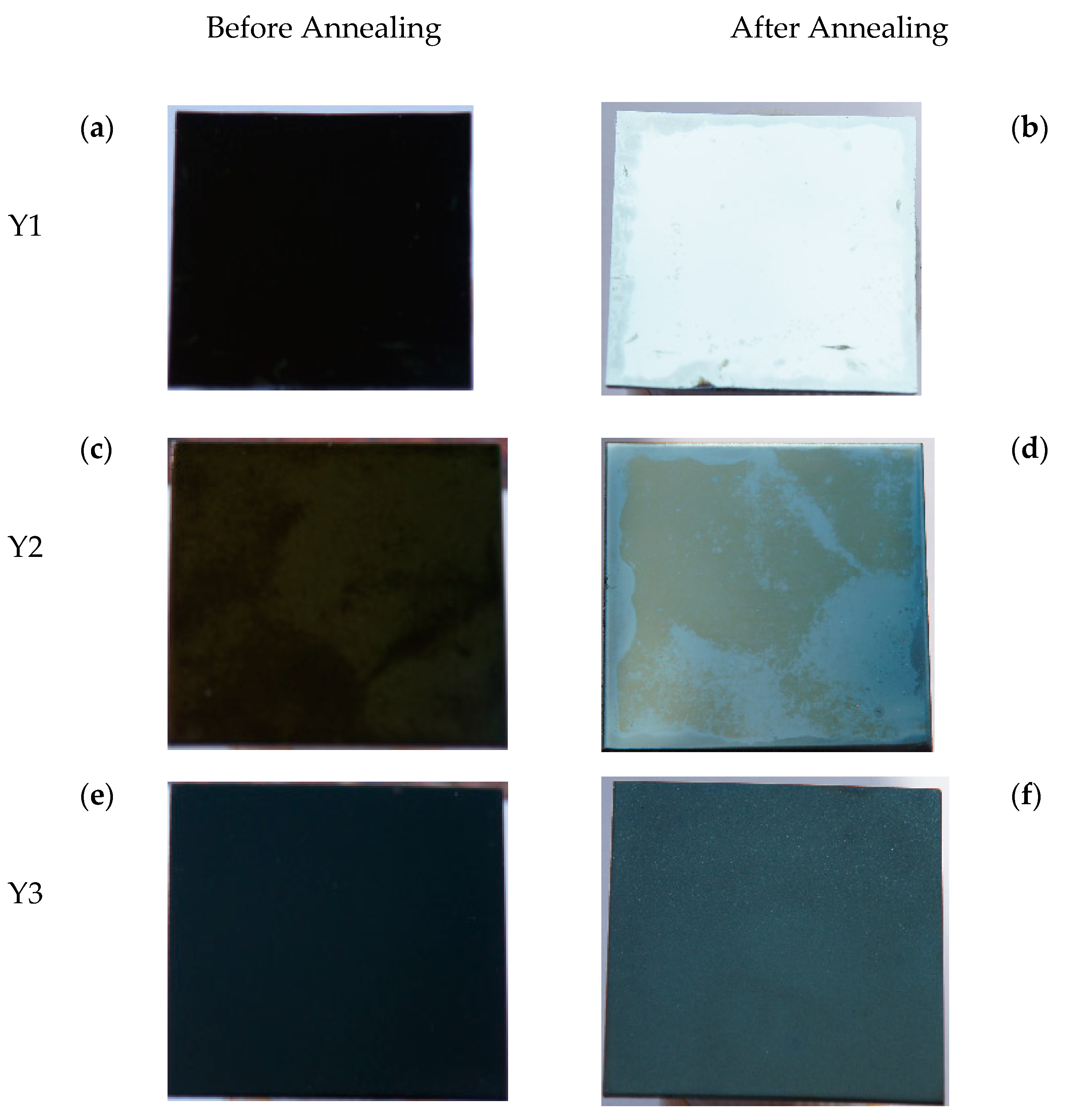
| ϕcorr (mV) | ⅈcorr (μA/cm2) | Corrosion Rate (×10−3 mmpy) | |
|---|---|---|---|
| Y0 | −445.16 | 0.58 | 4.398 |
| Y2 | −161.12 | 0.33 | 2.539 |
| Y3 | −4.65 | 0.24 | 1.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, F.-D.; Lai, Y.-T.; Chen, C.-S. Big Data Analysis, Design, Effect Fabrication, and Properties Analysis of SiO2/Cr/SiO2 Colored Solar Selective Absorbers with High PTCE and Chromaticity for Building Applications. Materials 2024, 17, 5810. https://doi.org/10.3390/ma17235810
Lai F-D, Lai Y-T, Chen C-S. Big Data Analysis, Design, Effect Fabrication, and Properties Analysis of SiO2/Cr/SiO2 Colored Solar Selective Absorbers with High PTCE and Chromaticity for Building Applications. Materials. 2024; 17(23):5810. https://doi.org/10.3390/ma17235810
Chicago/Turabian StyleLai, Fu-Der, Yen-Ting Lai, and Chang-Song Chen. 2024. "Big Data Analysis, Design, Effect Fabrication, and Properties Analysis of SiO2/Cr/SiO2 Colored Solar Selective Absorbers with High PTCE and Chromaticity for Building Applications" Materials 17, no. 23: 5810. https://doi.org/10.3390/ma17235810
APA StyleLai, F.-D., Lai, Y.-T., & Chen, C.-S. (2024). Big Data Analysis, Design, Effect Fabrication, and Properties Analysis of SiO2/Cr/SiO2 Colored Solar Selective Absorbers with High PTCE and Chromaticity for Building Applications. Materials, 17(23), 5810. https://doi.org/10.3390/ma17235810







