3D-Printed Lithium-Ion Battery Electrodes: A Brief Review of Three Key Fabrication Techniques
Abstract
1. Introduction
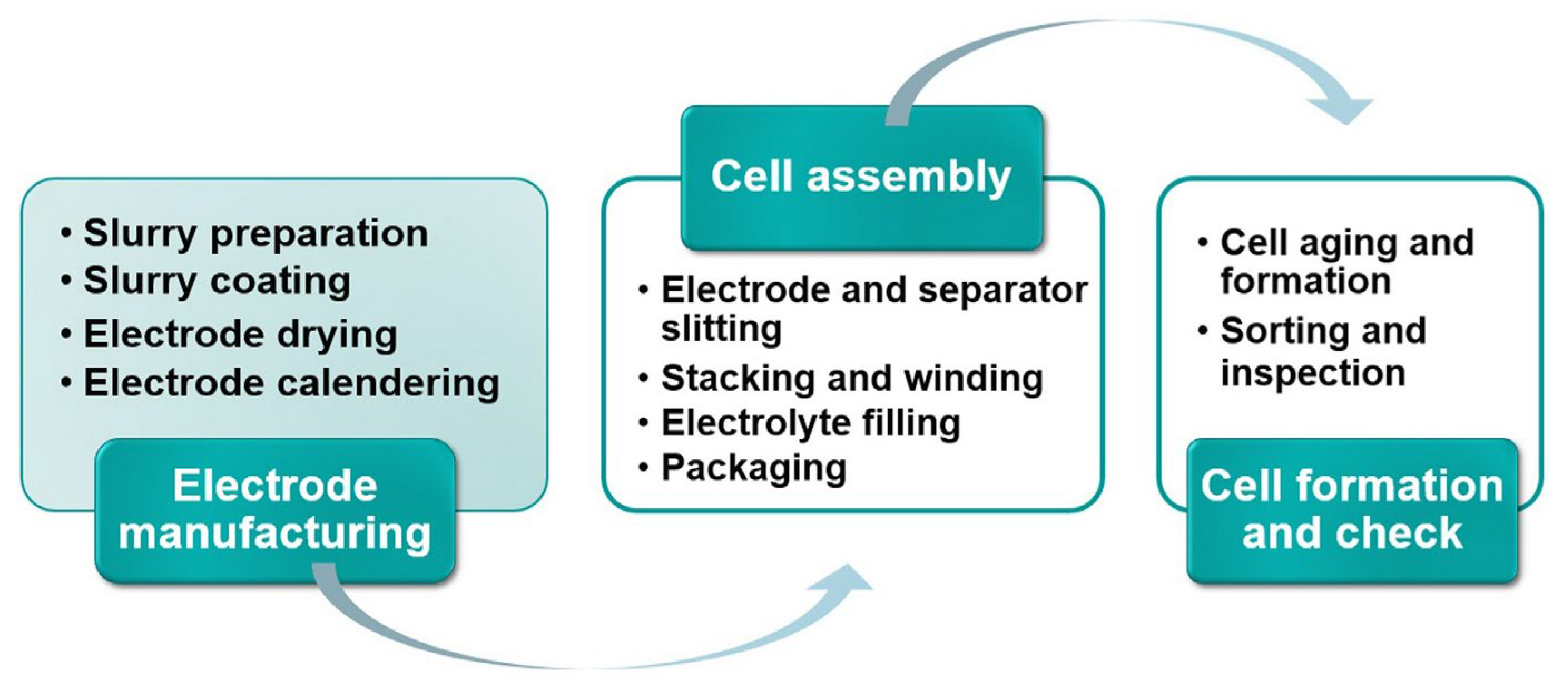
2. Three-Dimensional Printing in Battery Manufacturing: A Step Forward from Traditional Methods
3. Material Extrusion
3.1. Fused Deposition Modeling
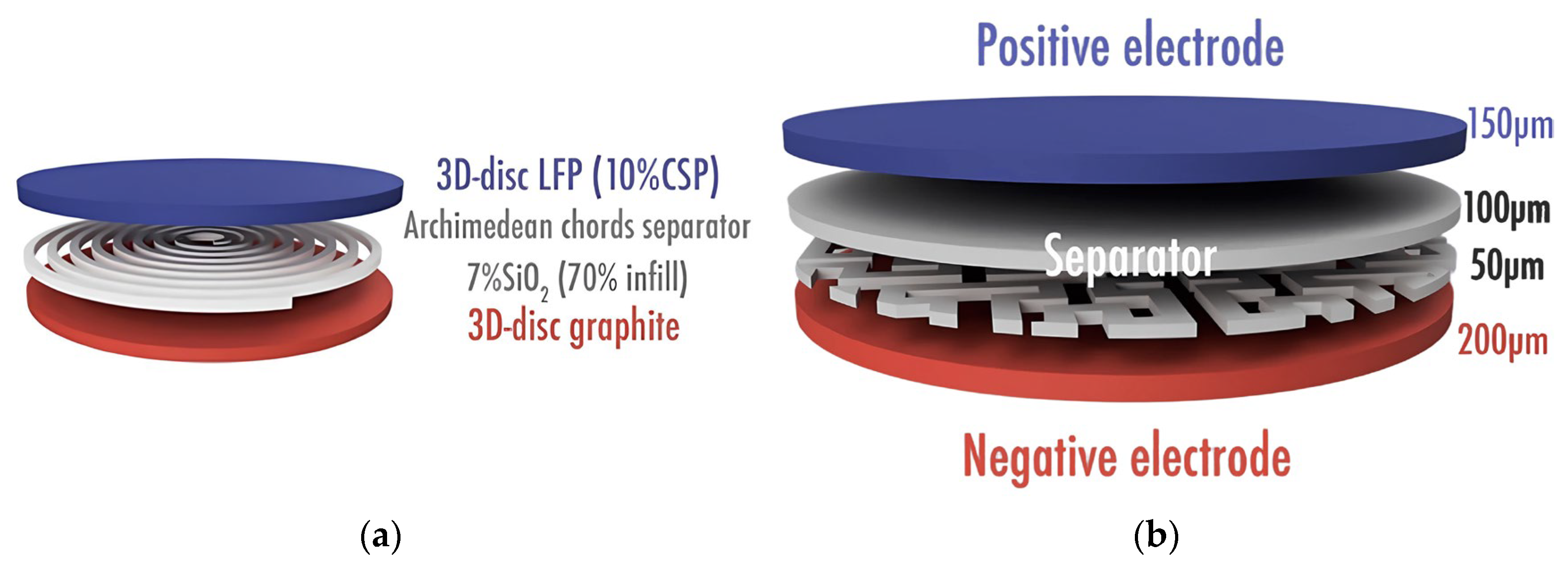
3.2. Direct Ink Writing
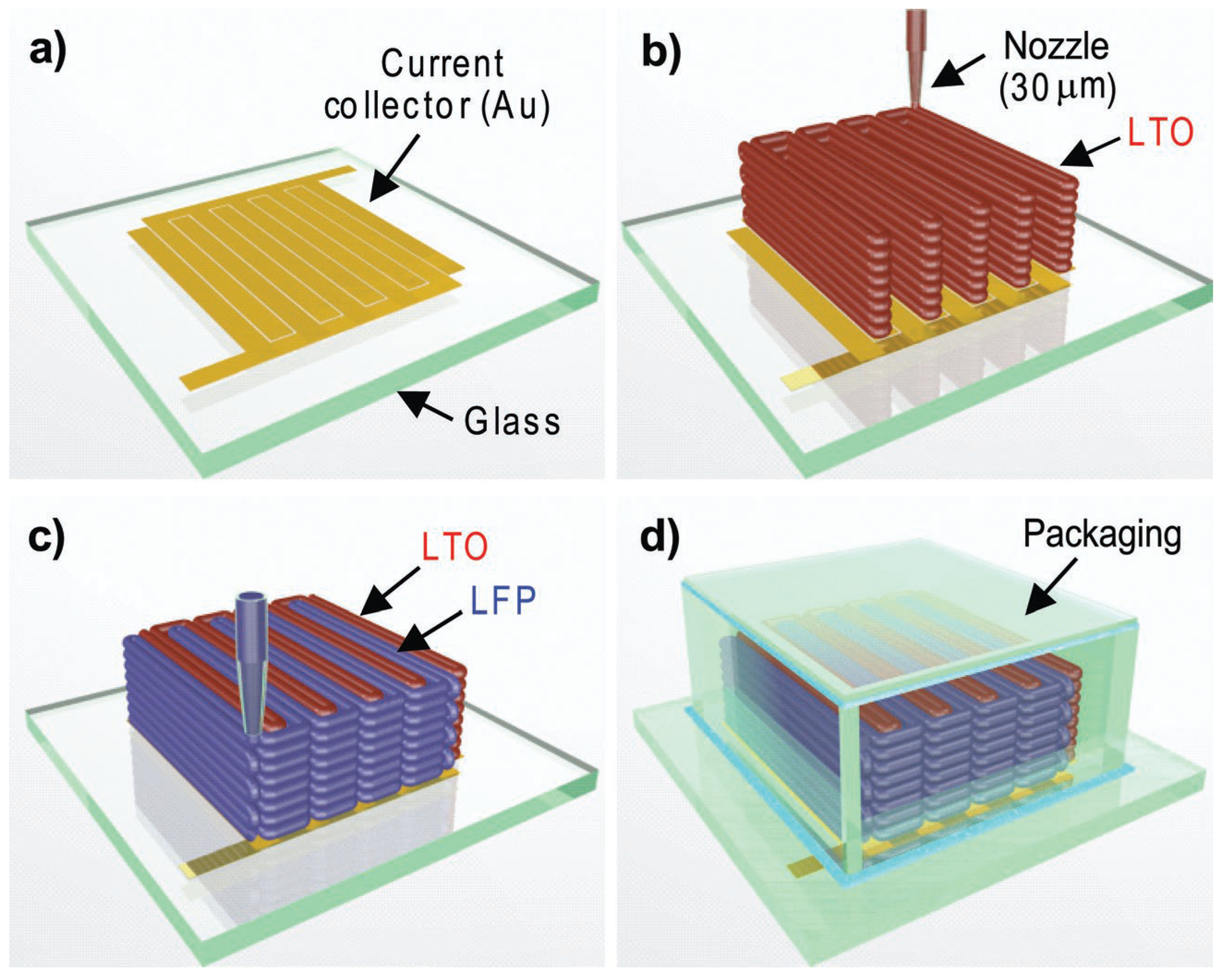
4. Material Jetting
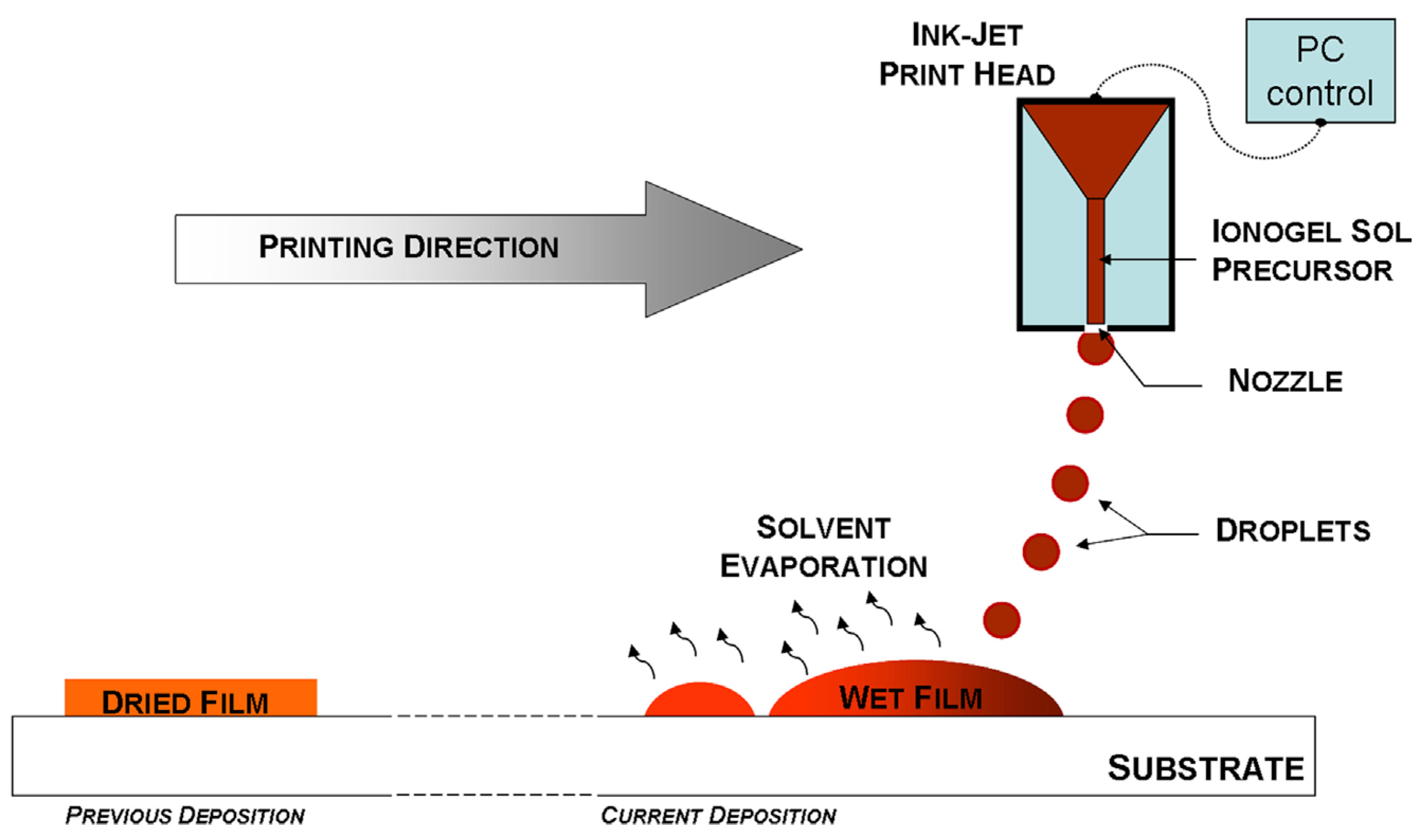
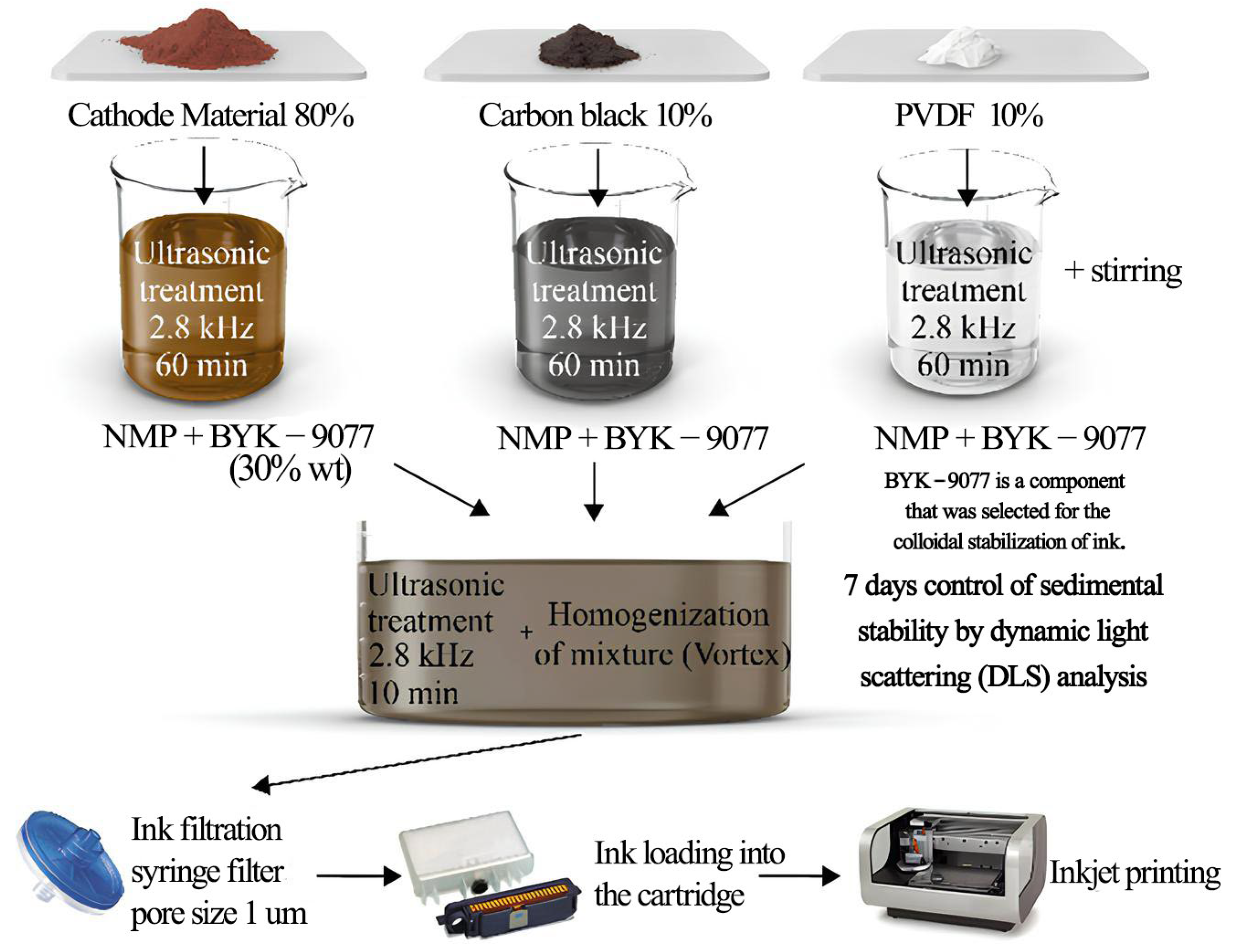
4.1. Inkjet Printing
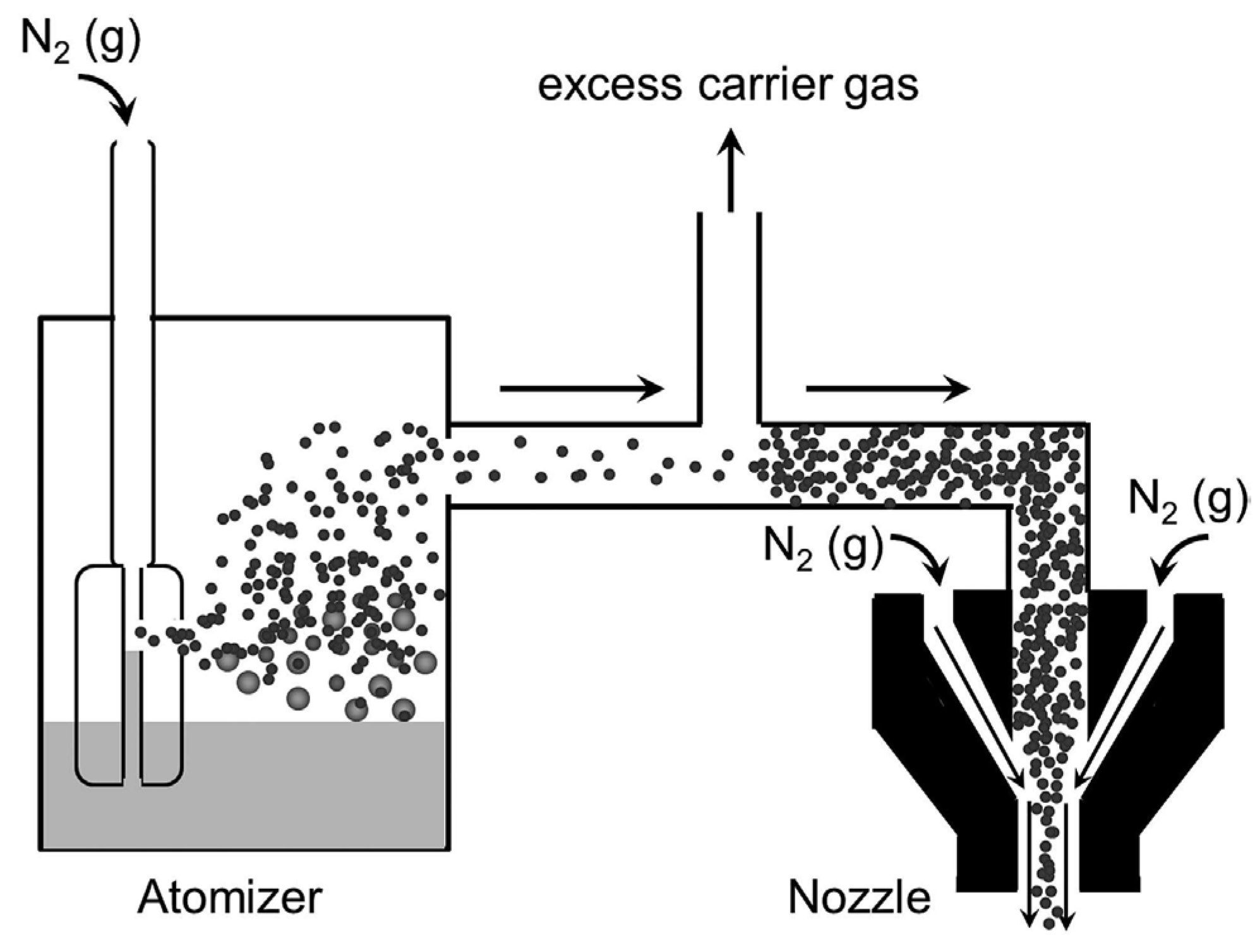
4.2. Aerosol Jet Printing
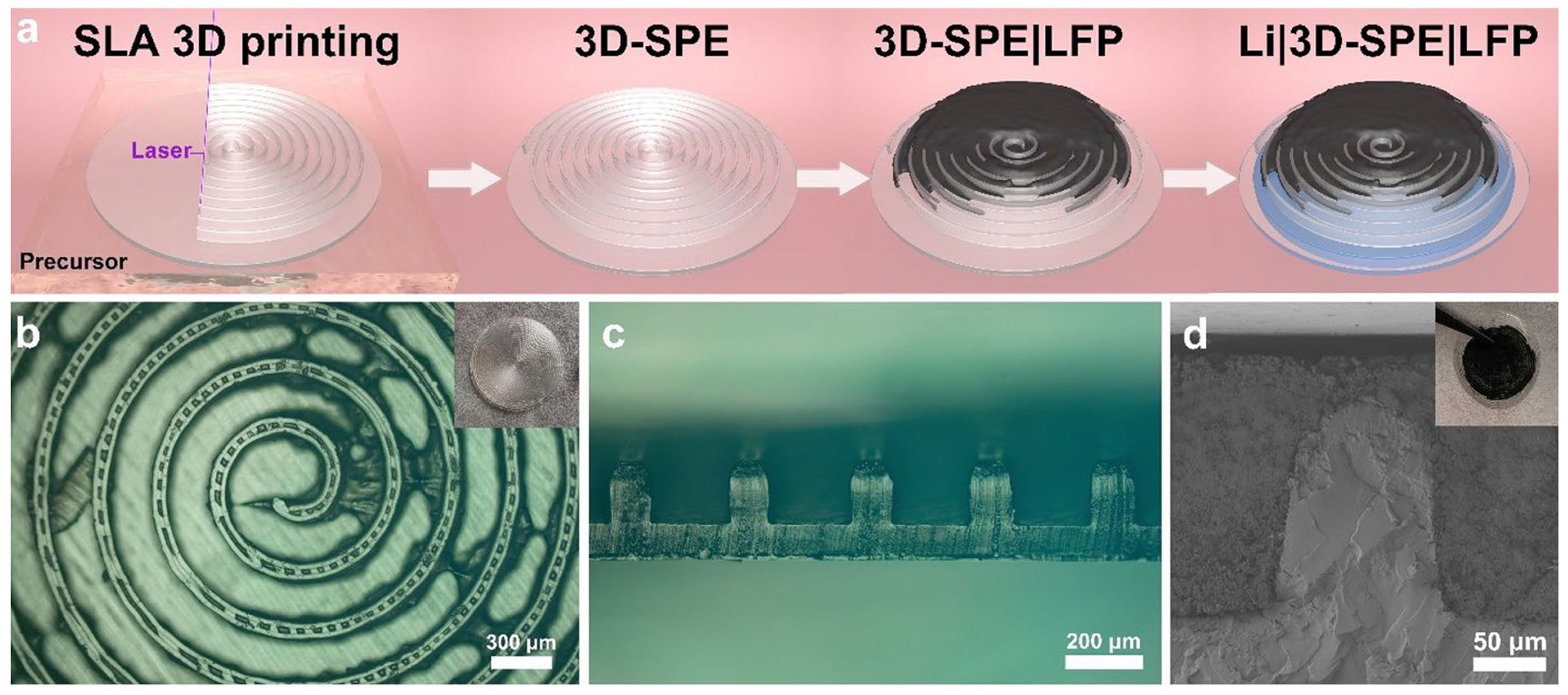
5. Vat Photopolymerization
- The lower viscosity of the material, which can affect the stability and integrity of printed layers;
- The light sensitivity of the material, which may pose difficulties during the curing or solidification step when a laser is used;
- The slower scan rate of the laser, which can reduce the speed of the printing process and increase fabrication time;
- The laser beam’s size, which influences the resolution of the printed patterns, thereby affecting the overall quality of the 3D-printed Li-ion batteries.
6. Performance Comparison, Advantages, and Limitations of the Three Key 3D Printing Techniques
7. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, P.; Fan, H.J. Inkjet and Extrusion Printing for Electrochemical Energy Storage: A Minireview. Adv. Mater. Technol. 2020, 5, 2000217. [Google Scholar] [CrossRef]
- Deiner, L.J.; Reitz, T.L. Inkjet and Aerosol Jet Printing of Electrochemical Devices for Energy Conversion and Storage. Adv. Eng. Mater. 2017, 19, 1600878. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Diouf, B.; Pode, R. Potential of Lithium-Ion Batteries in Renewable Energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Lee, S.; Woo Choi, H.; Lopes Figueiredo, C.; Shin, D.-W.; Mañosa Moncunill, F.; Ullrich, K.; Sinopoli, S.; Jovančić, P.; Yang, J.; Lee, H.; et al. Truly Form-Factor–Free Industrially Scalable System Integration for Electronic Textile Architectures with Multifunctional Fiber Devices. Sci. Adv. 2023, 9, eadf4049. [Google Scholar] [CrossRef]
- Shi, X.; Das, P.; Wu, Z.-S. Digital Microscale Electrochemical Energy Storage Devices for a Fully Connected and Intelligent World. ACS Energy Lett. 2022, 7, 267–281. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering Three-Dimensional Hybrid Supercapacitors and Microsupercapacitors for High-Performance Integrated Energy Storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and Lithium Ion Batteries for Applications in Microelectronic Devices: A Review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, X. Flexible Rechargeable Lithium Ion Batteries: Advances and Challenges in Materials and Process Technologies. J. Mater. Chem. A 2014, 2, 10712–10738. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Integration Issues of Lithium-Ion Battery into Electric Vehicles Battery Pack. J. Clean. Prod. 2016, 113, 1032–1045. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K.; et al. 3D-Printed All-Fiber Li-Ion Battery toward Wearable Energy Storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Deiner, L.J.; Jenkins, T.; Powell, A.; Howell, T.; Rottmayer, M. High Capacity Rate Capable Aerosol Jet Printed Li-ion Battery Cathode. Adv. Eng. Mater. 2019, 21, 1801281. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D Printing Technologies for Electrochemical Energy Storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, Z.-S.; Zhou, F.; Wang, X.; Ma, J.; Liu, C.; He, Y.-B.; Bao, X. All-Solid-State Planar Integrated Lithium Ion Micro-Batteries with Extraordinary Flexibility and High-Temperature Performance. Nano Energy 2018, 51, 613–620. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, F.; Wang, W.; Alexandridis, P.; Zhou, C.; Wu, G. 3D Direct Writing Fabrication of Electrodes for Electrochemical Storage Devices. J. Power Sources 2017, 354, 134–147. [Google Scholar] [CrossRef]
- Cobb, C.L.; Ho, C.C. Additive Manufacturing: Rethinking Battery Design. Electrochem. Soc. Interface 2016, 25, 75. [Google Scholar] [CrossRef]
- Ferrari, S.; Loveridge, M.; Beattie, S.D.; Jahn, M.; Dashwood, R.J.; Bhagat, R. Latest Advances in the Manufacturing of 3D Rechargeable Lithium Microbatteries. J. Power Sources 2015, 286, 25–46. [Google Scholar] [CrossRef]
- Chang, P.; Mei, H.; Zhou, S.; Dassios, K.G.; Cheng, L. 3D Printed Electrochemical Energy Storage Devices. J. Mater. Chem. A 2019, 7, 4230–4258. [Google Scholar] [CrossRef]
- Zhu, P.; Slater, P.R.; Kendrick, E. Insights into Architecture, Design and Manufacture of Electrodes for Lithium-Ion Batteries. Mater. Des. 2022, 223, 111208. [Google Scholar] [CrossRef]
- Heubner, C.; Nikolowski, K.; Reuber, S.; Schneider, M.; Wolter, M.; Michaelis, A. Recent Insights into Rate Performance Limitations of Li-Ion Batteries. Batter. Supercaps 2021, 4, 268–285. [Google Scholar] [CrossRef]
- Gören, A.; Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. State of the Art and Open Questions on Cathode Preparation Based on Carbon Coated Lithium Iron Phosphate. Compos. Part B Eng. 2015, 83, 333–345. [Google Scholar] [CrossRef]
- Hein, S.; Danner, T.; Westhoff, D.; Prifling, B.; Scurtu, R.; Kremer, L.; Hoffmann, A.; Hilger, A.; Osenberg, M.; Manke, I.; et al. Influence of Conductive Additives and Binder on the Impedance of Lithium-Ion Battery Electrodes: Effect of Morphology. J. Electrochem. Soc. 2020, 167, 013546. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Cao, L. Effect of Anode Binders on Low-Temperature Performance of Automotive Lithium-Ion Batteries. J. Power Sources 2019, 441, 227178. [Google Scholar] [CrossRef]
- Maurel, A.; Courty, M.; Fleutot, B.; Tortajada, H.; Prashantha, K.; Armand, M.; Grugeon, S.; Panier, S.; Dupont, L. Highly Loaded Graphite–Polylactic Acid Composite-Based Filaments for Lithium-Ion Battery Three-Dimensional Printing. Chem. Mater. 2018, 30, 7484–7493. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Xu, W.; Zhu, Y.; Kim, D.; Fan, Y.; Yu, B.; Chen, Y. 3D-printed Thermoplastic Polyurethane Electrodes for Customizable, Flexible Lithium-ion Batteries with an Ultra-long Lifetime. Small 2023, 19, 2301604. [Google Scholar] [CrossRef]
- Foster, C.W.; Down, M.P.; Zhang, Y.; Ji, X.; Rowley-Neale, S.J.; Smith, G.C.; Kelly, P.J.; Banks, C.E. 3D Printed Graphene Based Energy Storage Devices. Sci. Rep. 2017, 7, 42233. [Google Scholar] [CrossRef]
- Martinez, A.C.; Aranzola, A.P.; Schiaffino, E.; MacDonald, E.; Maurel, A. Additive Manufacturing of LiCoO2 Electrodes via Vat Photopolymerization for Lithium Ion Batteries. Energy Adv. 2024, 3, 1009–1018. [Google Scholar] [CrossRef]
- Vernardou, D.; Vasilopoulos, K.C.; Kenanakis, G. 3D Printed Graphene-Based Electrodes with High Electrochemical Performance. Appl. Phys. A 2017, 123, 623. [Google Scholar] [CrossRef]
- Foster, C.W.; Zou, G.-Q.; Jiang, Y.; Down, M.P.; Liauw, C.M.; Garcia-Miranda Ferrari, A.; Ji, X.; Smith, G.C.; Kelly, P.J.; Banks, C.E. Next-Generation Additive Manufacturing: Tailorable Graphene/Polylactic(Acid) Filaments Allow the Fabrication of 3D Printable Porous Anodes for Utilisation within Lithium-Ion Batteries. Batter. Supercaps 2019, 2, 448–453. [Google Scholar] [CrossRef]
- He, W.; Chen, C.; Jiang, J.; Chen, Z.; Liao, H.; Dou, H.; Zhang, X. 3D Printed Multilayer Graphite@SiO Structural Anode for High-Loading Lithium-Ion Battery. Batter. Supercaps 2022, 5, e202100258. [Google Scholar] [CrossRef]
- Drews, M.; Tepner, S.; Haberzettl, P.; Gentischer, H.; Beichel, W.; Breitwieser, M.; Vierrath, S.; Biro, D. Towards 3D-Lithium Ion Microbatteries Based on Silicon/Graphite Blend Anodes Using a Dispenser Printing Technique. RSC Adv. 2020, 10, 22440–22448. [Google Scholar] [CrossRef]
- Maurel, A.; Grugeon, S.; Fleutot, B.; Courty, M.; Prashantha, K.; Tortajada, H.; Armand, M.; Panier, S.; Dupont, L. Three-Dimensional Printing of a LiFePO4/Graphite Battery Cell via Fused Deposition Modeling. Sci. Rep. 2019, 9, 18031. [Google Scholar] [CrossRef]
- Ragones, H.; Menkin, S.; Kamir, Y.; Gladkikh, A.; Mukra, T.; Kosa, G.; Golodnitsky, D. Towards Smart Free Form-Factor 3D Printable Batteries. Sustain. Energy Fuels 2018, 2, 1542–1549. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Q.; Liu, L.; Xu, J.; Yan, M.; Jiang, Z. A Novel and Facile Route of Ink-Jet Printing to Thin Film SnO2 Anode for Rechargeable Lithium Ion Batteries. Electrochim. Acta 2006, 51, 2639–2645. [Google Scholar] [CrossRef]
- Xu, F.; Wang, T.; Li, W.; Jiang, Z. Preparing Ultra-Thin Nano-MnO2 Electrodes Using Computer Jet-Printing Method. Chem. Phys. Lett. 2003, 375, 247–251. [Google Scholar] [CrossRef]
- Lawes, S.; Sun, Q.; Lushington, A.; Xiao, B.; Liu, Y.; Sun, X. Inkjet-Printed Silicon as High Performance Anodes for Li-Ion Batteries. Nano Energy 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, A.; Sohn, H.; Nicoletti, C.; Iqbal, Z.; Federici, J.F. Fabrication of Rechargeable Lithium Ion Batteries Using Water-Based Inkjet Printed Cathodes. J. Manuf. Process. 2015, 20, 198–205. [Google Scholar] [CrossRef]
- Delannoy, P.-E.; Riou, B.; Brousse, T.; Le Bideau, J.; Guyomard, D.; Lestriez, B. Ink-Jet Printed Porous Composite LiFePO4 Electrode from Aqueous Suspension for Microbatteries. J. Power Sources 2015, 287, 261–268. [Google Scholar] [CrossRef]
- Lee, J.-H.; Wee, S.-B.; Kwon, M.-S.; Kim, H.-H.; Choi, J.-M.; Song, M.S.; Park, H.B.; Kim, H.; Paik, U. Strategic Dispersion of Carbon Black and Its Application to Ink-Jet-Printed Lithium Cobalt Oxide Electrodes for Lithium Ion Batteries. J. Power Sources 2011, 196, 6449–6455. [Google Scholar] [CrossRef]
- Huang, J.; Yang, J.; Li, W.; Cai, W.; Jiang, Z. Electrochemical Properties of LiCoO2 Thin Film Electrode Prepared by Ink-Jet Printing Technique. Thin Solid Films 2008, 516, 3314–3319. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.-S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of Interdigitated Li-Ion Microbattery Architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef]
- Delannoy, P.-E.; Riou, B.; Lestriez, B.; Guyomard, D.; Brousse, T.; Le Bideau, J. Toward Fast and Cost-Effective Ink-Jet Printing of Solid Electrolyte for Lithium Microbatteries. J. Power Sources 2015, 274, 1085–1090. [Google Scholar] [CrossRef]
- Mouraliraman, D.; Thiagarajan, A.; Deepa, S.; Sriram, G.; Aruchamy, K.; Oh, T.H.; Shin, D. 3D Printed Lithium-Ion Batteries: An in-Depth Examination of the Advancements in Flexibility and Stand-Alone Capability. J. Energy Storage 2024, 81, 110395. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Fu, Y.; Wang, X.; Qin, J.; Wu, Z.-S. The Status and Challenging Perspectives of 3D-Printed Micro-Batteries. Chem. Sci. 2024, 15, 5451–5481. [Google Scholar] [CrossRef]
- Luong, D.X.; Subramanian, A.K.; Silva, G.A.L.; Yoon, J.; Cofer, S.; Yang, K.; Owuor, P.S.; Wang, T.; Wang, Z.; Lou, J.; et al. Laminated Object Manufacturing of 3D-Printed Laser-Induced Graphene Foams. Adv. Mater. 2018, 30, 1707416. [Google Scholar] [CrossRef]
- Krinitcyn, M.; Fu, Z.; Harris, J.; Kostikov, K.; Pribytkov, G.A.; Greil, P.; Travitzky, N. Laminated Object Manufacturing of in-Situ Synthesized MAX-Phase Composites. Ceram. Int. 2017, 43, 9241–9245. [Google Scholar] [CrossRef]
- Azhari, A.; Marzbanrad, E.; Yilman, D.; Toyserkani, E.; Pope, M.A. Binder-Jet Powder-Bed Additive Manufacturing (3D Printing) of Thick Graphene-Based Electrodes. Carbon 2017, 119, 257–266. [Google Scholar] [CrossRef]
- Acord, K.A.; Dupuy, A.D.; Scipioni Bertoli, U.; Zheng, B.; West, W.C.; Chen, Q.N.; Shapiro, A.A.; Schoenung, J.M. Morphology, Microstructure, and Phase States in Selective Laser Sintered Lithium Ion Battery Cathodes. J. Mater. Process. Technol. 2021, 288, 116827. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Wu, B.; Brandon, N.P. Electrical Conductivity and Porosity in Stainless Steel 316L Scaffolds for Electrochemical Devices Fabricated Using Selective Laser Sintering. Mater. Des. 2016, 106, 51–59. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of Selective Laser Melting: Materials and Applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Chong, Y.; Yuan, H.; Huang, J.-Q.; Zhang, Q. Advanced Electrode Processing of Lithium Ion Batteries: A Review of Powder Technology in Battery Fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]
- Lippke, M.; Ohnimus, T.; Frankenberg, F.; Schilde, C.; Kwade, A. Drying and Calendering of Lithium Ion Battery Electrodes: A Combined Simulation Approach. Powder Technol. 2024, 444, 119984. [Google Scholar] [CrossRef]
- Mottaghi, M.; Pearce, J.M. A Review of 3D Printing Batteries. Batteries 2024, 10, 110. [Google Scholar] [CrossRef]
- Fonseca, N.; Thummalapalli, S.V.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Patil, D.; Thippanna, V.; Ramanathan, A.; Xu, W.; Guo, S.; et al. 3D Printing-Enabled Design and Manufacturing Strategies for Batteries: A Review. Small 2023, 19, 2302718. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Rueda, J.R.M.; Fernández-González, R. Three Dimensional Printing of Components and Functional Devices for Energy and Environmental Applications. Energy Environ. Sci. 2017, 10, 846–859. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Q.; Gao, X.; Wang, C.; Li, W.; Holness, F.B.; Zheng, M.; Li, R.; Price, A.D.; Sun, X.; et al. Toward High Areal Energy and Power Density Electrode for Li-Ion Batteries via Optimized 3D Printing Approach. ACS Appl. Mater. Interfaces 2018, 10, 39794–39801. [Google Scholar] [CrossRef]
- Mao, M.; He, J.; Li, X.; Zhang, B.; Lei, Q.; Liu, Y.; Li, D. The Emerging Frontiers and Applications of High-Resolution 3D Printing. Micromachines 2017, 8, 113. [Google Scholar] [CrossRef]
- Lawes, S.; Riese, A.; Sun, Q.; Cheng, N.; Sun, X. Printing Nanostructured Carbon for Energy Storage and Conversion Applications. Carbon 2015, 92, 150–176. [Google Scholar] [CrossRef]
- Håkansson, K.M.O.; Henriksson, I.C.; de la Peña Vázquez, C.; Kuzmenko, V.; Markstedt, K.; Enoksson, P.; Gatenholm, P. Solidification of 3D Printed Nanofibril Hydrogels into Functional 3D Cellulose Structures. Adv. Mater. Technol. 2016, 1, 1600096. [Google Scholar] [CrossRef]
- Bradley, D. Printing a Tiny 3D Battery. Mater. Today 2013, 16, 256. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, R.; He, Z.; Zhao, K.; Pan, L. Printing 3D Gel Polymer Electrolyte in Lithium-Ion Microbattery Using Stereolithography. J. Electrochem. Soc. 2017, 164, A1852. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Asher, E.; Deng, S.; Sun, X.; Yang, J. Paper with Power: Engraving 2D Materials on 3D Structures for Printed, High-Performance, Binder-Free, and All-Solid-State Supercapacitors. Adv. Funct. Mater. 2018, 28, 1803600. [Google Scholar] [CrossRef]
- Cohen, E.; Menkin, S.; Lifshits, M.; Kamir, Y.; Gladkich, A.; Kosa, G.; Golodnitsky, D. Novel Rechargeable 3D-Microbatteries on 3D-Printed-Polymer Substrates: Feasibility Study. Electrochim. Acta 2018, 265, 690–701. [Google Scholar] [CrossRef]
- MacDonald, E.; Wicker, R. Multiprocess 3D Printing for Increasing Component Functionality. Science 2016, 353, aaf2093. [Google Scholar] [CrossRef]
- Rocha, V.G.; García-Tuñón, E.; Botas, C.; Markoulidis, F.; Feilden, E.; D’Elia, E.; Ni, N.; Shaffer, M.; Saiz, E. Multimaterial 3D Printing of Graphene-Based Electrodes for Electrochemical Energy Storage Using Thermoresponsive Inks. ACS Appl. Mater. Interfaces 2017, 9, 37136–37145. [Google Scholar] [CrossRef]
- Zekoll, S.; Marriner-Edwards, C.; Ola Hekselman, A.K.; Kasemchainan, J.; Kuss, C.; Armstrong, D.E.J.; Cai, D.; Wallace, R.J.; Richter, F.H.; Thijssen, J.H.J.; et al. Hybrid Electrolytes with 3D Bicontinuous Ordered Ceramic and Polymer Microchannels for All-Solid-State Batteries. Energy Environ. Sci. 2018, 11, 185–201. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, M.; Wang, S.; Hill, C.; Joshi, P.; Kuruganti, T.; Hu, A. Laser Sintering of Printed Anodes for Al-Air Batteries. J. Electrochem. Soc. 2018, 165, A584. [Google Scholar] [CrossRef]
- Pröll, J.; Kim, H.; Piqué, A.; Seifert, H.J.; Pfleging, W. Laser-Printing and Femtosecond-Laser Structuring of LiMn2O4 Composite Cathodes for Li-Ion Microbatteries. J. Power Sources 2014, 255, 116–124. [Google Scholar] [CrossRef]
- Kim, S.W.; Yun, J.H.; Son, B.; Lee, Y.-G.; Kim, K.M.; Lee, Y.M.; Cho, K.Y. Graphite/Silicon Hybrid Electrodes Using a 3D Current Collector for Flexible Batteries. Adv. Mater. 2014, 26, 2977–2982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chi, B.; Li, B.; Gao, Z.; Du, Y.; Guo, J.; Wei, J. Fabrication of Highly Conductive Graphene Flexible Circuits by 3D Printing. Synth. Met. 2016, 217, 79–86. [Google Scholar] [CrossRef]
- Pei, M.; Shi, H.; Yao, F.; Liang, S.; Xu, Z.; Pei, X.; Wang, S.; Hu, Y. 3D Printing of Advanced Lithium Batteries: A Designing Strategy of Electrode/Electrolyte Architectures. J. Mater. Chem. A 2021, 9, 25237–25257. [Google Scholar] [CrossRef]
- Costa, C.M.; Gonçalves, R.; Lanceros-Méndez, S. Recent Advances and Future Challenges in Printed Batteries. Energy Storage Mater. 2020, 28, 216–234. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Manoj Kumar, K.P.; Bupathi Ram, P.M.; Ravichandran, M.; Vinayagamoorthy, M. A Review on Recent Advancements in Fused Deposition Modeling. Mater. Today Proc. 2020, 27, 752–756. [Google Scholar] [CrossRef]
- Rajan, K.; Samykano, M.; Kadirgama, K.; Harun, W.S.W.; Rahman, M. Fused Deposition Modeling: Process, Materials, Parameters, Properties, and Applications. Int. J. Adv. Manuf. Technol. 2022, 120, 1531–1570. [Google Scholar] [CrossRef]
- Nikzad, M.; Syed, H.M.; Sbarski, I.; Groth, A. A Study of Melt Flow Analysis of an ABS-Iron Composite in Fused Deposition Modelling Process. Tsinghua Sci. Technol. 2009, 14, 29–37. [Google Scholar] [CrossRef]
- Masood, S.H.; Song, W.Q. Development of New Metal/Polymer Materials for Rapid Tooling Using Fused Deposition Modelling. Mater. Des. 2004, 25, 587–594. [Google Scholar] [CrossRef]
- Boudeville, V.; Grugeon, S.; Maurel, A.; Lesieur, R.; Louati, M.; Cayla, A.; Ursescu, S.; Campagne, C.; Panier, S.; Dupont, L. Solvent-Free Extrusion of a LiFePO4-Based Monofilament for Three-Dimensional Printing of a Lithium-Ion Battery Positive Electrode. J. Power Sources 2024, 593, 233973. [Google Scholar] [CrossRef]
- Reyes, C.; Somogyi, R.; Niu, S.; Cruz, M.A.; Yang, F.; Catenacci, M.J.; Rhodes, C.P.; Wiley, B.J. Three-Dimensional Printing of a Complete Lithium Ion Battery with Fused Filament Fabrication. ACS Appl. Energy Mater. 2018, 1, 5268–5279. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Mattevi, C. Mesoscale Design of Multifunctional 3D Graphene Networks. Mater. Today 2016, 19, 428–436. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, Y.; Fang, F.; Fan, Z. Recent Progress on Printable Power Supply Devices and Systems with Nanomaterials. Nano Res. 2018, 11, 3065–3087. [Google Scholar] [CrossRef]
- Sztymela, K. Fabrication of 3D Electrodes for Modern Lithium-Ion Batteries by Inkjet Printing: Opportunities and Challenges. Ph.D. Thesis, University of Limoges, Limoges, France, 2023. [Google Scholar]
- Zhao, J.; Lu, H.; Zhao, X.; Malyi, O.I.; Peng, J.; Lu, C.; Li, X.; Zhang, Y.; Zeng, Z.; Xing, G.; et al. Printable Ink Design towards Customizable Miniaturized Energy Storage Devices. ACS Mater. Lett. 2020, 2, 1041–1056. [Google Scholar] [CrossRef]
- Lacey, S.D.; Kirsch, D.J.; Li, Y.; Morgenstern, J.T.; Zarket, B.C.; Yao, Y.; Dai, J.; Garcia, L.Q.; Liu, B.; Gao, T.; et al. Extrusion-based 3D Printing of Hierarchically Porous Advanced Battery Electrodes. Adv. Mater. 2018, 30, 1705651. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; Liou, F.; Park, J. Macro-/Micro-Controlled 3D Lithium-Ion Batteries via Additive Manufacturing and Electric Field Processing. Sci. Rep. 2018, 8, 1846. [Google Scholar] [CrossRef]
- Li, J.; Leu, M.C.; Panat, R.; Park, J. A Hybrid Three-Dimensionally Structured Electrode for Lithium-Ion Batteries via 3D Printing. Mater. Des. 2017, 119, 417–424. [Google Scholar] [CrossRef]
- Kohlmeyer, R.R.; Blake, A.J.; Hardin, J.O.; Carmona, E.A.; Carpena-Núñez, J.; Maruyama, B.; Berrigan, J.D.; Huang, H.; Durstock, M.F. Composite Batteries: A Simple yet Universal Approach to 3D Printable Lithium-Ion Battery Electrodes. J. Mater. Chem. A 2016, 4, 16856–16864. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, Y.; Kuang, Y.; Fang, D.; Li, T. 3D-Printed Highly Deformable Electrodes for Flexible Lithium Ion Batteries. Energy Storage Mater. 2020, 33, 55–61. [Google Scholar] [CrossRef]
- Izumi, A.; Sanada, M.; Furuichi, K.; Teraki, K.; Matsuda, T.; Hiramatsu, K.; Munakata, H.; Kanamura, K. Development of High Capacity Lithium-Ion Battery Applying Three-Dimensionally Patterned Electrode. Electrochim. Acta 2012, 79, 218–222. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A. Inkjet Printing. In Printing on Polymers; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 231–246. ISBN 978-0-323-37468-2. [Google Scholar]
- Smith, P.J.; Stringer, J. Applications in Inkjet Printing. In Fundamentals of Inkjet Printing; Hoath, S.D., Ed.; VCH Verlag GmbH & CO. KGaA: Weinheim, Germany, 2016; pp. 397–418. [Google Scholar]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing—Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, G.; Liu, L.; Jiang, Z. High-Performance Thin-Film Li4Ti5O12 Electrodes Fabricated by Using Ink-Jet Printing Technique and Their Electrochemical Properties. J. Solid State Electrochem. 2009, 13, 705–711. [Google Scholar] [CrossRef]
- Arcila-Velez, M.R.; Zhu, J.; Childress, A.; Karakaya, M.; Podila, R.; Rao, A.M.; Roberts, M.E. Roll-to-Roll Synthesis of Vertically Aligned Carbon Nanotube Electrodes for Electrical Double Layer Capacitors. Nano Energy 2014, 8, 9–16. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Taberna, P.-L.; Simon, P.; Fabre, N.; Mesnilgrente, F.; Conédéra, V.; Durou, H. Elaboration of a Microstructured Inkjet-Printed Carbon Electrochemical Capacitor. J. Power Sources 2010, 195, 1266–1269. [Google Scholar] [CrossRef]
- Zapka, W. Pros and Cons of Inkjet Technology in Industrial Inkjet Printing. In Handbook of Industrial Inkjet Printing: A Full System Approach; Zapka, W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 1–6. [Google Scholar]
- Goth, C.; Putzo, S.; Franke, J. Aerosol Jet Printing on Rapid Prototyping Materials for Fine Pitch Electronic Applications. In Proceedings of the 2011 IEEE 61st Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 31 May–3 June 2011; pp. 1211–1216. [Google Scholar]
- Nir, M.M.; Zamir, D.; Haymov, I.; Ben-Asher, L.; Cohen, O.; Faulkner, B.; De La Vega, F. Electrically Conductive Inks for Inkjet Printing. In The Chemistry of Inkjet Inks; Magdassi, S., Ed.; World Scientific Publishing Co. Pte. Ltd.: Hackensack, NJ, USA, 2010; pp. 225–254. ISBN 978-981-4470-92-6. [Google Scholar]
- Derby, B. Additive Manufacture of Ceramics Components by Inkjet Printing. Engineering 2015, 1, 113–123. [Google Scholar] [CrossRef]
- Kolchanov, D.S.; Mitrofanov, I.; Kim, A.; Koshtyal, Y.; Rumyantsev, A.; Sergeeva, E.; Vinogradov, A.; Popovich, A.; Maximov, M.Y. Inkjet Printing of Li-Rich Cathode Material for Thin-Film Lithium-Ion Microbatteries. Energy Technol. 2020, 8, 1901086. [Google Scholar] [CrossRef]
- Lawes, S.D. Inkjet Printed Thin Film Electrodes for Lithium-Ion Batteries. Master’s Thesis, The University of Western Ontario, London, ONT, Canada, 2015. [Google Scholar]
- Kushwaha, A.; Jangid, M.K.; Bhatt, B.B.; Mukhopadhyay, A.; Gupta, D. Inkjet-Printed Environmentally Friendly Graphene Film for Application as a High-Performance Anode in Li-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 7911–7921. [Google Scholar] [CrossRef]
- Maximov, M.; Kolchanov, D.; Mitrofanov, I.; Vinogradov, A.; Koshtyal, Y.; Rymyantsev, A.; Popovich, A. Inks Development for 3D Printing Cathode of Li-Ion Microbatteries. Proceedings 2019, 3, 7. [Google Scholar] [CrossRef]
- Viviani, P.; Gibertini, E.; Iervolino, F.; Levi, M.; Magagnin, L. Carbon Additive Effect on the Electrochemical Performances of Inkjet Printed Thin-Film Li4Ti5O12 Electrodes. J. Manuf. Process. 2021, 72, 411–418. [Google Scholar] [CrossRef]
- Wang, Y.; Lubbers, T.; Xia, R.; Zhang, Y.-Z.; Mehrali, M.; Huijben, M.; Elshof, J.E. Ten Printable Two-Dimensional V2O5/MXene Heterostructure Cathode for Lithium-Ion Battery. J. Electrochem. Soc. 2021, 168, 020507. [Google Scholar] [CrossRef]
- Fu, K.; Wang, Y.; Yan, C.; Yao, Y.; Chen, Y.; Dai, J.; Lacey, S.; Wang, Y.; Wan, J.; Li, T.; et al. Graphene Oxide-based Electrode Inks for 3D-printed Lithium-ion Batteries. Adv. Mater. 2016, 28, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Sharma, A.; Bhatt, B.B.; Mukhopadhyay, A.; Gupta, D. Inkjet-Printed Graphene-Modified Aluminum Current Collector for High-Voltage Lithium-Ion Battery. ACS Appl. Energy Mater. 2023, 6, 4168–4178. [Google Scholar] [CrossRef]
- Zhou, S.; Usman, I.; Wang, Y.; Pan, A. 3D Printing for Rechargeable Lithium Metal Batteries. Energy Storage Mater. 2021, 38, 141–156. [Google Scholar] [CrossRef]
- Arora, S.; Abkenar, A.; Jayasinghe, S.; Tammi, K. Chapter 4—Materials and Manufacturing Methods for Advanced Li-Ion Batteries. In Heavy-Duty Electric Vehicles; Butterworth-Heinemann: Oxford, UK, 2021; pp. 69–104. ISBN 978-0-12-818126-3. [Google Scholar]
- Clement, B.; Lyu, M.; Sandeep Kulkarni, E.; Lin, T.; Hu, Y.; Lockett, V.; Greig, C.; Wang, L. Recent Advances in Printed Thin-Film Batteries. Engineering 2022, 13, 238–261. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Xu, J.; Liu, L.; Yang, J.; Jiang, Z.-Y. Thin film LiCoO2 cathode prepared by using ink-jet printing technique and its electrochemical properties. Chem. J. Chin. Univ. 2007, 28, 1122–1125. [Google Scholar]
- Park, S.; Nenov, N.S.; Ramachandran, A.; Chung, K.; Hoon Lee, S.; Yoo, J.; Yeo, J.; Bae, C.-J. Development of Highly Energy Densified Ink for 3D Printable Batteries. Energy Technol. 2018, 6, 2058–2064. [Google Scholar] [CrossRef]
- McOwen, D.W.; Xu, S.; Gong, Y.; Wen, Y.; Godbey, G.L.; Gritton, J.E.; Hamann, T.R.; Dai, J.; Hitz, G.T.; Hu, L.; et al. 3D-printing Electrolytes for Solid-state Batteries. Adv. Mater. 2018, 30, 1707132. [Google Scholar] [CrossRef]
- Wei, T.-S.; Ahn, B.Y.; Grotto, J.; Lewis, J.A. 3D Printing of Customized Li-ion Batteries with Thick Electrodes. Adv. Mater. 2018, 30, 1703027. [Google Scholar] [CrossRef]
- Ko, S.H. Low Temperature Thermal Engineering of Nanoparticle Ink for Flexible Electronics Applications. Semicond. Sci. Technol. 2016, 31, 073003. [Google Scholar] [CrossRef]
- Wilkinson, N.J.; Smith, M.A.A.; Kay, R.W.; Harris, R.A. A Review of Aerosol Jet Printing—A Non-Traditional Hybrid Process for Micro-Manufacturing. Int. J. Adv. Manuf. Technol. 2019, 105, 4599–4619. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Pham, H.; Sarkar, S.; Ludwig, B.; Chen, I.-M.; Everhart, W.; Park, J.; Wang, Y.; Pan, H. Customizable Nonplanar Printing of Lithium-ion Batteries. Adv. Mater. Technol. 2019, 4, 1900645. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Chartrain, N.; Meenakshisundaram, V.; Feller, K.D.; Williams, C.B.; Long, T.E. 110th Anniversary: Vat Photopolymerization-Based Additive Manufacturing: Current Trends and Future Directions in Materials Design. Ind. Eng. Chem. Res. 2019, 58, 15109–15118. [Google Scholar] [CrossRef]
- Martinez, A.C.; Schiaffino, E.M.; Aranzola, A.P.; Fernandez, C.A.; Seol, M.-L.; Sherrard, C.G.; Jones, J.; Huddleston, W.H.; Dornbusch, D.A.; Sreenivasan, S.T.; et al. Multiprocess 3D Printing of Sodium-Ion Batteries via Vat Photopolymerization and Direct Ink Writing. J. Phys. Energy 2023, 5, 045010. [Google Scholar] [CrossRef]
- Chartier, T.; Chaput, C.; Doreau, F.; Loiseau, M. Stereolithography of Structural Complex Ceramic Parts. J. Mater. Sci. 2002, 37, 3141–3147. [Google Scholar] [CrossRef]
- He, Y.; Chen, S.; Nie, L.; Sun, Z.; Wu, X.; Liu, W. Stereolithography Three-Dimensional Printing Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. Nano Lett. 2020, 20, 7136–7143. [Google Scholar] [CrossRef]
- Sabato, A.G.; Eroles, M.N.; Anelli, S.; Sierra, C.D.; Gonzalez-Rosillo, J.C.; Torrell, M.; Pesce, A.; Accardo, G.; Casas-Cabanas, M.; López-Aranguren, P.; et al. 3D Printing of Self-Supported Solid Electrolytes Made of Glass-Derived Li1.5Al0.5Ge1.5P3O12 for All-Solid-State Lithium-Metal Batteries. J. Mater. Chem. A 2023, 11, 13677–13686. [Google Scholar] [CrossRef]
- Hensleigh, R.M.; Cui, H.; Oakdale, J.S.; Ye, J.C.; Campbell, P.G.; Duoss, E.B.; Spadaccini, C.M.; Zheng, X.; Worsley, M.A. Additive Manufacturing of Complex Micro-Architected Graphene Aerogels. Mater. Horiz. 2018, 5, 1035–1041. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Xu, X.; Zhou, C.; Lin, D. Three-Dimensional Printing Hollow Polymer Template-Mediated Graphene Lattices with Tailorable Architectures and Multifunctional Properties. ACS Nano 2018, 12, 1096–1106. [Google Scholar] [CrossRef]
- Wang, K.; Zou, W.; Quan, B.; Yu, A.; Wu, H.; Jiang, P.; Wei, Z. An All-Solid-State Flexible Micro-supercapacitor on a Chip. Adv. Energy Mater. 2011, 1, 1068–1072. [Google Scholar] [CrossRef]
- Truby, R.L.; Lewis, J.A. Printing Soft Matter in Three Dimensions. Nature 2016, 540, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Deivanayagam, R.; Shahbazian-Yassar, R. 3D Printing of Electrochemical Energy Storage Devices: A Review of Printing Techniques and Electrode/Electrolyte Architectures. Batter. Supercaps 2020, 3, 130–146. [Google Scholar] [CrossRef]
- Maurel, A.; Martinez, A.C.; Grugeon, S.; Panier, S.; Dupont, L.; Cortes, P.; Sherrard, C.G.; Small, I.; Sreenivasan, S.T.; Macdonald, E. Toward High Resolution 3D Printing of Shape-Conformable Batteries via Vat Photopolymerization: Review and Perspective. IEEE Access 2021, 9, 140654–140666. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat Photopolymerization of Polymers and Polymer Composites: Processes and Applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
| Technique | Characteristics in Battery and Component Fabrication |
|---|---|
| Traditional Methods | Complex, multi-step process Time-consuming and labor-intensive Limited control over microstructure and active material loading High variability in performance consistency Requires separate equipment for each step Limited design flexibility for complex shapes |
| 3D Printing (AM) | Simplifies fabrication with a single setup Reduces time and labor Precise control over microstructure and material loading Enhances performance consistency Minimal waste, eco-friendly Enables complex, customizable geometries |
| Printed Structural Component | Ink Composition | The Electrochemical Properties | Printer | Refs Year | |
|---|---|---|---|---|---|
| Cathode | LCO thin-film electrode | LCO (active cathode material) + AB (conducting agent) + CH10B/CH12B (polymeric hyper dispersants) + SCMC (binder). Mixed solvent was used. | Initial discharge capacity of 81 mAhg−1 at a discharge current of 20 A/cm2 between 3.0 and 4.2 V; 87% capacity retention after 50 charge–discharge cycles. | Canon BJC-1000sp (Canon Inc., Tokyo, Japan) | [114] 2007 |
| Round-shaped thin-film LCO electrode with a printed layer thickness of 1.2 μm (30 printing bands) | 300 mg LCO (active cathode material) + 10 mL deionized water (solvent) + 1.5 mL of a 2 mg/mL commercial surfactant solution + 15 mg of conductive carbon black + 1 mL of monoethanolamine (PH adjustment) + 1.5 mg of SCMC (binder). | Initial discharge capacity of 120 mAhg−1 at a discharge current density of 180 μA/cm2; 95% capacity retention after 100 charge–discharge cycles. | Canon BJC-1000sp (Canon Inc., Tokyo, Japan) | [43] 2008 | |
| LCO thin-film electrode | 93 wt. % of active cathode material, 3 wt. % CB and 4 wt. % PVDF binder. | The discharge capacities for the electrodes using conventional, UV/ozone, and UV/ozone–TETA-treated CB were 121.6, 128.2, and 140.8 mAhg−1, respectively. | An ink-jet printer (Fujifilm Dimatix Inc., Santa Clara, CA, USA) | [42] 2011 | |
| LCO electrode | LCO + Cured binder + Super P (80:10:10 wt. %). Novel acrylate-based curable inks was used. | Initial discharge capacity of 147.8 mAhg−1 at a discharge current of 0.1C. | − | [115] 2018 | |
| Printed LFP electrodes with a thickness of 20 μm, including the current collector | LFP/C (active cathode material) + CB (conductive agent) + SCMC (binder) (80:10:10 wt. %) + buffer solution (HCl + NaOH) used as solvent + triton X-100 (surfactant) + glycerin (viscosity adjustment). | Initial discharge capacities of 129.9 mAhg−1 and 151.3 mAhg−1 at a discharge current rate of 0.1C (1C = 150 mAhg−1), using Al and CNT paper current collectors, respectively. | Dimatix-2800 (Fujifilm Dimatix, Inc., Santa Clara, CA, USA) | [40] 2015 | |
| LFP-based composite porous electrodes with a thickness of 4 μm (40 printing bands) | LFP/C (active cathode material) + CB (conductive agent) + PAMA (binder) (85:10:5 wt. %). Deionized water was used as a solvent. | Discharge capacities of 80 mAhg−1 at a current rate of 9C and 70 mAhg−1 at 90C using organic electrolyte. Discharge capacity of 63 mAhg−1 at a current rate of 9C using the ionic liquid-based electrolyte. | Piezoelectric ink-jet printer (Fujifilm Dimatix, Inc., Santa Clara, CA, USA) | [41] 2015 | |
| Printed 1.20 NCM and 1.25 NCM electodes | Li-rich cathode active material + CB (conductive agent) + PVDF (binder) (80:10:10 wt. %). NMP was used as a solvent. Different additives such as ethylene glycol, diethylene glycol, propylene glycol were studied. | Both electrodes have a discharge capacity of more than 250 mAhg−1 at a current rate of 0.1C between 2.5 and 4.8V. | Dimatix DMP-2831 inkjet printer (Fujifilm Dimatix, Inc., Santa Clara, CA, USA) | [106] 2019 | |
| LMR-K with a thickness of 7 μm | Li-rich cathode active material + CB (conductive agent) + PVDF (binder) (80:10:10 wt. %). NMP was used as a solvent. | Initial discharge capacity of 240 mAhg−1 at a current rate of 0.01C. | Dimatix DMP-2831 inkjet printer (Fujifilm Di-matix, Inc., Santa Clara, CA, USA) | [103] 2020 | |
| 2D heterostructure electrode V2O5 /MXene | V2O5: conductive agent: binder = 70:20:10 wt. %. A mass ratio of 80:20 for V2O5/ Ti3C2Tx was used. | Initial discharge capacity of 321 mAhg−1 at a current rate of 1C; 91.8% capacity retention after 680 charge–discharge cycles. | Dimatix DMP-2800 inkjet printer (Fujifilm Dimatix, Inc., Santa Clara, CA, USA) | [108] 2021 | |
| Solid Electrolyte | Li7La3Zr2O12 solid electrolyte | LLZ active material + n-butanol + alpha-terpineol + PVB binder + BBP plasticizer (30% solid loading). | Average overpotential about 2.3 mV at a current density of 0.1 mAcm−2; area-specific resistance of 22 Ω cm−2. | nScrypt 3Dn-300 printer (Tabletop-3Dn, nScrypt Inc., Orlando, FL, USA) | [116] 2018 |
| Full cell | 3D interdigitated microbattery architectures (3D-IMA) | LTO/LFP + glycerol, ethylene glycol, hydroxypropyl cellulose, hydroxyethyl cellulose (solid loading: 55-65 wt. %). | The areal capacity of the packaged 3D-IMA was 1.2 mAhcm−2 at a current rate of 0.5C. | − | [44] 2013 |
| Fully 3D printed and packaged LIBs | Cathode: 30 vol. % LFP + 1.25 vol. % KB (conductive agent) + 1 wt. % PVP (nonionic dispersant) + LiTFSI/PC; Anode: 30 vol. % LTO + 1.35 vol. % KB (conductive agent) + 1 wt. % PVP (nonionic dispersant) + LiTFSI/PC; Separator: PC + triton TX-100 + Al2O3 + HMMP solution + LiTFSI/PC; Package: SiO2 + epoxy (4:96 vol. %). | The areal capacity of 4.45 mAhcm−2 at a current density of 0.14 mAcm−2. | Custom-made 3D printer | [117] 2018 | |
| Cathode: LFP/rGO (70:30 wt. %); Anode: LTO/rGO (70:30 wt. %); Electrolyte: PVDF-co-HFP + Al2O3. | ~100 mAhg−1 at 50 mAg−1. | - | [109] 2016 | ||
| Printing Technique | Resolution (µm) | Advantages | Limitations |
|---|---|---|---|
| Material Extrusion |
50–200 (FDM) 1–250 (DIW) |
|
|
| Material Jetting | 5–200 (IJP) |
|
|
| Vat Photopolymerization | 10–25 (SLA) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlovskii, A.A.; Pushnitsa, K.; Kosenko, A.; Novikov, P.; Popovich, A.A. 3D-Printed Lithium-Ion Battery Electrodes: A Brief Review of Three Key Fabrication Techniques. Materials 2024, 17, 5904. https://doi.org/10.3390/ma17235904
Pavlovskii AA, Pushnitsa K, Kosenko A, Novikov P, Popovich AA. 3D-Printed Lithium-Ion Battery Electrodes: A Brief Review of Three Key Fabrication Techniques. Materials. 2024; 17(23):5904. https://doi.org/10.3390/ma17235904
Chicago/Turabian StylePavlovskii, Alexander A., Konstantin Pushnitsa, Alexandra Kosenko, Pavel Novikov, and Anatoliy A. Popovich. 2024. "3D-Printed Lithium-Ion Battery Electrodes: A Brief Review of Three Key Fabrication Techniques" Materials 17, no. 23: 5904. https://doi.org/10.3390/ma17235904
APA StylePavlovskii, A. A., Pushnitsa, K., Kosenko, A., Novikov, P., & Popovich, A. A. (2024). 3D-Printed Lithium-Ion Battery Electrodes: A Brief Review of Three Key Fabrication Techniques. Materials, 17(23), 5904. https://doi.org/10.3390/ma17235904






