Efficient Management of Asbestos Waste Through Utilization as Mineral Additives in Portland Cement Production
Highlights
- Asbestos waste can be effectively used as mineral additives in cement production, supporting waste management.
- Cement enriched with asbestos additives shows durable mechanical properties similar to traditional cements.
- The use of processed asbestos waste reduces raw material consumption and emissions in cement production.
- Innovative use of asbestos supports eco-friendly construction development and safe waste disposal.
- This method of asbestos utilization ensures complete health safety, fully eliminating toxicity risks.
Abstract
1. Introduction
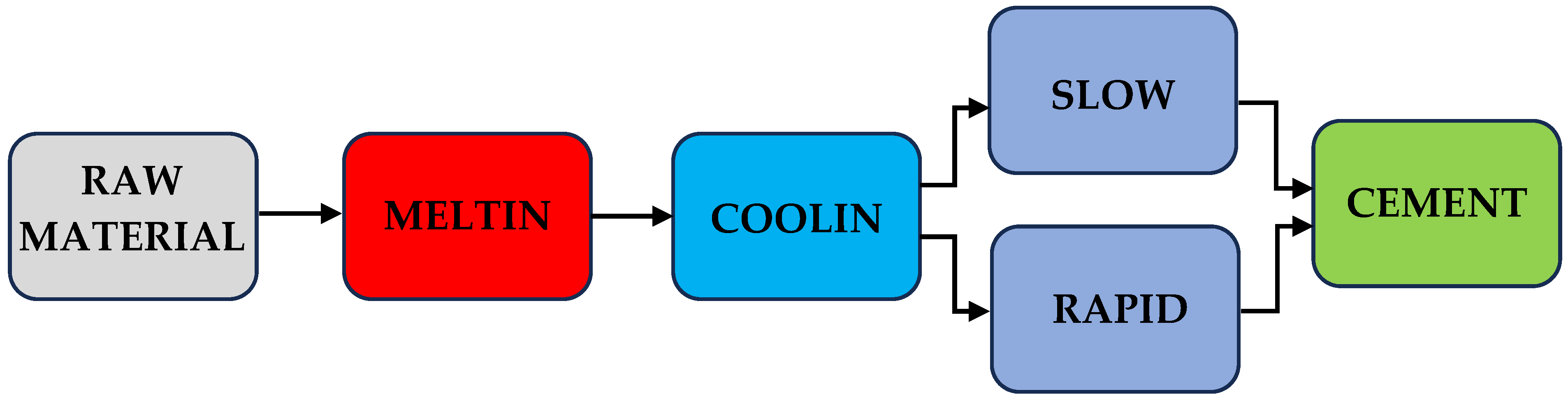
2. Methods
2.1. Cement Synthesis
2.2. XRD Analysis
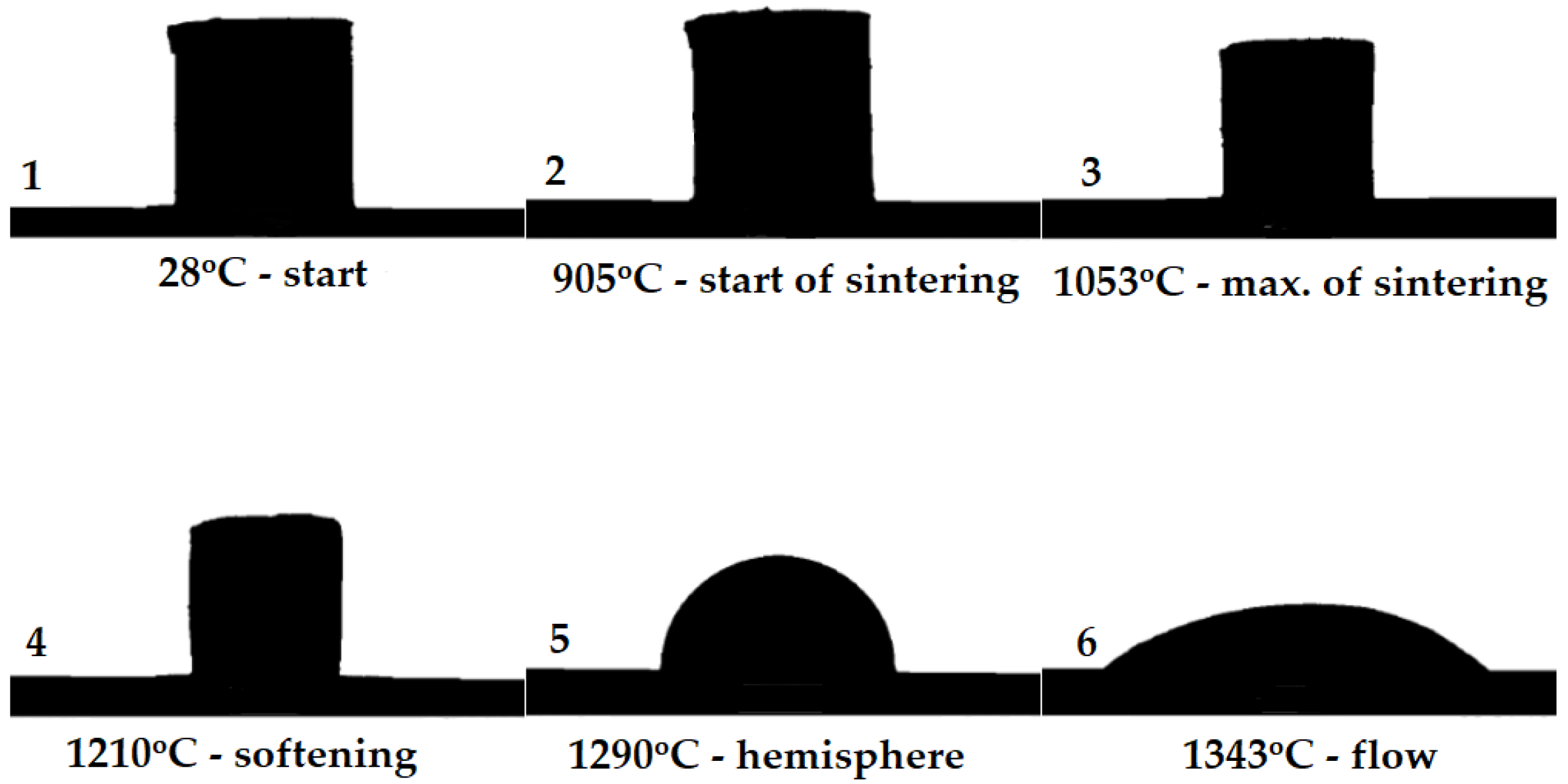
2.3. High-Temperature Microscopy
2.4. Compressive Strength of Cements
2.5. Studies on the Shrinkage and Expansion of Cements
3. Results and Discussion
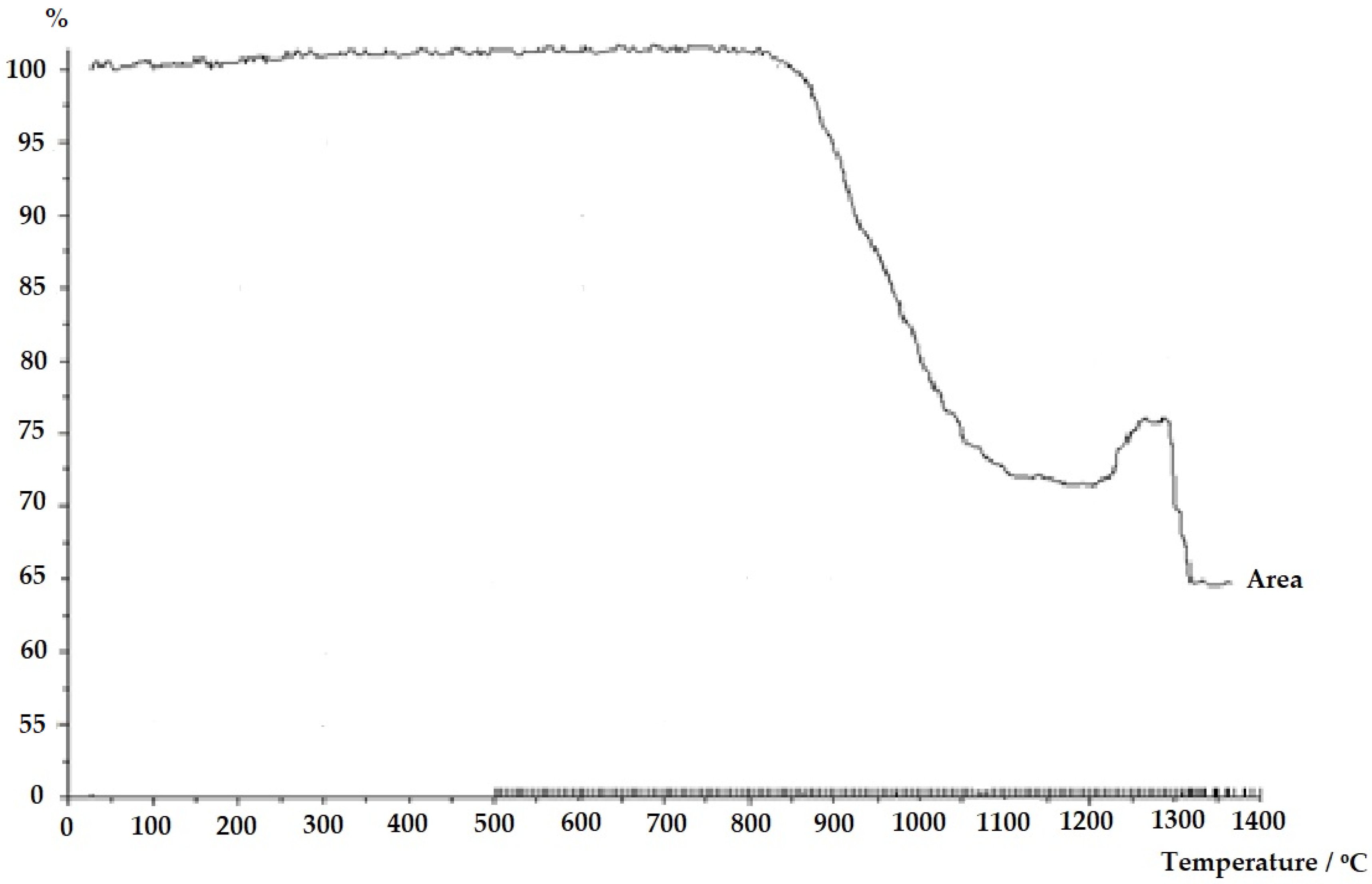
3.1. Results of Chemical Analysis of Raw Materials
- CaCO3—43.73%, due to the decomposition of calcium carbonate into calcium oxide (CaO) and carbon dioxide (CO2),
- SiO2—1.44%, indicating minimal losses primarily associated with the presence of adsorbed moisture or minor impurities,
- Asbestos cement—29.4%, suggesting significant mass loss due to the thermal decomposition of asbestos and other volatile components in the material.
3.2. Results of High-Temperature Microscopy
3.3. Chemical Analysis of Clinker
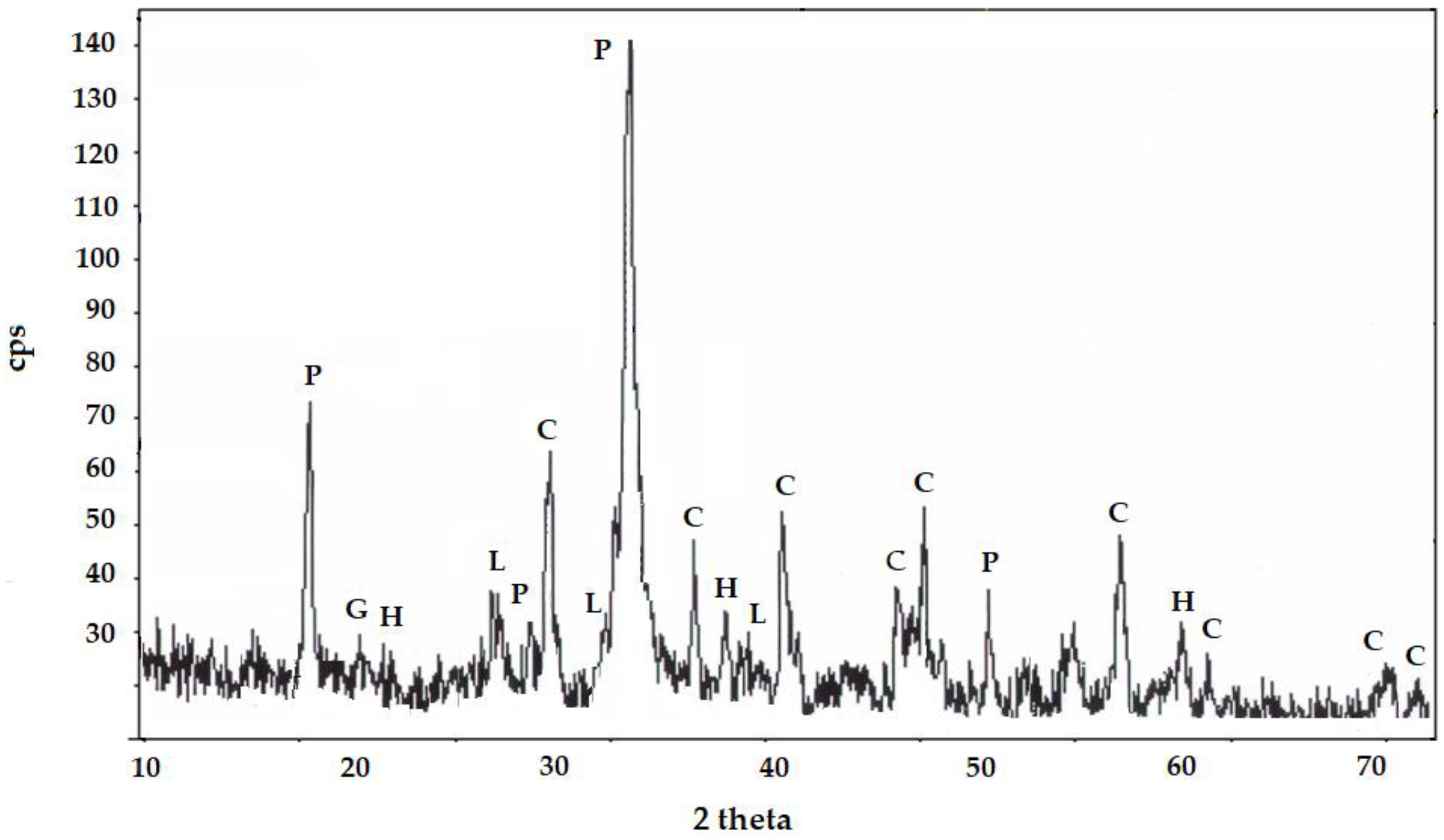
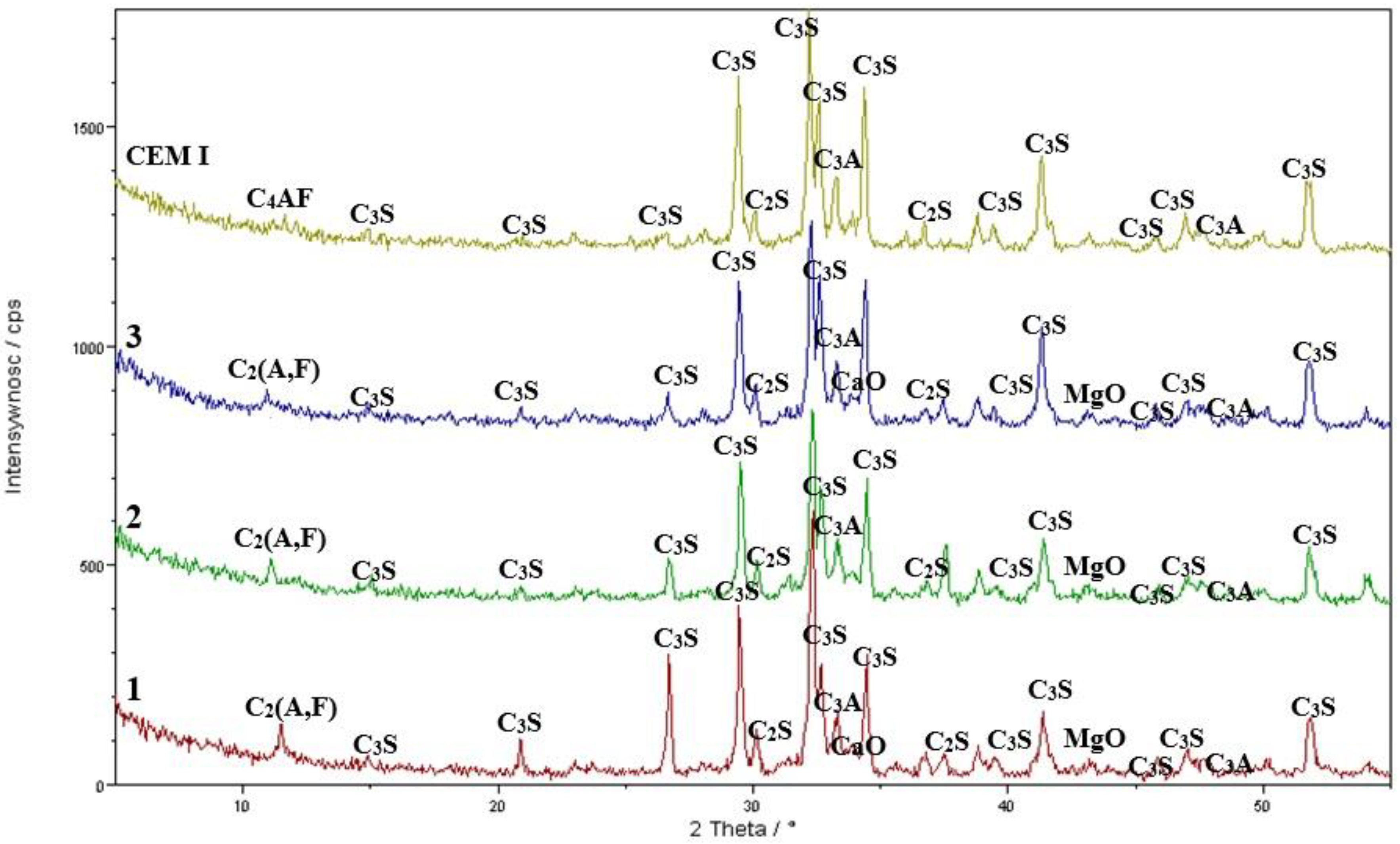
3.4. XRD Results—Cement Synthesis
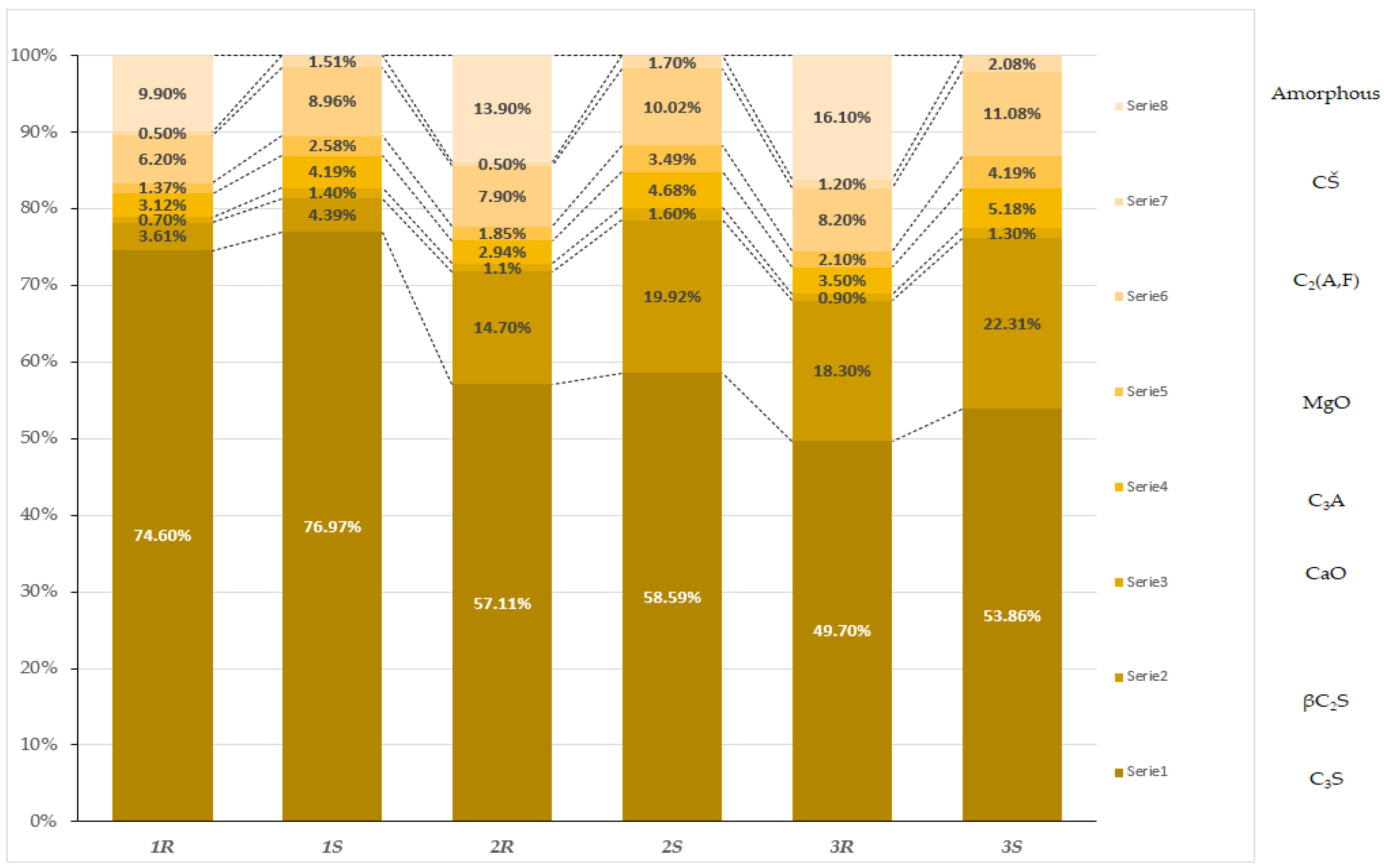
Quantitative Composition Analysis
3.5. Results of Physicochemical Properties of Cements
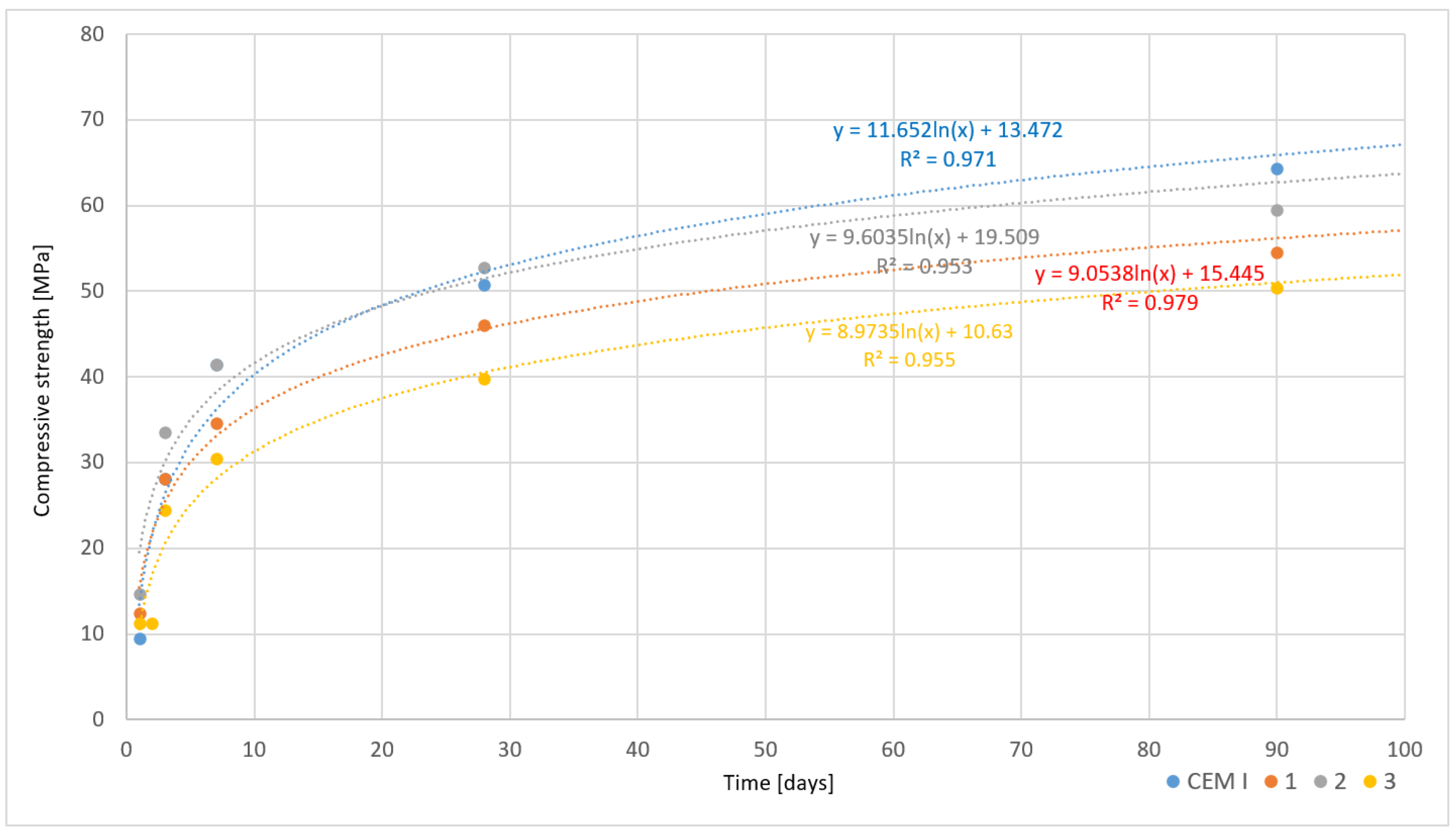
3.5.1. Slowly Cooled Samples
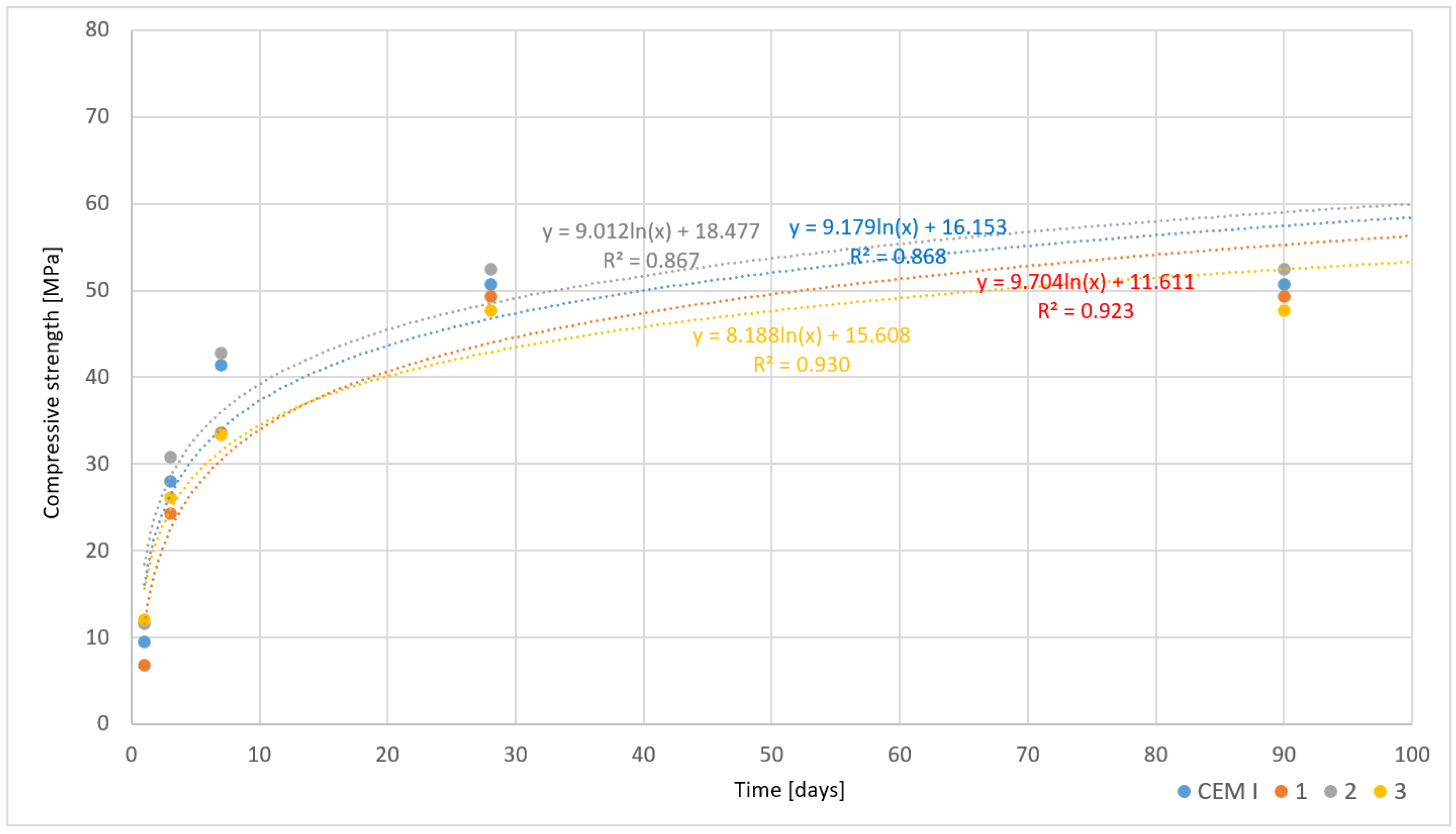
3.5.2. Rapidly Cooled Samples
4. Conclusions
- Cements modified with asbestos waste achieved a compressive strength comparable to CEM I cements. The compressive strength after 90 days was close to the values for CEM I, being lower by 2–15% depending on the cooling method applied. Additionally, cements containing asbestos demonstrated potential for use in construction due to maintaining standard physico-chemical parameters.
- The cooling method of the clinkers significantly affected the hydraulic and mineralogical properties of the cements. Slow cooling promoted a higher content of tricalcium silicate (C3S), reaching up to 76.97%, while rapid cooling favored a higher content of amorphous phases (ranging from 9.9% to 16.1%). Amorphous phases can intensify the hydration process, leading to distinct mechanical and volumetric properties of the cement.
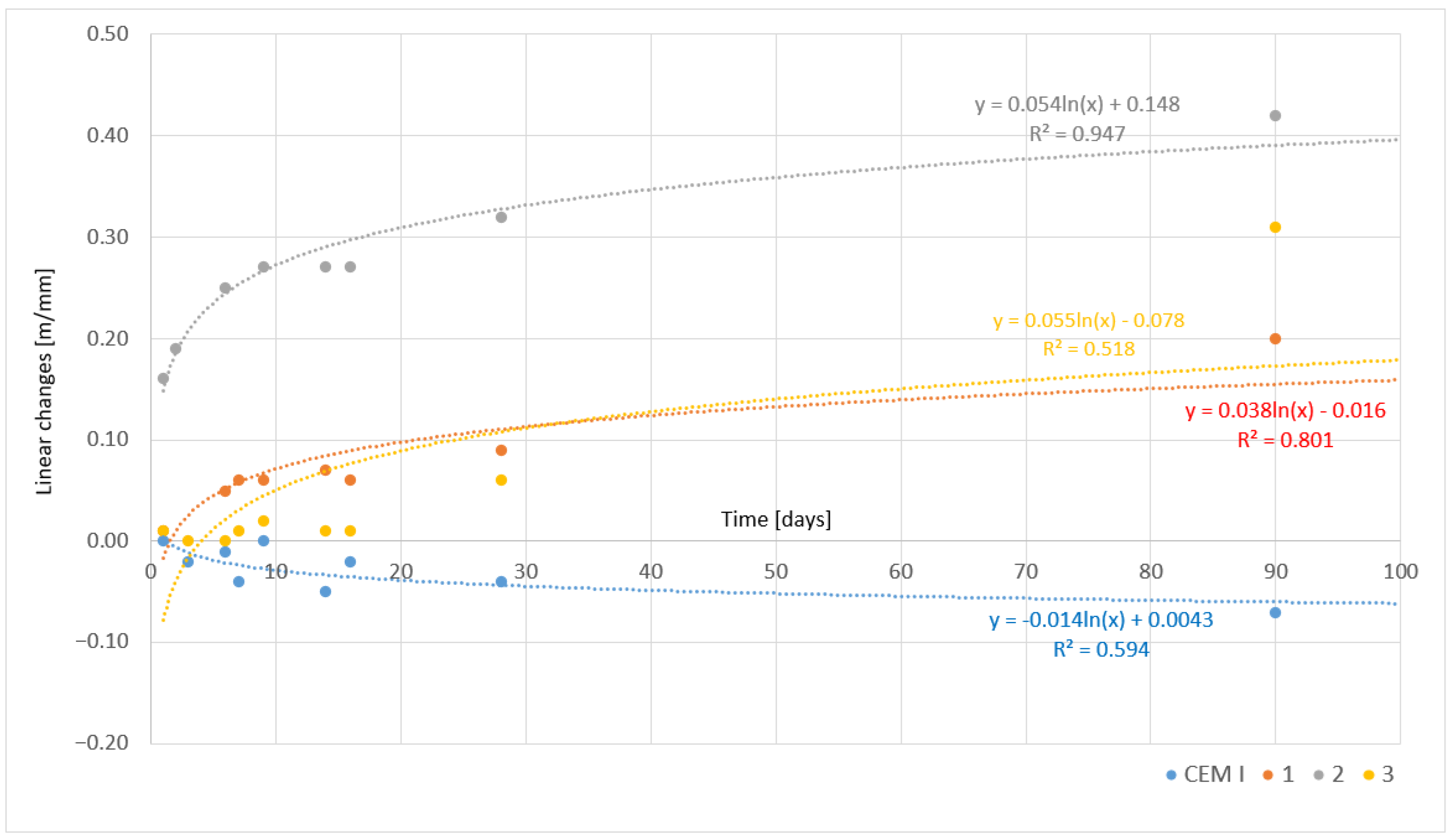
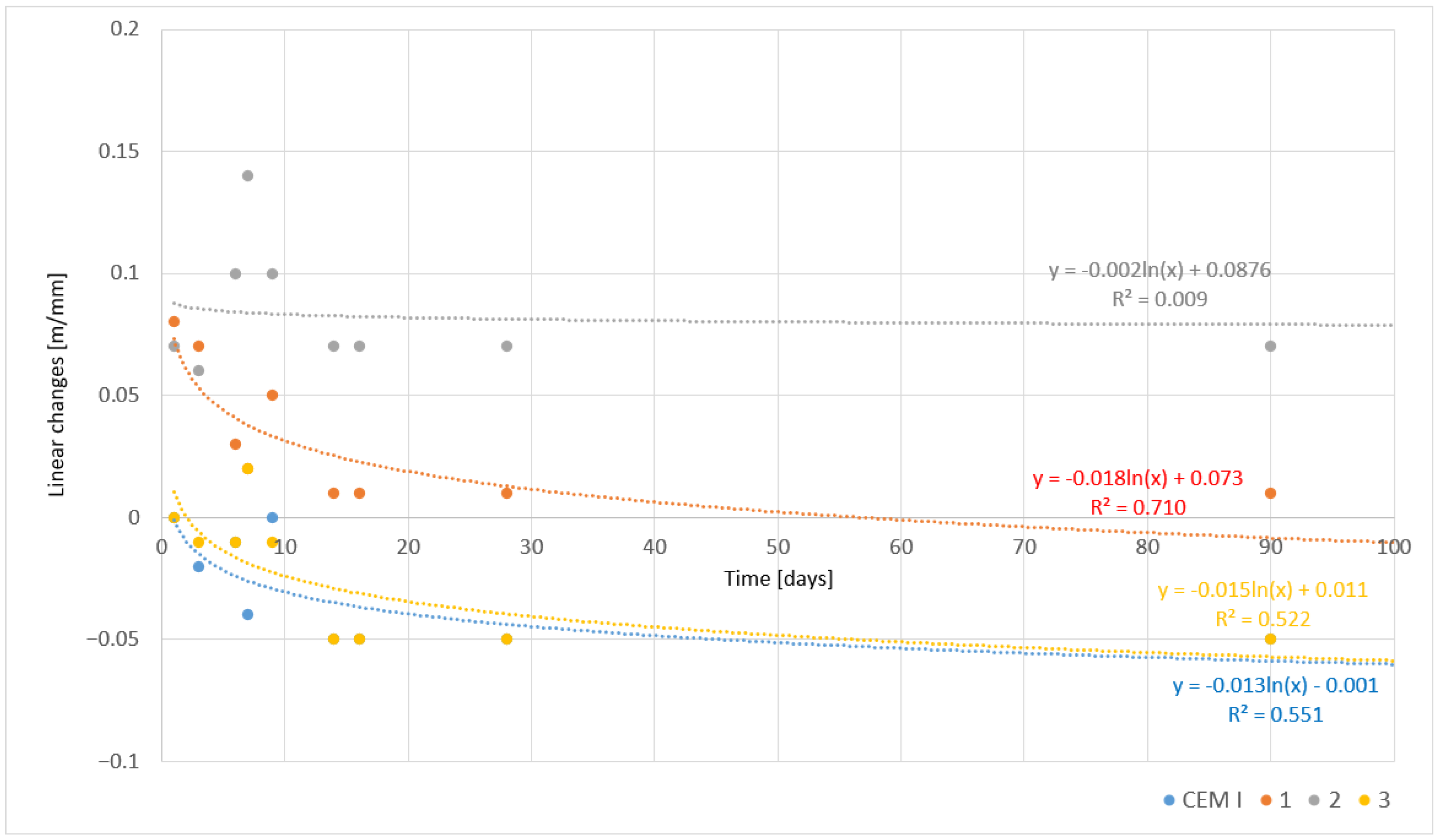
- Asbestos-modified cements exhibited varying linear changes, from shrinkage of −0.07 mm/m (CEM I) to expansion of 0.42 mm/m (sample 2). This phenomenon resulted from the use of different clinker cooling methods, which influenced the phase composition and hydration processes.
- The implementation of asbestos waste, particularly chrysotile, in Portland cement production contributes to reducing the demand for natural raw materials and lowering CO2 emissions, making this process environmentally and economically beneficial. Research confirms that asbestos waste can be effectively utilized as a mineral additive, enabling the production of cements with mechanical properties comparable to conventional cements. Furthermore, the elimination of asbestos fibers from circulation significantly improves health safety while supporting sustainable development and effective waste management.
- It is recommended to conduct detailed studies on the hydraulic and chemical activity of cements modified with asbestos waste, as well as their long-term environmental and health impacts. Analyzing calorimetric and hydration properties will allow for a deeper understanding of the potential of these materials in various industrial applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kusiorowski, R.; Gerle, A.; Kujawa, M.; Antonovič, V.; Boris, R. Structural Characterisation of End-of-Life Cement–Asbestos Materials from Lithuania. Fibers 2024, 12, 37. [Google Scholar] [CrossRef]
- Pollastri, S.; Perchiazzi, N.; Lezzerini, M.; Plaisier, J.R.; Cavallo, A.; Dalconi, M.C.; Bursi Gandolfi, N.; Gualtieri, A.F. The Crystal Structure of Mineral Fibres: Chrysotile. Period. Mineral. 2016, 85, 249–259. [Google Scholar] [CrossRef]
- Bajno, D.; Bednarz, Ł.; Grzybowska, A. The Role and Place of Traditional Chimney System Solutions in Environmental Progress and in Reducing Energy Consumption. Energies 2021, 14, 4720. [Google Scholar] [CrossRef]
- Henderson, D.W. Asbestos-Related Pleuropulmonary Diseases: Asbestosis, Mesothelioma and Lung Cancer. Pathology 1982, 14, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Porzio, A.; Feola, A.; Parisi, G.; Lauro, A.; Campobasso, C.P. Colorectal Cancer: 35 Cases in Asbestos-Exposed Workers. Healthcare 2023, 11, 3077. [Google Scholar] [CrossRef]
- Militello, G.M.; Gaggero, L.; La Maestra, S. Asbestiform Amphiboles and Cleavage Fragments Analogues: Overview of Critical Dimensions, Aspect Ratios, Exposure and Health Effects. Minerals 2021, 11, 525. [Google Scholar] [CrossRef]
- Lin, R.-T.; Chien, L.C.; Jimba, M.; Furuya, S.; Takahashi, K. Implementation of National Policies for a Total Asbestos Ban: A Global Comparison. Lancet Planet. Health 2019, 3, e341–e348. Available online: https://www.thelancet.com/journals/lanplh/article/PIIS2542-5196(19)30109-3/fulltext (accessed on 1 January 2019). [CrossRef]
- Radvanec, M.; Tuček, Ľ.; Derco, J.; Čechovská, K.; Németh, Z. Change of Carcinogenic Chrysotile Fibers in Asbestos Cement (Eternit) to Harmless Waste by Artificial Carbonatization: Petrological and Technological Results. J. Hazard. Mater. 2013, 252–253, 390–400. [Google Scholar] [CrossRef]
- Paolini, V.; Tomassetti, L.; Segreto, M.; Borin, D.; Liotta, F.; Torre, M.; Petracchini, F. Asbestos Treatment Technologies. J. Mater. Cycles Waste Manag. 2019, 21, 205–226. [Google Scholar] [CrossRef]
- Bolan, S.; Kempton, L.; McCarthy, T.; Wijesekara, H.; Piyathilake, U.; Jasemizad, T.; Padhye, L.P.; Zhang, T.; Rinklebe, J.; Wang, H.; et al. Sustainable Management of Hazardous Asbestos-Containing Materials: Containment, Stabilization, and Inertization. Sci. Total Environ. 2023, 881, 163456. [Google Scholar] [CrossRef]
- Durczak, K.; Pyzalski, M.; Brylewski, T.; Juszczyk, M.; Leśniak, A.; Libura, M.; Ustinovičius, L.; Vaišnoras, M. Modern Methods of Asbestos Waste Management as Innovative Solutions for Recycling and Sustainable Cement Production. Sustainability 2024, 16, 8798. [Google Scholar] [CrossRef]
- Durczak, K.; Pyzalski, M.; Brylewski, T.; Sujak, A. Effect of Variable Synthesis Conditions on the Formation of Ye’elimite-Aluminate-Calcium (YAC) Cement and Its Hydration in the Presence of Portland Cement (OPC) and Several Accessory Additives. Materials 2023, 16, 6052. [Google Scholar] [CrossRef]
- Singh, R.; Sontakke, M.; Vivek, J.M.; Rao, B.; Asolekar, S.R. Environmental Sound Management of Asbestos-Containing Wastes Generated from Industries in India. In Waste Management and Resource Efficiency; Ghosh, S., Ed.; Springer: Singapore, 2019. [Google Scholar]
- Ilgren, E. Review: Coalinga Chrysotile: Dissolution, Concentration, Regulation and General Relevance. Indoor Built Environ. 2008, 17, 42–57. [Google Scholar] [CrossRef]
- PN-EN 196-2:2006; Cement Test Methods-Part 2: Chemical Analysis of Cement. Polski Komitet Normalizacyjny: Warsaw, Poland, 2006.
- Pyzalski, M.; Brylewski, T.; Sujak, A.; Durczak, K. Changes in the Phase Composition of Calcium Aluminoferrites Based on the Synthesis Condition and Al2O3/Fe2O3 Molar Ratio. Materials 2023, 16, 4234. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Hamann, B. Review In Situ high-temperature optical microscopy. J. Mater. Sci. 1999, 34, 5419–5436. [Google Scholar] [CrossRef]
- PN-EN 196-1; Cement—Part 1. Cement Test Methods—Part 1: Determination of Strength. Polish Committee for Standardization: Warsaw, Poland, 2018.
- ASTM C 845-96; Standard Specification for Expansive Hydraulic Cement. ASTM International: West Conshohocken, PA, USA, 1996.
- Durczak, K.; Pyzalski, M.; Pilarski, K.; Brylewski, T.; Sujak, A. The Effect of Liquid Slurry-Enhanced Corrosion on the Phase Composition of Selected Portland Cement Pastes. Materials 2021, 14, 1707. [Google Scholar] [CrossRef] [PubMed]
- Pyzalski, M.; Bialoskórski, J.; Walasek, E. Reaction between Carbon Fibres and Molten Silicon: Heat Determination Using DTA. J. Therm. Anal. 1986, 31, 1193–1196. [Google Scholar] [CrossRef]
- Witek, J.; Kusiorowski, R. Neutralization of Cement-Asbestos Waste by Melting in an Arc-Resistance Furnace. Waste Manag. 2017, 69, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Babafemi, A.J.; Šavija, B.; Paul, S.C.; Anggraini, V. Engineering Properties of Concrete with Waste Recycled Plastic: A Review. Sustainability 2018, 10, 3875. [Google Scholar] [CrossRef]
- Johnson, R.A.; Miller, I.; Freund, J.E. Probability and Statistics for Engineers, 9th ed.; Pearson: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Navidi, W.C. Statistics for Engineers and Scientists, 4th ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Akmalaiuly, K.; Berdikul, N.; Pundienė, I.; Pranckevičienė, J. The Effect of Mechanical Activation of Fly Ash on Cement-Based Materials Hydration and Hardened State Properties. Materials 2023, 16, 2959. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Lura, P.; Müller, H.S.; Han, S.; Zhao, T. Application of Neutron Imaging to Investigate Fundamental Aspects of Durability of Cement-Based Materials: A Review. Cem. Concr. Res. 2018, 108, 152–166. [Google Scholar] [CrossRef]
| Component | Content [% by Mass] |
|---|---|
| CaCO3 | 99 |
| HCI | 0.005 |
| N | 0.04 |
| Cl | 0.001 |
| SO4 | 0.05 |
| Mg | 0.05 |
| K | 0.01 |
| Na | 0.05 |
| Fe | 0.001 |
| Component | Content [% by Mass] |
|---|---|
| SiO2 | 99.7 |
| Na2O | 0.1 |
| Al2O3 | 0.05 |
| Fe2O3 | 0.03 |
| TiO2 | <0.01 |
| Organic impurities | <0.01 |
| Moisture (H2O) | 0.1 |
| Component | Content [% by Mass] |
|---|---|
| SiO2 | 25.82 |
| Al2O3 | 4.11 |
| Fe2O3 | 3.52 |
| TiO2 | 0.31 |
| CaO | 56.87 |
| MgO | 6.93 |
| K2O | 0.59 |
| Na2O | 0.15 |
| SO3 | 1.70 |
| Component | Type of Portland Cement | ||
|---|---|---|---|
| “CHEŁM” CEM I 42.5 N-HSR/NA | “GÓRAŻDŻE” CEM I 42.5R | “ODRA” CEM I 42.5R | |
| Content [% by Mass] | |||
| SiO2 | 21.10 | 21.48 | 18.50 |
| Al2O3 | 3.23 | 5.10 | 5.50 |
| Fe2O3 | 4.51 | 2.75 | 2.70 |
| CaO | 64.65 | 64.90 | 64.00 |
| MgO | 1.13 | 1.44 | 1.60 |
| SO3 | 3.08 | 2.53 | 2.10 |
| K2O | 0.43 | 0.83 | 1.00 |
| Na2O | 0.20 | 0.14 | 0.10 |
| Cl− | 0.047 | 0.08 | 0.028 |
| Na2Oeq | 0.48 | - | - |
| Modified Raw Material Samples (Formulations for Cement Clinker) | |||
|---|---|---|---|
| Symbol | 1 | 2 | 3 |
| Composition | Eternit—65% CaO—30% SiO2—5% | Eternit—75% CaO—22% SiO2—3% | Eternit—85% CaO—15% |
| Clinkers | ||||||
|---|---|---|---|---|---|---|
| Type of Cooling | 1 | 2 | 3 | |||
| Rapid | Slow | Rapid | Slow | Rapid | Slow | |
| Chemical Composition [Mass %] | ||||||
| SiO2 | 21.29 | 20.83 | 21.02 | 18.84 | 19.99 | 21.44 |
| Al2O3 | 5.57 | 5.39 | 6.27 | 4.09 | 4.18 | 2.22 |
| Fe2O3 | 1.68 | 1.66 | 3.14 | 2.42 | 2.20 | 1.43 |
| TiO2 | 0.22 | 0.23 | 0.24 | 0.18 | 0.18 | 0.12 |
| CaO | 67.19 | 68.62 | 62.20 | 68.77 | 63.74 | 69.38 |
| MgO | 3.75 | 2.68 | 7.03 | 5.65 | 9.67 | 5.32 |
| K2O | <0.01 | 0.02 | 0.03 | <0.02 | <0.01 | <0.01 |
| Na2O | 0.20 | 0.49 | <0.01 | <0.02 | <0.01 | <0.01 |
| Clinkers | ||||||
|---|---|---|---|---|---|---|
| Type of Cooling | 1 | 2 | 3 | |||
| Rapid | Slow | Rapid | Slow | Rapid | Slow | |
| Phase Composition [Mass %] | ||||||
| C3S | 74.60 | 76.97 | 57.11 | 58.59 | 49.7 | 53.86 |
| βC2S | 3.61 | 4.39 | 14.7 | 19.92 | 18.3 | 22.31 |
| CaO | 0.7 | 1.4 | 1.1 | 1.6 | 0.9 | 1.3 |
| C3A | 3.12 | 4.19 | 2.94 | 4.68 | 3.5 | 5.18 |
| MgO | 1.37 | 2.58 | 1.85 | 3.49 | 2.1 | 4.19 |
| C2(A,F) | 6.2 | 8.96 | 7.9 | 10.02 | 8.2 | 11.08 |
| CŜ | 0.5 | 1.51 | 0.5 | 1.7 | 1.2 | 2.08 |
| Amorphous | 9.9 | - | 13.9 | - | 16.1 | - |
| Sample | Flexural/Compressive Strength in MPa [Days] | Setting Time [hours] | Flow [mm] | W/C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 28 | 90 | Start | End | |||
| CEM I | 3.2/9.5 | - | 6.4/28.1 | 8.5/41.4 | 9.2/50.8 | 9.3/64.3 | 2.45 | 5.0 | 172 | 0.45 |
| 1 | 3.7/12.4 | - | 5.9/28.1 | 7.6/34.6 | 9.0/46.1 | 9.0/54.5 | 2.0 | 4.0 | 190 | 0.50 |
| 2 | 4.0/14.7 | - | 6.5/33.5 | 7.8/41.5 | 9.2/52.8 | 9.3/59.5 | 1.0 | 2.55 | 165 | 0.45 |
| 3 | 3.0/11.2 | 3.0/11.2 | 5.3/24.5 | 6.5/30.5 | 7.5/39.8 | 7.5/50.4 | 2.0 | 3.45 | 160 | 0.50 |
| Sample | Linear Changes [mm/m] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | ||||||||||
| 1 | 2 | 3 | 6 | 7 | 9 | 14 | 16 | 28 | 90 | |
| CEM I | 0.00 | - | −0.02 | −0.01 | −0.04 | 0.00 | −0.05 | −0.02 | −0.04 | −0.07 |
| 1 | 0.01 | - | - | 0.05 | 0.06 | 0.06 | 0.07 | 0.06 | 0.09 | 0.20 |
| 2 | 0.16 | 0.19 | - | 0.25 | - | 0.27 | 0.27 | 0.27 | 0.32 | 0.42 |
| 3 | 0.01 | - | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.01 | 0.06 | 0.31 |
| Sample | Flexural/Compressive Strength in MPa [Days] | Setting Time [hours] | Flow [mm] | W/C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 7 | 28 | 90 | Start | End | |||
| CEM I | 3.2/9.5 | - | 6.4/28.1 | 8.5/41.4 | 9.2/50.8 | 9.2/50.8 | 2.45 | 5.00 | 172 | 0.45 |
| 1 | 2.4/6.9 | - | 5.3/24.3 | 7.4/33.6 | 9.2/49.4 | 9.2/49.4 | 2.30 | 4.50 | 162 | 0.45 |
| 2 | 3.1/11.7 | - | 6.0/30.0 | 8.2/42.1 | 9.5/52.5 | 9.5/52.5 | 2.20 | 4.15 | 183 | 0.45 |
| 3 | 3.6/12.1 | - | 5.6/26.2 | 6.9/33.4 | 8.8/47.7 | 8.8/47.7 | 3.20 | 4.50 | 161 | 0.45 |
| Sample | Linear Changes [mm/m] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | ||||||||||
| 1 | 2 | 3 | 6 | 7 | 9 | 14 | 16 | 28 | 90 | |
| CEM I | 0.00 | - | −0.02 | −0.01 | −0.04 | 0.00 | −0.05 | −0.05 | −0.05 | −0.05 |
| 1 | 0.08 | - | 0.07 | 0.03 | 0.02 | 0.05 | 0.01 | 0.01 | 0.01 | 0.01 |
| 2 | 0.07 | - | 0.06 | 0.10 | 0.14 | 0.10 | 0.07 | 0.07 | 0.07 | 0.07 |
| 3 | 0.00 | - | −0.01 | −0.01 | 0.02 | −0.01 | −0.05 | −0.05 | −0.05 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durczak, K.; Pyzalski, M.; Sujak, A.; Juszczyk, M.; Sala, D.; Ustinovichius, L. Efficient Management of Asbestos Waste Through Utilization as Mineral Additives in Portland Cement Production. Materials 2024, 17, 5793. https://doi.org/10.3390/ma17235793
Durczak K, Pyzalski M, Sujak A, Juszczyk M, Sala D, Ustinovichius L. Efficient Management of Asbestos Waste Through Utilization as Mineral Additives in Portland Cement Production. Materials. 2024; 17(23):5793. https://doi.org/10.3390/ma17235793
Chicago/Turabian StyleDurczak, Karol, Michał Pyzalski, Agnieszka Sujak, Michał Juszczyk, Dariusz Sala, and Leonas Ustinovichius. 2024. "Efficient Management of Asbestos Waste Through Utilization as Mineral Additives in Portland Cement Production" Materials 17, no. 23: 5793. https://doi.org/10.3390/ma17235793
APA StyleDurczak, K., Pyzalski, M., Sujak, A., Juszczyk, M., Sala, D., & Ustinovichius, L. (2024). Efficient Management of Asbestos Waste Through Utilization as Mineral Additives in Portland Cement Production. Materials, 17(23), 5793. https://doi.org/10.3390/ma17235793










