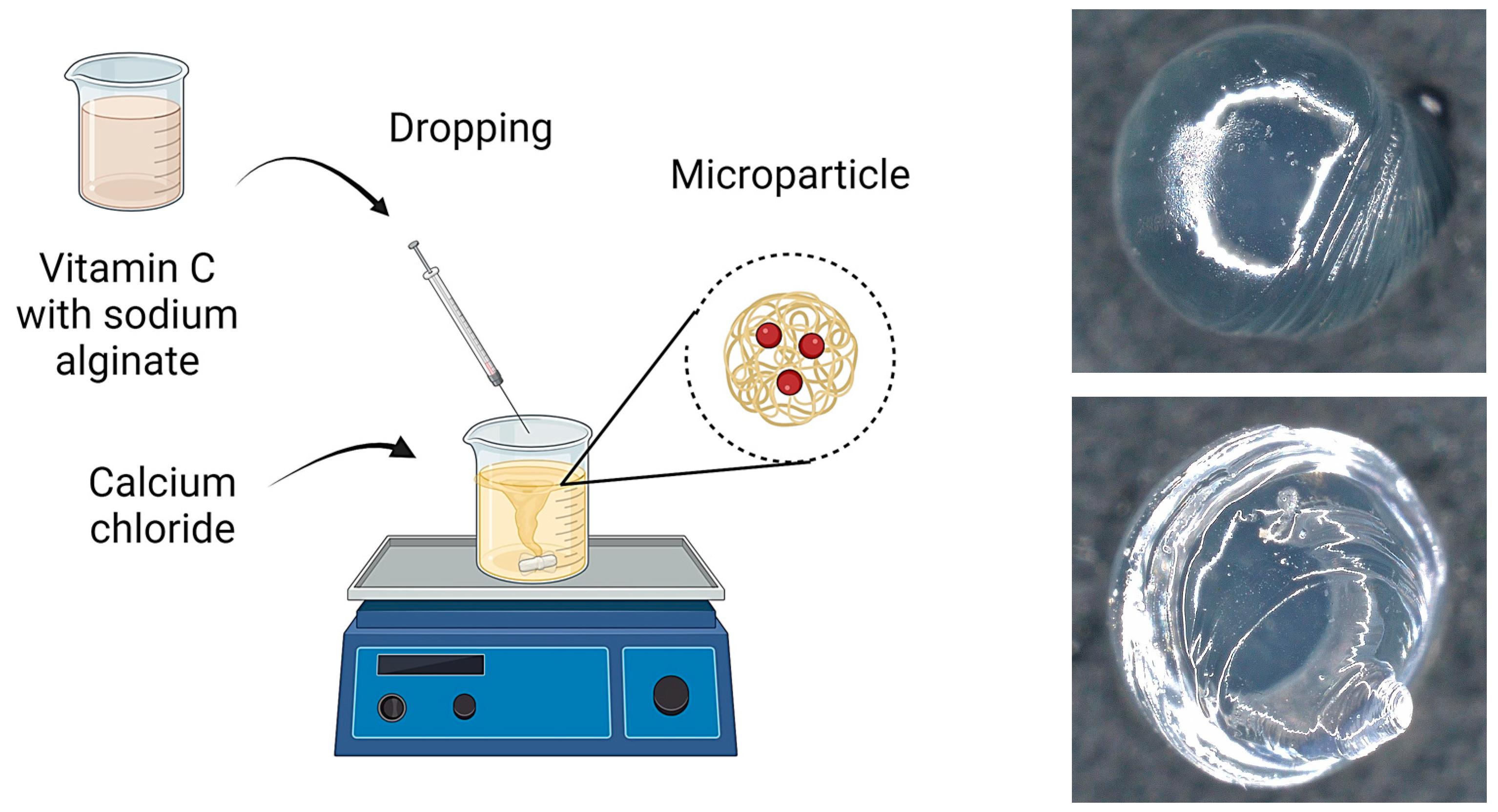
3.1. Physicochemical Characterization
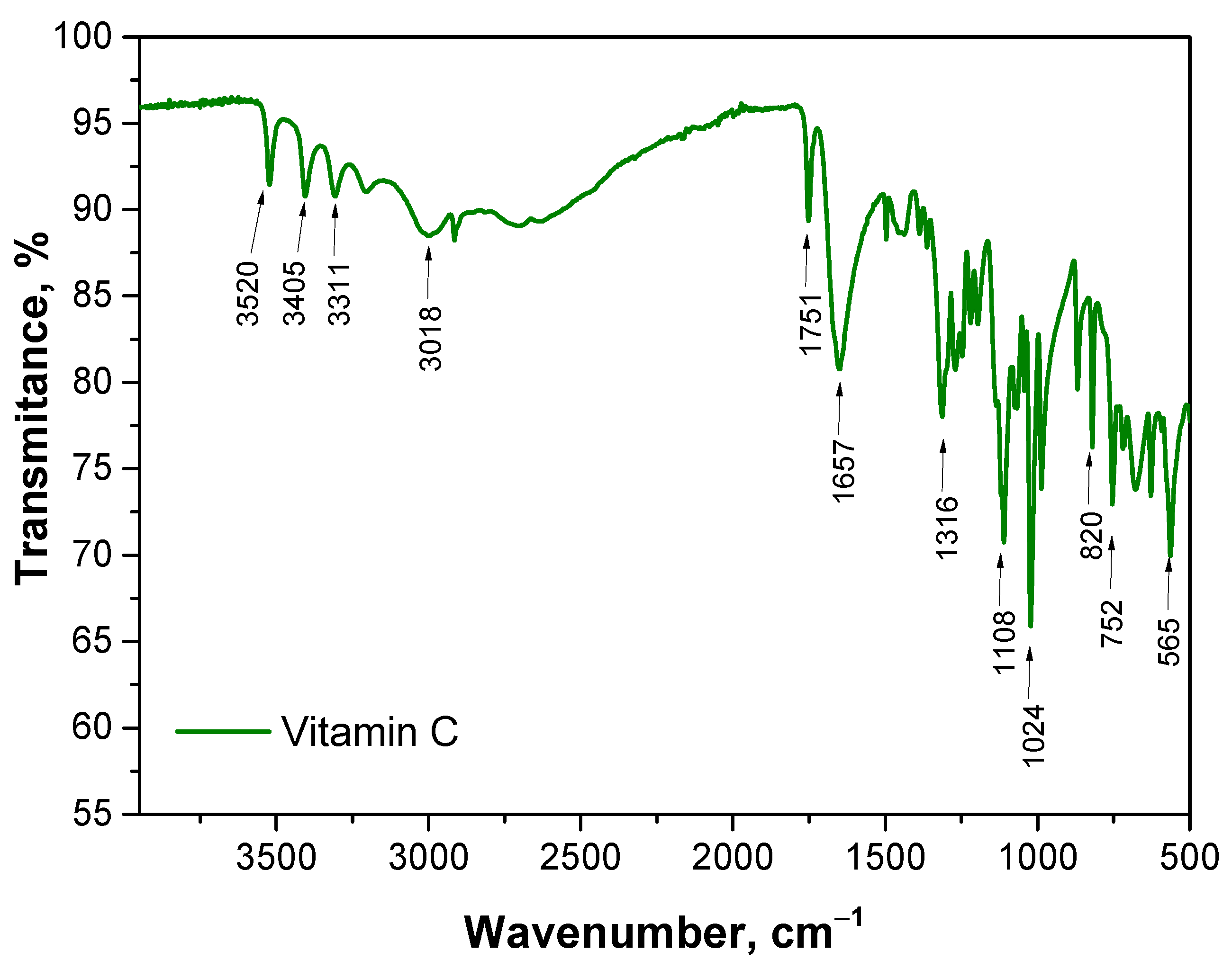
The aim of this study was to analyze the chemical structure of various samples using FT-IR spectroscopy to identify key functional groups and assess differences due to the presence of vitamin C. The FT-IR spectrum of vitamin C is presented in
Figure 2. The results obtained are consistent with the results of other researchers [
26,
27]. The spectra of polymeric materials are presented in
Figure 3.
For the Microcapsules_0 sample, which consists of sodium alginate-based microcapsules without vitamin C, the FT-IR spectrum showed characteristic peaks for alginate. A broad band at wavenumber 3342 cm−1 was observed, which can be attributed to the stretching vibrations of hydroxyl (O-H) groups. This is typical for polysaccharides such as alginate, which contains numerous hydroxyl groups responsible for hydrogen bonding. The intense absorption band at 1640 cm−1 corresponds to the asymmetric stretching of carboxylate groups (COO−), indicating the presence of alginate salts. Additionally, a peak at 1428 cm−1 is assigned to the symmetric stretching of COO−, which is also characteristic of alginate. The absence of other significant peaks confirms that the sample does not contain any additional substances, such as vitamin C. In the case of the Microcapsules_Vit.C sample, which contains microcapsules with vitamin C, the FT-IR spectrum shows not only the characteristic peaks for alginate but also additional bands indicating the presence of vitamin C. Notably, a distinct peak appears at 1753 cm−1, which can be attributed to the stretching of the carbonyl group (C=O), characteristic of ascorbic acid. The appearance of this band confirms the presence of vitamin C in the alginate matrix. The presence of vitamin C also affects the bands at wavenumbers 3522 cm−1, 3405 cm−1, 3310 cm−1 and 3012 cm−1, indicating potential interactions between the hydroxyl groups of vitamin C and alginate, which may suggest hydrogen bonding between these components.
The FT-IR spectra for the hydrogel samples also reveal differences depending on the presence of vitamin C. For the Hydrogel_0 sample, which is a PVA-based hydrogel without vitamin C, the characteristic bands of PVA are clearly visible. The main peak at wavenumber 3388 cm−1 corresponds to the stretching of hydroxyl (O-H) groups, which is typical for PVA, a polymer rich in hydroxyl groups responsible for forming hydrogen bonds. A peak at 1448 cm−1 corresponds to the stretching of C-H bonds, characteristic of polyvinyl alcohol. The absence of additional peaks in the spectrum confirms that this sample does not contain active substances like vitamin C. In the Hydrogel_Vit.C sample, where the hydrogel contains vitamin C, additional peaks are observed compared to the hydrogel without vitamin C. A peak at 1725 cm−1, attributed to the carbonyl group (C=O) of ascorbic acid, is a key indicator of the presence of vitamin C in the sample. Similar to the microcapsules, the band at 3388 cm−1 is altered, suggesting interactions between vitamin C and PVA, likely in the form of hydrogen bonds. The final sample, the hybrid system, which is a hydrogel containing microcapsules with vitamin C, exhibits bands characteristic of both the hydrogel and the microcapsules containing vitamin C. The spectrum shows a peak at 1725 cm−1, confirming the presence of vitamin C in the hybrid system, although the intensity of this band is lower compared to the Hydrogel_Vit.C sample. This suggests that vitamin C is encapsulated within the microcapsules, limiting its direct interaction with the hydrogel matrix. Additionally, the spectrum shows bands associated with both alginate and PVA, confirming the complex structure of this system.
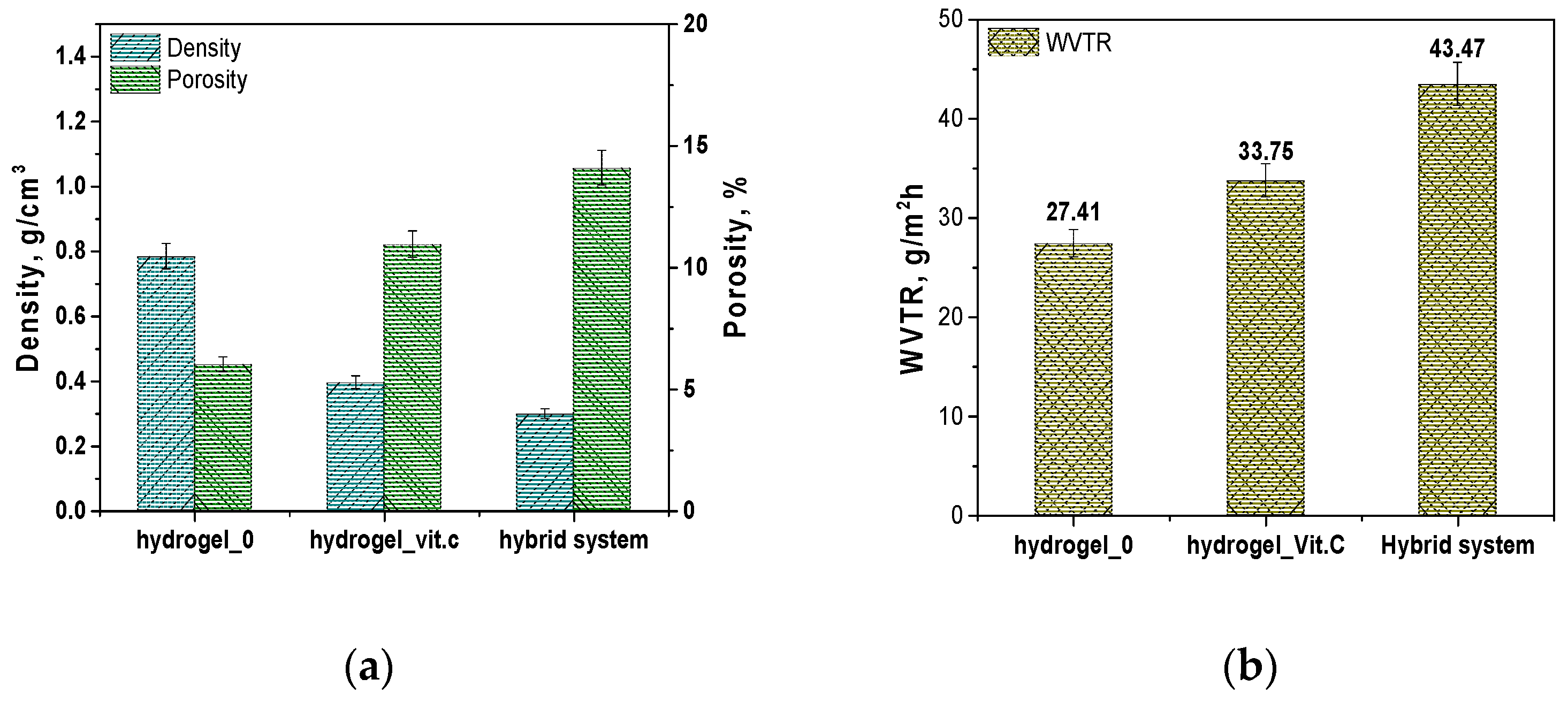
Next, results are presented to determine the porosity density and vapor permeability of the hydrogel systems and the hybrid carrier. The results are presented in
Figure 4.
The results regarding density and porosity show significant differences between the three systems analyzed. The base hydrogel (Hydrogel_0) exhibits the highest density, measuring 0.78356 g/cm
3, and the lowest porosity, at 6.015%. The introduction of vitamin C into the hydrogel leads to a significant decrease in density—Hydrogel_Vit.C has a density of 0.3955 g/cm
3, indicating a reduction in the compactness of the hydrogel structure. The porosity of this system nearly doubles compared to the base hydrogel, reaching 10.946%. The lowest density was observed in the hybrid system, which contains microcapsules with vitamin C, with a value of 0.29851 g/cm
3, indicating the most open structure. The porosity of this system is the highest, at 14.094%. These results suggest that the addition of vitamin C, whether in dissolved form in the hydrogel or encapsulated in microcapsules, leads to significant structural changes in the hydrogel. The increase in porosity may be due to the presence of a greater amount of internal spaces in the material, which may facilitate better release of active substances. Vitamin C, as an acidic substance, can affect the crosslinking of the polymer by interacting with functional groups in the hydrogel matrix, which can lead to the formation of additional spaces in the structure of the material. Increased porosity may result from such interactions, which promote the formation of internal pores and channels. The presence of vitamin C can also affect the flexibility and organization of the hydrogel network, resulting in changes in its internal structure. This kind of structure promotes easier release of active substances, as more free spaces allow for better transport and diffusion of molecules. Similar structural relationships, related to the presence of vitamin C, were observed in a study by Guo et al. In their work, chitosan hydrogels containing vitamin C (CSVC) were characterized by pores of larger size compared to pure chitosan hydrogel. The authors suggest that vitamin C affected the dispersion of chitosan throughout the hydrogel system, which promoted the formation of larger pores. In addition, CSVC hydrogels lost moisture more easily during freeze-drying, leading to an expansion of the branching structure and an increase in pore size. These observations are consistent with our results, indicating that the addition of vitamin C can affect the hydrogel structure by increasing its porosity and available internal spaces [
28].
The results of the water vapor transmission rate (WVTR) tests indicate that the base hydrogel (Hydrogel_0) has the lowest WVTR value, measuring 27.41 g/m2/h. After the addition of vitamin C (Hydrogel_Vit.C), the WVTR increases to 33.75 g/m2/h, suggesting that the presence of vitamin C enhances the permeability of the hydrogel. The highest permeability was observed in the hybrid system, with a WVTR value of 43.47 g/m2/h. This high water vapor permeability in the hybrid system may result from the presence of microcapsules, which further contribute to the structural openness and porosity of the system, promoting more efficient moisture exchange. As mentioned above, this result is due to the effect of vitamin C on the structure of the hydrogel. The addition of vitamin C increases the porosity of the hydrogel, creating more internal spaces in its matrix. The additional pores and channels allow water vapor to penetrate more easily, resulting in higher permeability. In other words, the structural modifications associated with the presence of vitamin C increase the hydrogel’s ability to transmit water vapor by expanding the available pathways for its movement. The notable decrease in density and increase in porosity in both the sample with vitamin C and the hybrid system suggest that these materials may interact more effectively with their environment, especially in terms of the controlled release of active substances. The increased water vapor transmission rate (WVTR) also indicates potentially improved functional properties in these materials, particularly in medical applications such as dressings, where proper moisture exchange with the surrounding environment is required.
The relationship between density, porosity, and water vapor permeability (WVTR) is widely recognized as a key factor for materials such as hydrogels and microcapsules, which have applications in controlled release and medical applications such as dressings. The observed decrease in the density of hydrogel samples with a concomitant increase in porosity and an increase in WVTR values is due to the fact that materials with lower density have more empty spaces (pores), allowing water vapor and active substances to flow more easily. Structural changes in cellulose-based polymer films were demonstrated by Niu et al. The different composition affecting the hydrophilic nature of the materials was associated with a change in physicochemical properties. Again, a close relationship of a decrease in density with an increase in porosity was demonstrated. In addition, these relationships were also associated with water absorption and water vapor permeability, similarly to the results presented above [
29]. Hoch et al., in turn, proved that permeability is related to the degree of hydration of the material under study and its internal structure [
30]. Interestingly, internal structure parameters related to material density and permeability have also been studied in the packaging industry. Chalykh et al. proposed a model showing the relationship of permeability with water vapor diffusion coefficients in the pore volume [
31]. In turn, Chavda et al. proved that fluid sorption and permeability are closely related to the degree of crosslinking of the polymer material. These results can be applied to the obtained hydrogel materials with reduced density. As shown, the lower crosslinking density of polymer chains influences their looser arrangement, which is related to properties such as sorption and porosity. Thus, the obtained results are consistent with previously presented scientific reports [
32].
Next, the sorption capacity was analyzed in two different environments: distilled water and SBF fluid to reflect different biological conditions. The aim was to determine the degree of swelling of the tested samples at different incubation times (1 h, 6 h, 12 h, 24 h), depending on the type of media. The results of the analysis are presented in
Figure 5.
Sample microcapsules without vitamin C exhibited the lowest degree of swelling at all time points, which is consistent with expectations, as they do not contain vitamin C or other active substances that could affect fluid absorption. Their swelling degree in distilled water and SBF ranged from approximately 0.5 to 1.0 g/g after 24 h of incubation. Next, microcapsules_Vit.C showed a significantly higher degree of swelling compared to the microcapsules without vitamin C, which may be due to interactions between vitamin C and water, causing the alginate structure to expand. The degree of swelling of the microcapsules containing vitamin C reached approximately 1.5–2.0 g/g in both fluids after 24 h. In turn, hydrogel without vitamin C demonstrated a medium level of swelling, with values around 1.0–1.5 g/g after 24 h. Compared to the microcapsules, the hydrogel exhibited a greater ability to absorb fluids, which can be attributed to the hydrophilic properties of poly(vinyl alcohol) (PVA), which forms the hydrogel. Hydrogel modified with vitamin C exhibited a much higher degree of swelling than Hydrogel_0. Vitamin C, due to its hydrophilic nature, can further increase the material’s fluid absorption capacity, as reflected in the results, where the degree of swelling reached approximately 2.5–3.0 g/g in both fluids after 24 h. Interestingly, differences in the degree of swelling between Hydrogel_0 and the Hydrogel_Vit.C were not constant throughout the study period. At some time points, both samples showed similar absorption, which may suggest that structural changes caused by the presence of vitamin C, such as increased porosity, may act differently depending on the degree of water saturation of the hydrogel. The swelling values for Hydrogel_Vit.C eventually reached about 2.5–3.0 g/g after 24 h, with similar results for both samples at 12 h of testing, indicating the influence of the degree of saturation. This effect was observed only for the sample placed in distilled water. The hybrid system showed the highest degree of swelling among all tested samples, reaching values of around 3.0–3.5 g/g after 24 h. The high sorption capacity of this system results from the combined absorption properties of the hydrogel and the vitamin C-containing microcapsules.
The sorption capacity tests demonstrate that the introduction of vitamin C, both in dissolved form in the hydrogel and encapsulated in microcapsules, significantly increases the carriers’ ability to absorb fluids. The hybrid system showed the highest sorption capacity, making it potentially the most effective system for applications requiring intense absorption, such as wound dressings or controlled release systems for active substances.
The results of the hydrogels’ sorption capacity clearly correlate with the previous analyses of their density and porosity. The base hydrogel (Hydrogel_0), characterized by the highest density and lowest porosity, demonstrated a limited ability to swell, which can be attributed to its fewer pores available for fluid absorption. The introduction of vitamin C (Hydrogel_Vit.C) significantly reduced the density and simultaneously increased the porosity, which directly translated into an increased fluid absorption capacity—Hydrogel_Vit.C exhibited a much higher degree of swelling. The highest sorption capacities were observed in the hybrid system, which indicates a further increase in porosity and reduction in density, promoting even greater fluid absorption. Thus, the results suggest that lower density and higher porosity of hydrogels directly enhance their sorption capacity.
Moreover, the sorption coefficients for all tested samples were higher in distilled water compared to simulated body fluid (SBF). This is because distilled water does not contain ions or compounds that could interact with the hydrogel matrix. In contrast, SBF contains various ions, such as Na+, Cl−, and Ca2+, which can interact with the polymer network of the hydrogels and influence their fluid absorption capacity. These interactions may stabilize the hydrogel structure, limiting its ability to swell and absorb fluids compared to distilled water, where such restrictions are not present.
3.2. Incubation Analysis in Simulated Body Fluids
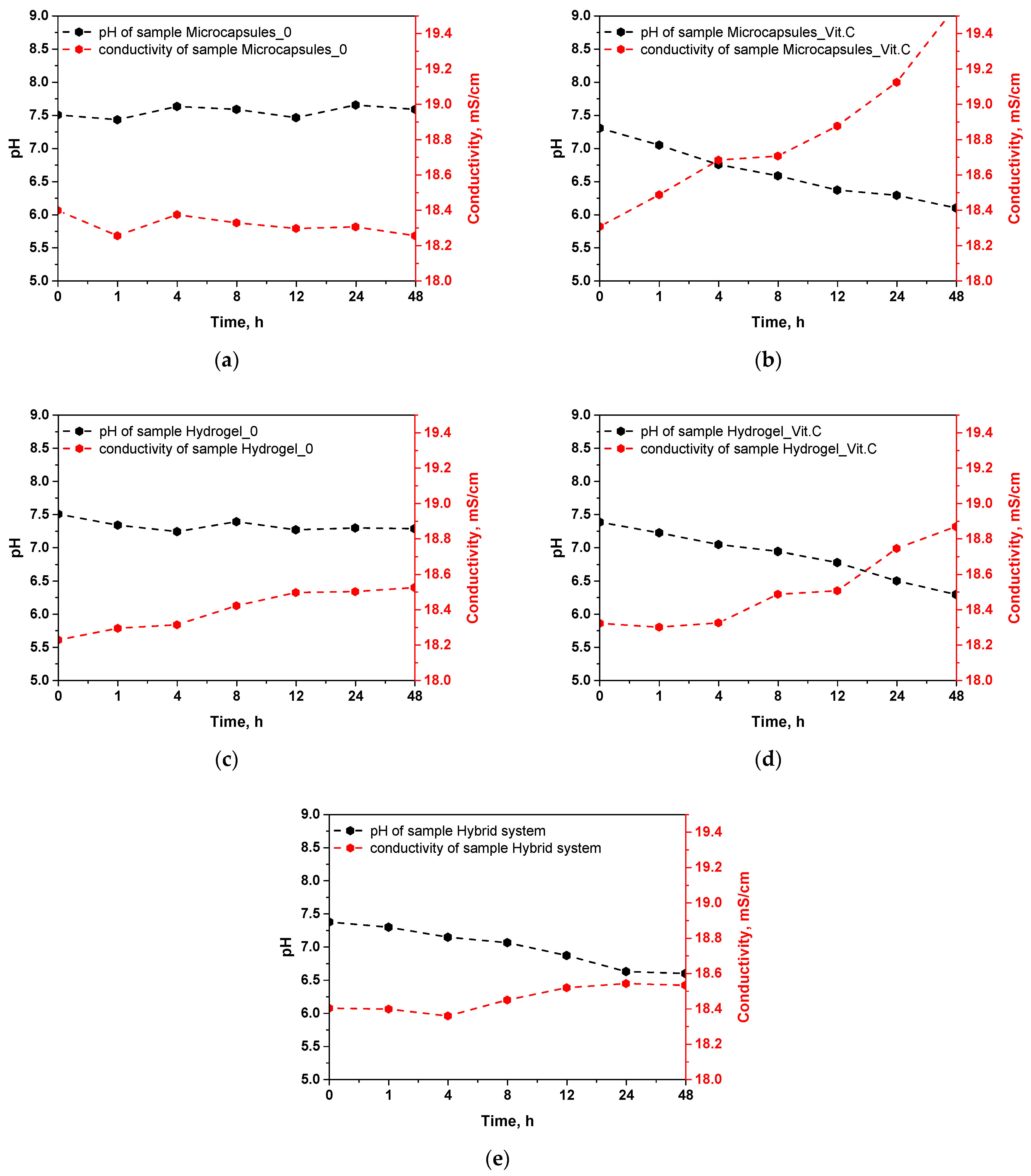
The results of the incubation of microcapsules and hydrogels in simulated body fluid (SBF) allow for an evaluation of the stability of these materials and their ability to release active substances, particularly vitamin C. The measured parameters, such as pH and ionic conductivity, reflect the changes occurring in the samples during incubation, providing insight into the behavior of these systems in conditions simulating a biological environment. The results of this analysis are presented in
Figure 6.
The pH stability during incubation for the samples of microcapsules without vitamin C and the base hydrogel indicates their relative chemical stability. Both the microcapsules and the hydrogel without vitamin C show no significant changes in pH, suggesting no notable interactions with the SBF fluid or the release of any soluble substances. This behavior is consistent with previous results regarding density and porosity, where it was found that these samples exhibit higher density and lower porosity. Therefore, it can be assumed that in these materials, the higher-density structure limits their interaction with the surrounding environment, thus reducing their ability to release ions or active substances. In the case of the samples containing vitamin C, a marked decrease in pH during incubation is observed. This is particularly evident in the microcapsules with vitamin C, where the pH drops to 6.097 after 48 h. A similar trend is observed in the vitamin C hydrogel and the hybrid system, where the pH drops to 6.293 and 6.597, respectively. The decrease in pH can be attributed to the gradual release of vitamin C, which is ascorbic acid, and upon dissolving in the medium, it leads to acidification of the environment. This behavior aligns with earlier results on sorption capacity, which showed that vitamin C-containing systems have an increased ability to absorb fluids, which may promote faster release of vitamin C.
Ionic conductivity reflects the release of ions from the tested materials or their degradation in the SBF environment. Samples of microcapsules without vitamin C and the base hydrogel exhibit stable conductivity in the range of 18.2–18.4 mS/cm, again suggesting their high chemical stability and lack of significant structural changes during incubation. Low conductivity is consistent with earlier results, indicating their low porosity and higher density, which limits interactions with the surrounding environment and prevents ion release. In the samples containing vitamin C, an increase in conductivity is observed over time, particularly for the microcapsules with vitamin C, where the conductivity rises to 19.553 mS/cm after 48 h. Increased conductivity indicates the release of ions from the microcapsules, which may be related to the release of vitamin C and the partial degradation of the capsule material. A similar increase in conductivity is noted for the vitamin C hydrogel (18.868 mS/cm) and the hybrid system (18.532 mS/cm), further confirming that vitamin C and other ionic substances are gradually being released from these systems. The increased conductivity in vitamin C-containing samples correlates with the results on porosity. Higher porosity, especially in the hybrid system, promotes greater ion exchange between the samples and the incubation fluid, leading to increased conductivity. In addition, the change in conductivity values in the tested incubation fluids confirms that the polymer systems were able to effectively release vitamin C and ion exchange with the environment, which confirms their potential in controlled release of the active substance.
The results of the incubation in simulated body fluid (SBF) clearly indicate differences in the behavior of the tested samples depending on their composition. Samples containing vitamin C show more significant changes in both pH and ionic conductivity, suggesting their ability to gradually release vitamin C and possibly other ionic compounds. On the other hand, samples without vitamin C placed in the incurable fluid do not change its pH and conductivity. Therefore, it can be concluded that the changes in these parameters are mainly related to the presence of vitamin C.
3.3. Microscopic Observations and Surface Roughness
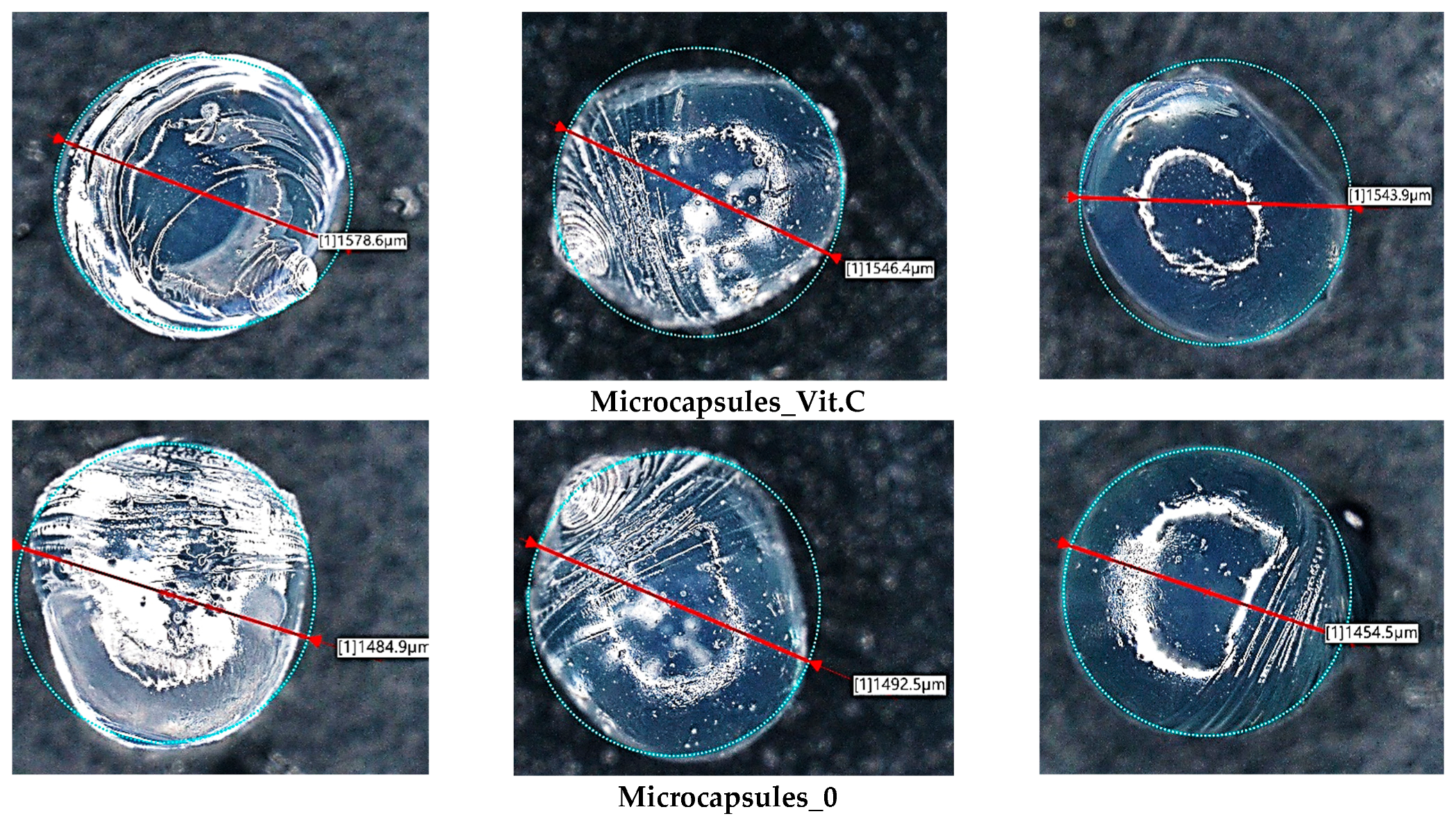
Subsequently, microscopic observations were made of the resulting systems. In the case of capsules, their average size was determined. The results are presented in
Figure 7. Then, the surface morphology of the hydrogel materials was visualized (
Figure 8).
The irregular rod-like and sphere-like structures seen in
Figure 6 are characteristic of the microcapsules formed when alginate is infused into calcium chloride solution. These irregularities are due to the rapid gelation process that occurs when a drop of alginate detaches from the tip of the needle. As each droplet detaches from the needle, its surface begins to crosslink immediately upon contact with the calcium ions in the solution, resulting in local rippling or minor deformations, particularly at the point of droplet detachment. Rapid crosslinking of the droplet surface can lead to the formation of small protuberances or ripples, which result in the observed irregularities in the microcapsule structure.
The basic hydrogel has a relatively smooth and uniform surface, suggesting the absence of defects created during photopolymerization. In contrast, the vitamin C-containing hydrogel appears rougher and more irregular, which may be related to interactions of vitamin C with the polymer matrix, leading to changes in the surface structure. The most pronounced changes are observed in the case of the hybrid system, which contains microcapsules with vitamin C. The photo clearly shows a more complex and rougher surface, indicating the presence of microcapsules inside the hydrogel structure. No major changes in the shape of the microcapsules were observed. They are mostly close to spherical in shape. The irregularities are due to the crosslinking process. Vitamin C-containing capsules are characterized by a larger diameter, with little change.
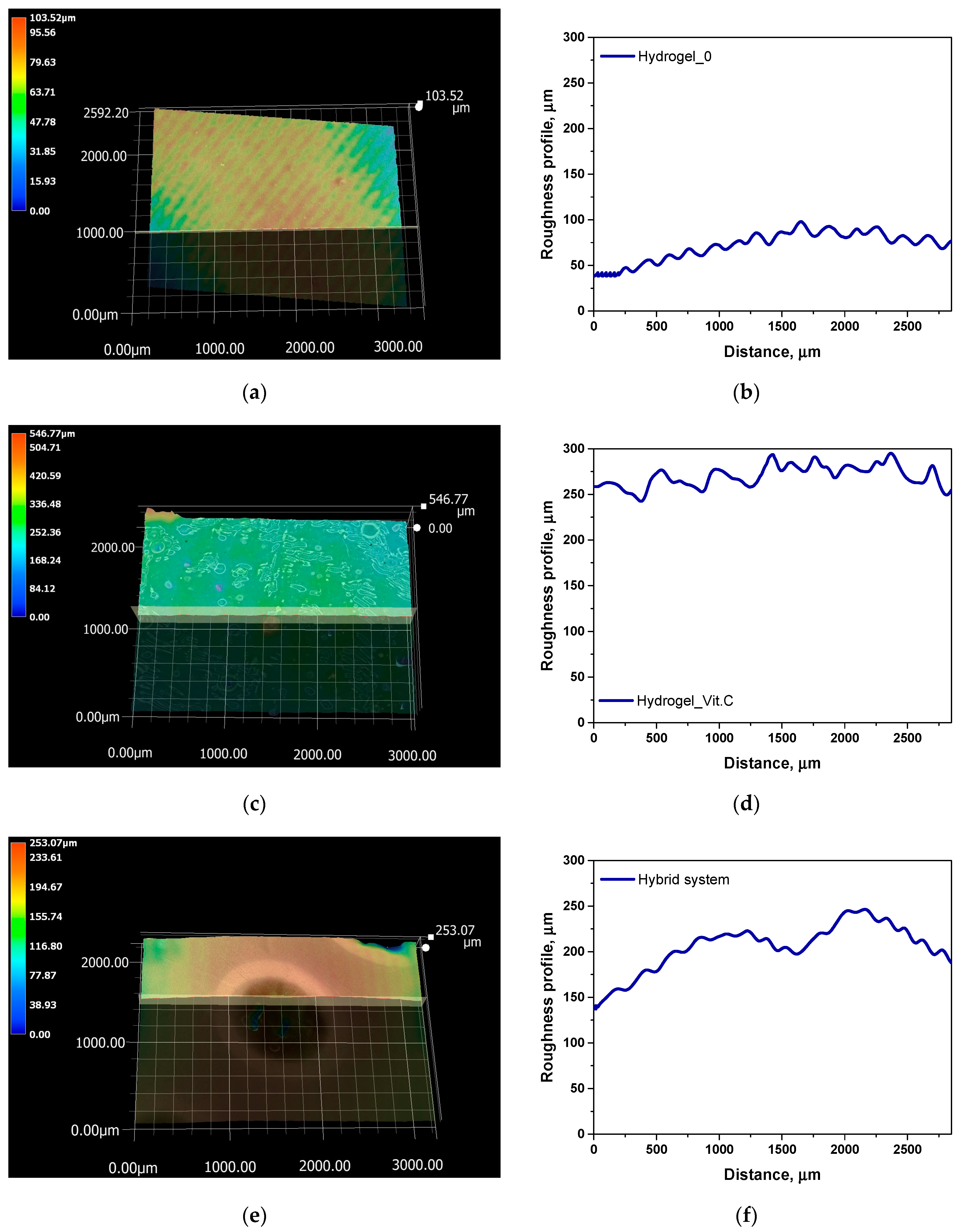
Next, a roughness profile analysis was conducted for the hydrogel samples. The results are presented in
Figure 9 and
Table 3.
The analysis of surface roughness measurements, combined with previous data on porosity and density, allows for conclusions regarding the relationship between surface structure and the functional properties of the studied systems. The average roughness (Ra) and maximum profile height (Rz) values revealed significant differences between the samples, reflecting the impact of the presence of vitamin C and microcapsules on the surface topography. Relatively low Ra and Rz values in the base hydrogel suggest that its surface is more smooth, likely due to the lower porosity of this system. The base hydrogel also exhibited the highest density and lowest porosity, which limited its ability to absorb fluids, as reflected in the lower roughness values. Reduced roughness and lower porosity lead to a smaller surface area available for interaction with the environment, which may be disadvantageous in applications requiring intensive fluid exchange, such as wound dressings. The addition of vitamin C in the hydrogel significantly increased both the surface roughness (Ra = 14.16 µm, Rz = 98.12 µm) and porosity, likely due to interactions between vitamin C and the hydrogel’s polymer network. The increased surface roughness is consistent with the porosity results, indicating a more open and branched structure in this hydrogel, which facilitates greater fluid absorption capacity. The higher porosity and surface roughness of the vitamin C-containing hydrogel may promote better interaction with the biological environment, which is critical for applications such as drug delivery systems or medical wound dressings. The highest Ra (20.15 µm) and Rz (89.51 µm) values were recorded for the hybrid system, which aligns with the presence of microcapsules within the hydrogel structure. The inclusion of microcapsules resulted in a significant increase in the porosity of this system, as confirmed by previous density and porosity measurements. The increased roughness in the hybrid system suggests that the surface of the microcapsule-containing hydrogel is much more uneven, which could provide a larger contact area with fluids and enable better control of active substance release. The high porosity and roughness of this system not only improve sorption capacity but may also influence the efficiency of active substance release, making this system promising for applications requiring controlled release, such as drug delivery or long-term wound dressings. In summary, the analysis indicates a strong correlation between surface roughness and the porosity of the studied systems. The increase in roughness, resulting from the modification of hydrogels with vitamin C and microcapsules, enhances sorption properties and potentially improves bioadhesion, which is crucial for medical applications.
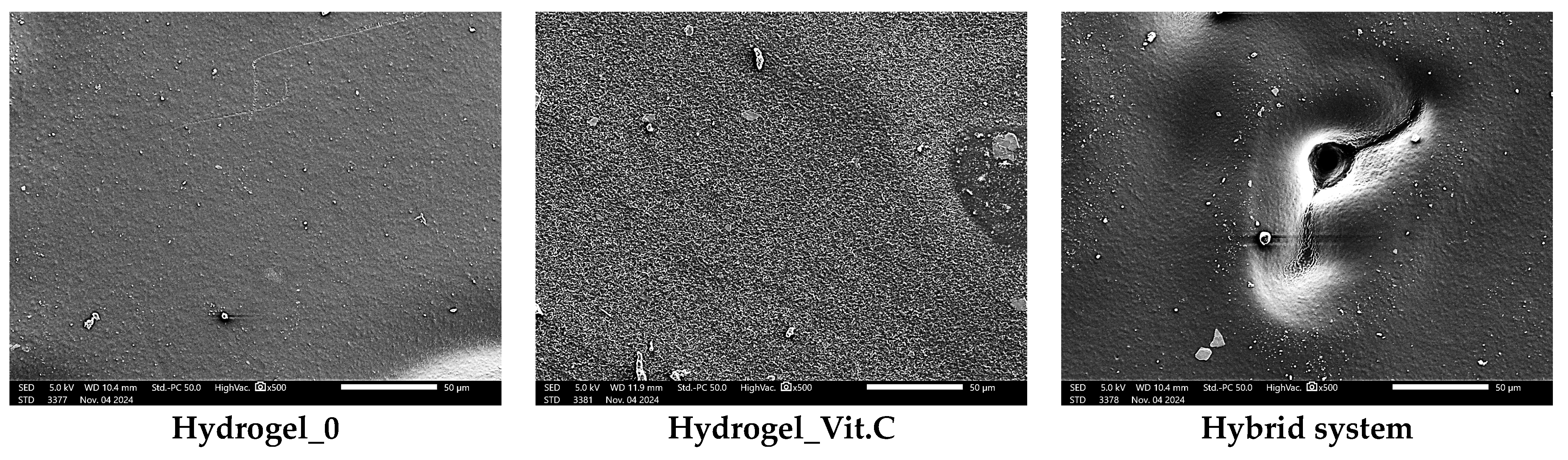
Figure 10 shows images obtained from SEM observations, which are consistent with digital microscope analysis and roughness measurements. The basic hydrogel shows a relatively smooth and uniform surface, indicating a structure without additional modifications. In contrast, the vitamin C-containing hydrogel shows increased texture irregularity, likely due to interactions between vitamin C and the polymer matrix, which alter the surface structure. At the same time, an increase in the corrugation of the entire surface can be seen. No defects in the polymer matrix were observed. The most noticeable changes are observed in the hybrid system containing microcapsules with vitamin C. SEM images reveal the presence of pores and a more irregular structure. These results are consistent with the observations presented above regarding surface roughness.
3.4. Evaluation of Release Kinetics Under Static and Dynamic Conditions
Subsequently, a study on the release of vitamin C from the microcapsules and hydrogels was conducted. To measure the release, a reaction with ferric chloride and phenanthroline was used.
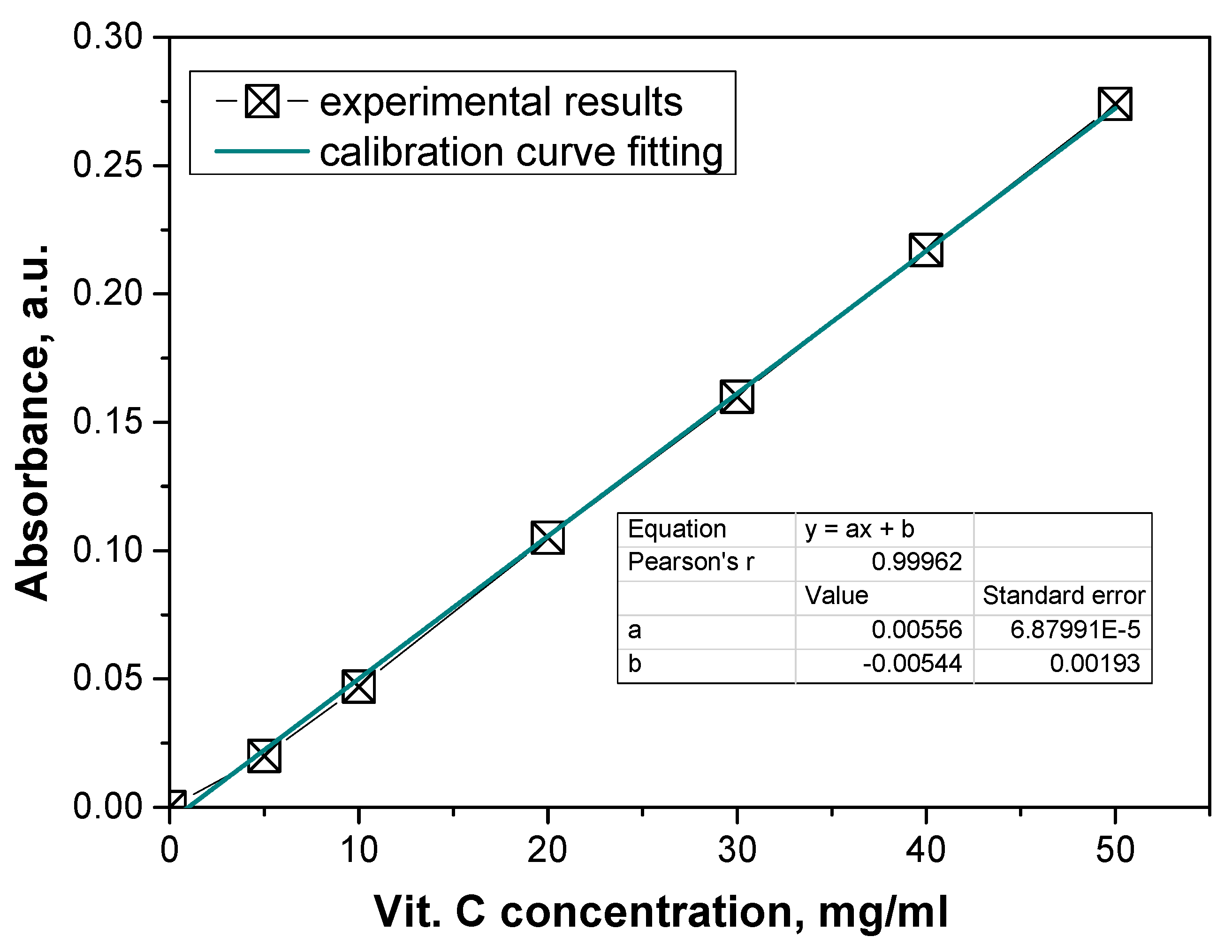
This study began by determining a calibration curve to quantify the release of vitamin C. The calibration curve was constructed by measuring the absorbance of solutions with known concentrations of vitamin C. The concentration of vitamin C (in mg/L) was plotted on the
x-axis, while the corresponding absorbance values (measured at the wavelength specific to the Fe
2+-phenanthroline complex) were plotted on the
y-axis (
Figure 11).
This calibration curve provides a linear relationship between the concentration of vitamin C and the absorbance, which allows for the determination of unknown concentrations of vitamin C in the release study based on their absorbance values.
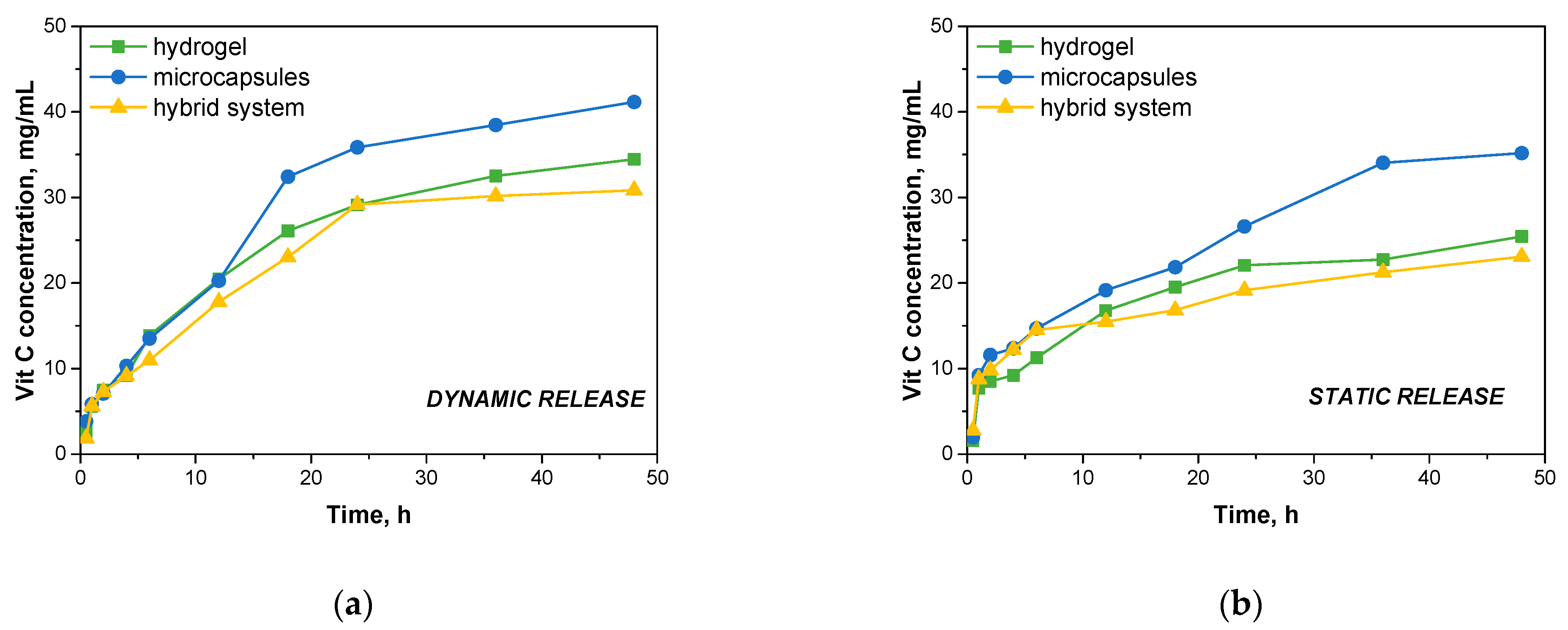
Figure 12 then presents the results of vitamin C release at specific time intervals in a dynamic and static process.
Under dynamic conditions, it was observed that the microcapsules showed the highest rate of vitamin C release. After 48 h, the microcapsules released 41.137 mg/mL of vitamin C, indicating rapid diffusion of the active ingredient from the microcapsule matrix. The rapid release can be explained by the relatively small structure of the microcapsules, which are subjected to continuous mechanical forces under dynamic conditions, promoting the swelling of the capsules and facilitating the release of vitamin C into the medium. Hydrogels showed a slightly slower release rate compared to microcapsules. After 48 h, the hydrogels released 34.448 mg/mL of vitamin C. The slower release from the hydrogels may be related to the more crosslinked structure of the hydrogel matrix, which controls the diffusion of vitamin C. However, the dynamic experimental conditions favored greater contact between the hydrogel surface and the incubation fluid, which improved the diffusion of the active ingredient, although it was still slower than with microcapsules. The lowest release values were recorded for the hybrid system, which released 30.836 mg/mL of vitamin C after 48 h. The hybrid system, consisting of microcapsules embedded in a hydrogel, showed the most controlled release of vitamin C. The hydrogel structure appears to provide an additional barrier that limits the rate of vitamin C release from the microcapsules. This composite structure provides a more gradual release of the active ingredient, which may be beneficial in applications that require prolonged action, such as dressings or drug delivery systems.
Under static conditions, where no mechanical mixing was used, the rate of vitamin C release was noticeably slower in all systems tested. After 48 h, the microcapsules released 35.177 mg/mL of vitamin C, which was still the highest value compared to the other systems. The lack of mechanical agitation slowed the diffusion process, but the microcapsules were able to release the active ingredient relatively quickly due to their small structure. The hydrogels released 25.437 mg/mL of vitamin C after 48 h, a lower value compared to the dynamic process. Under static conditions, where contact with the incubation fluid was more limited, the hydrogels showed slower release of the active ingredient, which may be explained by the higher density of the hydrogel matrix, which limited the migration of vitamin C into the environment. The hybrid system also showed the lowest release rate in the static process, with a result of 23.085 mg/mL of vitamin C after 48 h. Such a result confirms that the complex structure of the hybrid system, in which the hydrogel further surrounds the microcapsules, significantly delays the release of vitamin C. Under static conditions, where there are no additional mechanical forces to promote diffusion, the hydrogel barrier works even more effectively, leading to a slower and more controlled release.
The results show significant application potential in extended-release active ingredient delivery systems. Compared to other developed systems, a relatively prolonged release profile of vitamin C was achieved. Systems described in the literature are characterized by the undesirable effect of sudden ejection of the substance, which may limit their application where controlled, prolonged release of the active substance is required. In a study conducted by Calzado-Delgado on poly(vinyl alcohol) (PVA) nanofiber media, a rapid release of vitamin C was observed—as much as 63% of the total vitamin content was released within the first hour [
33]. Similarly, in the work of Shing et al., an orally dissolvable film (ODF) containing vitamin C and catechin was studied. The results of this study also indicate an intense rapid release effect, as more than 70% of vitamin C was released in simulated body fluid within the first 30 min. Such a rapid release profile makes this system more suitable for rapid delivery of antioxidants, but it does not meet the objectives for prolonged release [
34]. Also, in a study by Abdullach et al. on hydrogel systems containing avocado seed protein, there was a sudden ejection of vitamin C during the initial phases of release. Although the authors describe the use of this system as a potential carrier for bioactive compounds, the resulting release profile indicates the difficulty of achieving prolonged action without changing the composition and structure of the hydrogel [
24].
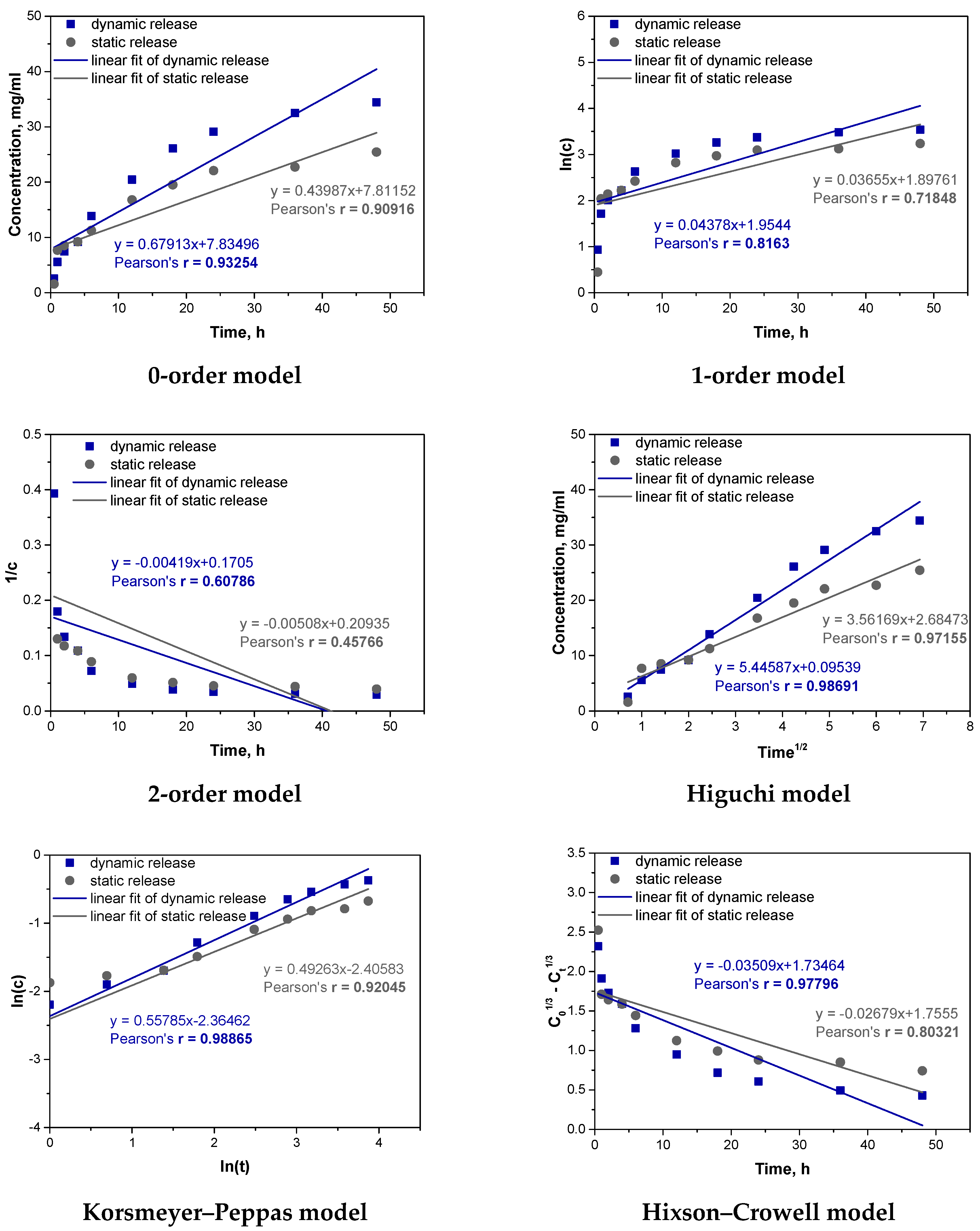
In order to better understand the process of vitamin C release from the obtained carriers, fitting of experimental results to different kinetic models was also carried out. The results of fitting the results for the Hydrogel_Vit.C sample are shown below in
Figure 13 and
Table 4.
The analysis of the kinetics of vitamin C release from the hydrogel containing vitamin C was performed using six kinetic models. The best fit to the experimental results was obtained for the Higuchi and Korsmeyer–Peppas models. The Higuchi model, which assumes that the release of the substance is primarily controlled by diffusion through a porous matrix, showed very high correlation coefficients for both dynamic (r = 0.98691) and static (r = 0.97155) conditions. This result suggests that diffusion is the dominant mechanism controlling the release of vitamin C from the hydrogel in both cases. Under dynamic conditions, where mechanical agitation is applied, the diffusion process is enhanced by the movement of the fluid, facilitating faster penetration of vitamin C through the hydrogel matrix. In contrast, in static conditions, diffusion occurs more slowly, as reflected by the slightly lower correlation coefficient, though diffusion still plays a key role in controlling the release process. The second model that well described the kinetics of vitamin C release from the hydrogel was the Korsmeyer–Peppas model. This model is useful for systems where the release mechanism is more complex, involving both diffusion and polymer matrix swelling. The correlation coefficient for dynamic release was r = 0.98865, and for static release, it was r = 0.92045, indicating that this model also fits the data very well. An important parameter in the Korsmeyer–Peppas model is the exponent “n”, which provides information about the mechanism controlling the release. When “n” is around 0.5, the process is dominated by Fickian diffusion (a simple diffusion mechanism). When “n” exceeds 0.5, it suggests that the release is controlled by both diffusion and swelling of the matrix, indicating a more complex mechanism. In the case of the vitamin C hydrogel, the value of “n” obtained from the Korsmeyer–Peppas model was greater than 0.5, suggesting that, in addition to diffusion, matrix swelling also plays a significant role in controlling the release. Mechanical agitation in the dynamic process likely enhanced this effect, which explains the better fit of the model under these conditions.
Both the Higuchi and Korsmeyer–Peppas models confirm that diffusion is the key mechanism controlling the release of vitamin C from the hydrogel, regardless of the conditions. However, differences in model fit between dynamic and static release indicate that these processes occur at different rates depending on the conditions. In dynamic conditions, where mechanical agitation facilitates swelling and increases contact between the hydrogel matrix and the medium, the release mechanism becomes more complex, involving both diffusion and swelling, which explains the better fit of the Korsmeyer–Peppas model. In static conditions, without mechanical assistance, the swelling process is slower, and diffusion is more strictly controlled by the porous structure of the hydrogel, which is better reflected by the Higuchi model.
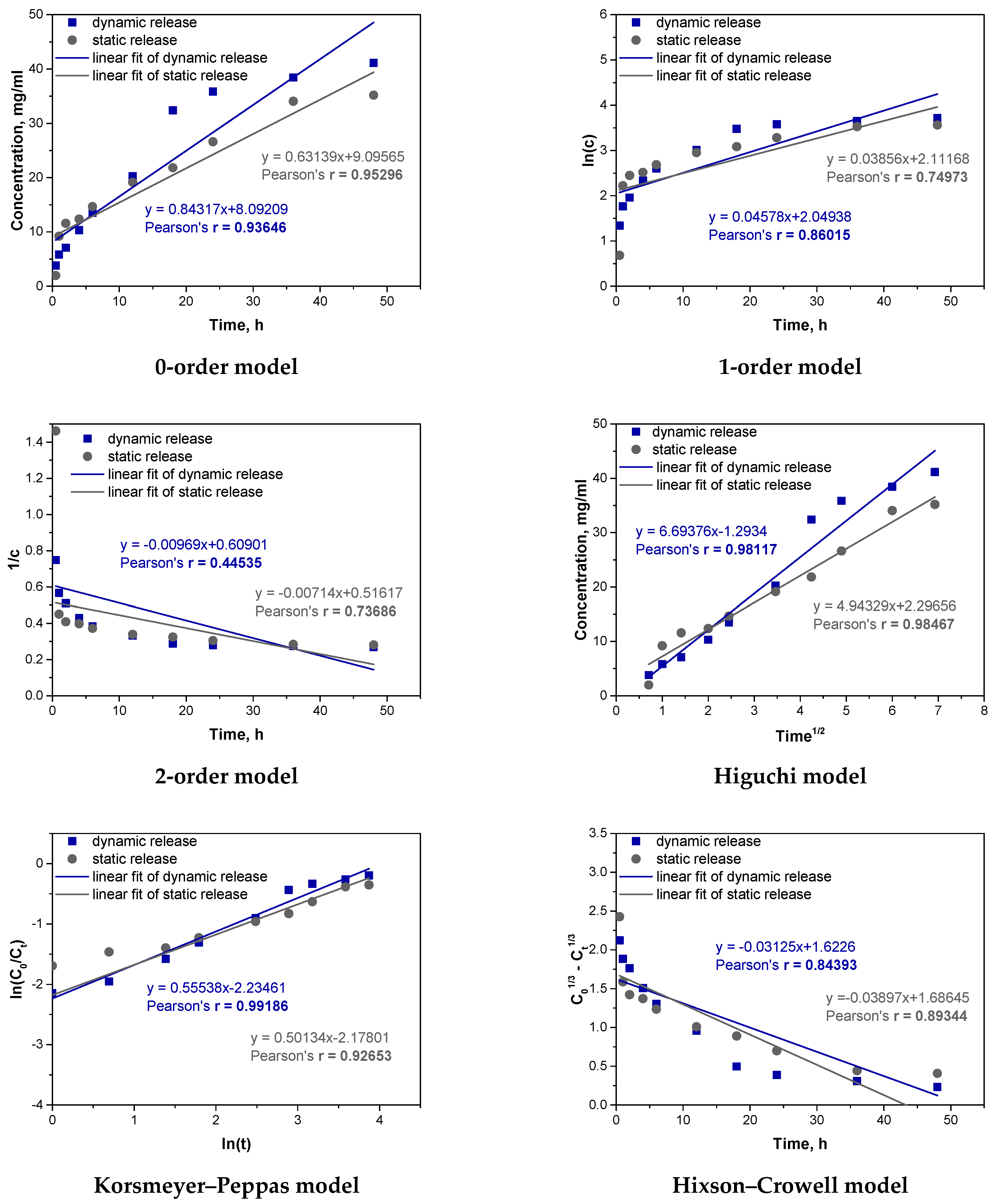
Analogous fitting was then carried out for the microcapsules_Vit.C sample. The results are presented in
Figure 14 and
Table 5.
For the microcapsules_Vit.C sample, the analysis of Pearson’s correlation coefficients indicates that under dynamic conditions, the Korsmeyer–Peppas model (r = 0.99186) and the Higuchi model (r = 0.98117) best describe the release of vitamin C. The high correlation for the Korsmeyer–Peppas model, with an “n” value above 0.5, suggests that both diffusion and swelling significantly influence release, as mechanical agitation enhances the swelling of the alginate matrix. The Higuchi model, though also showing high correlation, primarily reflects diffusion without accounting for swelling, explaining its slightly lower fit. In static conditions, the Higuchi model (r = 0.98467) and the zero-order model (r = 0.95296) provide the best fit. The high fit of the Higuchi model confirms that diffusion is the main mechanism without significant swelling due to the absence of mechanical agitation. The zero-order model indicates a stable release rate, suggesting a steady diffusion process, especially in the early stages. Comparing dynamic and static processes, mechanical agitation in dynamic conditions promotes swelling and a complex release mechanism, fitting well with the Korsmeyer–Peppas model. In contrast, static conditions favor diffusion as the dominant mechanism, consistent with the Higuchi model’s fit. Thus, release mechanisms vary with conditions: dynamic conditions involve both diffusion and swelling, while static conditions are dominated by diffusion alone.
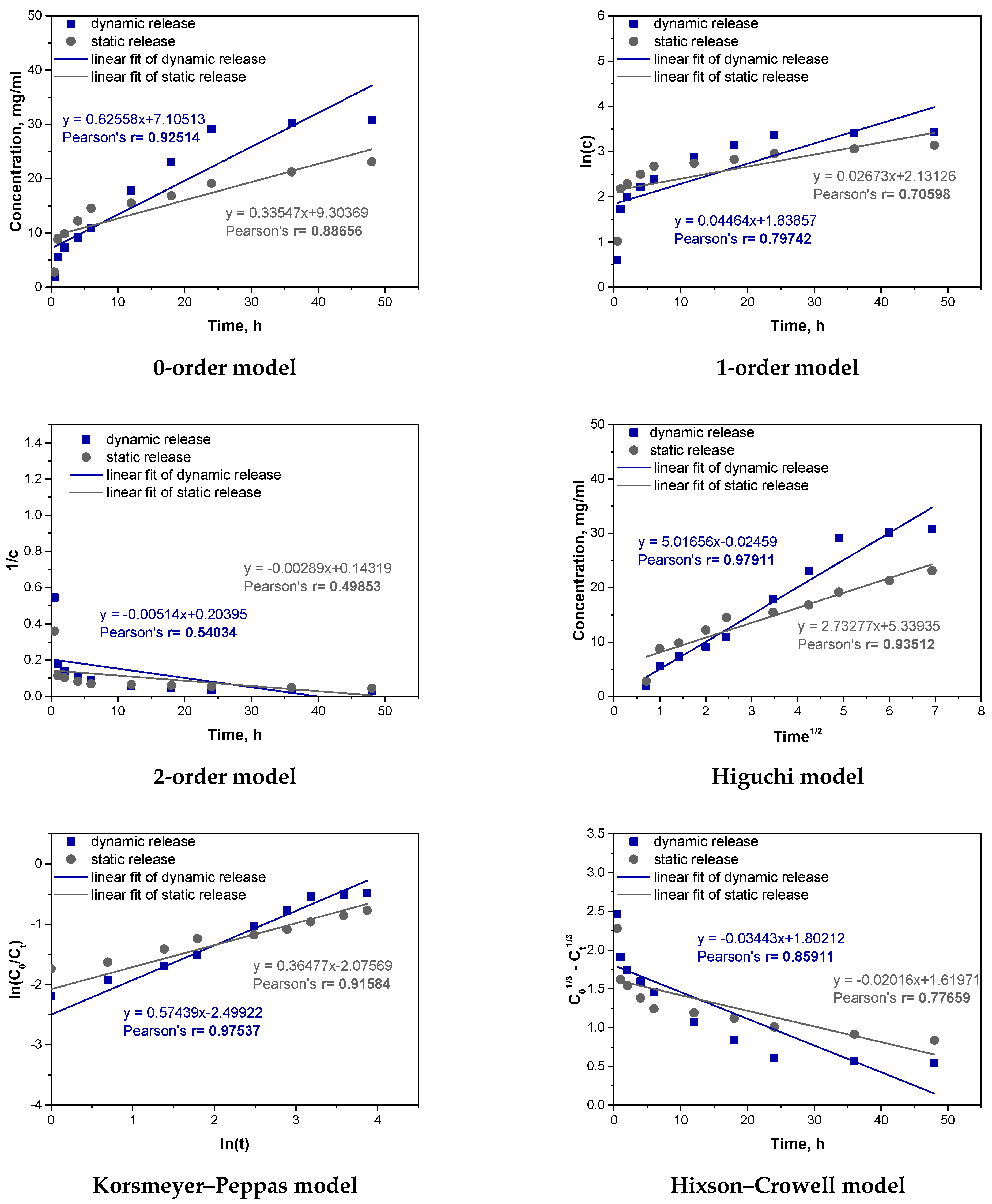
Next, similar fitting was carried out for a sample of the hybrid system. The results are presented in
Figure 15 and
Table 6.
For the hybrid system (a hydrogel containing vitamin C microcapsules), the best kinetic fit was obtained for the Higuchi model (r = 0.97911 dynamic, r = 0.93512 static) and the Korsmeyer–Peppas model (r = 0.97537 dynamic, r = 0.91584 static). This suggests that the release mechanism is complex, involving both diffusion and swelling. In dynamic conditions, where agitation enhances the interaction with the medium, swelling plays a larger role, as reflected by the Korsmeyer–Peppas model’s better fit. In static conditions, limited swelling emphasizes diffusion, aligning well with the Higuchi model. The release rate of vitamin C in the hybrid system was slower compared to the microcapsule and hydrogel samples, with 30.836 mg released in dynamic and 23.085 mg in static conditions, versus higher releases in the other samples. This slower, more controlled release results from the dual barriers of the hydrogel and microcapsules, creating additional resistance to diffusion. Compared to previous analyses, the hybrid system’s structure offers a slower, more sustained release, unlike the microcapsules, which provided a faster, diffusion-dominated release. The hybrid system’s dual-barrier structure makes it suitable for applications requiring prolonged, stable release of active compounds, valuable in drug delivery contexts.