The Thermomechanical, Functional and Biocompatibility Properties of a Au–Pt–Ge Alloy for PFM Dental Restorations
Abstract
1. Introduction
- High-noble alloys, characterised by a noblemetal content of a minimum of 60 wt% and a gold content of at least 40%.
- Noble alloys, containing a noblemetal content of at least 25%, with no specific requirement for gold content.
- Predominantly base metal alloys, possessing a noblemetal content below 25% [19].
2. Materials and Methods
2.1. Study Design for Determining Dental Alloy Properties for Clinical Use
2.2. Alloy Production Experiment Parameters
2.3. Biocompatibility Evaluation
2.3.1. Sample Preparation for Biocompatibility Testing
2.3.2. MTT Assay
2.4. Static Immersion Testing
2.4.1. Preparation of Solution—Artificial Saliva
2.4.2. Sample Preparation
2.4.3. Testing Procedure
2.5. DSC Analysis
2.6. Dilatometric Coefficient (CTE) Analysis
2.7. Hardness
2.8. Tensile Testing
2.9. Density Measurements
2.10. Microstructure Investigations
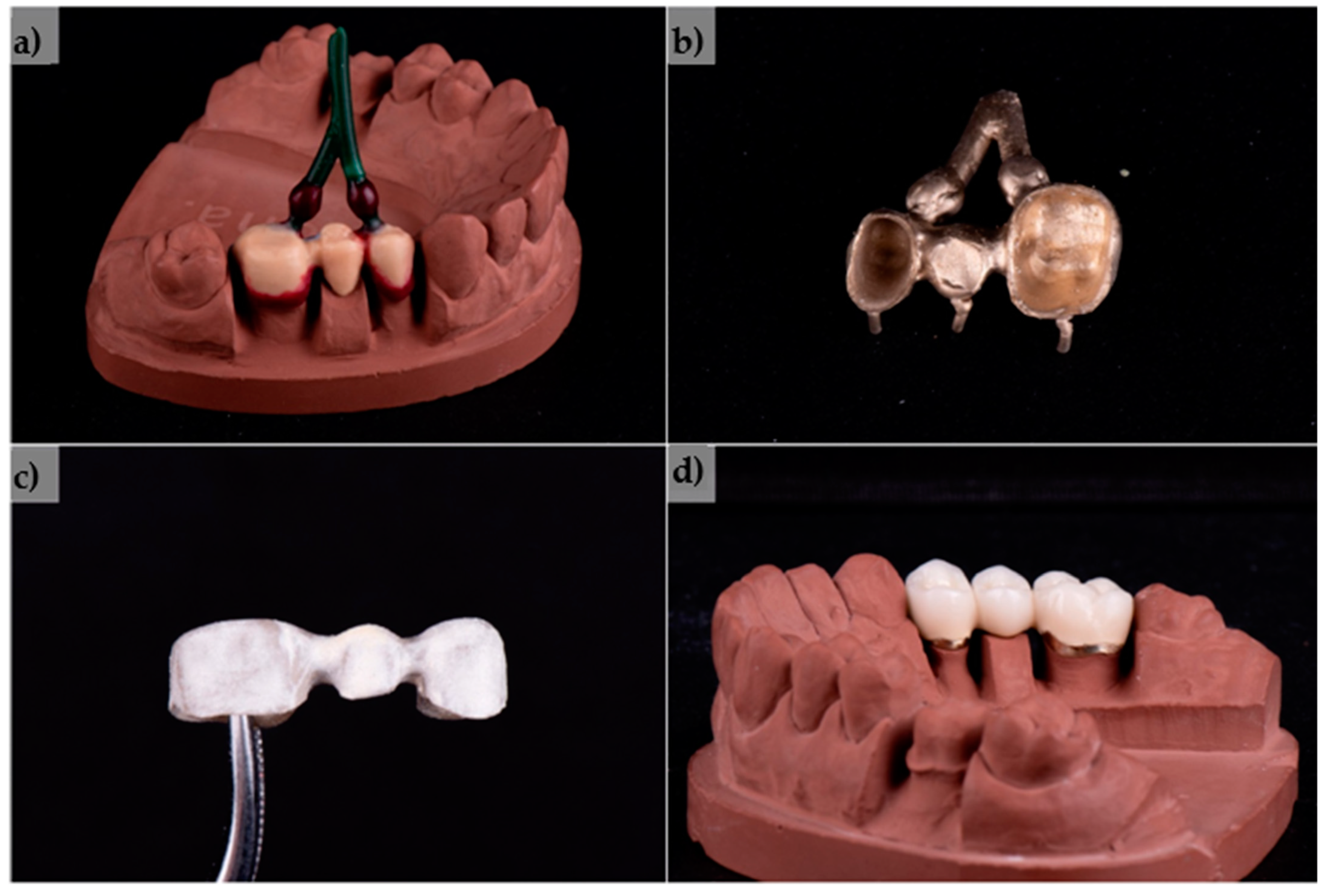
2.11. Production of a Test PFM Dental Bridge
2.12. Statistical Analysis
3. Results
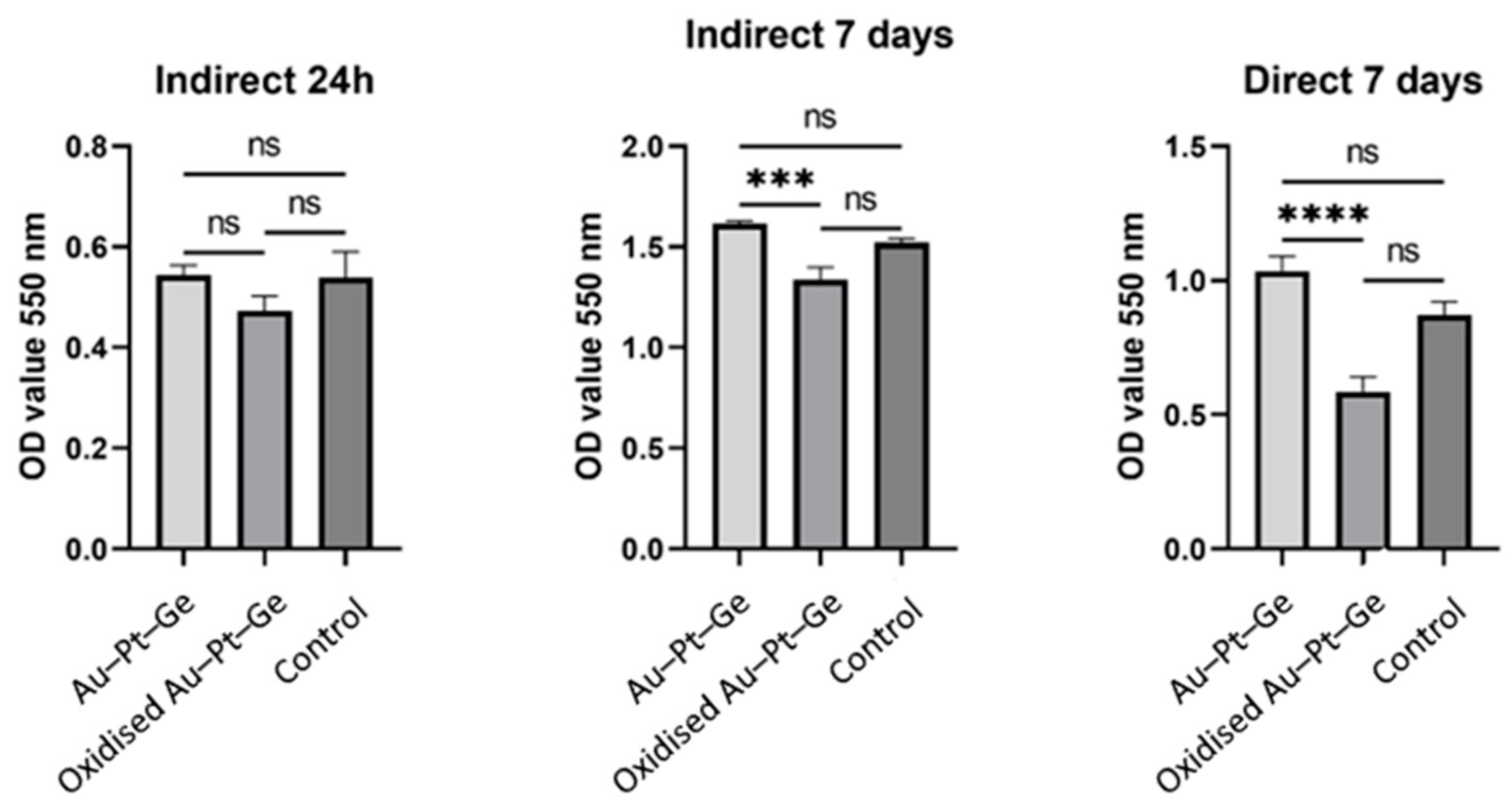
3.1. Biocompatibility
3.2. ICP Analysis of Solutions After Immersion Testing
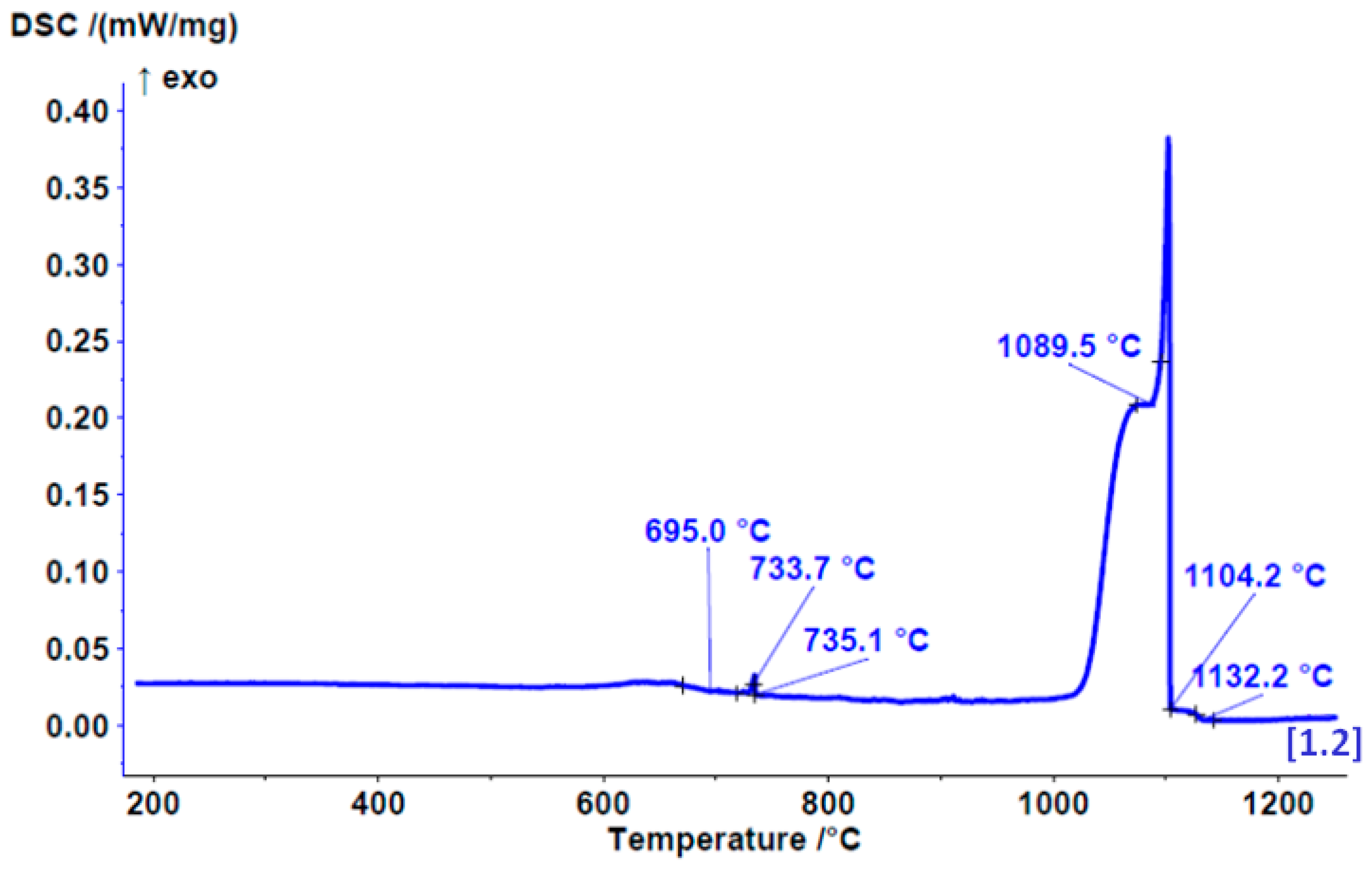
3.3. DSC Results
3.4. CTE Results
3.5. Microhardness
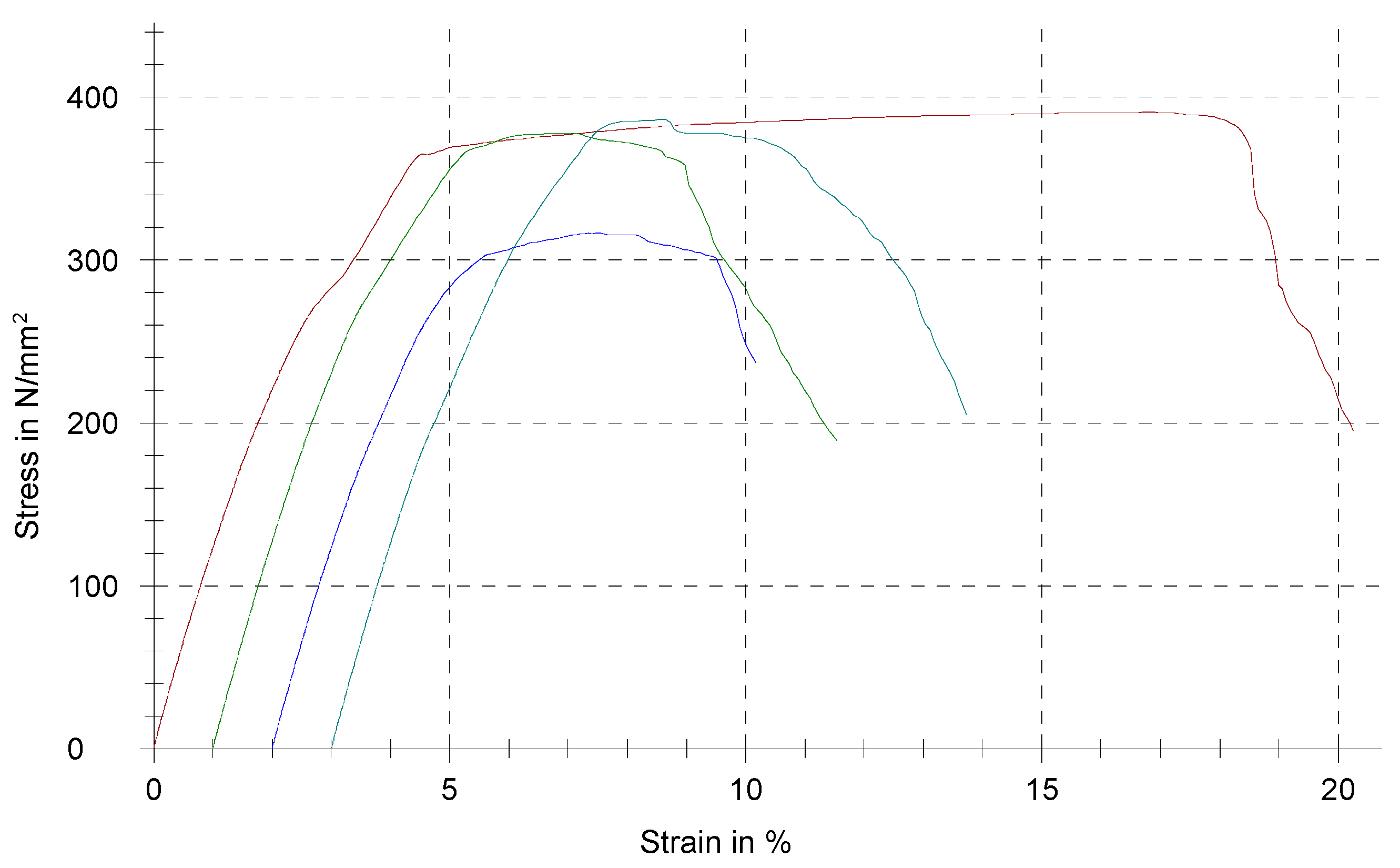
3.6. Tensile Testing Results
3.7. Density
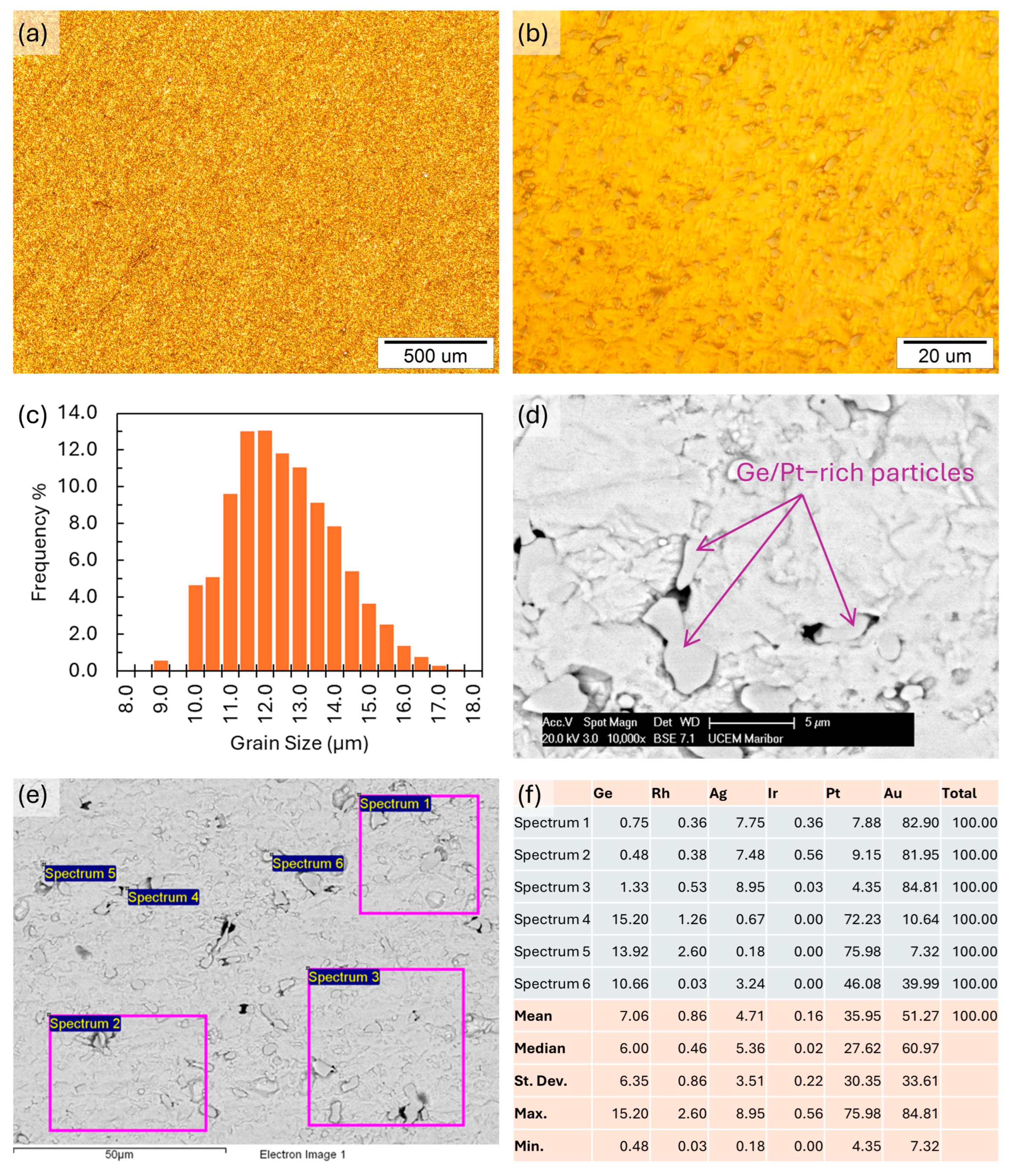
3.8. Microstructure Investigation Results
3.9. Produced Test PFM Dental Bridge
4. Discussion
5. Conclusions
- Based on the analyses of the manufactured alloy, its thermomechanical properties are suitable for applications in dentistry for prosthetic products as a type III alloy. It is suitable for multiple-unit fixed prostheses, while not being appropriate for extensive multiunit fixed restorations. The cast alloy has a finely grained microstructure with Ge segregations, which decrease its mechanical properties.
- A relatively low melting point and slightly higher CTE than other conventional dental alloys requires the careful selection of processing parameters and porcelain combinations for the production of PFM dental restorations.
- The measured metal ion release from immersion testing was minimal, demonstrating that the alloy is biologically safe for use in an oral environment and is highly suitable for patients prone to metal allergies.
- The production of the three-unit PFM dental bridge was performed with no major difficulties in processing, being less demanding and faster than with base metals and resulting in the production of a high-quality dental restoration.
- The cell viability examination for as-cast and polished alloy samples showed significantly higher viability ratings on the polished samples, demonstrating the improved biocompatibility of polished casting alloys compared to their as-cast counterparts. The results show that a dental substructure in direct contact with the surrounding tissue and susceptible to exposure to oral and subgingival fluids should be highly polished.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khmaj, M.R.; Khmaj, A.B.; Brantley, W.A.; Johnston, W.M.; Dasgupta, T. Comparison of the Metal-to-Ceramic Bond Strengths of Four Noble Alloys with Press-on-Metal and Conventional Porcelain Layering Techniques. J. Prosthet. Dent. 2014, 112, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Dolgov, N.; Dikova, T.; Dzhendov, D.; Pavlova, D.; Simov, M. Mechanical Properties of Dental Co-Cr Alloys Fabricated via Casting and Selective Laser Melting. Mater. Sci. Non-Equilib. Phase Transform. 2016, 2, 3–7. [Google Scholar]
- Nakano, T.; Hagihara, K.; Ribeiro, A.R.; Fujii, Y.; Todo, T.; Fukushima, R.; Rocha, L.A. Orientation Dependence of the Wear Resistance in the Co–Cr–Mo Single Crystal. Wear 2021, 478–479, 203758. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Gopalakrishnan, U.; Felicita, A.S.; Mahendra, L.; Kanji, M.A.; Varadarajan, S.; Raj, A.T.; Feroz, S.M.A.; Mehta, D.; Baeshen, H.A.; Patil, S. Assessing the Potential Association Between Microbes and Corrosion of Intra-Oral Metallic Alloy-Based Dental Appliances Through a Systematic Review of the Literature. Front Bioeng. Biotechnol. 2021, 9, 631103. [Google Scholar] [CrossRef]
- Urbutytė, K.; Barčiūtė, A.; Lopatienė, K. The Changes in Nickel and Chromium Ion Levels in Saliva with Fixed Orthodontic Appliances: A Systematic Review. Appl. Sci. 2023, 13, 4739. [Google Scholar] [CrossRef]
- Kulkarni, P.; Agrawal, S.; Bansal, A.; Jain, A.; Tiwari, U.; Anand, A. Assessment of Nickel Release from Various Dental Appliances Used Routinely in Pediatric Dentistry. Indian J. Dent. 2016, 7, 81. [Google Scholar] [CrossRef]
- Vastag, G.; Majerič, P.; Lazić, V.; Rudolf, R. Electrochemical Behaviour of an Au-Ge Alloy in an Artificial Saliva and Sweat Solution. Metals 2024, 14, 668. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Lazić, V.; Grgur, B. Development of a New AuCuZnGe Alloy and Determination of Its Corrosion Properties. Metals 2022, 12, 1284. [Google Scholar] [CrossRef]
- Kancyper, S.G.; Koka, S. The Influence of Intracrevicular Crown Margins on Gingival Health: Preliminary Findings. J. Prosthet. Dent. 2001, 85, 461–465. [Google Scholar] [CrossRef]
- Behr, M.; Zeman, F.; Baitinger, T.; Galler, J.; Koller, M.; Handel, G.; Rosentritt, M. The Clinical Performance of Porcelain-Fused-to-Metal Precious Alloy Single Crowns: Chipping, Recurrent Caries, Periodontitis, and Loss of Retention. Int. J. Prosthodont. 2014, 27, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Shiraishi, T.; Al-Salehi, S.K. Ion Release from Experimental Au–Pt-Based Metal–Ceramic Alloys. Dent. Mater. 2010, 26, 682–687. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Özcan, M. Fracture Reasons in Ceramic-Fused-to-Metal Restorations. J. Oral Rehabil. 2003, 30, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Haugen, H.J.; Soltvedt, B.M.; Nguyen, P.N.; Ronold, H.J.; Johnsen, G.F. Discrepancy in Alloy Composition of Imported and Non-Imported Porcelain-Fused-to-Metal (PFM) Crowns Produced by Norwegian Dental Laboratories. Biomater. Investig. Dent. 2020, 7, 41. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Boșca, A.B.; Băbțan, A.M.; Vermeșan, H.; Cristea, C.; Tertiș, M.; Pășcuță, P.; Borodi, G.; Suciu, M.; Barbu-Tudoran, L.; et al. Study on the Surface of Cobalt-Chromium Dental Alloys and Their Behavior in Oral Cavity as Cast Materials. Materials 2022, 15, 3052. [Google Scholar] [CrossRef]
- Lojen, G.; Stambolić, A.; Šetina Batič, B.; Rudolf, R. Experimental Continuous Casting of Nitinol. Metals 2020, 10, 505. [Google Scholar] [CrossRef]
- Lugscheider, E.; Knotek, O.; Klöhn, K. Melting Behaviour of Nickel—Chromium—Silicon Alloys. Thermochim. Acta 1979, 29, 323–326. [Google Scholar] [CrossRef]
- Chapter 10—Restorative Materials: Metals. In Craig’s Restorative Dental Materials, 14th ed.; Sakaguchi, R., Ferracane, J., Powers, J., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 171–208. ISBN 978-0-323-47821-2. [Google Scholar]
- Grgur, B.N.; Lazić, V.; Stojić, D.; Rudolf, R. Electrochemical Testing of Noble Metal Dental Alloys: The Influence of Their Chemical Composition on the Corrosion Resistance. Corros. Sci. 2021, 184, 109412. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 22674:2022 Dentistry Metallic Materials for Fixed and Removable Restorations and Appliances, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2022. [Google Scholar]
- Lazić, M.; Lazić, M.M.; Karišik, M.J.; Lazarević, M.; Jug, A.; Anžel, I.; Milašin, J. Biocompatibility Study of a Cu-Al-Ni Rod Obtained by Continuous Casting. Processes 2022, 10, 1507. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993-5:2009 Biological Evaluation of Medical Devices, Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. ISO 10271:2020 Dentistry—Corrosion Test Methods for Metallic Materials, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- International Organization for Standardization. ISO 6507-1:1997 Metallic Materials—Vickers Hardness Test, Part 1: Test Method, 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 1997. [Google Scholar]
- Lopes, S.C.; Pagnano, V.O.; De Almeida Rollo, J.M.D.; Leal, M.B.; Bezzon, O.L. Correlation between Metal-Ceramic Bond Strength and Coefficient of Linear Thermal Expansion Difference. J. Appl. Oral Sci. 2009, 17, 122–128. [Google Scholar] [CrossRef]
- Lazić, V.; Stamenković, D.; Todorović, A.; Rudolf, R.; Anžel, I. Investigation of Mechanical and Biomedical Properties of New Dental Alloy with High Content of Au. J. Metall. 2008, 14, 121–134. [Google Scholar]
- Faurschou, A.; Menné, T.; Johansen, J.D.; Thyssen, J.P. Metal Allergen of the 21st Century—A Review on Exposure, Epidemiology and Clinical Manifestations of Palladium Allergy. Contact Dermat. 2011, 64, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Bordel-Gómez, M.T.; Miranda-Romero, A. Palladium Allergy: A Frequent Sensitisation. Allergol. Immunopathol. 2008, 36, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Ahlström, M.G.; Thyssen, J.P.; Menné, T.; Johansen, J.D. Prevalence of Nickel Allergy in Europe Following the EU Nickel Directive—A Review. Contact Dermat. 2017, 77, 193–200. [Google Scholar] [CrossRef]
- Muris, J.; Scheper, R.J.; Kleverlaan, C.J.; Rustemeyer, T.; Van Hoogstraten, I.M.W.; Von Blomberg, M.E.; Feilzer, A.J. Palladium-Based Dental Alloys Are Associated with Oral Disease and Palladium-Induced Immune Responses. Contact Dermat. 2014, 71, 82–91. [Google Scholar] [CrossRef]
- Shigematsu, H.; Kumagai, K.; Suzuki, M.; Eguchi, T.; Matsubara, R.; Nakasone, Y.; Nasu, K.; Yoshizawa, T.; Ichikawa, H.; Mori, T.; et al. Cross-Reactivity of Palladium in a Murine Model of Metal-Induced Allergic Contact Dermatitis. Int. J. Mol. Sci. 2020, 21, 4061. [Google Scholar] [CrossRef]
- Thompson, V.P.; Rekow, E.D. 23—Dental Ceramics. In Bioceramics and Their Clinical Applications; Kokubo, T., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 518–547. ISBN 978-1-84569-204-9. [Google Scholar]
- Knosp, H.; Nawaz, M.; Stümke, M. Dental Gold Alloys: Composition, Properties and Applications. Gold Bull. 1981, 14, 57–64. [Google Scholar] [CrossRef]
- Farzin, M.; Giti, R.; Asalforush-Rezaiye, A. The Effect of Multiple Firings on the Shear Bond Strength of Porcelain to a New Millable Alloy and a Conventional Casting Alloy. Materials 2018, 11, 478. [Google Scholar] [CrossRef]
- Rudolf, R.; Lazić, V.; Majerič, P.; Ivanič, A.; Kravanja, G.; Raić, K.T. Dental Gold Alloys and Gold Nanoparticles for Biomedical Applications; SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-98745-9. [Google Scholar]
- Peretti, D.; Di Siro, M.; Di Siro, S. Nickel—And Palladium-Free Master Alloys for All Karats of White Gold—The Santa Fe Symposium. In Proceedings of the Santa Fe Symposium on Jewelry Manufacturing Technology, Albuquerque, NM, USA, 21–24 May 2017; pp. 343–362. [Google Scholar]
- Haugli, K.H.; Syverud, M.; Samuelsen, J.T. Ion Release from Three Different Dental Alloys—Effect of Dynamic Loading and Toxicity of Released Elements. Biomater. Investig. Dent. 2020, 7, 71–79. [Google Scholar] [CrossRef]
- Tomova, Z.; Vlahova, A.; Zlatev, S.; Stoeva, I.; Tomov, D.; Davcheva, D.; Hadzhigaev, V. Clinical Evaluation of Corrosion Resistance, Ion Release, and Biocompatibility of CoCr Alloy for Metal-Ceramic Restorations Produced by CAD/CAM Technologies. Dent. J. 2023, 11, 166. [Google Scholar] [CrossRef]
- Keith, L.S.; Maples-Reynolds, N. Germanium. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 289–316. [Google Scholar]
- Craig, R.G.; Hanks, C.T. Cytotoxicity of Experimental Casting Alloys Evaluated by Cell Culture Tests. J. Dent. Res. 1990, 69, 1539–1542. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.G.; Hanks, C.T. Reaction of Fibroblasts to Various Dental Casting Alloys. J. Oral Pathol. 1988, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kansu, G.; Aydin, A.K. Evaluation of the Biocompatibility of Various Dental Alloys: Part I—Toxic Potentials. Eur. J. Prosthodont. Restor. Dent. 1996, 4, 129–136. [Google Scholar] [PubMed]
- Rutkunas, V.; Bukelskiene, V.; Sabaliauskas, V.; Balciunas, E.; Malinauskas, M.; Baltriukiene, D. Assessment of Human Gingival Fibroblast Interaction with Dental Implant Abutment Materials. J. Mater. Sci. Mater. Med. 2015, 26, 1–9. [Google Scholar] [CrossRef]
- Bergman, M. Corrosion in the Oral Cavity--Potential Local and Systemic Effects. Int. Dent. J. 1986, 36, 41–44. [Google Scholar]
- Berstein, A.; Bemauer, I.; Marx, R.; Geurtsen, W. Human Cell Culture Studies with Dental Metallic Materials. Biomaterials 1992, 13, 98–100. [Google Scholar] [CrossRef]
- Schmalz, G. Biological Interactions of Dental Cast Alloys with Oral Tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- Schmalz, G.; Langer, H.; Schweikl, H. Cytotoxicity of Dental Alloy Extracts and Corresponding Metal Salt Solutions. J. Dent. Res. 1998, 77, 1772–1778. [Google Scholar] [CrossRef]
- Celinski, A.I. The Influence of Oxide-Forming Elements on Corrosion and Biocompatibility of Experimental PFM Alloys; Eberhard Karls University of Tübingen: Tübingen, Germany, 2006. [Google Scholar]
- Wataha, J.C.; Lockwood, P.E.; Nelson, S.K. Initial versus Subsequent Release of Elements from Dental Casting Alloys. J. Oral Rehabil. 1999, 26, 798–803. [Google Scholar] [CrossRef]
- Wataha, J.C.; Malcolm, C.T. Effect of Alloy Surface Composition on Release of Elements from Dental Casting Alloys. J. Oral Rehabil. 1996, 23, 583–589. [Google Scholar] [CrossRef]




| Ag | Au | Be | Cd | Ge | In | Ir | Ni | Pb | Pd | Pt | Rh | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 1.622 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.622 |
| Measurement | Microhardness HV5 |
|---|---|
| 1 | 130.1 |
| 2 | 125.4 |
| 3 | 129.6 |
| 4 | 128.1 |
| 5 | 124.9 |
| 6 | 124.9 |
| Mean | 127.17 |
| Median | 126.75 |
| Min. | 124.9 |
| Max. | 130.1 |
| St. Dev. | 2.19 |
| Measurement | Rp 0.2 (N/mm2) | L0 (mm) | E-Modulus (N/mm2) | Rm (N/mm2) | e Break (%) |
|---|---|---|---|---|---|
| 1 | 266.12 | 15.00 | 10,846.07 | 390.39 | 20.26 |
| 2 | 277.65 | 15.00 | 11,397.78 | 377.50 | 10.53 |
| 3 | 266.50 | 15.00 | 10,665.14 | 316.24 | 8.17 |
| 4 | 286.68 | 15.00 | 10,931.50 | 386.20 | 10.72 |
| Mean | 274.24 | 15.00 | 10,960.12 | 367.58 | 12.42 |
| Median | 272.08 | 15.00 | 10,888.79 | 381.85 | 10.63 |
| Standard Dev. | 8.55 | 0.00 | 270.36 | 30.01 | 4.64 |
| Measurement | Mass of Pycnometer and Liquid Without Sample (g) | Mass of Pycnometer and Liquid with Sample (g) | Alloy Density (g/cm3) |
|---|---|---|---|
| 1 | 40.4202 | 43.2638 | 16.1659 |
| 2 | 40.4197 | 43.2659 | 16.3937 |
| 3 | 40.4171 | 43.2676 | 16.7849 |
| 4 | 40.4173 | 43.2640 | 16.4383 |
| 5 | 40.4182 | 43.2661 | 16.5462 |
| Mean | 40.4185 | 43.2655 | 16.4633 |
| Median | 40.4182 | 43.2659 | 16.4383 |
| Standard Dev. | 0.0014 | 0.0016 | 0.2259 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majerič, P.; Lazić, M.M.; Mitić, D.; Lazić, M.; Lazić, E.K.; Vastag, G.; Anžel, I.; Lazić, V.; Rudolf, R. The Thermomechanical, Functional and Biocompatibility Properties of a Au–Pt–Ge Alloy for PFM Dental Restorations. Materials 2024, 17, 5491. https://doi.org/10.3390/ma17225491
Majerič P, Lazić MM, Mitić D, Lazić M, Lazić EK, Vastag G, Anžel I, Lazić V, Rudolf R. The Thermomechanical, Functional and Biocompatibility Properties of a Au–Pt–Ge Alloy for PFM Dental Restorations. Materials. 2024; 17(22):5491. https://doi.org/10.3390/ma17225491
Chicago/Turabian StyleMajerič, Peter, Minja Miličić Lazić, Dijana Mitić, Marko Lazić, Ema Krdžović Lazić, Gyöngyi Vastag, Ivan Anžel, Vojkan Lazić, and Rebeka Rudolf. 2024. "The Thermomechanical, Functional and Biocompatibility Properties of a Au–Pt–Ge Alloy for PFM Dental Restorations" Materials 17, no. 22: 5491. https://doi.org/10.3390/ma17225491
APA StyleMajerič, P., Lazić, M. M., Mitić, D., Lazić, M., Lazić, E. K., Vastag, G., Anžel, I., Lazić, V., & Rudolf, R. (2024). The Thermomechanical, Functional and Biocompatibility Properties of a Au–Pt–Ge Alloy for PFM Dental Restorations. Materials, 17(22), 5491. https://doi.org/10.3390/ma17225491









