Poly-Amino-β-Cyclodextrin Microparticles for the Reduction of Xenobiotics and Emerging Contaminants, Including Pharmaceuticals, from the Natural Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Encapsulation Process
2.2. Adsorption Experiment
2.3. High-Perfprmance Liquid Chromatografy (HPLC) Methods
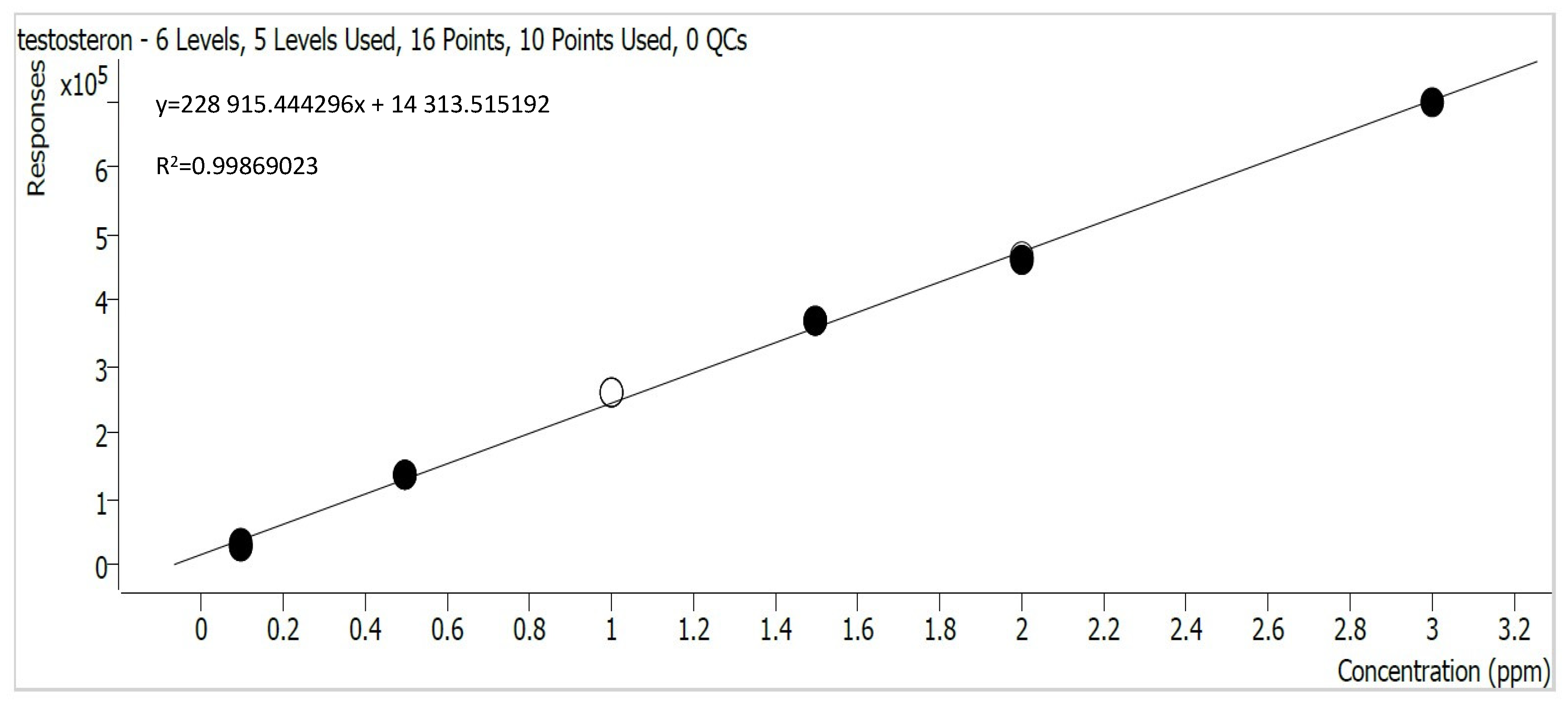
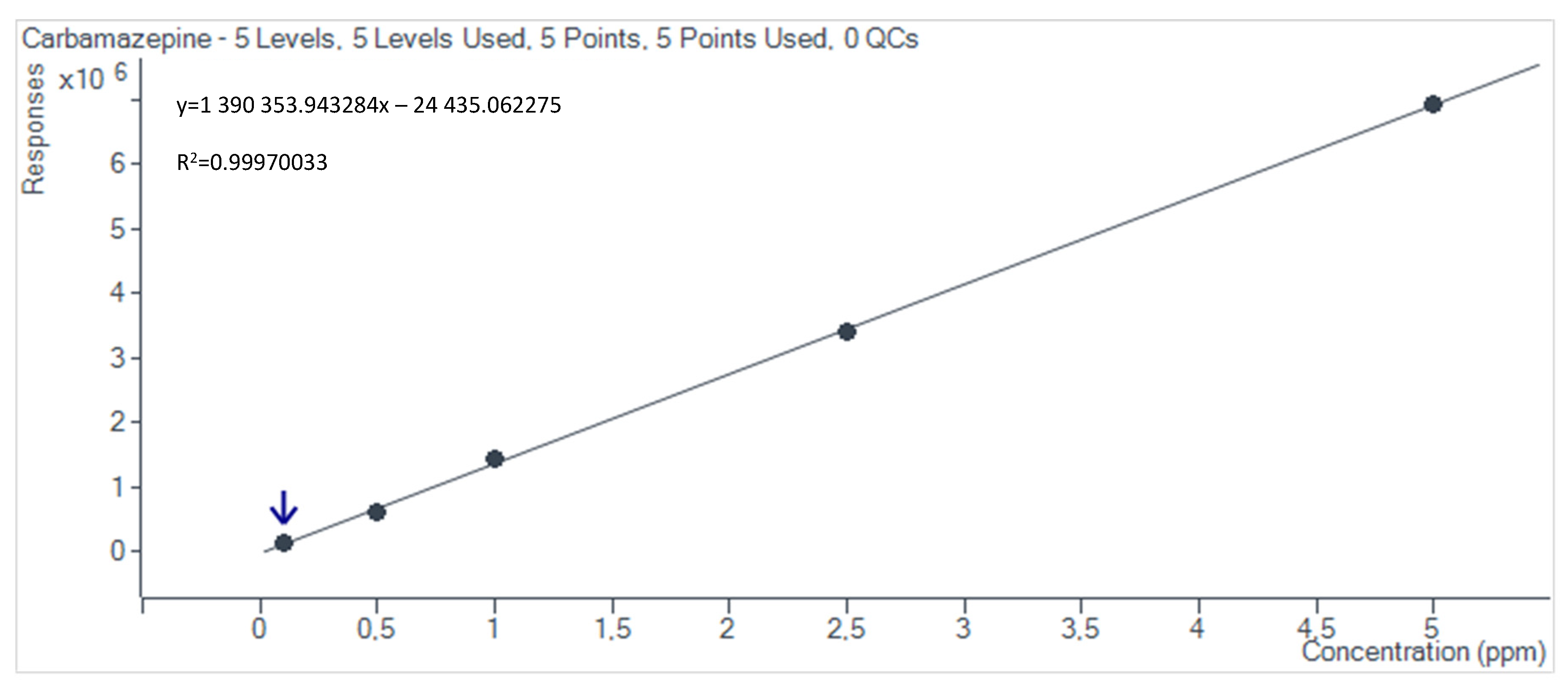
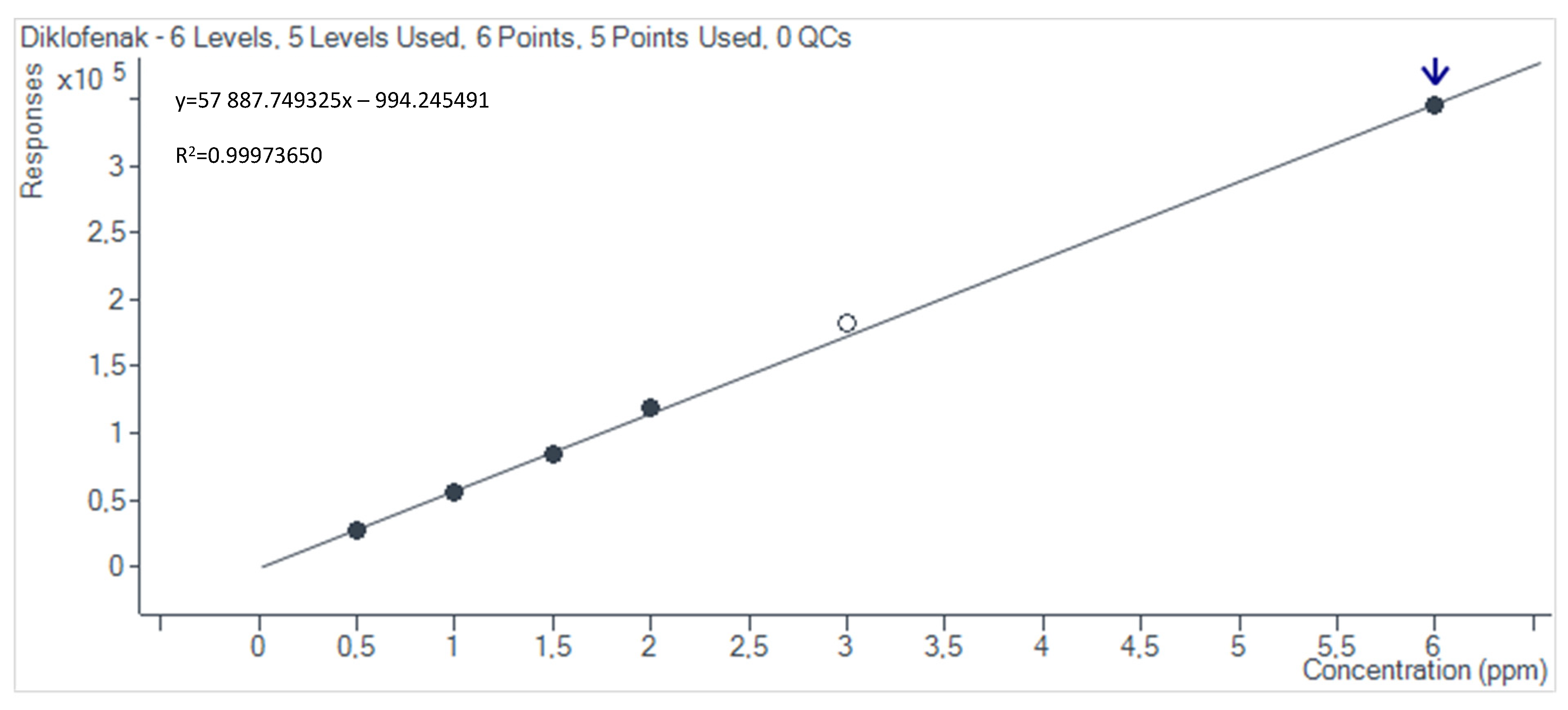
2.4. Preparation of HPLC Calibration Curves
3. Results and Discussion
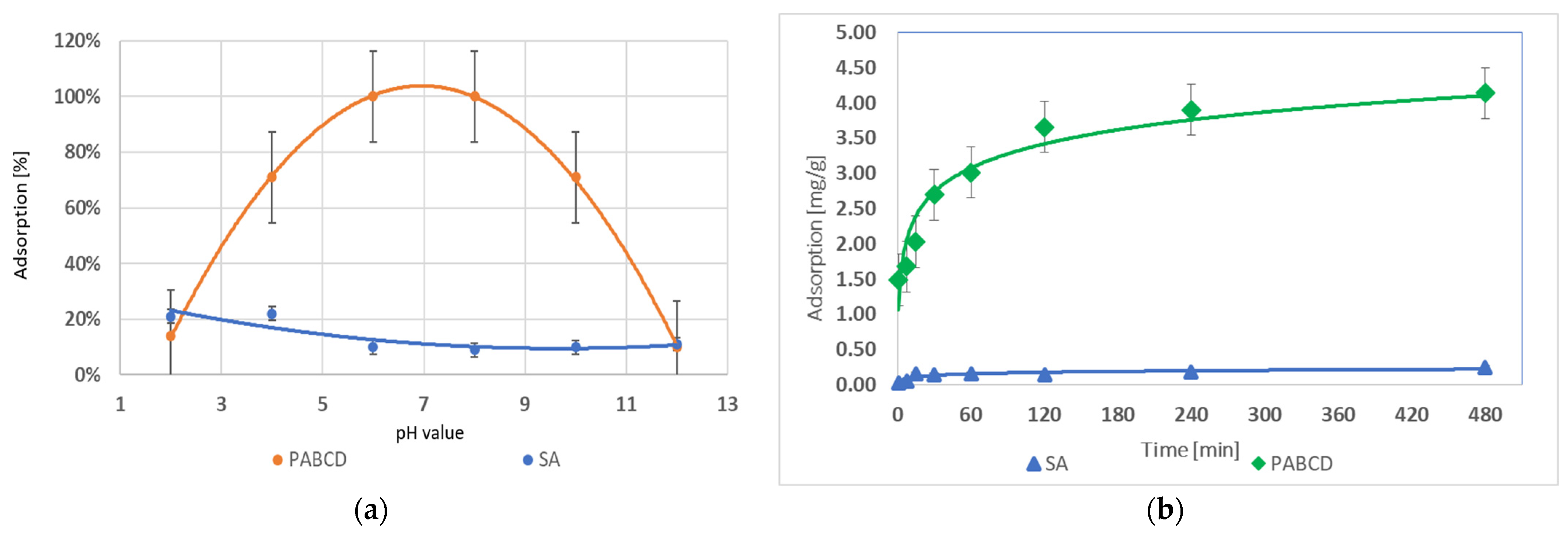
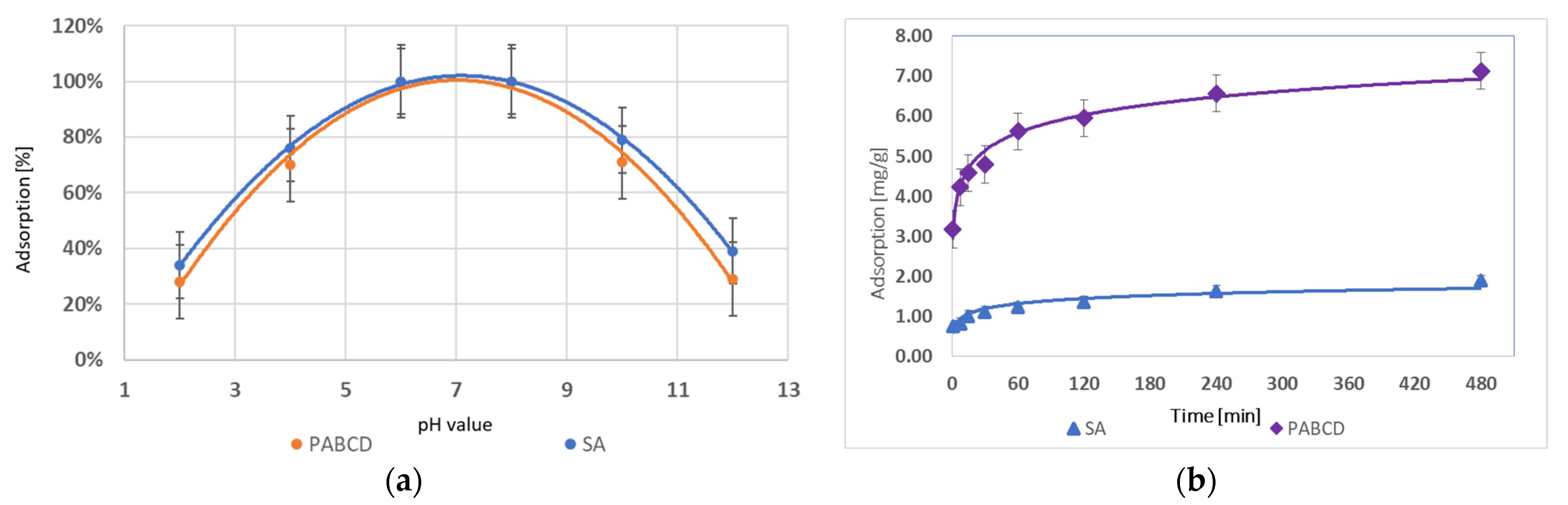
3.1. Testosterone and Progesterone Adsorption
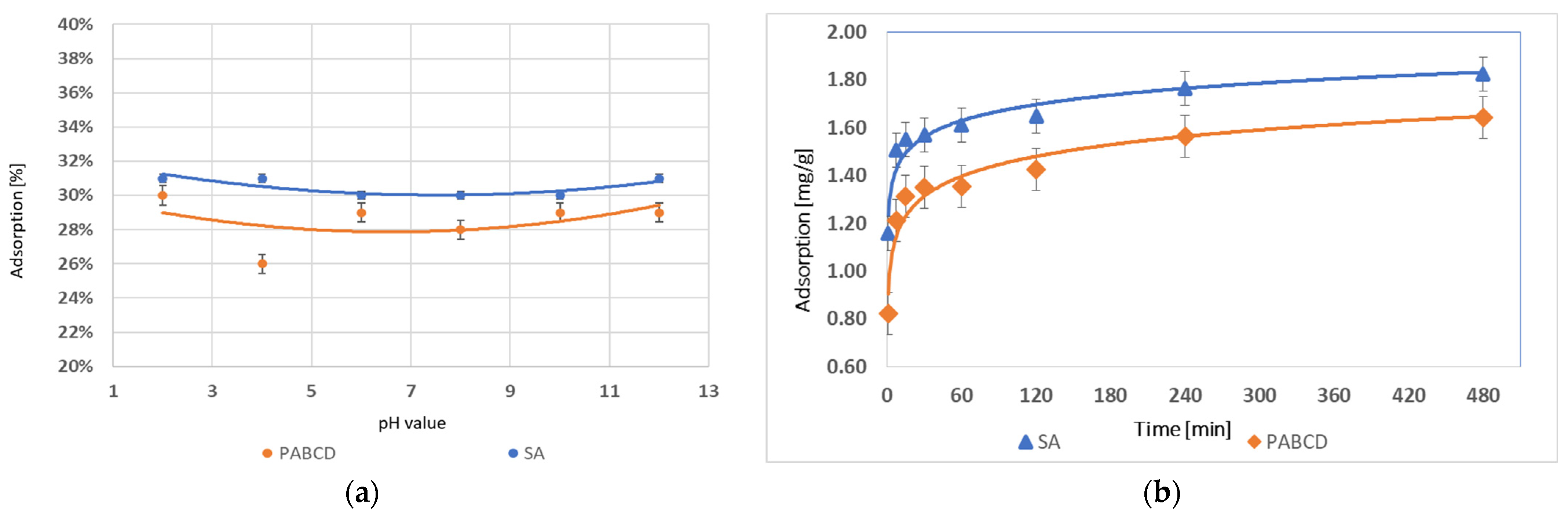
3.2. Non-Steroidal Anti-Inflammatory Drug Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, W.; Jin, X.; Cong, Y.; Liu, Y.; Fu, J. Degradable natural polymer hydrogels for articular cartilage tissue engineering. J. Chem. Technol. Biotechnol. 2013, 88, 327–339. [Google Scholar] [CrossRef]
- Shetye, S.P.; Godbole, D.A.M.; Bhilegaokar, D.S.; Gajare, P.S. Hydrogels: Introduction, Preparation, Characterization and Applications. Hum. J. 2015, 1, 47–71. [Google Scholar]
- Gao, X.; Guo, C.; Hao, J.; Zhao, Z.; Long, H.; Li, M. Adsorption of heavy metal ions by sodium alginate based adsorbent-a review and new perspectives. Int. J. Biol. Macromol. 2020, 164, 4423–4434. [Google Scholar] [CrossRef]
- Shao, Z.-J.; Huang, X.-L.; Yang, F.; Zhao, W.-F.; Zhou, X.-Z.; Zhao, C.-S. Engineering sodium alginate-based cross-linked beads with high removal ability of toxic metal ions and cationic dyes. Carbohydr. Polym. 2018, 187, 85–93. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Dodziuk, H. Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Wiley-VCH: Weinheim, Germany, 2006; pp. 1–489. [Google Scholar]
- Sliwa, W.; Girek, T. Cyclodextrins: Properties and Applications; Wiley-VCH: Weinheim, Germany, 2017; p. 336. [Google Scholar]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zhong, N.; Huang, Y.; He, K.; Ye, X. Injectable in situ dual-crosslinking hyaluronic acid and sodium alginate based hydrogels for drug release. J. Biomater. Sci. Polym. Ed. 2019, 30, 995–1007. [Google Scholar] [CrossRef]
- Batool, S.R.; Nazeer, M.A.; Ekinci, D.; Sahin, A.; Kizilel, S. Multifunctional alginate-based hydrogel with reversible crosslinking for controlled therapeutics delivery. Int. J. Biol. Macromol. 2020, 150, 315–325. [Google Scholar] [CrossRef]
- Siboro, S.A.P.; Anugrah, D.S.B.; Ramesh, K.; Park, S.-H.; Kim, H.-R.; Lim, K.T. Tunable porosity of covalently crosslinked alginate-based hydrogels and its significance in drug release behavior. Carbohydr. Polym. 2021, 260, 117779. [Google Scholar] [CrossRef]
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Lucas-Abellán, C.; Mercader-Ros, M.T.; López-Miranda, S.; Gómez-López, V.M.; Semeraro, P.; Cosma, P.; Fini, P. Adsorption properties of β-and hydroxypropyl-β-cyclodextrins cross-linked with epichlorohydrin in aqueous solution. A sustainable recycling strategy in textile dyeing process. Polymers 2019, 11, 252. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakamitsu, Y.; Zheng, Y.; Takashima, Y.; Yamaguchi, H.; Harada, A. Preparation of cyclodextrin-based porous polymeric membrane by bulk polymerization of ethyl acrylate in the presence of cyclodextrin. Polymer 2019, 177, 208–213. [Google Scholar] [CrossRef]
- Rojas-Aguirre, Y.; Torres-Mena, M.A.; López-Méndez, L.J.; Alcaraz-Estrada, S.L.; Guadarrama, P.; Urucha-Ortíz, J.M. PEGylated β-cyclodextrins: Click synthesis and in vitro biological insights. Carbohydr. Polym. 2019, 223, 115113. [Google Scholar] [CrossRef] [PubMed]
- Malanga, M.; Seggio, M.; Kirejev, V.; Fraix, A.; Di Bari, I.; Fenyvesi, E.; Ericson, M.B.; Sortino, S. A phototherapeutic fluorescent β-cyclodextrin branched polymer delivering nitric oxide. Biomater. Sci. 2019, 7, 2272–2276. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Verma, A.; Raizada, P.; Gunduz, O.; Janas, D.; Alsanie, W.F.; Scarpa, F.; Thakur, V.K. Bentonite-based sodium alginate/dextrin cross-linked poly (acrylic acid) hydrogel nanohybrids for facile removal of paraquat herbicide from aqueous solutions. Chemosphere 2022, 291, 133002. [Google Scholar] [CrossRef]
- Girek, T.; Koziel, K.; Girek, B.; Ciesielski, W. CD oxyanions as a tool for synthesis of highly anionic cyclodextrin polymers. Polymers 2020, 12, 2845. [Google Scholar] [CrossRef]
- Ciesielska, A.; Ciesielski, W.; Girek, B.; Girek, T.; Koziel, K.; Kulawik, D.; Lagiewka, J. Biomedical application of cyclodextrin polymers cross-linked via dianhydrides of carboxylic acids. Appl. Sci. 2020, 10, 8463. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef]
- Cecone, C.; Zanetti, M.; Anceschi, A.; Caldera, F.; Trotta, F.; Bracco, P. Microfibers of microporous carbon obtained from the pyrolysis of electrospun β-cyclodextrin/pyromellitic dianhydride nanosponges. Polym. Degrad. Stab. 2019, 161, 277–282. [Google Scholar] [CrossRef]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems-A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389–1460. [Google Scholar] [CrossRef] [PubMed]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: Current applications and future prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, K.; Łagiewka, J.; Girek, B.; Folentarska, A.; Girek, T.; Ciesielski, W. Synthesis of New Amino-β-Cyclodextrin Polymer, Cross-Linked with Pyromellitic Dianhydride and Their Use for the Synthesis of Polymeric Cyclodextrin Based Nanoparticles. Polymers 2021, 13, 1332. [Google Scholar] [CrossRef]
- Kozieł-Trąbska, K.; Żarska, S.; Girek, T.; Ciesielski, W. Characterization of New Polymer Material of Amino-β-Cyclodextrin and Sodium Alginate for Environmental Purposes. Membranes 2023, 13, 447. [Google Scholar] [CrossRef]
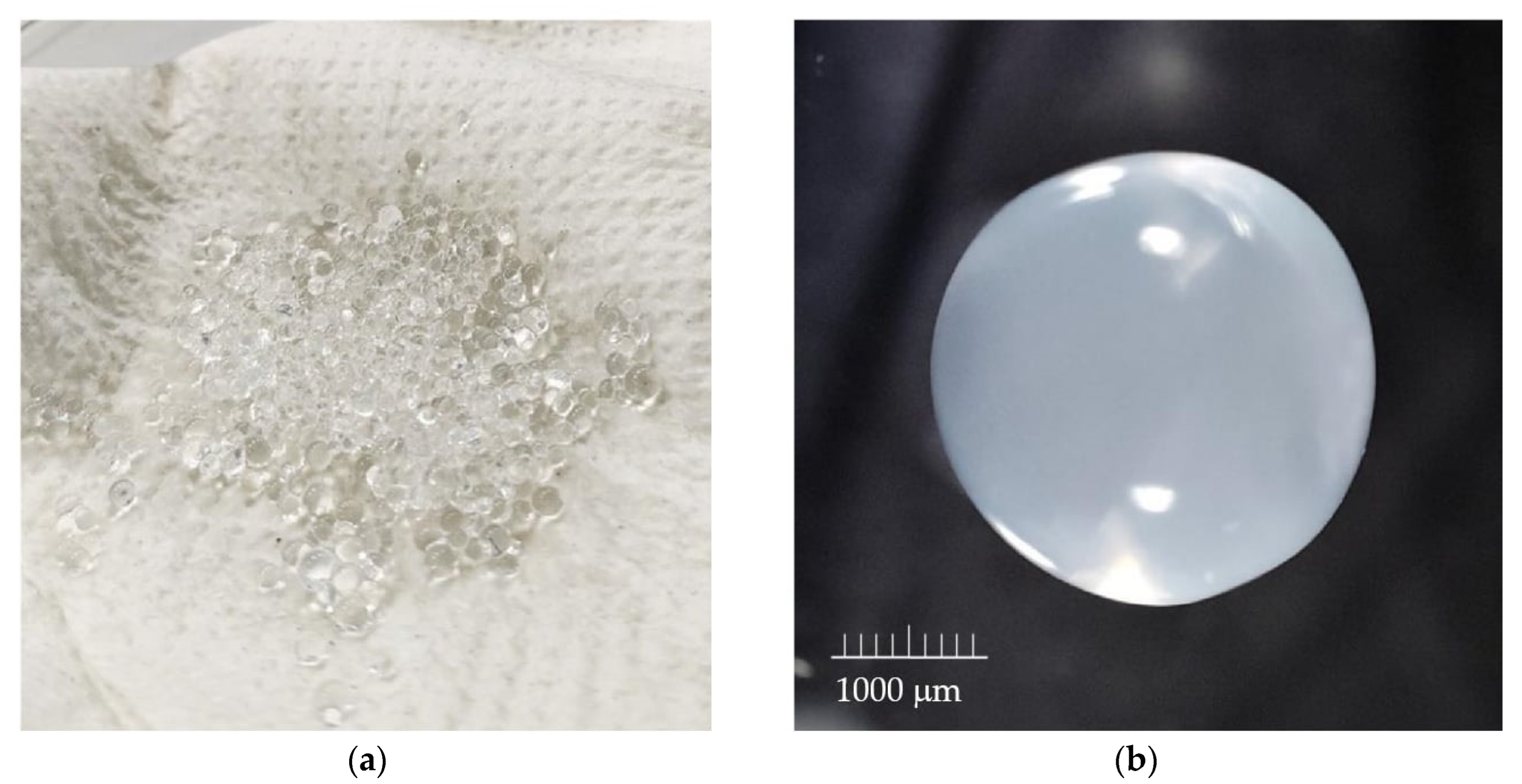


| Nozzle Diameter (µm) | Concentration of Sodium Alginate [%] | Flow Rate [mL/min] | Frequency [Hz] | Voltage [V] | Amplitude |
|---|---|---|---|---|---|
| 1000 | 2 | 30 | 100 | 500 | 0,4 |
| 450 | 1.5 | 9 | 300 | 500 | 0,5 |
| 80 | 1 | 1 | 2000 | 500 | 0,6 |
| Testosterone | Progesterone | Carbamazepine | Diclofenac | |
|---|---|---|---|---|
| Initial concentrations [ppm] | 2.3829 | 4.5496 | 3.5364 | 5.2319 |
| Solvent | 70% MeOH/30% H2O | 70% MeOH/30% H2O | 70% MeCN/30% H2O | 75% MeCN/25% H2O |
| pH Values Used for Analysis | ||||||
|---|---|---|---|---|---|---|
| pH | 2 | 3 | 4 | 5 | 10 | 12 |
| Adsorption Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time [min] | 1 | 7.5 | 15 | 30 | 60 | 120 | 240 | 360 | 480 |
| Compound | Eluent | Flow Rate [ml/min] | Injection Volume [µL] | Column Temp. [°C] | |
|---|---|---|---|---|---|
| Testosterone | A | H2O + 20 mM AF * + 0.1% FA ** | 0.7 | 5 | 50 |
| B | MeOH + 0.1% FA | ||||
| Progesterone | A | H2O + 20 mM AF + 0.1% FA | 0.7 | 5 | 50 |
| B | MeOH + 0.1% FA | ||||
| Carbamazepine | A | H2O + 5 mM AF + 0.1% FA | 0.8 | 5 | 40 |
| B | 90% MeCN + 5 mM AF + 0.1% FA | ||||
| Diclofenac | A | H2O + 5 mM AF + 0.1% FA | 0.8 | 5 | 40 |
| B | MeCN + 5 mM AF + 0.1% FA |
| Compound | RT (min) | Molecular Weight | Polarity | MRM Transition (m/z) | Collision Energy (V) |
|---|---|---|---|---|---|
| Testosterone | 5.86 | 288.4 | Positive | 289.3 > 97.1 | 23 |
| Progesterone | 7.44 | 314.5 | Positive | 315.2 > 97.1 | 25 |
| Carbamazepine | 2.13 | 236.3 | Positive | 237.1 > 194.1; 237.1 >194.1 | 16; 32 |
| Diclofenac | 2.32 | 296.2 | Positive | 296 > 250; 296 > 215 | 8; 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciesielski, W.; Kulawik, D.; Girek, B.; Kozieł-Trąbska, K.; Zawierucha, I.; Girek, T. Poly-Amino-β-Cyclodextrin Microparticles for the Reduction of Xenobiotics and Emerging Contaminants, Including Pharmaceuticals, from the Natural Environment. Materials 2024, 17, 5424. https://doi.org/10.3390/ma17225424
Ciesielski W, Kulawik D, Girek B, Kozieł-Trąbska K, Zawierucha I, Girek T. Poly-Amino-β-Cyclodextrin Microparticles for the Reduction of Xenobiotics and Emerging Contaminants, Including Pharmaceuticals, from the Natural Environment. Materials. 2024; 17(22):5424. https://doi.org/10.3390/ma17225424
Chicago/Turabian StyleCiesielski, Wojciech, Damian Kulawik, Beata Girek, Kinga Kozieł-Trąbska, Iwona Zawierucha, and Tomasz Girek. 2024. "Poly-Amino-β-Cyclodextrin Microparticles for the Reduction of Xenobiotics and Emerging Contaminants, Including Pharmaceuticals, from the Natural Environment" Materials 17, no. 22: 5424. https://doi.org/10.3390/ma17225424
APA StyleCiesielski, W., Kulawik, D., Girek, B., Kozieł-Trąbska, K., Zawierucha, I., & Girek, T. (2024). Poly-Amino-β-Cyclodextrin Microparticles for the Reduction of Xenobiotics and Emerging Contaminants, Including Pharmaceuticals, from the Natural Environment. Materials, 17(22), 5424. https://doi.org/10.3390/ma17225424








