Long-Term Storage of Ti3C2Tx Aqueous Dispersion with Stable Electrochemical Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of MXenes
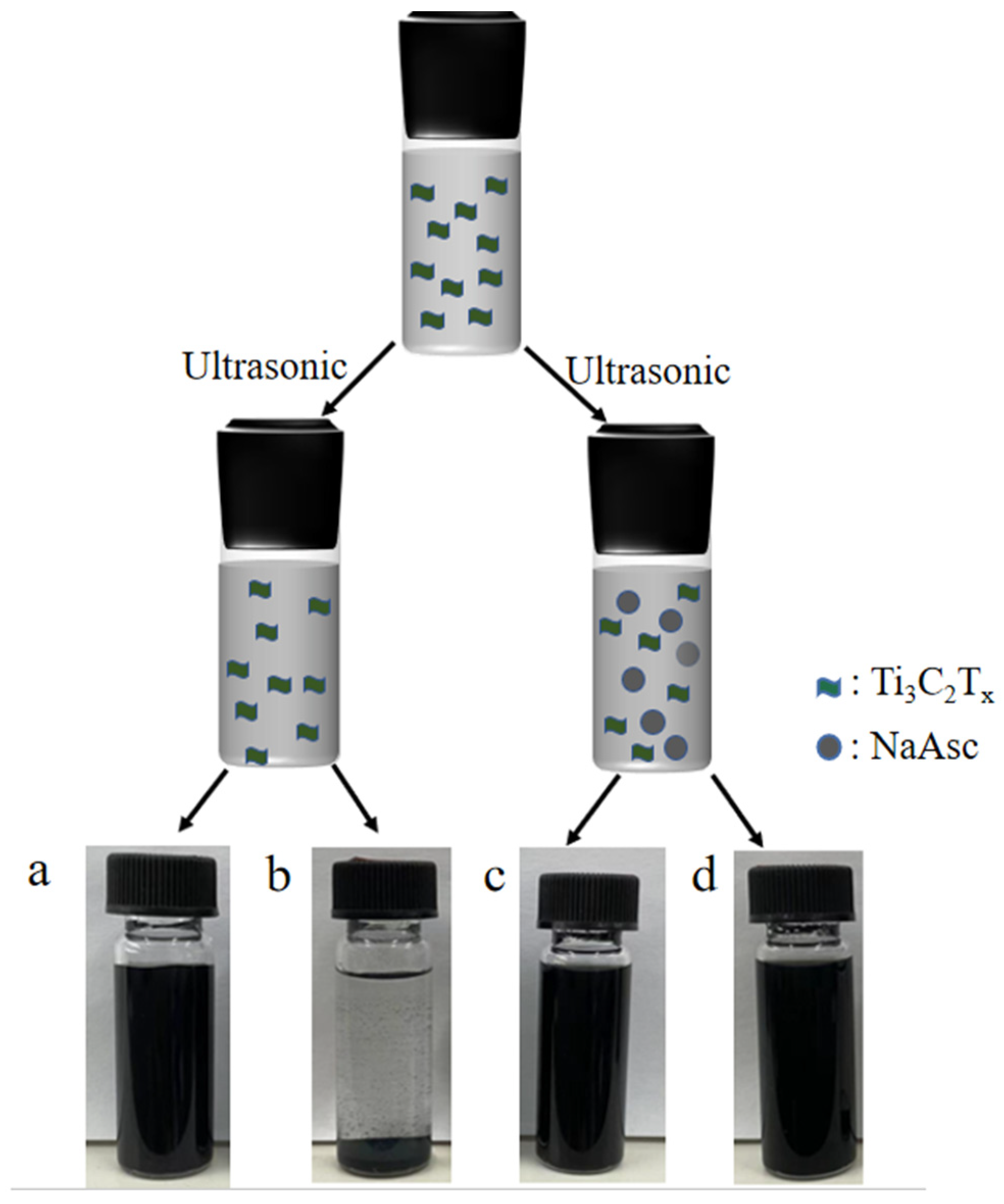
2.3. Preparation of MXene-NaAsc Aqueous Dispersion
2.4. Characterizations
2.5. Electrochemical Measurement
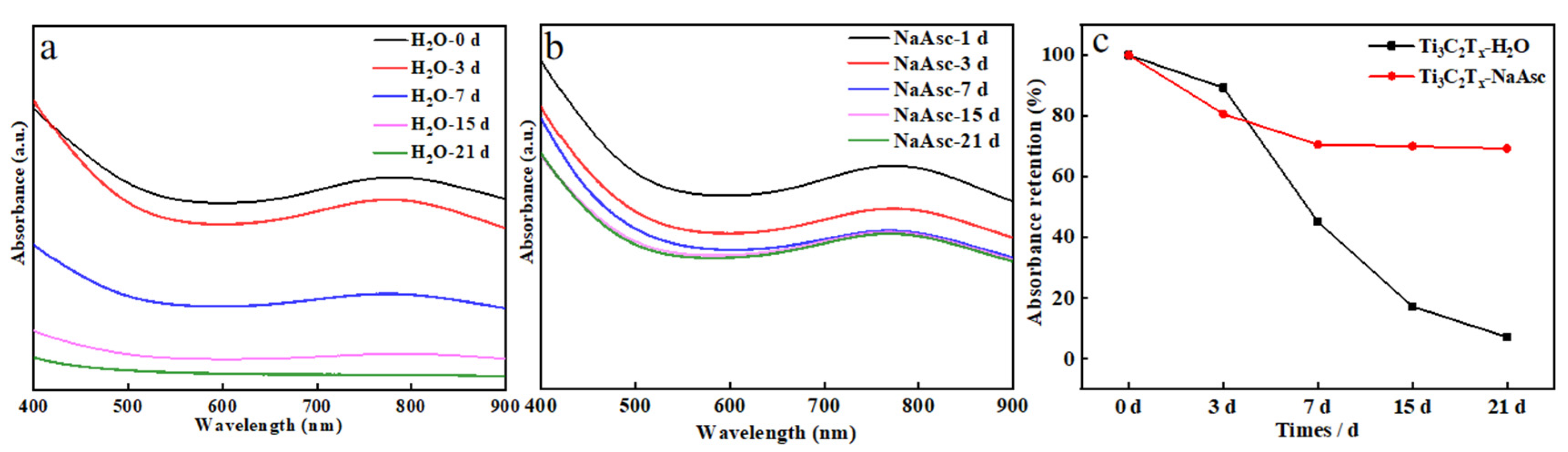
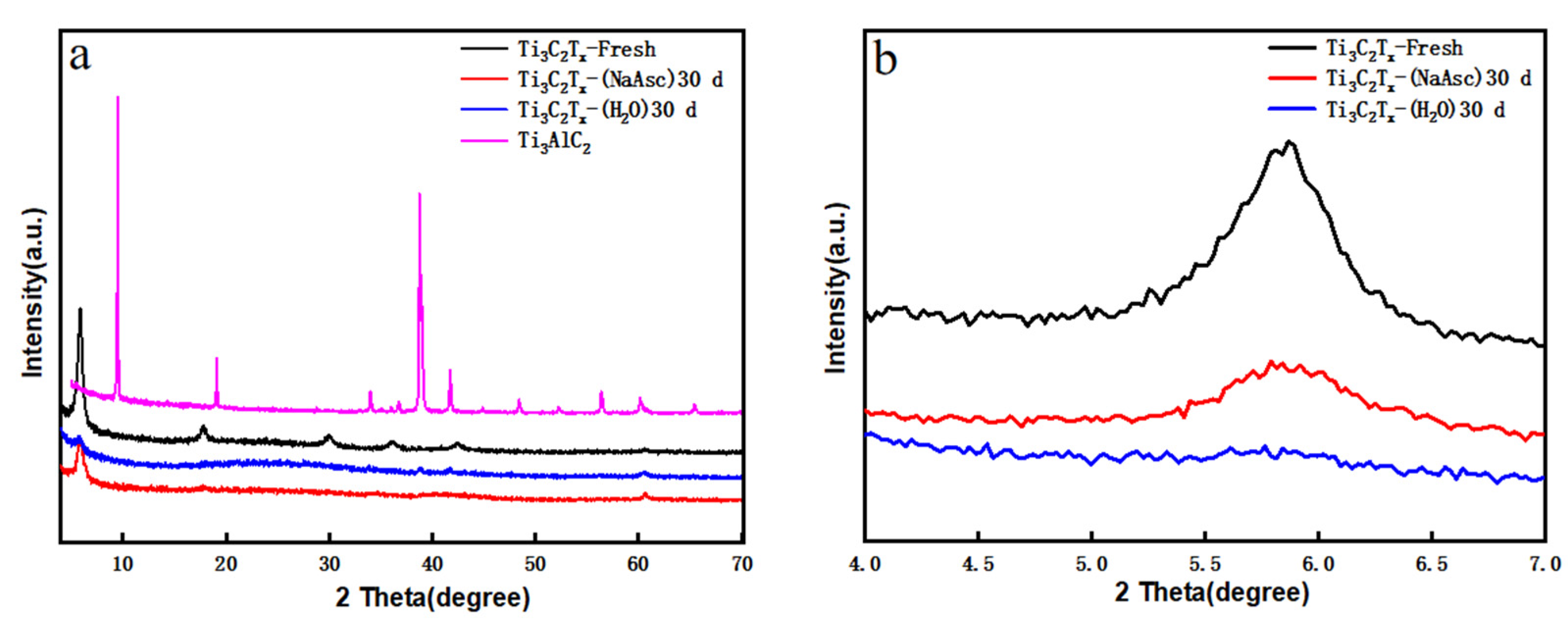
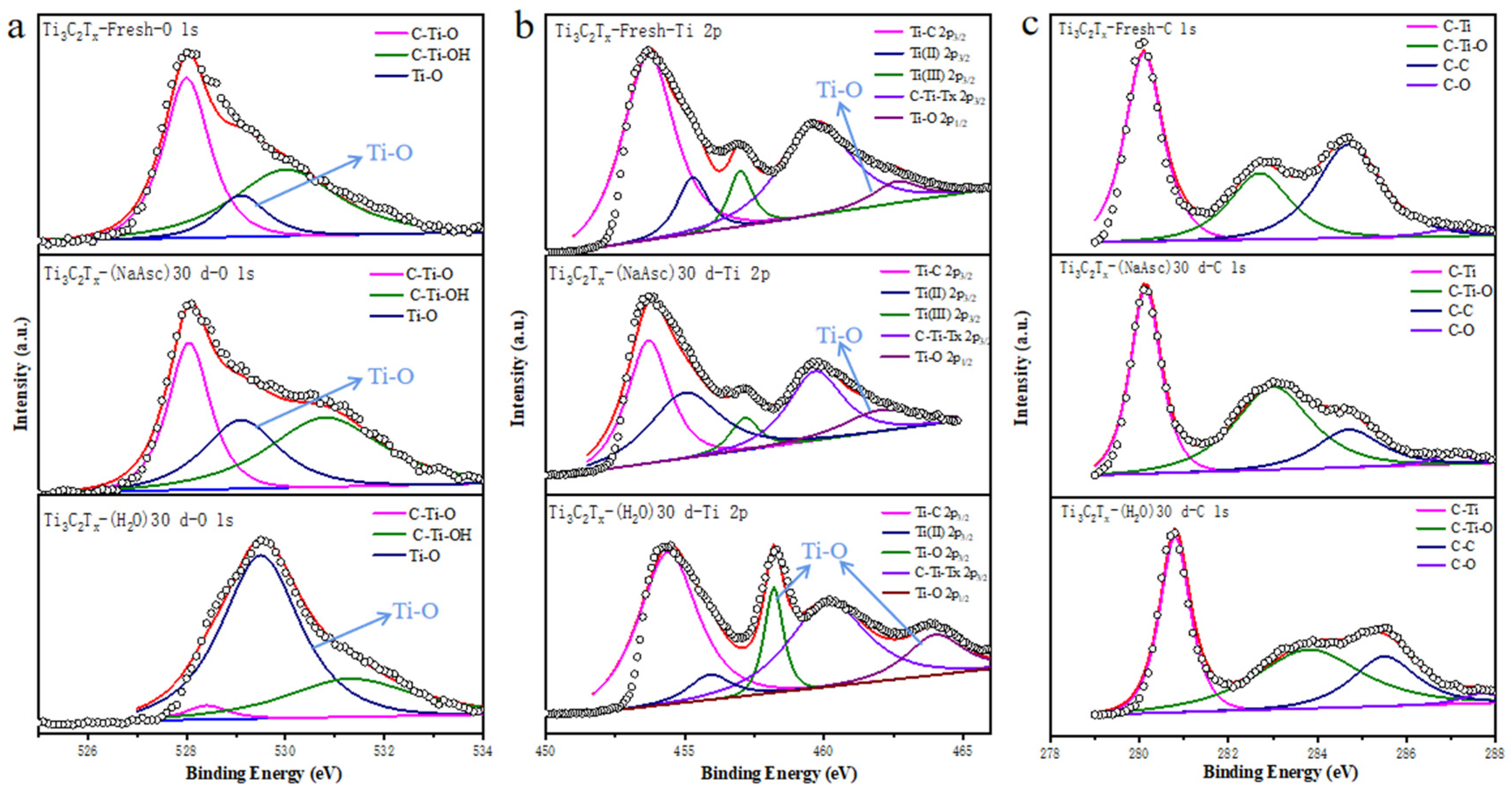
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary Article: MXenes: A New Family of Two-Dimensional Materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Z.; Shuck, C.E.; Liang, G.; Gogotsi, Y.; Zhi, C. MXene Chemistry, Electrochemistry and Energy Storage Applications. Nat. Rev. Chem. 2022, 6, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, T.; Qin, X.; Zhang, Q.; Liu, T.; Dai, W.; Chen, B.; Yu, H.; Shi, S. Recent Advances in 2D MXenes: Preparation, Intercalation and Applications in Flexible Devices. J. Mater. Chem. A 2021, 9, 14147–14171. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive Two-Dimensional Titanium Carbide “clay” with High Volumetric Capacitance. Nature 2015, 516, 78–81. [Google Scholar] [CrossRef]
- Li, T.; Yao, L.; Liu, Q.; Gu, J.; Luo, R.; Li, J.; Yan, X.; Wang, W.; Liu, P.; Chen, B.; et al. Fluorine-Free Synthesis of High-Purity Ti3C2Tx (T=OH, O) via Alkali Treatment. Angew. Chem.-Int. Ed. 2018, 57, 6115–6119. [Google Scholar] [CrossRef]
- Hart, J.L.; Hantanasirisakul, K.; Lang, A.C.; Anasori, B.; Pinto, D.; Pivak, Y.; van Omme, J.T.; May, S.J.; Gogotsi, Y.; Taheri, M.L. Control of MXenes’ Electronic Properties through Termination and Intercalation. Nat. Commun. 2019, 10, 522. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes Stretch Hydrogel Sensor Performance to New Limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Li, L.; Hu, X.; Zou, Z.; Wang, J.; Luo, S.; Gao, Y. A Highly Flexible and Sensitive Piezoresistive Sensor Based on MXene with Greatly Changed Interlayer Distances. Nat. Commun. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Si, W.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Highly Stretchable, Elastic, and Sensitive MXene-Based Hydrogel for Flexible Strain and Pressure Sensors. Research 2020, 2020, 2038560. [Google Scholar] [CrossRef]
- Gao, L.; Wang, M.; Wang, W.; Xu, H.; Wang, Y.; Zhao, H.; Cao, K.; Xu, D.; Li, L. Highly Sensitive Pseudocapacitive Iontronic Pressure Sensor with Broad Sensing Range. Nano-Micro Lett. 2021, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chacon, B.; Park, H.; Hantanasirisakul, K.; Kim, T.; Shevchuk, K.; Lee, J.; Kang, H.; Cho, S.Y.; Kim, J.; et al. N-p-Conductor Transition of Gas Sensing Behaviors in Mo2CTx MXene. ACS Sens. 2022, 7, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, L.; Yan, H.; Tian, B.; Zhu, X. Black Graphitic Carbon Nitride Nanosheets with Mid-Gap States Realizing Highly Efficient Near-Infrared Photo-Thermal Conversion for Photoacoustic Imaging. J. Mater. Chem. B 2022, 47, 9923–9930. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.; Zhao, D.; Zhang, X.; Zhuo, J.; Yin, Y.; Wang, X.; Yang, G.; Yi, F. Ti3C2Tx: MXene for Electrode Materials of Supercapacitors. J. Mater. Chem. A 2021, 9, 11501–11529. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, J.; Yang, D.; Soomro, R.A.; Xu, B. Flexible Carbon Dots-Intercalated MXene Film Electrode with Outstanding Volumetric Performance for Supercapacitors. Adv. Funct. Mater. 2023, 33, 2209918. [Google Scholar] [CrossRef]
- Feng, S.; Wang, X.; Wang, M.; Bai, C.; Cao, S.; Kong, D. Crumpled MXene Electrodes for Ultrastretchable and High-Area-Capacitance Supercapacitors. Nano Lett. 2021, 21, 7561–7568. [Google Scholar] [CrossRef]
- Xu, J.; Peng, T.; Zhang, Q.; Zheng, H.; Yu, H.; Shi, S. Intercalation Effects on the Electrochemical Properties of Ti3C2Tx MXene Nanosheets for High-Performance Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 8794–8803. [Google Scholar] [CrossRef]
- Yu, Z.; Feng, W.; Lu, W.; Li, B.; Yao, H.; Zeng, K.; Ouyang, J. MXenes with Tunable Work Functions and Their Application as Electron- and Hole-Transport Materials in Non-Fullerene Organic Solar Cells. J. Mater. Chem. A 2019, 7, 11160–11169. [Google Scholar] [CrossRef]
- Sun, B.; Lu, Q.; Chen, K.; Zheng, W.; Liao, Z.; Lopatik, N.; Li, D.; Hantusch, M.; Zhou, S.; Wang, H.I.; et al. Redox-Active Metaphosphate-Like Terminals Enable High-Capacity MXene Anodes for Ultrafast Na-Ion Storage. Adv. Mater. 2022, 34, 2108682. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wang, J.; Sun, Z.; Cui, G.; Chen, Y.; Wang, T.; Zheng, L.; Zhao, Y.; Shui, L.; et al. Strain Engineering of a MXene/CNT Hierarchical Porous Hollow Microsphere Electrocatalyst for a High-Efficiency Lithium Polysulfide Conversion Process. Angew. Chem.-Int. Ed. 2021, 60, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Shi, H.; Wu, Z.S. Recent Advances and Promise of MXene-Based Nanostructures for High-Performance Metal Ion Batteries. Adv. Funct. Mater. 2020, 30, 2000706. [Google Scholar] [CrossRef]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye Adsorption and Decomposition on Two-Dimensional Titanium Carbide in Aqueous Media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-Dimensional Molybdenum Carbide (MXene) as an Efficient Electrocatalyst for Hydrogen Evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Sun, Z. Novel 2D Transition-Metal Carbides: Ultrahigh Performance Electrocatalysts for Overall Water Splitting and Oxygen Reduction. Adv. Funct. Mater. 2020, 30, 2000570. [Google Scholar] [CrossRef]
- Morales-Garciá, Á.; Calle-Vallejo, F.; Illas, F. MXenes: New Horizons in Catalysis. ACS Catal. 2020, 10, 13487–13503. [Google Scholar] [CrossRef]
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; Kim, M.K.; Kwon, J.; Hong, J.; Kim, H.; Kim, D.; Gogotsi, Y.; Koo, C.M. Anomalous Absorption of Electromagnetic Waves by 2D Transition Metal Carbonitride Ti3CNTx (MXene). Science 2020, 369, 446–450. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic Interference Shielding with 2D Transition Metal Carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, Flexible, and Lightweight MXene Foams for High-Performance Electromagnetic-Interference Shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Chen, W.; Liu, L.-X.; Zhang, H.-B.; Yu, Z.-Z. Flexible, Transparent, and Conductive Ti3C2Tx MXene-Silver Nanowire Films with Smart Acoustic Sensitivity for High-Performance Electromagnetic Interference Shielding. ACS Nano 2020, 14, 16643–16653. [Google Scholar] [CrossRef]
- Han, M.; Shuck, C.E.; Singh, A.; Yang, Y.; Foucher, A.C.; Goad, A.; McBride, B.; May, S.J.; Shenoy, V.B.; Stach, E.A.; et al. Efficient Microwave Absorption with Vn+1CnTx MXenes. Cell Rep. Phys. Sci. 2022, 3, 101073. [Google Scholar] [CrossRef]
- Zhang, H.; Wan, J.; Wu, R.; Chen, Y.; Yu, H.; Shi, S. MXenes for Electromagnetic Interference Shielding: Insights from Structural Design. Carbon 2024, 218, 118716. [Google Scholar] [CrossRef]
- Hou, C.; Huang, C.; Yu, H.; Shi, S. Surface-Engineered Ti3C2Tx with Tunable Work Functions for Highly Efficient Polymer Solar Cells. Small 2022, 18, 2201046. [Google Scholar] [CrossRef]
- Huang, C.; Shi, S.; Yu, H. Work Function Adjustment of Nb2CTx Nanoflakes as Hole and Electron Transport Layers in Organic Solar Cells by Controlling Surface Functional Groups. ACS Energy Lett. 2021, 6, 3464–3472. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, C.; Sun, Y.; Yu, H. Amino-Functionalized Niobium-Carbide MXene Serving as Electron Transport Layer and Perovskite Additive for the Preparation of High-Performance and Stable Methylammonium-Free Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2113367. [Google Scholar] [CrossRef]
- Zhou, H.; Han, S.J.; Lee, H.; Zhang, D.; Anayee, M.; Jo, S.H.; Gogotsi, Y.; Lee, T. Overcoming the Limitations of MXene Electrodes for Solution-Processed Optoelectronic Devices. Adv. Mater. 2022, 34, 2206377. [Google Scholar] [CrossRef]
- Ahn, S.; Han, T.H.; Maleski, K.; Song, J.; Kim, Y.H.; Park, M.H.; Zhou, H.; Yoo, S.; Gogotsi, Y.; Lee, T.W. A 2D Titanium Carbide MXene Flexible Electrode for High-Efficiency Light-Emitting Diodes. Adv. Mater. 2020, 32, 2000919. [Google Scholar] [CrossRef]
- Driscoll, N.; Erickson, B.; Murphy, B.B.; Richardson, A.G.; Robbins, G.; Apollo, N.V.; Mentzelopoulos, G.; Mathis, T.; Hantanasirisakul, K.; Bagga, P.; et al. MXene-Infused Bioelectronic Interfaces for Multiscale Electrophysiology and Stimulation. Sci. Transl. Med. 2021, 13, eabf8629. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Li, L.; Wang, J. Insights into the Photothermal Conversion of 2D MXene Nanomaterials: Synthesis, Mechanism, and Applications. Adv. Funct. Mater. 2020, 30, 2000712. [Google Scholar] [CrossRef]
- Hazan, A.; Ratzker, B.; Zhang, D.; Katiyi, A.; Sokol, M.; Gogotsi, Y.; Karabchevsky, A. MXene-Nanoflakes-Enabled All-Optical Nonlinear Activation Function for On-Chip Photonic Deep Neural Networks. Adv. Mater. 2023, 35, 2210216. [Google Scholar] [CrossRef]
- Sokolov, A.; Ali, M.; Li, H.; Jeon, Y.R.; Ko, M.J.; Choi, C. Partially Oxidized MXene Ti3C2Tx Sheets for Memristor Having Synapse and Threshold Resistive Switching Characteristics. Adv. Electron. Mater. 2021, 7, 2000866. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Qiu, J. Stabilizing MXene by Hydration Chemistry in Aqueous Solution. Angew. Chem.-Int. Ed. 2021, 60, 26587–26591. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhang, Y.; Wang, H.; Khan, K.; Tareen, A.K.; Qian, W.; Zhang, H.; Ågren, H. Recent Advances in Oxidation Stable Chemistry of 2D MXenes. Adv. Mater. 2022, 34, 2107554. [Google Scholar] [CrossRef]
- Huang, S.; Mochalin, V.N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Shuck, C.E.; Han, M.; Maleski, K.; Hantanasirisakul, K.; Kim, S.J.; Choi, J.; Reil, W.E.B.; Gogotsi, Y. Effect of Ti3AlC2 MAX Phase on Structure and Properties of Resultant Ti3C2Tx MXene. ACS Appl. Nano Mater. 2019, 2, 3368–3376. [Google Scholar] [CrossRef]
- Mathis, T.S.; Maleski, K.; Goad, A.; Sarycheva, A.; Anayee, M.; Foucher, A.C.; Hantanasirisakul, K.; Shuck, C.E.; Stach, E.A.; Gogotsi, Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. [Google Scholar] [CrossRef]
- Zhang, C.J.; Pinilla, S.; McEvoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, N.; Hegh, D.; Usman, K.A.S.; Guan, G.; Qin, S.; Jurewicz, I.; Yang, W.; Razal, J.M. Freezing Titanium Carbide Aqueous Dispersions for Ultra-Long-Term Storage. ACS Appl. Mater. Interfaces 2020, 12, 34032–34040. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, S.J.; Cho, S.Y.; Choi, J.; Maleski, K.; Lee, B.J.; Jung, H.T.; Gogotsi, Y.; Lee, Y.; Ahn, C.W. An Investigation into the Factors Governing the Oxidation of Two-Dimensional Ti3C2 MXene. Nanoscale 2019, 11, 8387–8393. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, H.; Fan, R.; Ji, P.; Fu, X.; Li, H. High Concentration of Ti3C2Tx MXene in Organic Solvent. ACS Nano 2021, 15, 5249–5262. [Google Scholar] [CrossRef]
- Maleski, K.; Mochalin, V.N.; Gogotsi, Y. Dispersions of Two-Dimensional Titanium Carbide MXene in Organic Solvents. Chem. Mater. 2017, 29, 1632–1640. [Google Scholar] [CrossRef]
- Seyedin, S.; Zhang, J.; Usman, K.A.S.; Qin, S.; Glushenkov, A.M.; Yanza, E.R.S.; Jones, R.T.; Razal, J.M. Facile Solution Processing of Stable MXene Dispersions towards Conductive Composite Fibers. Glob. Chall. 2019, 3, 1900037. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.; Kim, S.K.; Park, S.; Song, W.; Myung, S.; Jung, H.K.; Lee, S.S.; Yoon, D.H.; An, K.S. Chemically Stabilized and Functionalized 2D-MXene with Deep Eutectic Solvents as Versatile Dispersion Medium. Adv. Funct. Mater. 2021, 31, 2008722. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.J.; Kim, Y.J.; Lim, Y.; Chae, Y.; Lee, B.J.; Kim, Y.T.; Han, H.; Gogotsi, Y.; Ahn, C.W. Oxidation-Resistant Titanium Carbide MXene Films. J. Mater. Chem. A 2020, 8, 573–581. [Google Scholar] [CrossRef]
- Zhao, X.; Holta, D.E.; Tan, Z.; Oh, J.H.; Echols, I.J.; Anas, M.; Cao, H.; Lutkenhaus, J.L.; Radovic, M.; Green, M.J. Annealed Ti3C2Tx MXene Films for Oxidation-Resistant Functional Coatings. ACS Appl. Nano Mater. 2020, 3, 10578–10585. [Google Scholar] [CrossRef]
- Natu, V.; Hart, J.L.; Sokol, M.; Chiang, H.; Taheri, M.L.; Barsoum, M.W. Edge Capping of 2D-MXene Sheets with Polyanionic Salts To Mitigate Oxidation in Aqueous Colloidal Suspensions. Angew. Chem.-Int. Ed. 2019, 58, 12655–12660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Vashisth, A.; Blivin, J.W.; Tan, Z.; Holta, D.E.; Kotasthane, V.; Shah, S.A.; Habib, T.; Liu, S.; Lutkenhaus, J.L.; et al. PH, Nanosheet Concentration, and Antioxidant Affect the Oxidation of Ti3C2Tx and Ti2CTx MXene Dispersions. Adv. Mater. Interfaces 2020, 7, 2000845. [Google Scholar] [CrossRef]
- Zhao, X.; Vashisth, A.; Prehn, E.; Sun, W.; Shah, S.A.; Habib, T.; Chen, Y.; Tan, Z.; Lutkenhaus, J.L.; Radovic, M.; et al. Antioxidants Unlock Shelf-Stable Ti3C2Tx (MXene) Nanosheet Dispersions. Matter 2019, 1, 513–526. [Google Scholar] [CrossRef]
- Doo, S.; Chae, A.; Kim, D.; Oh, T.; Ko, T.Y.; Kim, S.J.; Koh, D.Y.; Koo, C.M. Mechanism and Kinetics of Oxidation Reaction of Aqueous Ti3C2Tx Suspensions at Different PHs and Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 22855–22865. [Google Scholar] [CrossRef]
- Habib, T.; Zhao, X.; Shah, S.A.; Chen, Y.; Sun, W.; An, H.; Lutkenhaus, J.L.; Radovic, M.; Green, M.J. Oxidation Stability of Ti3C2Tx MXene Nanosheets in Solvents and Composite Films. NPJ 2D Mater. Appl. 2019, 3, 8. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, X.; Zhang, P.; Wei, Y.; Qiao, N.; Yang, B.; Soomro, R.A.; Zhang, R.; Xu, B. Effect of Sodium Dodecyl Sulfate on Stability of MXene Aqueous Dispersion. Adv. Sci. 2023, 10, 2300273. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Fan, B.; Zhang, P.; Wei, Y.; Soomro, R.A.; Zhao, X.; Kumar, J. Ultralong Stability of Ti3C2Tx MXene Dispersion Through Synergistic Regulation of Storage Environment and Defect Capping with Tris-HCl Buffering. Small Methods 2024, 8, 2301689. [Google Scholar] [CrossRef] [PubMed]
- Raisi, B.; Huang, L.; Ye, Z. Modification of Ti3C2Tx MXene with Hyperbranched Polyethylene Ionomers: Stable Dispersions in Nonpolar/Low-Polarity Organic Solvents, Oxidation Protection, and Potential Application in Supercapacitors. J. Mater. Chem. A 2023, 11, 17167–17187. [Google Scholar] [CrossRef]
- Wan, J.; Wu, R.; Chen, Y.; Zhang, H.; Li, H.; Wang, B.; Liskiewicz, T.; Shi, S. Amino Modification of Ti3C2 MXenes for High-Performance Supercapacitors. Appl. Surf. Sci. 2024, 678, 161154. [Google Scholar] [CrossRef]


| 5 mV/s | 10 mV/s | 20 mV/s | 50 mV/s | 100 mV/s | 200 mV/s | |
|---|---|---|---|---|---|---|
| Ti3C2Tx-Fresh | 411.4 | 394.2 | 368.5 | 303.5 | 211.1 | 100.7 |
| Ti3C2Tx-NaAsc-15 | 396.3 | 382.7 | 360.5 | 299.2 | 208.2 | 102.5 |
| Ti3C2Tx-NaAsc-30 | 391.3 | 374.7 | 352.4 | 302.6 | 233.4 | 133.6 |
| Ti3C2Tx-NaAsc-60 | 381.1 | 364.7 | 342.5 | 288.5 | 213.5 | 112.8 |
| Antioxidant | Time (Day) | Electrolyte | Rate (mV/s) | Capacitance (F/g) | Retention | Ref |
|---|---|---|---|---|---|---|
| SDS | 35 | 3 M H2SO4 | - | ~300 a | 95.4% | [61] |
| Tris-HCl | 35 | 3 M H2SO4 | - | 251.6 a | 94.6% | [62] |
| HPI | - | [EMIM]+ [BF4]− | 2 | 220 b | - | [63] |
| NaAsc | 60 | 1 M H2SO4 | 5 | 381.1 b | 92.6% | Here |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.; Wu, R.; Wang, B.; Liskiewicz, T.; Shi, S. Long-Term Storage of Ti3C2Tx Aqueous Dispersion with Stable Electrochemical Properties. Materials 2024, 17, 5414. https://doi.org/10.3390/ma17225414
Peng T, Wu R, Wang B, Liskiewicz T, Shi S. Long-Term Storage of Ti3C2Tx Aqueous Dispersion with Stable Electrochemical Properties. Materials. 2024; 17(22):5414. https://doi.org/10.3390/ma17225414
Chicago/Turabian StylePeng, Ting, Ruiqing Wu, Bohai Wang, Tomasz Liskiewicz, and Shengwei Shi. 2024. "Long-Term Storage of Ti3C2Tx Aqueous Dispersion with Stable Electrochemical Properties" Materials 17, no. 22: 5414. https://doi.org/10.3390/ma17225414
APA StylePeng, T., Wu, R., Wang, B., Liskiewicz, T., & Shi, S. (2024). Long-Term Storage of Ti3C2Tx Aqueous Dispersion with Stable Electrochemical Properties. Materials, 17(22), 5414. https://doi.org/10.3390/ma17225414







