Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
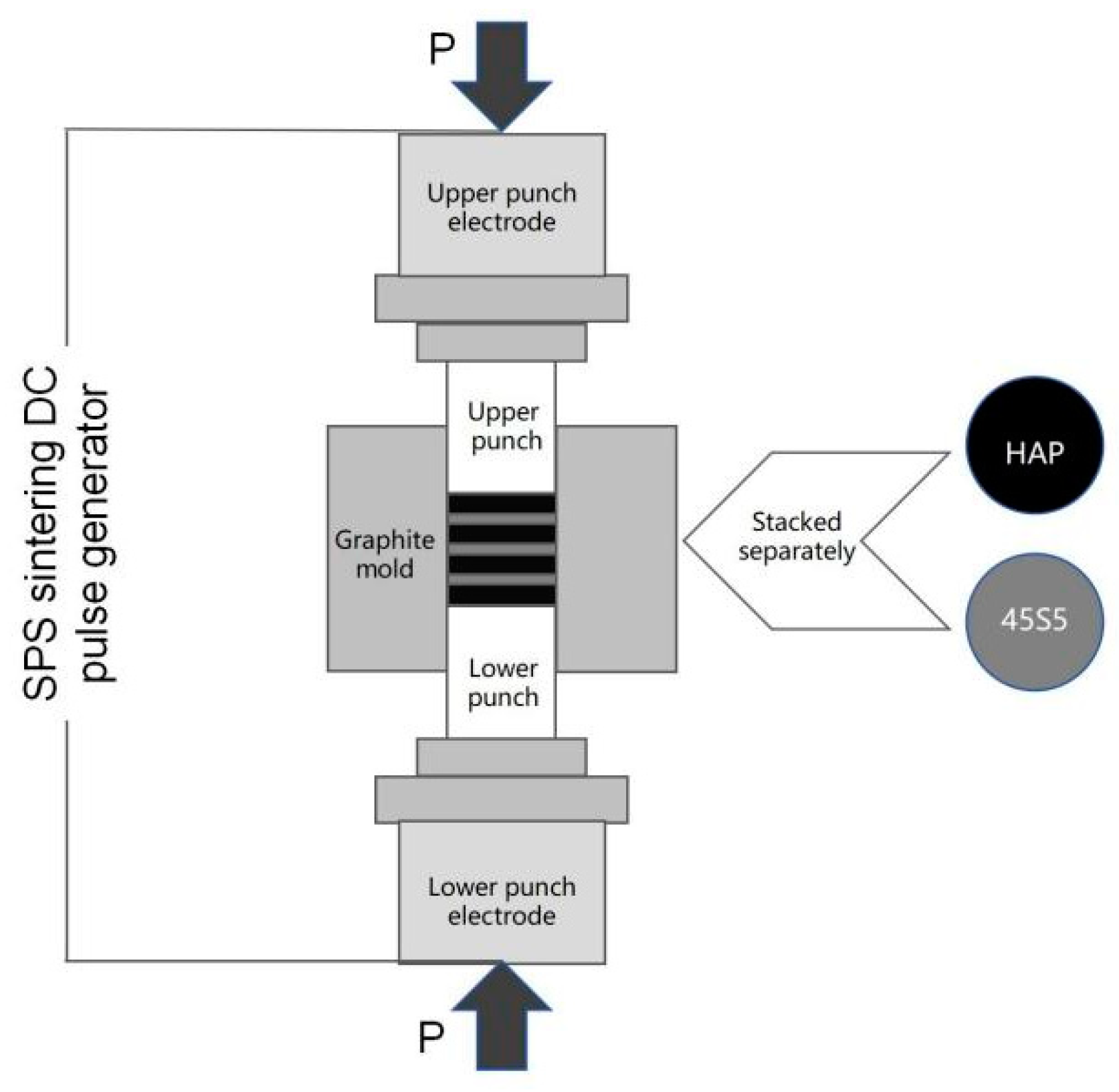
2.1. Manufacturing Process
2.2. Characterization
3. Results and Discussion
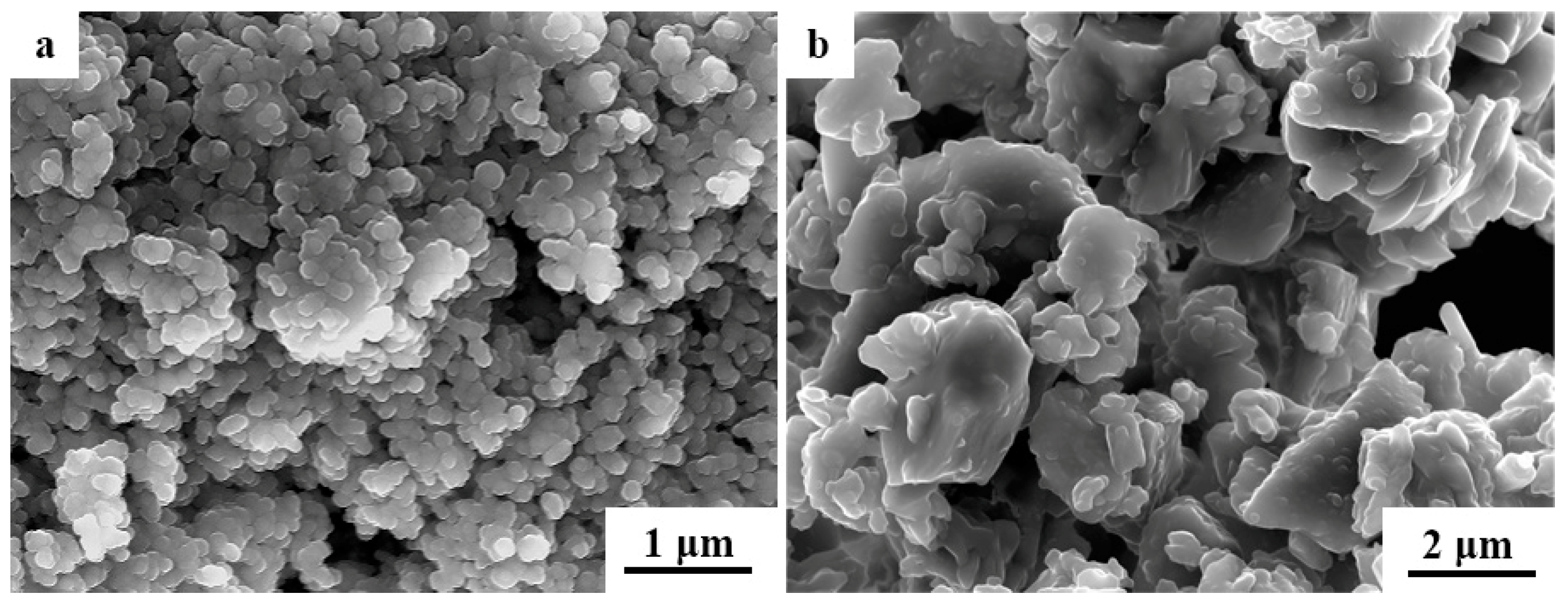
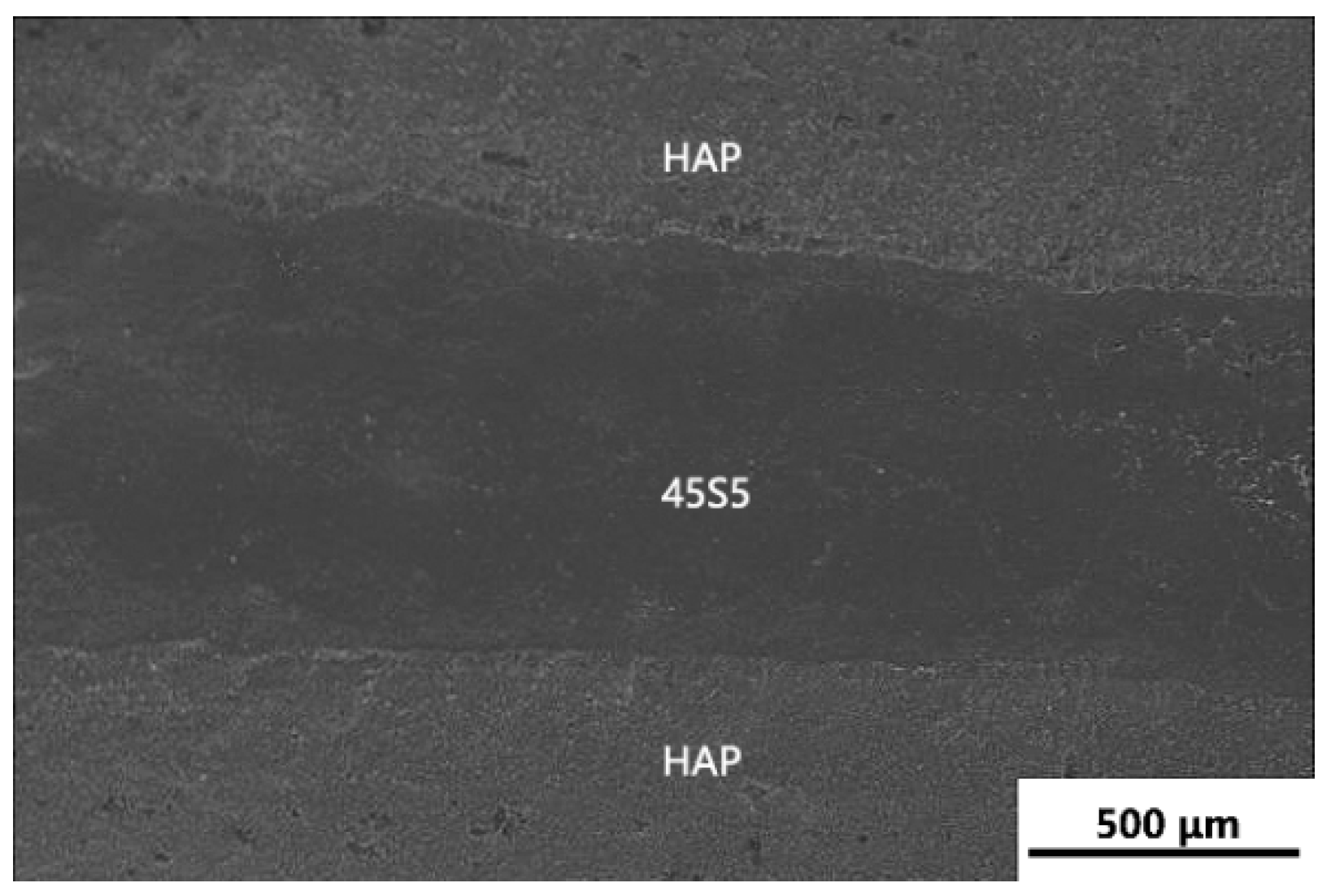
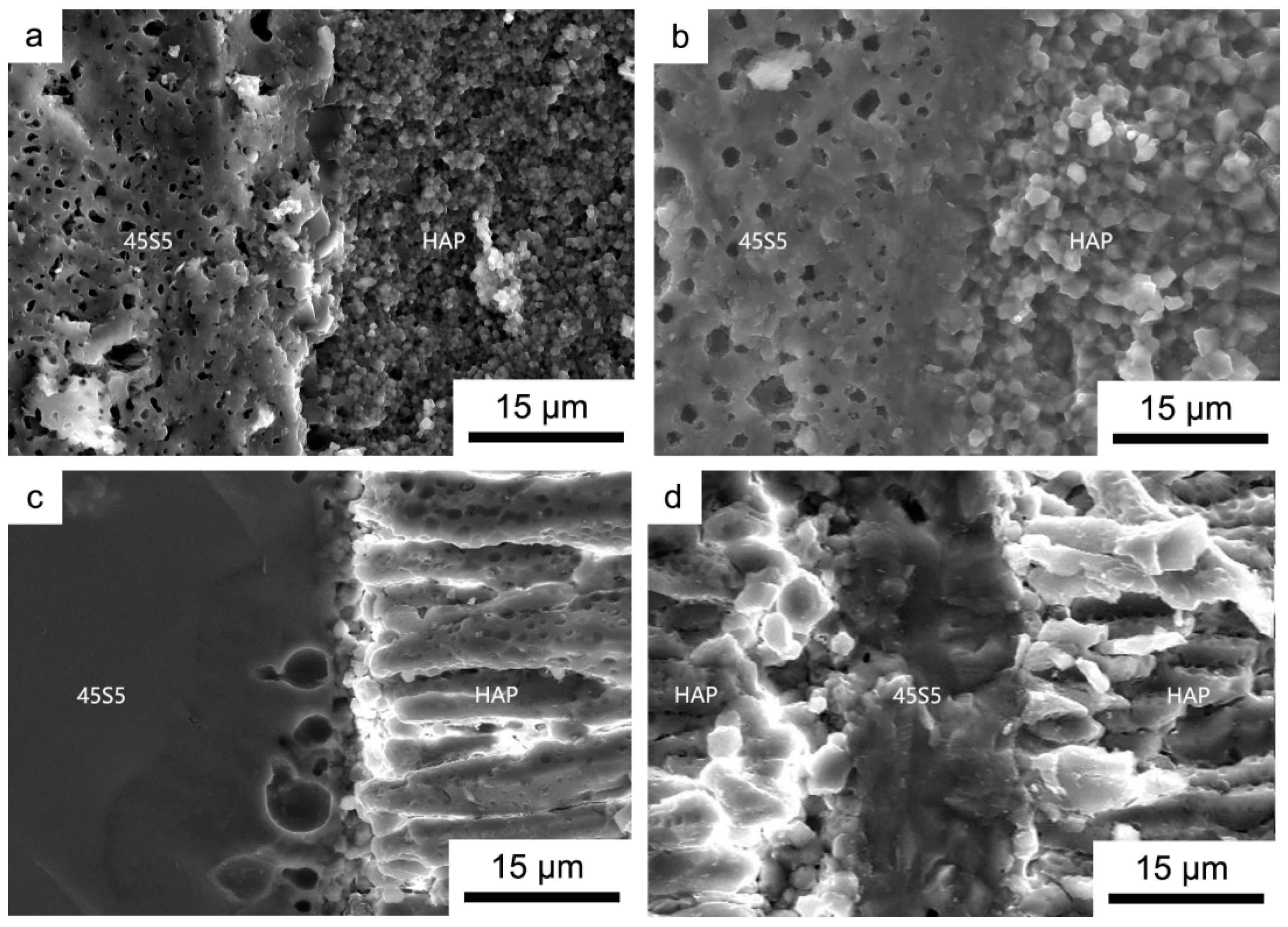
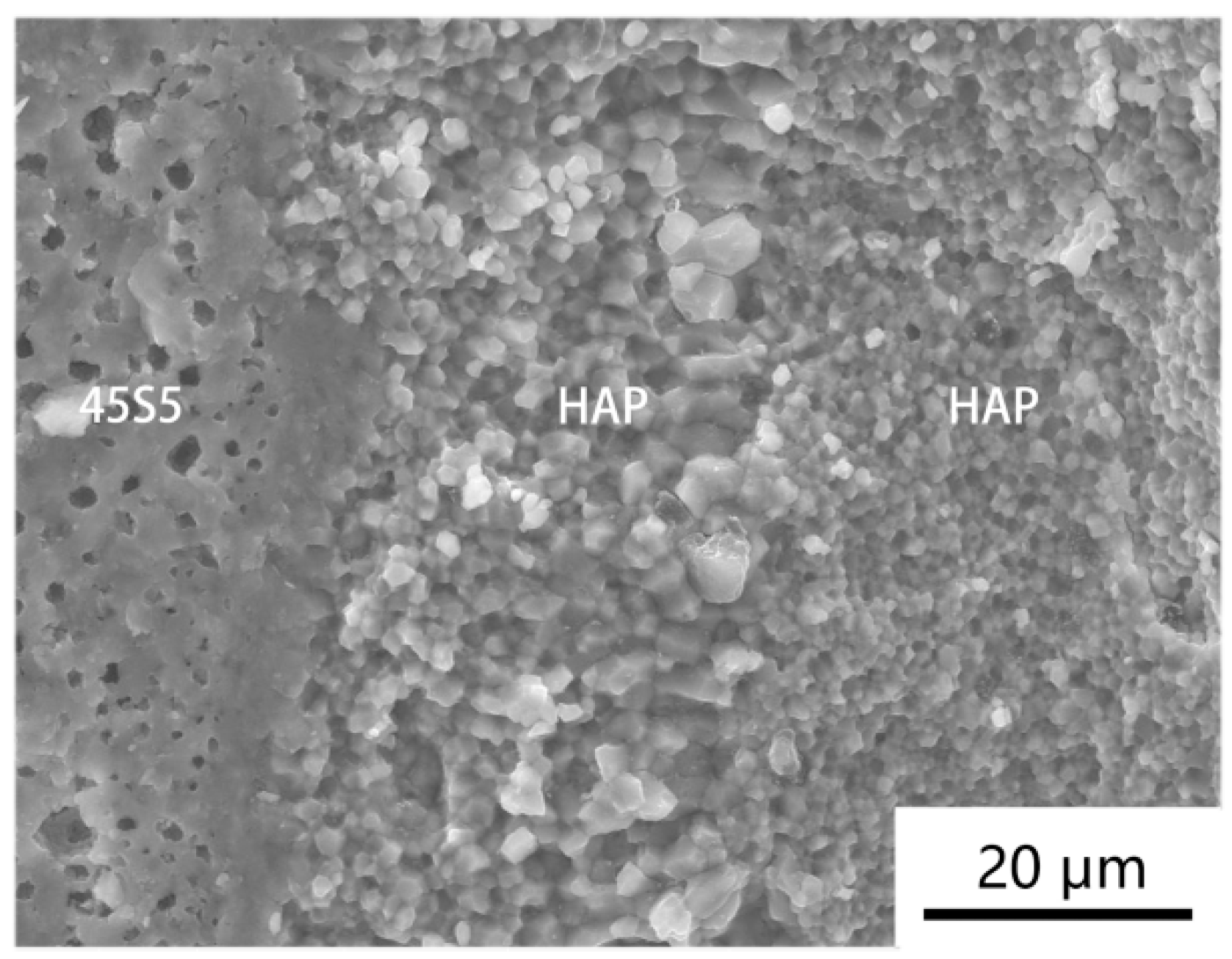
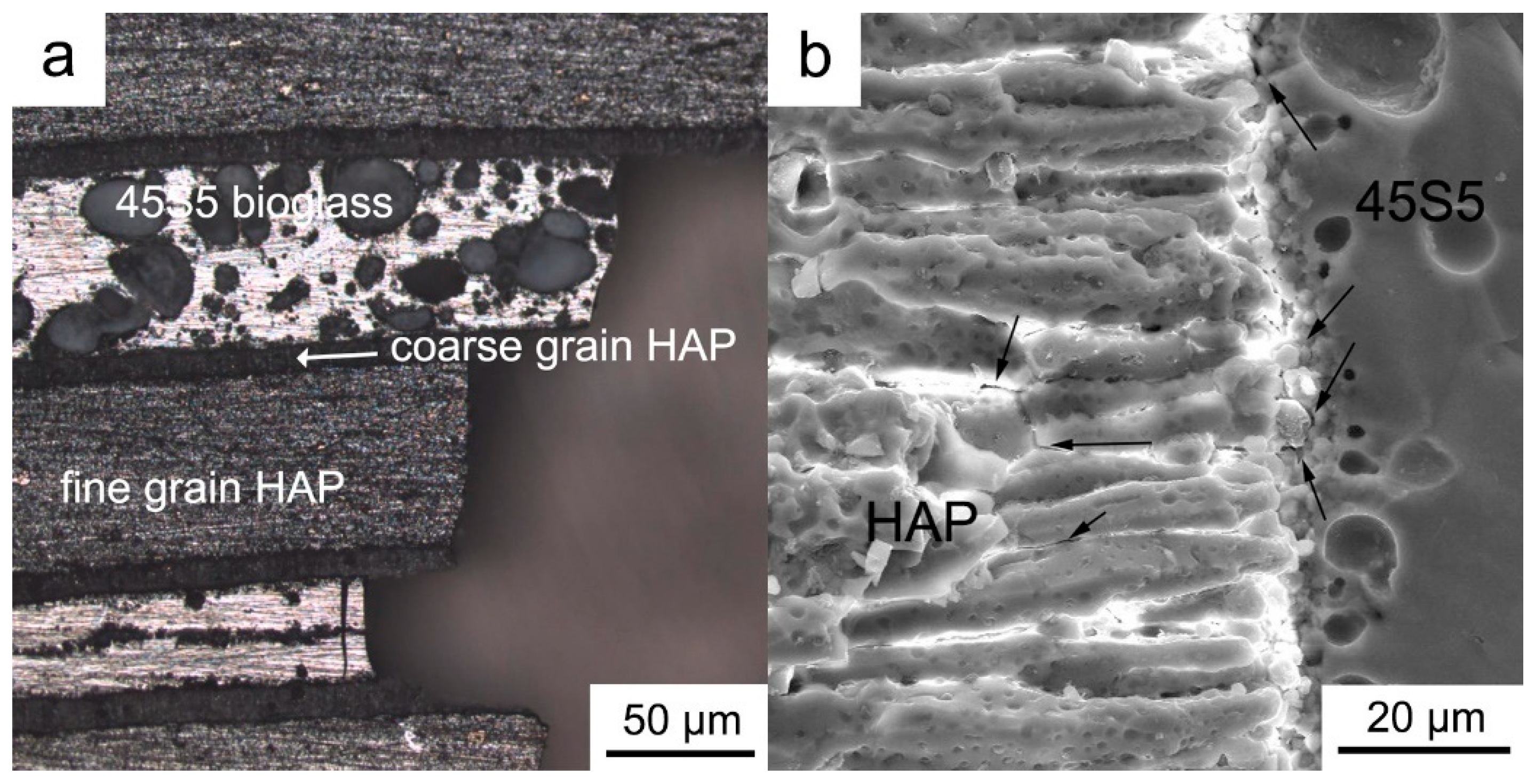
3.1. Microstructure Analysis
3.2. The Impact of the Layered Structure on Performance
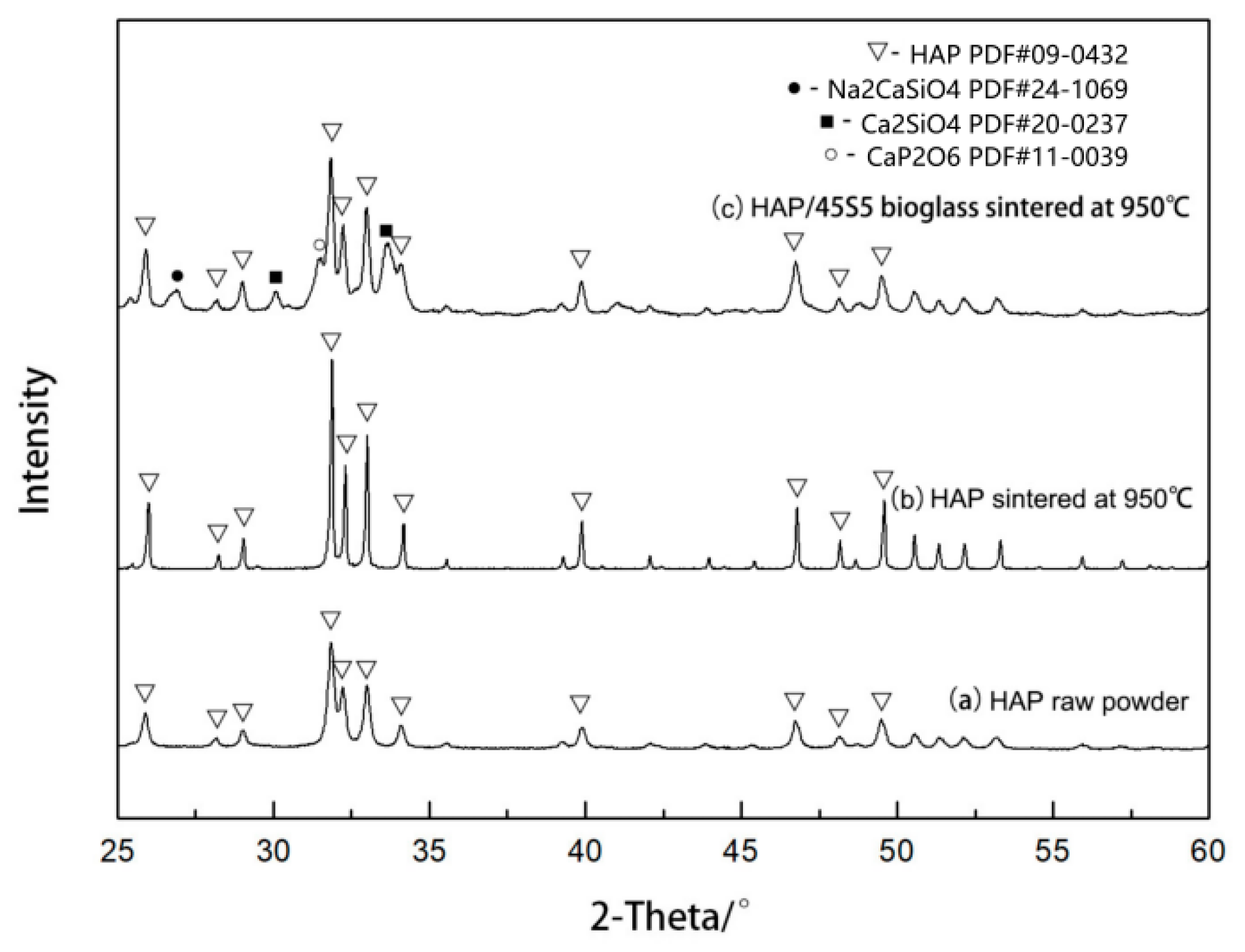
3.2.1. Phase Analysis
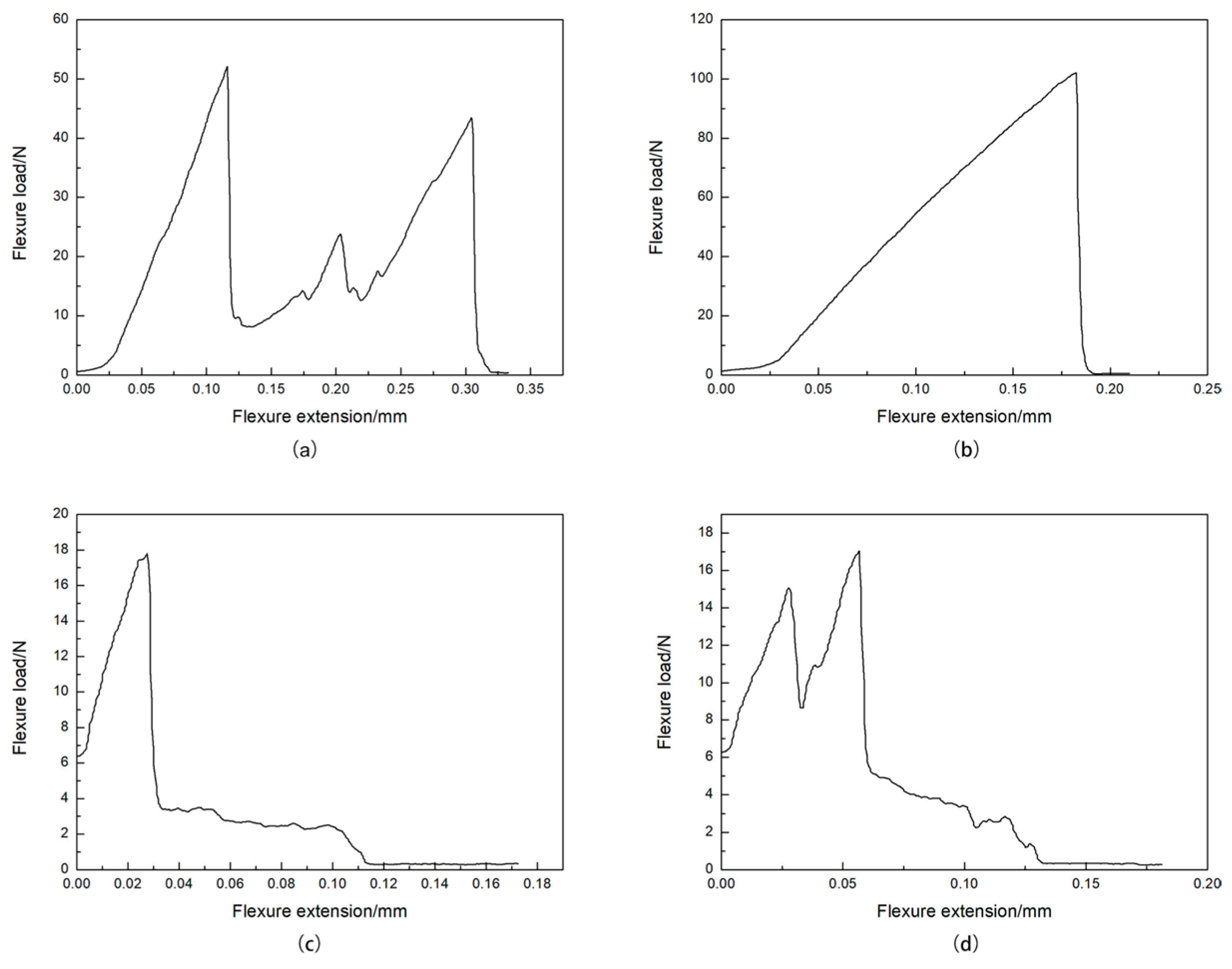
3.2.2. Load-Displacement Curve Analysis
3.2.3. Analysis of Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, C.; Zhang, Z.; Ma, X.; Liu, L.; Wu, Y.; Li, D.; Wang, P.; Duan, X. Mechanical and ablation properties of laminated ZrB2-SiC ceramics with Si3N4 whisker interface. Corros. Sci. 2022, 197, 110051. [Google Scholar] [CrossRef]
- Li, S.; Luo, X.; Wei, C.; Gao, P.; Wang, P.; Zhou, L. Enhanced strength and toughness of silicon carbide ceramics by graphene platelet-derived laminated reinforcement. J. Alloys Compd. 2020, 834, 155252. [Google Scholar] [CrossRef]
- Xiang, L.; Cheng, L.; Hou, Y.; Wang, F.; Li, L.; Zhang, L. Fabrication and mechanical properties of laminated HfC-SiC/BN ceramics. J. Eur. Ceram. Soc. 2014, 34, 3635–3640. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z.; Song, P.; Wang, S.; Wang, Y.; Mao, K. Laminated structure optimization and drawing performance of Al2O3-TiC/Al2O3-TiC-CaF2 self-lubricating laminated ceramic conical die. Ceram. Int. 2015, 41, 12480–12489. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Z.; Zhang, Z.; Ma, X.; Wang, P.; Li, S.; Liu, L. High toughness and R-curve behaviour of laminated SiC/graphite ceramics. Ceram. Int. 2020, 46, 22973–22979. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, M.; Ye, F.; Bai, Y.; Li, M.; Fan, S.; Zhang, L. Structure design, fabrication, properties of laminated ceramics: A review. Int. J. Lightweight Mater. Manuf. 2018, 1, 126–141. [Google Scholar] [CrossRef]
- Naga, S.M.; Kenawy, S.H.; Awaad, M.; Abd El-Wahab, H.S.; Greil, P.; Abadir, M.F. Synthesis and characterization of laminated Si/SiC composites. J. Adv. Res. 2013, 4, 75–82. [Google Scholar] [CrossRef]
- Krstic, Z.; Krstic, V.D. Crack propagation and residual stress in laminated Si3N4/BN composite structures. J. Eur. Ceram. Soc. 2011, 31, 1841–1847. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Wu, J.; Li, Q.; Wu, C.; Li, Y. Effects of Nb on the elements diffusion and mechanical properties of laminated Ti/Al2O3 composites. Mater. Sci. Eng. A 2015, 636, 263–268. [Google Scholar] [CrossRef]
- Hu, S.; Song, J.; Gao, J.; Liu, J.; Meng, W.; Wang, Y. Effect of layer thickness ratio on microstructure and mechanical properties of TiCN-HfN/TiCN-WC laminated ceramics. Ceram. Int. 2023, 49, 20763–20771. [Google Scholar] [CrossRef]
- Zhao, Q.; Ju, B.; Guo, T.; Yang, W.; Kang, P.; Xue, W.; Liu, H.; Xiu, Z.; Chen, G.; Jiang, L.; et al. Preparation and fracture behavior of bionic layered SiCp/Al composites by tape casting and pressure infiltration. Ceram. Int. 2023, 49, 9060–9068. [Google Scholar] [CrossRef]
- Maleki, M.; Sheikh-Al-Eslamian, S.M.; Hasani, E.; Ghasemi, A. Comparative study on the microstructure and mechanical behavior of monolithic ceramic and laminated composite of high strength 3Y-TZP and high fracture toughness 12Ce-TZP. J. Alloys Compd. 2019, 776, 166–171. [Google Scholar] [CrossRef]
- Derakhshani, B.; Parvin, N.; Khodaei, M. Influence of sintering temperature on the mechanical properties and biocompatibility of titanium-nano hydroxyapatite functionally graded materials. J. Mater. Res. Technol. 2023, 27, 2538–2547. [Google Scholar] [CrossRef]
- Wu, R.; Zeng, T.; Fan, M.; Cui, Y.; Xu, G.; Wang, X.; Cheng, S. Microstructure and mechanical properties of 3D printed porous Al2O3-ZrO2 laminated ceramics with tailored porosity. Ceram. Int. 2023, 49, 33369–33381. [Google Scholar] [CrossRef]
- Shi, M.; Han, Q.; Liu, X.; Cheng, X.; Han, Z. Coupling bionic design and application of flow curved surface for carbon fiber composite fan blade. J. Mater. Res. Technol. 2023, 27, 1797–1807. [Google Scholar] [CrossRef]
- Tang, Z.; Li, G.; Lu, S.; Wang, J.; Chi, L. Enhance mechanical damping behavior of RHA-cement mortar with bionic inorganic-organic laminated structures. Constr. Build. Mater. 2022, 323, 126521. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, Z.; Huang, S.; Cheng, X. Fabrication of laminated ZrB2-SiC ceramics via tape casting and vacuum hot-pressing sintering and their mechanical properties. Ceram. Int. 2015, 41, 11555–11561. [Google Scholar] [CrossRef]
- Wang, C.; Huang, Y.; Zan, Q.; Guo, H.; Cai, S. Biomimetic structure design—A possible approach to change the brittleness of ceramics in nature. Mater. Sci. Eng. C 2000, 11, 9–12. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, K.; Chen, G.; Wang, Z.; Han, W. Numerical simulation and experimental research on superplasticity of ceramic/ceramic laminated composite. Ceram. Int. 2005, 31, 923–927. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.; Yang, Z.; Liu, Y. Orientation of carbon fiber in magnesium-doped hydroxyapatite and its effect on mechanical and tribological properties of carbon fiber reinforced composites. Mater. Chem. Phys. 2023, 306, 128078. [Google Scholar] [CrossRef]
- Mansoor, P.; Dasharath, S.M. Synthesis and characterization of wollastonite (CaSio3)/titanium oxide (TiO2) and hydroxyapatite (HA) ceramic composites for bio-medical applications fabricated by spark plasma sintering technology. Mater. Today Proc. 2021, 45, 332–337. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cacciotti, I.; Bartoli, C.; Gazzarri, M.; Bianco, A.; Chiellini, F.; Cannillo, V. Mg- and/or Sr-doped tricalcium phosphate/bioactive glass composites: Synthesis, microstructure and biological responsiveness. Mater. Sci. Eng. C 2014, 42, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Ait Said, H.; Mabroum, H.; Lahcini, M.; Oudadesse, H.; Barroug, A.; Ben Youcef, H.; Noukrati, H. Manufacturing methods, properties, and potential applications in bone tissue regeneration of hydroxyapatite-chitosan biocomposites: A review. Int. J. Biol. Macromol. 2023, 243, 125150. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Lee, B.; Lee, C.; Gain, A.K.; Song, H. Microstructures and material properties of fibrous HAp/Al2O3-ZrO2 composites fabricated by multi-pass extrusion process. J. Eur. Ceram. Soc. 2007, 27, 157–163. [Google Scholar] [CrossRef]
- He, J.Y.; Shen, M.J.; Wan, Q.Q.; Zhang, Y.S.; Xiao, Y.H.; Wei, J.H.; Liu, Y.; Yan, J.F.; Wan, M.C.; Xu, K.H.; et al. The Janus Nature of Nanohydroxyapatite in Tumor Progression. Adv. Funct. Mater. 2022, 32, 2107599. [Google Scholar] [CrossRef]
- Cacciotti, I. Multisubstituted hydroxyapatite powders and coatings: The influence of the codoping on the hydroxyapatite performances. Int. J. Appl. Ceram. Technol. 2019, 16, 1864–1884. [Google Scholar] [CrossRef]
- Bystrov, V.; Bystrova, A.; Dekhtyar, Y. HAP nanoparticle and substrate surface electrical potential towards bone cells adhesion: Recent results review. Adv. Colloid Interface Sci. 2017, 249, 213–219. [Google Scholar] [CrossRef]
- Prihanto, A.; Muryanto, S.; Ismail, R.; Jamari, J.; Bayuseno, A.P. Batch hydrothermal synthesis of nanocrystalline, thermostable hydroxyapatite at various pH and temperature levels. Inorg. Chem. Commun. 2023, 157, 111301. [Google Scholar] [CrossRef]
- Abdollahi, S.; Ma, A.C.; Cerruti, M. Surface transformations of Bioglass 45S5 during scaffold synthesis for bone tissue engineering. Langmuir 2013, 29, 1466–1474. [Google Scholar] [CrossRef]
- Clupper, D. Crystallization kinetics of tape cast bioactive glass 45S5. J. Non Cryst. Solids 2003, 318, 43–48. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci.-Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Spirandeli, B.R.; Ribas, R.G.; Amaral, S.S.; Martins, E.F.; Esposito, E.; Vasconcellos, L.; Campos, T.; Thim, G.P.; Triches, E.S. Incorporation of 45S5 bioglass via sol-gel in beta-TCP scaffolds: Bioactivity and antimicrobial activity evaluation. Mater. Sci. Eng. C-Mater. 2021, 131, 112453. [Google Scholar] [CrossRef] [PubMed]
- Abad-Javier, M.E.; Cajero-Juárez, M.; Nuñez-Anita, R.E.; Contreras-García, M.E. Effect of collagen type I and vitamin D3 functionalization of biomimetic bioglass scaffolds on hydroxyapatite condensation. J. Eur. Ceram. Soc. 2019, 39, 3505–3512. [Google Scholar] [CrossRef]
- Lei, Q.; Chen, Y.; Gao, S.; Li, J.; Xiao, L.; Huang, H.; Zhang, Q.; Zhang, T.; Yan, F.; Cai, L. Enhanced magnetothermal effect of high porous bioglass for both bone repair and antitumor therapy. Mater. Des. 2023, 227, 111754. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Ojaghi-Ilkhchi, M.; Farrokhi-Rad, M. Evaluation of bioglass and hydroxyapatite based nanocomposite coatings obtained by electrophoretic deposition. Ceram. Int. 2020, 46, 26069–26077. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Electrophoretic deposition of Fe3O4 nanoparticles incorporated hydroxyapatite-bioglass-chitosan nanocomposite coating on AZ91 Mg alloy. Mater. Today Commun. 2021, 26, 101870. [Google Scholar] [CrossRef]
- Li, C.; Lin, X.; Jiang, X.; Zhou, Z.; Luo, Z.; Lu, A. Preparation, structure, osteogenic differentiation ability, and biosafety of bioglass for root canal disinfection. Ceram. Int. 2024, 50, 45453–45464. [Google Scholar] [CrossRef]
- Owoeye, S.S.; Folorunso, D.O.; Aramide, F.; Okotie, B. Microwave energy assisted fabrication and characterization of 45S5 bioglass-ceramics using bio-wastes as alternative resources for biomedical applications. Ceram. Int. 2024, 50, 12746–12762. [Google Scholar] [CrossRef]
- Veláquez-González, C.S.; Aguilar-Reyes, E.A.; León-Patiño, C.A. Effect of Ta2O5 content on the microstructural properties of 45S5 bioglass glass-ceramic scaffolds. Boletín Soc. Española Cerámica Vidr. 2024, 63, 304–315. [Google Scholar] [CrossRef]
- Taveri, G.; Hanzel, O.; Sedláček, J.; Toušek, J.; Neščaková, Z.; Michálek, M.; Dlouhý, I.; Hnatko, M. Consolidation of Bioglass® 45S5 suspension through cold isostatic pressing. Ceram. Int. 2021, 47, 4090–4096. [Google Scholar] [CrossRef]
- Demirkiran, H.; Hu, Y.; Zuin, L.; Appathurai, N.; Aswath, P.B. XANES analysis of calcium and sodium phosphates and silicates and hydroxyapatite–Bioglass®45S5 co-sintered bioceramics. Mater. Sci. Eng. C 2011, 31, 134–143. [Google Scholar] [CrossRef]
- Goller, G.; Demirkıran, H.; Oktar, F.N.; Demirkesen, E. Processing and characterization of bioglass reinforced hydroxyapatite composites. Ceram. Int. 2003, 29, 721–724. [Google Scholar] [CrossRef]
- Bellucci, D.; Desogus, L.; Montinaro, S.; Orrù, R.; Cao, G.; Cannillo, V. Innovative hydroxyapatite/bioactive glass composites processed by spark plasma sintering for bone tissue repair. J. Eur. Ceram. Soc. 2017, 37, 1723–1733. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J.; Kondoh, K.; Umeda, J. Low pressure spark plasma sintered hydroxyapatite and Bioglass® composite scaffolds for bone tissue repair. Ceram. Int. 2018, 44, 23052–23062. [Google Scholar] [CrossRef]
- Sundarabharathi, L.; Parangusan, H.; Ponnamma, D.; Al-Maadeed, M.A.A.; Chinnaswamy, M. In-vitro biocompatibility, bioactivity and photoluminescence properties of Eu3+/Sr2+ dual-doped nano-hydroxyapatite for biomedical applications. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 2191–2201. [Google Scholar] [CrossRef]
- Bushra, A.; Subhani, A.; Islam, N. A comprehensive review on biological and environmental applications of chitosan-hydroxyapatite biocomposites. Compos. Part. C Open Access 2023, 12, 100402. [Google Scholar] [CrossRef]
- Cuccu, A.; Montinaro, S.; Orrù, R.; Cao, G.; Bellucci, D.; Sola, A.; Cannillo, V. Consolidation of different hydroxyapatite powders by SPS: Optimization of the sintering conditions and characterization of the obtained bulk products. Ceram. Int. 2015, 41, 725–736. [Google Scholar] [CrossRef]
- Dubey, A.K.; Sitesh, G.; Nath, S.; Basu, B. Spark plasma sintering to restrict sintering reactions and enhance properties of hydroxyapatite-mullite biocomposites. Ceram. Int. 2011, 37, 2755–2761. [Google Scholar] [CrossRef]
- Figueiredo, A.; Coimbra, P.; Cabrita, A.; Guerra, F.; Figueiredo, M. Comparison of a xenogeneic and an alloplastic material used in dental implants in terms of physico-chemical characteristics and in vivo inflammatory response. Mater. Sci. Eng. C 2013, 33, 3506–3513. [Google Scholar] [CrossRef]
- GB/T 4741-1999; Standard Test Method for Bending Strength of Ceramic Materials. National Standard of the People’s Republic of China: Beijing, China, 1999.
- Canales, D.A.; Pinones, N.; Saavedra, M.; Loyo, C.; Palza, H.; Peponi, L.; Leones, A.; Baier, R.V.; Boccaccini, A.R.; Grunelwald, A.; et al. Fabrication and assessment of bifunctional electrospun poly(l-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 2023, 228, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Han, H.; Zhao, J.; Deng, H.; Cai, P.; Sun, Q.; Yu, Q.; Zhuang, W.; Huang, Z.; Zhou, Y. Ti3AlC2 preceramic paper derived in-situ laminated TiC/Ni-Ni3(Al,Ti) composite: Microstructure, mechanical properties and fracture behavior. J. Alloys Compd. 2024, 1004, 175818. [Google Scholar] [CrossRef]
- Sglavo, V.M.; Bellettati, N. Ceramic laminates with improved mechanical reliability by tailoring the porosity of the constituting layers. J. Eur. Ceram. Soc. 2017, 37, 1643–1650. [Google Scholar] [CrossRef]
- Broz, J.J.; Simske, S.J.; Corley, W.D.; Greenberg, A.R. Effects of deproteinization and ashing on site-specific properties of cortical bone. J. Mater. Sci. Mater. Med. 1997, 8, 395–401. [Google Scholar] [CrossRef]
- Ming, K.; Jiehua, H.; Lixuan, Z.; Xinguang, W.; Hanming, G.; Ruixuan, H. Mechanical properties and effect on osteodifferentiation of induced pluripotent stemcells of chitosan/whisker/calcium phosphate cement composite biomaterial. Chin. J. Reparative Reconstr. Surg. 2018, 32, 959–967. [Google Scholar]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef]
- Ghomi, H.; Fathi, M.H.; Edris, H. Effect of the composition of hydroxyapatite/bioactive glass nanocomposite foams on their bioactivity and mechanical properties. Mater. Res. Bull. 2012, 47, 3523–3532. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Xu, J.L.; Yu, L.G.; Fang, X.Y.; Khor, K.A. Spark plasma sintering of sol-gel derived 45S5 Bioglass®-ceramics: Mechanical properties and biocompatibility evaluation. Mater. Sci. Eng. C 2012, 32, 494–502. [Google Scholar] [CrossRef]


| Temperature/°C | Pressure/MPa | Soaking Time/min | Heating Rate/°C·min−1 | Vacuum Degree/Pa |
|---|---|---|---|---|
| 850–1050 | 40 | 5 | 100 | 15 |
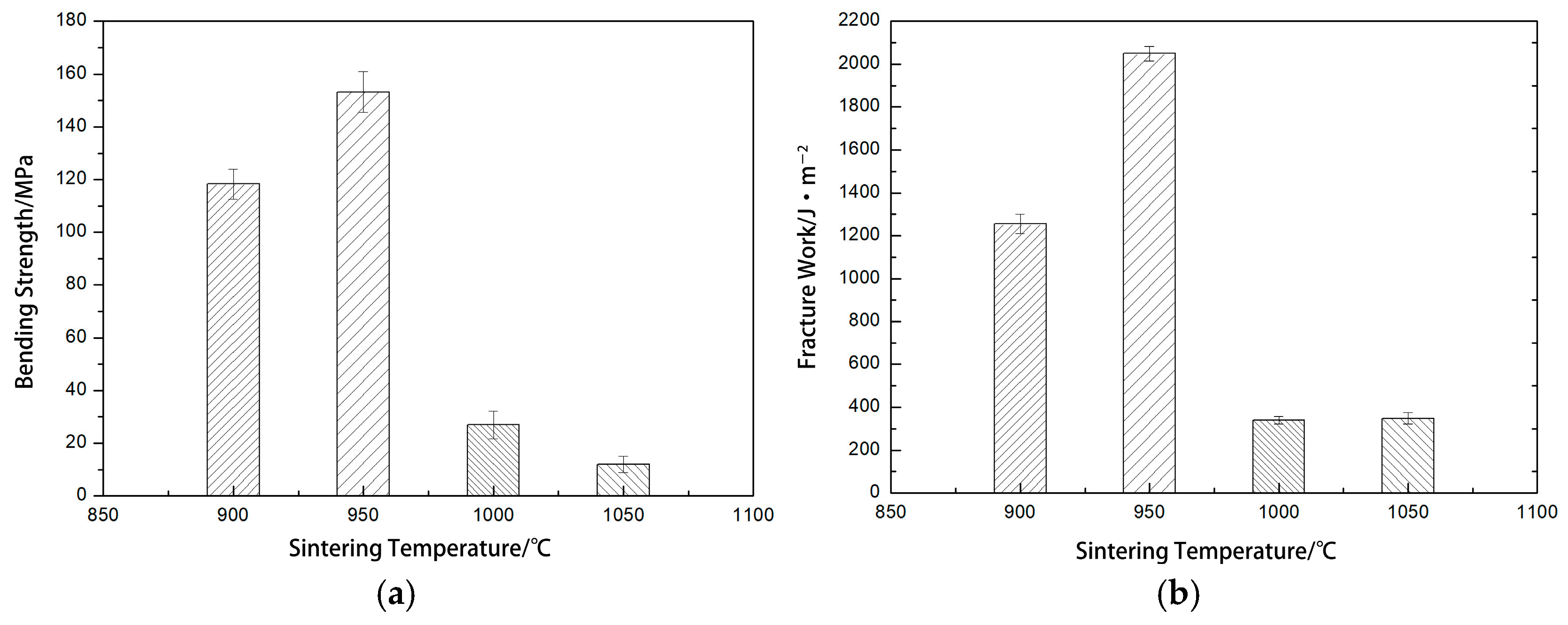
| Serial Number | Sinter Temperature /°C | Flexure Strength /MPa | Fracture Work /J·m−2 |
|---|---|---|---|
| F1 | 900 | 118.28 ± 5.6 | 1255 ± 46 |
| F2 | 950 | 153.22 ± 7.7 | 2049 ± 34 |
| F3 | 1000 | 26.9 ± 5.2 | 340.58 ± 18 |
| F4 | 1050 | 11.9 ± 3.1 | 347.62 ± 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Li, X.; Yun, B. Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering. Materials 2024, 17, 5413. https://doi.org/10.3390/ma17225413
Meng Y, Li X, Yun B. Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering. Materials. 2024; 17(22):5413. https://doi.org/10.3390/ma17225413
Chicago/Turabian StyleMeng, Ye, Xinge Li, and Bing Yun. 2024. "Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering" Materials 17, no. 22: 5413. https://doi.org/10.3390/ma17225413
APA StyleMeng, Y., Li, X., & Yun, B. (2024). Preparation and Characterization of Mechanical Properties of HAP/45S5 Bioglass Laminated Ceramic Composites via Spark Plasma Sintering. Materials, 17(22), 5413. https://doi.org/10.3390/ma17225413





