Fixation of Tripotassium Citrate Flame Retardant Using a Sorbitol and Citric Acid Wood-Modification Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Wood Materials
2.2. Modification Process
2.3. Dimensional Stability
2.3.1. Solution Uptake (SU)
2.3.2. Cell Wall Bulking Coefficient (CWB)
2.3.3. Weight Percent Gain (WPG)
2.3.4. Leaching Rate (LR)
2.3.5. Anti-Swelling Efficiency (ASE)
2.3.6. Equilibrium Moisture Content (EMC)
2.4. Flame Retardant Measurement
2.4.1. Burner Test
2.4.2. Single-Flame Test
2.4.3. Cone Calorimeter
2.5. Mechanical Properties
2.5.1. Three-Point Bending Test
2.5.2. Impact Bending Strength Test
2.6. Chemical Property Analysis
3. Results and Discussion
3.1. Dimensional Stability
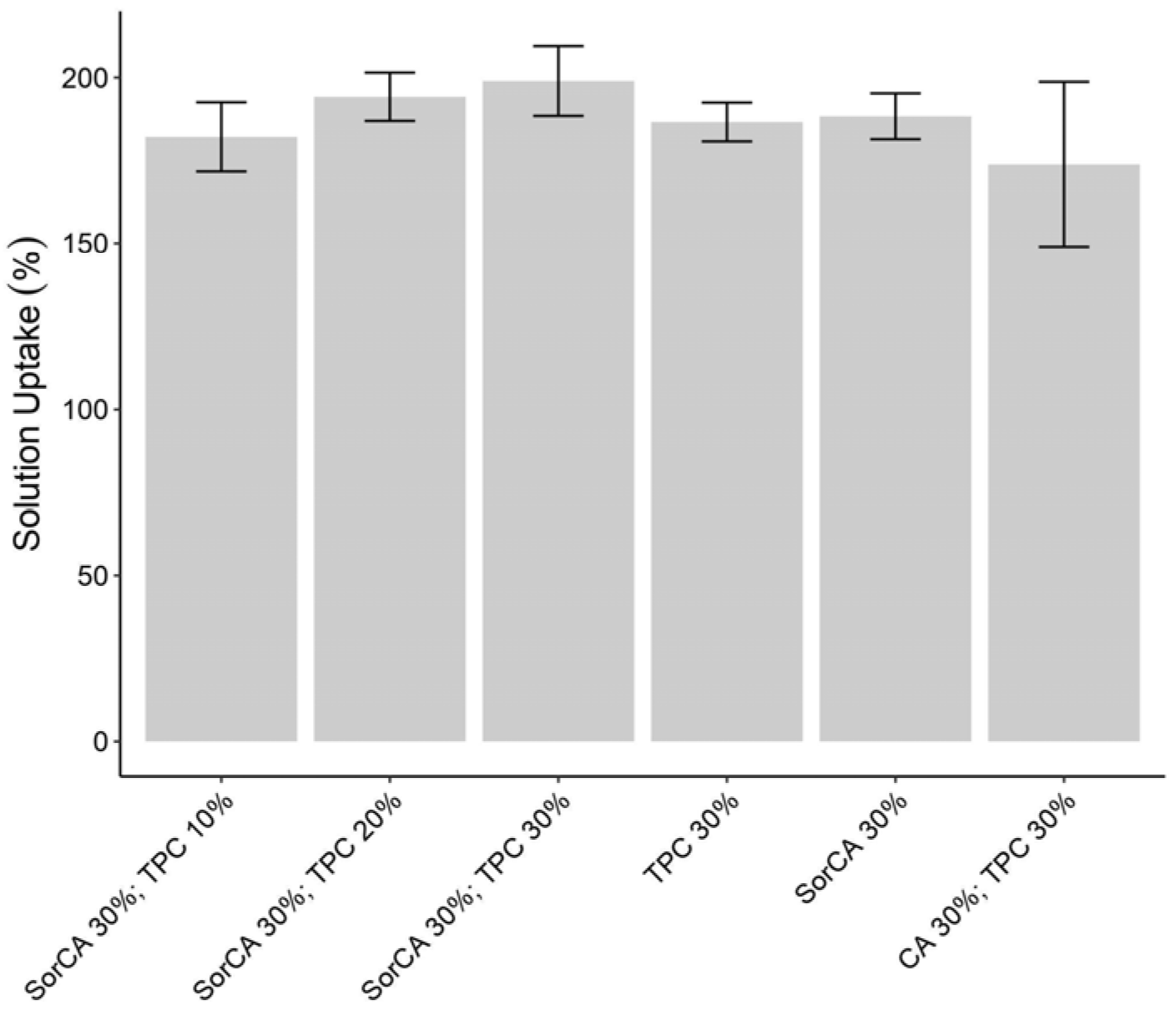
3.1.1. Solution Uptake (SU)
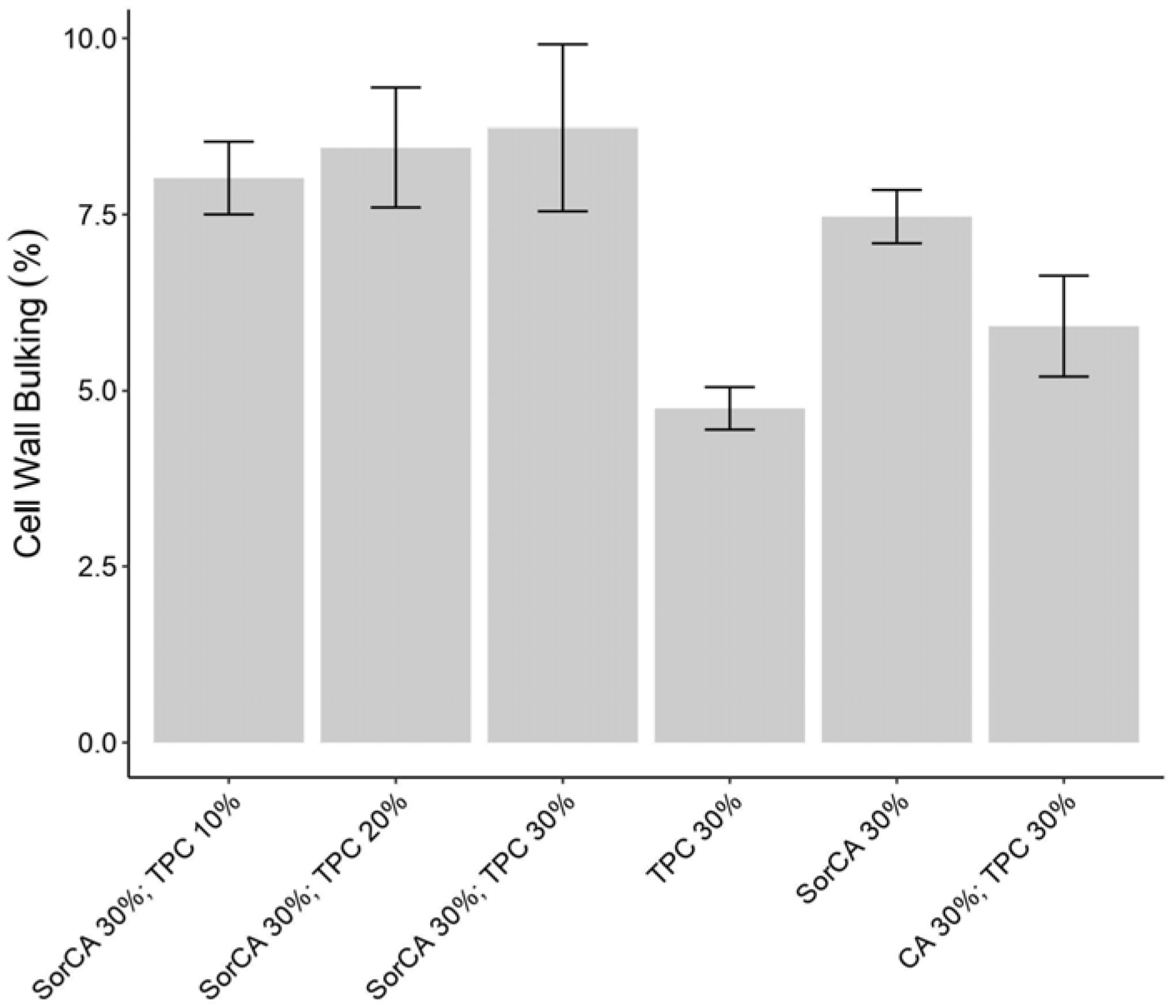
3.1.2. Cell Wall Bulking Coefficient (CWB)
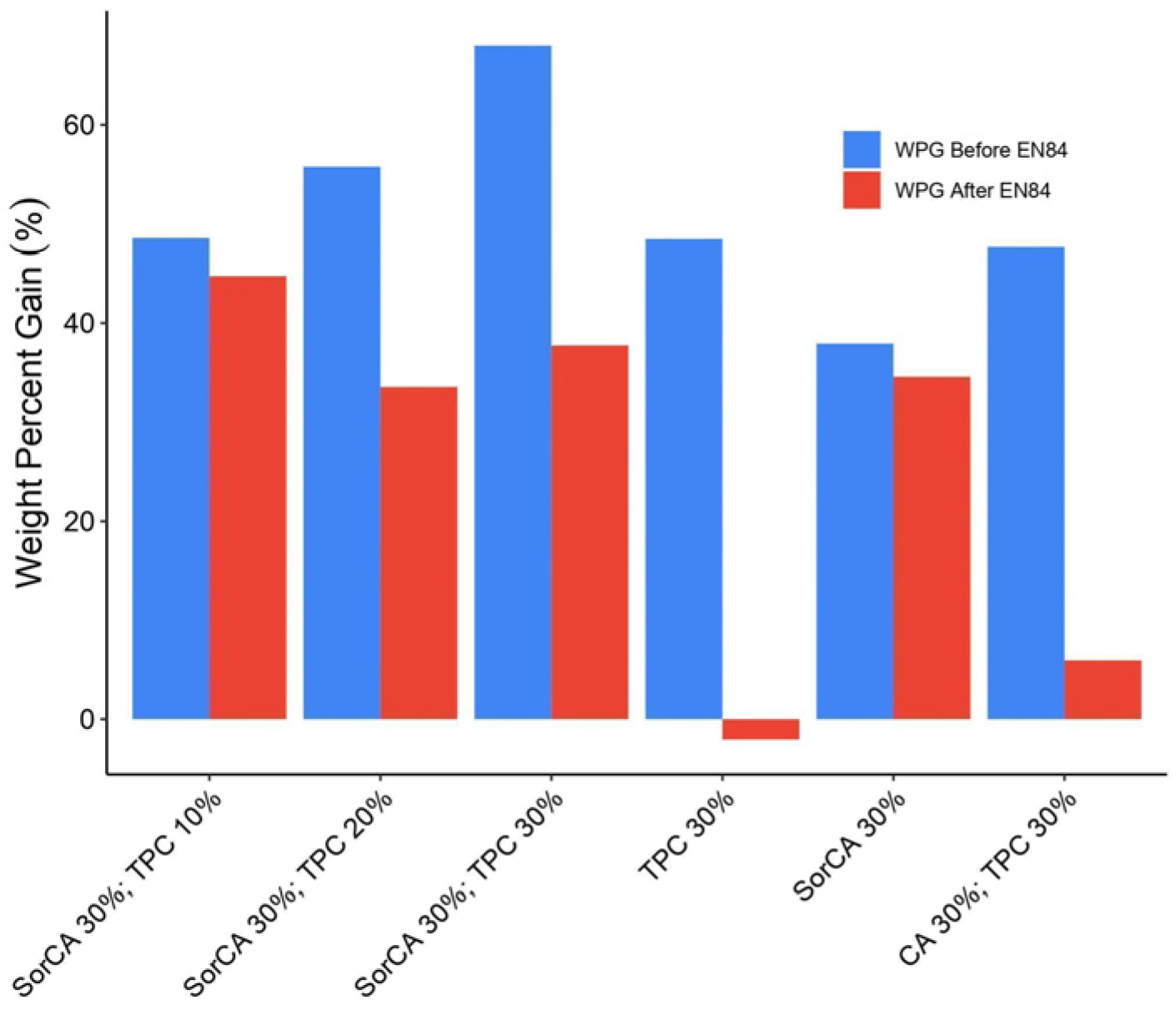
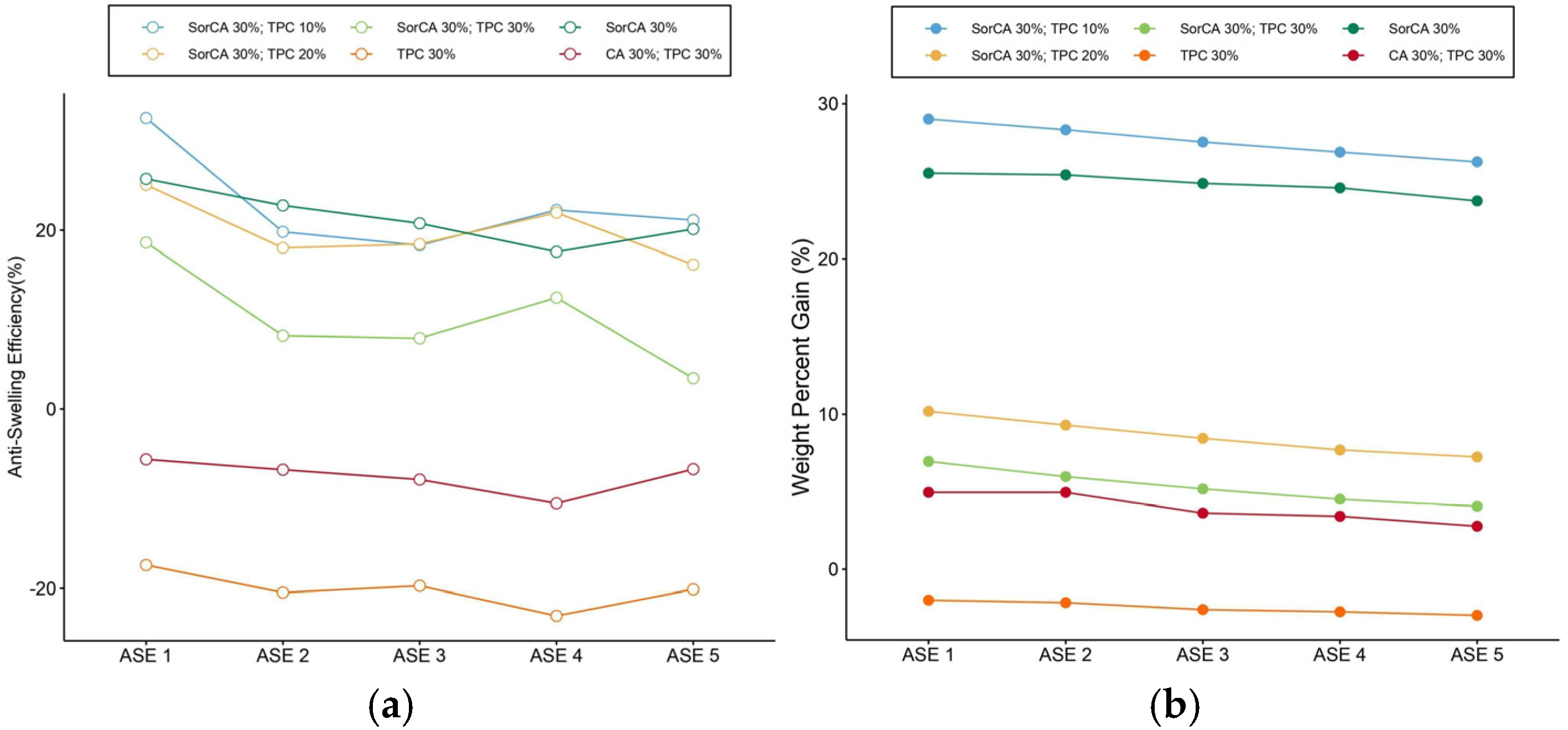
3.1.3. Weight Percent Gain (WPG)
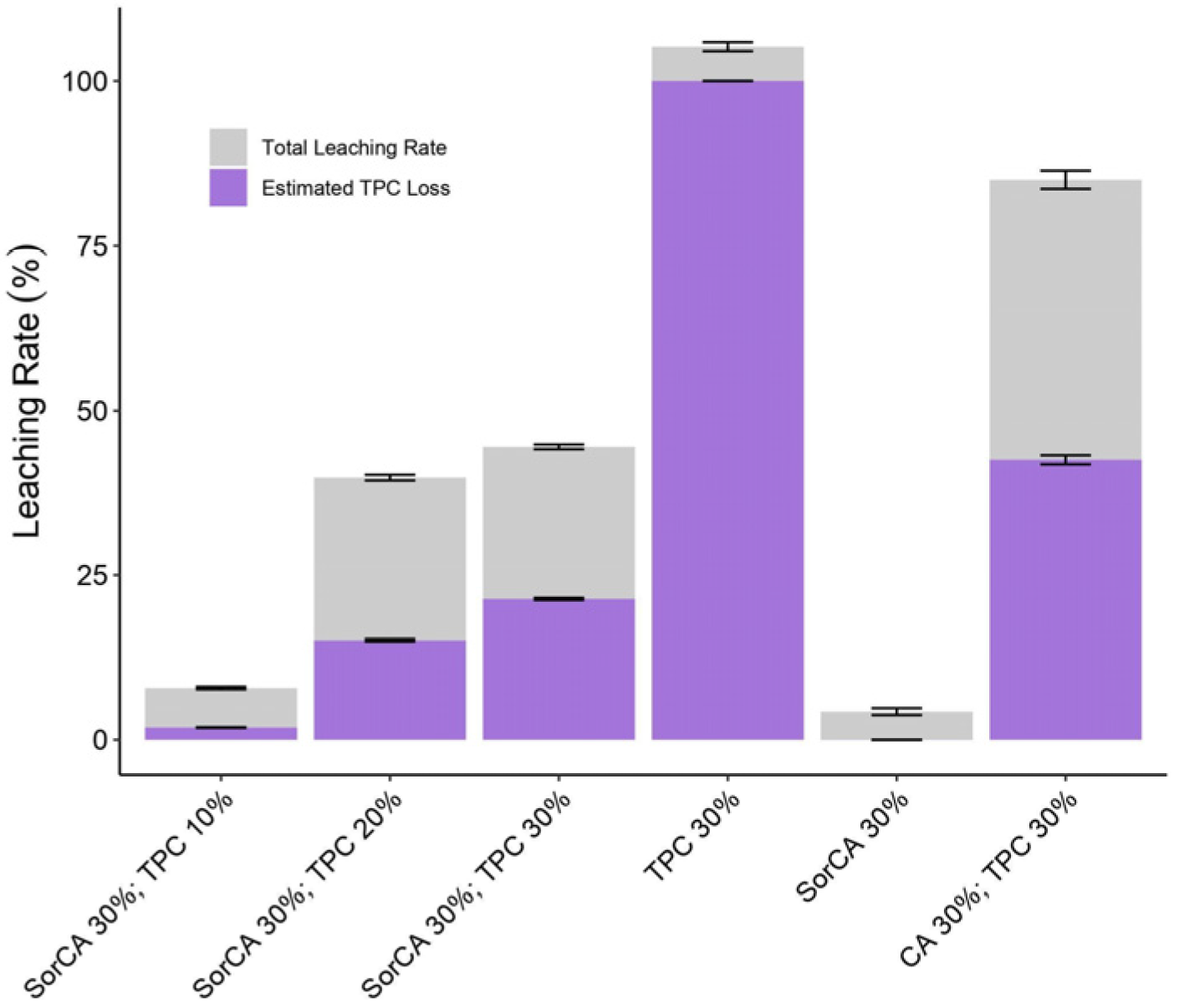
3.1.4. Leaching Rate (LR)
3.1.5. Anti-Swelling Efficiency (ASE)
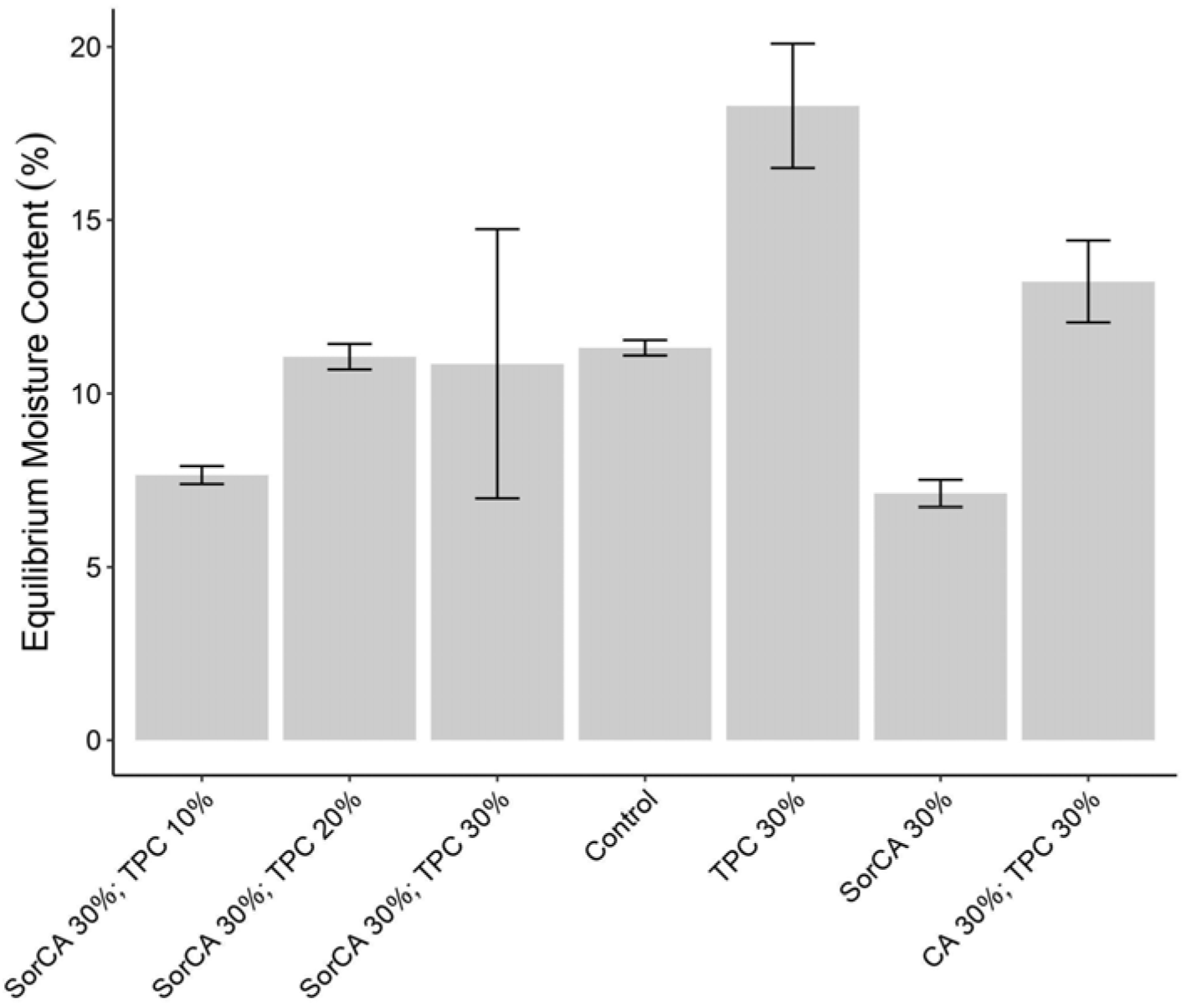
3.1.6. Equilibrium Moisture Content (EMC)
3.2. Flame Retardant Measurement
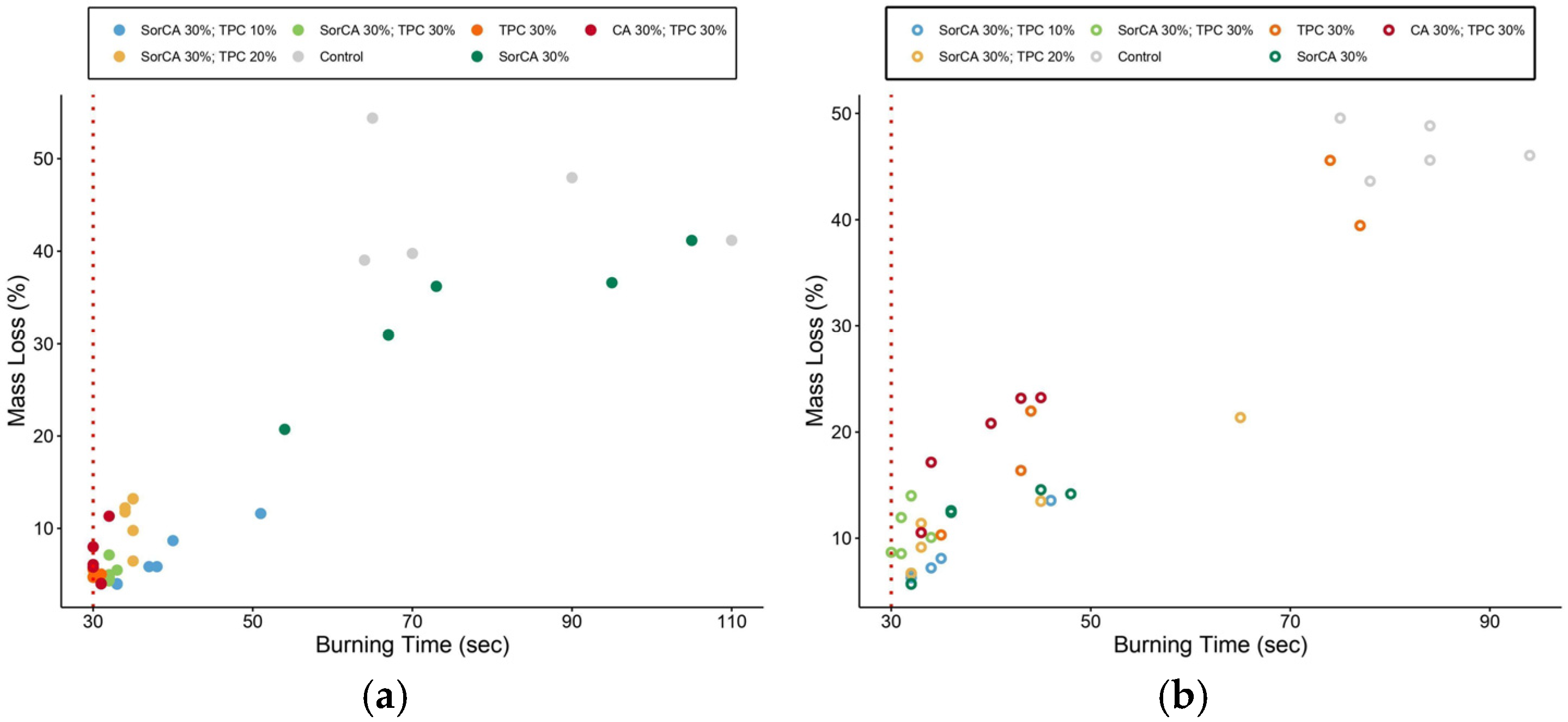
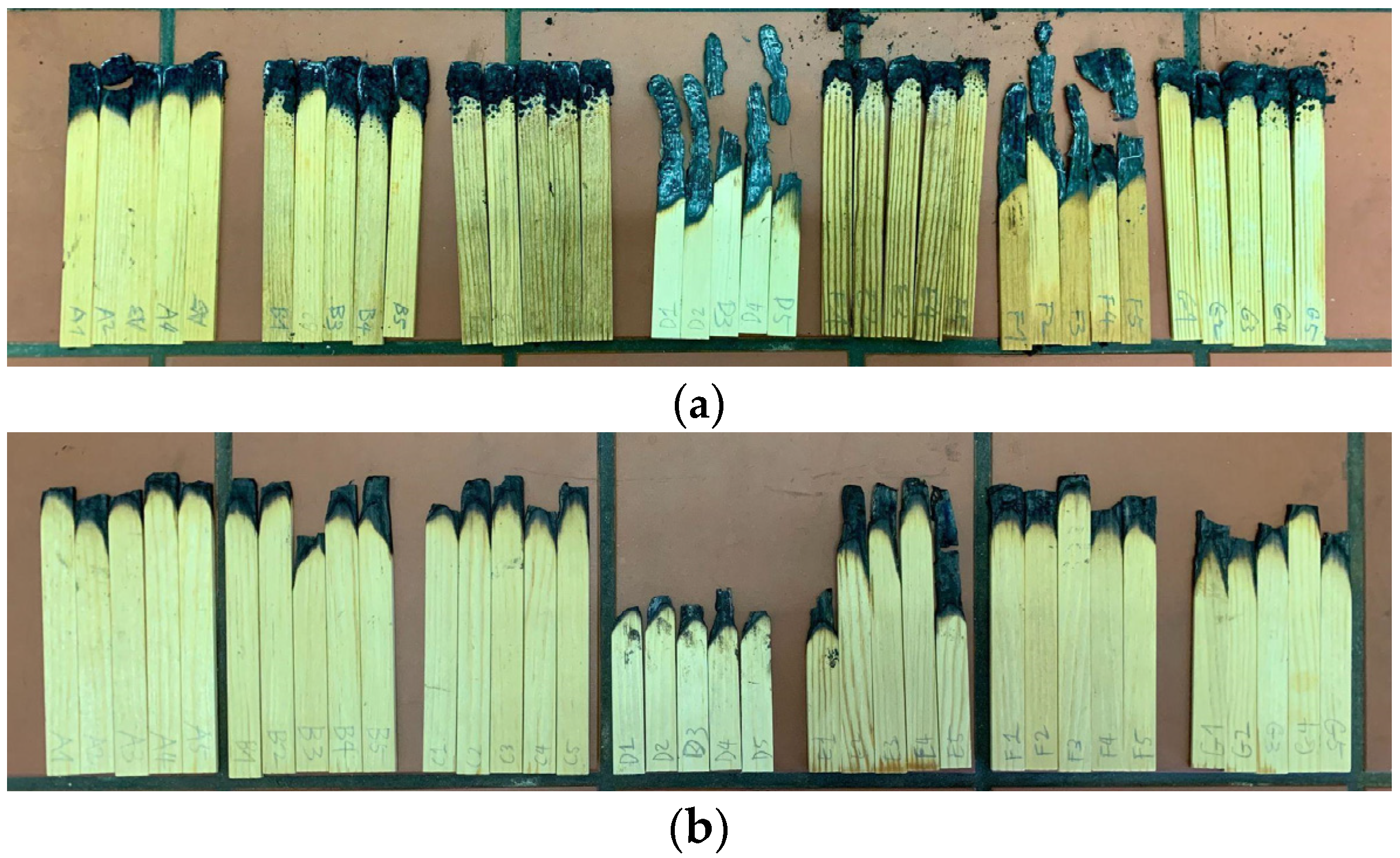
3.2.1. Burner Test
3.2.2. Single-Flame Test
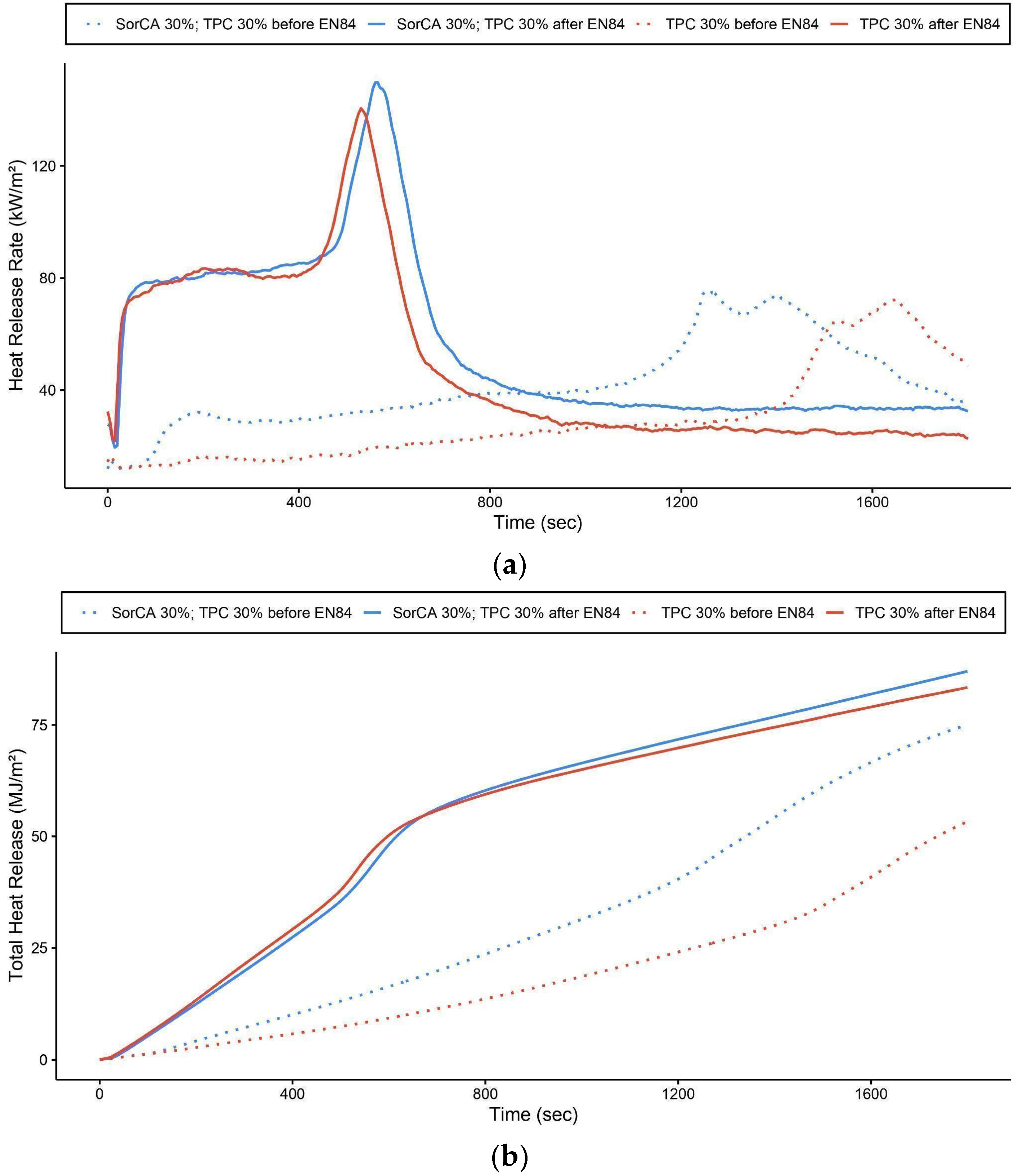
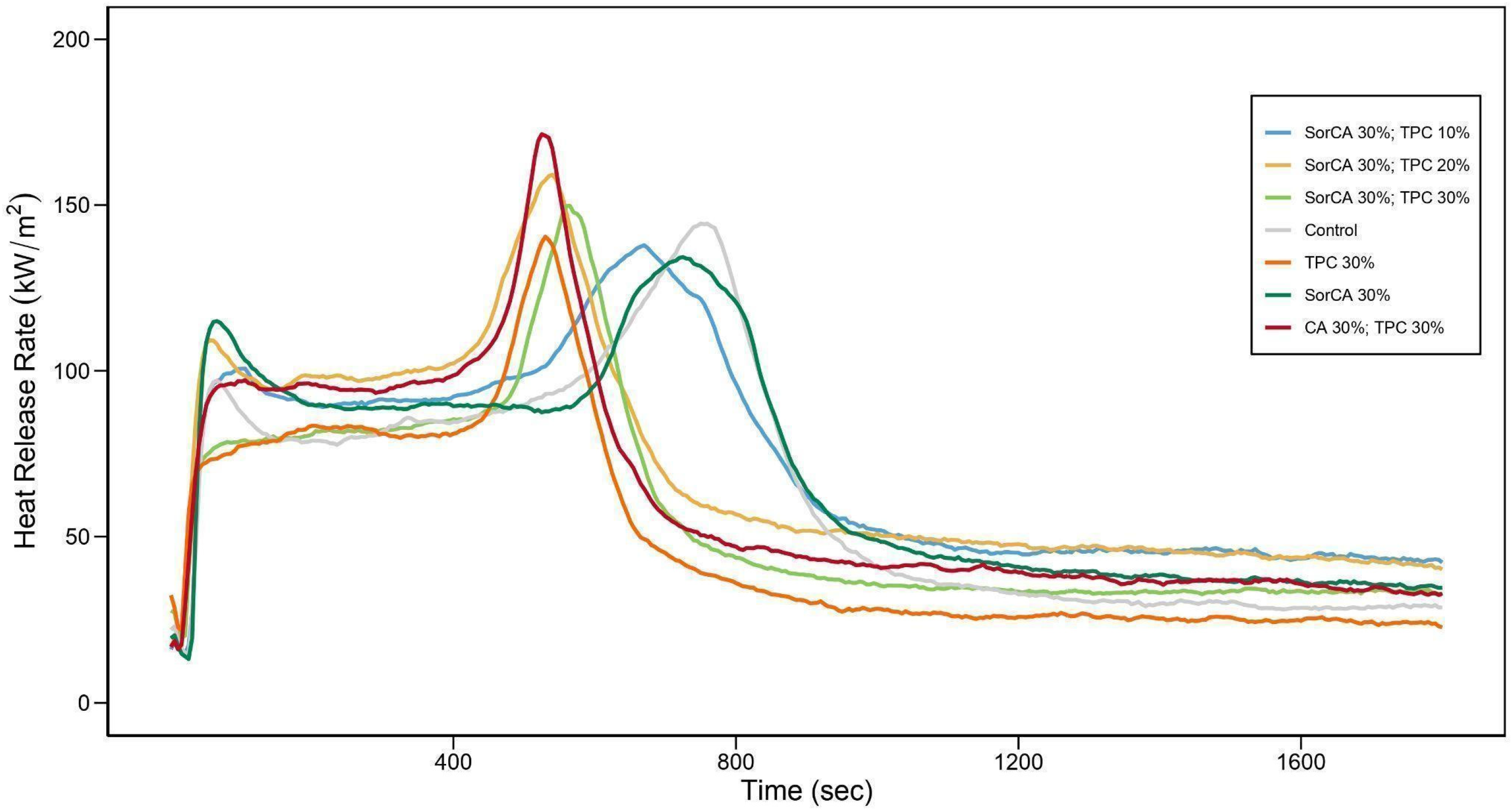
3.2.3. Cone Calorimeter
3.3. Mechanical Properties
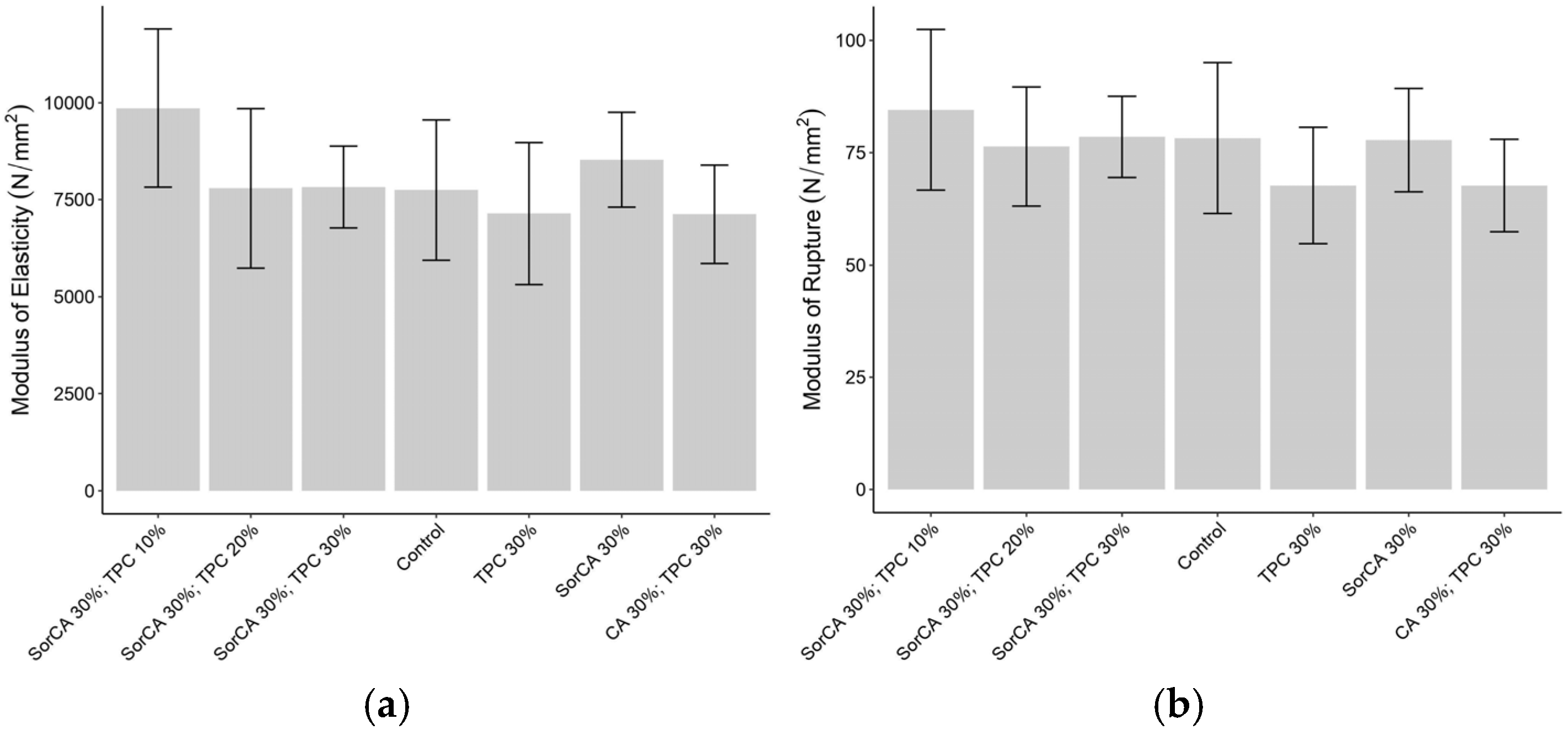
3.3.1. Three-Point Bending Test
3.3.2. Impact Bending Strength Test
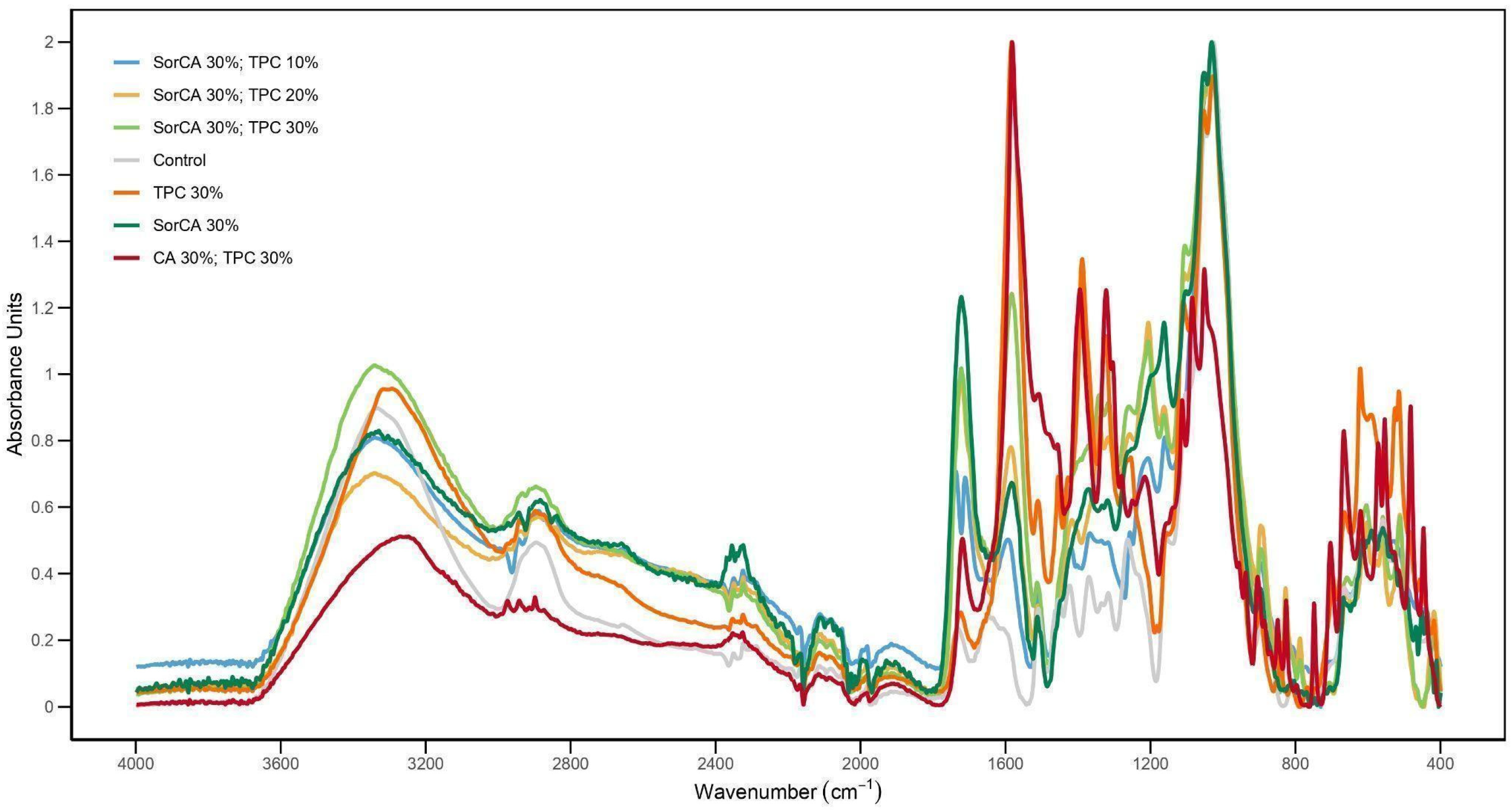
3.4. Chemical Property Analysis
FTIR-ATR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mai, C.; Schmitt, U.; Niemz, P. A brief overview on the development of wood research. Holzforschung 2022, 76, 102–119. [Google Scholar] [CrossRef]
- Hu, J.; Fu, Z.; Wang, X.; Chai, Y. Manufacturing and characterization of modified wood with in situ polymerization and cross-linking of water-soluble monomers on wood cell walls. Polymers 2022, 14, 3299. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Karlsson, O.; Kim, I.; Myronycheva, O.; Mensah, R.A.; Försth, M.; Sandberg, D. Fire retardancy and leaching resistance of furfurylated pine wood (Pinus sylvestris L.) treated with guanyl-urea phosphate. Polymers 2022, 14, 1829. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Wood chemical modification based on bio-based polycarboxylic acid and polyols—Status quo and future perspectives. Wood Mater. Sci. Eng. 2022, 17, 1040–1054. [Google Scholar] [CrossRef]
- Jones, D.; Sandberg, D.; Goli, G.; Todaro, L. Wood Modification in Europe: A State-Of-The-Art about Processes, Products and Applications; Firenze University Press: Firenze, Italy, 2019; p. 123. [Google Scholar]
- Guo, W.; Xiao, Z.; Wentzel, M.; Emmerich, L.; Xie, Y.; Militz, H. Modification of Scots Pine with Activated Glucose and Citric Acid: Physical and Mechanical Properties. BioResources 2019, 14, 3445–3458. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, P.; Chen, Z.; Li, L.; Huang, Y. Flame retardancy, thermal stability, and hygroscopicity of wood materials modified with melamine and amino trimethylene phosphonic acid. Constr. Build. Mater. 2021, 267, 121042. [Google Scholar] [CrossRef]
- Xie, Y.; Fu, Q.; Wang, Q.; Xiao, Z.; Militz, H. Effects of chemical modification on the mechanical properties of wood. Eur. J. Wood Wood Prod. 2013, 71, 401–416. [Google Scholar] [CrossRef]
- Pečnik, J.G.; Kutnar, A.; Militz, H.; Schwarzkopf, M.; Schwager, H. Fatigue behavior of beech and pine wood modified with low molecular weight phenol-formaldehyde resin. Holzforschung 2021, 75, 37–47. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Biological durability and wood–water interactions of sorbitol and citric acid (SorCA) modified wood. J. Wood Sci. 2023, 69, 34. [Google Scholar] [CrossRef]
- Wu, M.; Emmerich, L.; Kurkowiak, K.; Militz, H. Combined treatment of wood with thermosetting resins and phosphorous flame retardants. Eur. J. Wood Wood Prod. 2024, 82, 167–174. [Google Scholar] [CrossRef]
- Augustina, S.; Dwianto, W.; Wahyudi, I.; Syafii, W.; Gérardin, P.; Marbun, S.D. Wood impregnation in relation to its mechanisms and properties enhancement. BioResources 2023, 18, 4332. [Google Scholar] [CrossRef]
- Homan, W.; Tjeerdsma, B.; Beckers, E.; Jorissen, A. Structural and other properties of modified wood. In Proceedings of the World Conference on Timber Engineering, Whistler, BC, Canada, 31 July– 3 August 2000; Volume 5. [Google Scholar]
- Bollmus, S.; Beeretz, C.; Militz, H. Tensile and impact bending properties of chemically modified Scots pine. Forests 2020, 11, 84. [Google Scholar] [CrossRef]
- Rabe, S.; Klack, P.; Bahr, H.; Schartel, B. Assessing the fire behavior of woods modified by N-methylol crosslinking, thermal treatment, and acetylation. Fire Mater. 2020, 44, 530–539. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification—Chemical, Thermal and Other Processes; Wiley Series in Renewable Resources; Wiley and Sons: Chichester, UK, 2006. [Google Scholar]
- Waeber, P.O.; Schuurman, D.; Ramamonjisoa, B.; Langrand, M.; Barber, C.V.; Innes, J.L.; Wilmé, L. Uplisting of Malagasy precious woods critical for their survival. Biol. Conserv. 2019, 235, 89–92. [Google Scholar] [CrossRef]
- Militz, H.; Lande, S. Challenges in wood modification technology on the way to practical applications. Wood Mater. Sci. Eng. 2009, 4, 23–29. [Google Scholar] [CrossRef]
- Chabert, A.J.; Fredon, E.; Rémond, R. Improving the stability of beech wood with polyester treatment based on malic acid. Holzforschung 2022, 76, 268–275. [Google Scholar] [CrossRef]
- Liu, C.; Yao, A.; Chen, K.; Shi, Y.; Feng, Y.; Zhang, P.; Chen, Z. MXene based core-shell flame retardant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part B Eng. 2021, 226, 109363. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood. For. Prod. Abstr. 1983, 6, 363–382. [Google Scholar]
- Dong, Y.; Xue, Q.; Fu, Z.; Yan, Y.; Lu, Y.; Liu, Y.; Li, J. Enhancing wood stability and fire retardancy through citric acid and phosphorylated sucrose stearate cross-linking modification. Constr. Build. Mater. 2023, 393, 131946. [Google Scholar] [CrossRef]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech wood modification based on in situ esterification with sorbitol and citric acid. Wood Sci. Technol. 2020, 54, 479–502. [Google Scholar] [CrossRef]
- Doll, K.M.; Shogren, R.L.; Willett, J.L.; Swift, G. Solvent-free polymerization of citric acid and D-sorbitol. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 4259–4267. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Sorption behavior and swelling of citric acid and sorbitol (SorCA) treated wood. Holzforschung 2021, 75, 1136–1149. [Google Scholar] [CrossRef]
- L’hostis, C.; Thévenon, M.F.; Fredon, E.; Gérardin, P. Improvement of beech wood properties by in situ formation of polyesters of citric and tartaric acid in combination with glycerol. Holzforschung 2018, 72, 291–299. [Google Scholar] [CrossRef]
- Schorr, D.; Blanchet, P.; Essoua Essoua, G.G. Glycerol and citric acid treatment of lodgepole pine. J. Wood Chem. Technol. 2018, 38, 123–136. [Google Scholar] [CrossRef]
- Essoua Essoua, G.G.; Landry, V.; Beauregard, L.R.; Blanchet, P. Pine wood treated with a citric acid and glycerol mixture: Biomaterial performance improved by a bio-byproduct. BioResources 2016, 11, 3049–3072. [Google Scholar] [CrossRef]
- Berube, M.A.; Schorr, D.; Ball, R.J.; Landry, V.; Blanchet, P. Determination of in situ esterification parameters of citric acid-glycerol based polymers for wood impregnation. J. Polym. Environ. 2018, 26, 970–979. [Google Scholar] [CrossRef]
- He, X.; Xiao, Z.; Feng, X.; Sui, S.; Wang, Q.; Xie, Y. Modification of poplar wood with glucose crosslinked with citric acid and 1,3-dimethylol-4,5-dihydroxy ethyleneurea. Holzforschung 2016, 70, 47–53. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of wood using sorbitol and citric acid under aqueous conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Alfredsen, G.; Larnøy, E.; Beck, G.; Biørnstad, J.; Gobakken, L.R.; Treu, A. A summary of decay performance with citric acid and sorbitol modification. In Proceedings of the IRG Annual Meeting, IRG/WP 20-40898, Online Webinar, 10–11 June 2020. [Google Scholar]
- Treu, A.; Nunes, L.; Larnøy, E. Macrobiological degradation of esterified wood with sorbitol and citric acid. Forests 2020, 11, 776. [Google Scholar] [CrossRef]
- Noël, M.; Mougel, E.; Fredon, E.; Masson, D.; Masson, E. Lactic acid/wood-based composite material. Part 2: Physical and mechanical performance. Bioresour. Technol. 2009, 100, 4717–4722. [Google Scholar] [CrossRef]
- Chabert, A. Traitement Biosourcé du Hêtre par la Formation in situ de Polyesters à base d’Acide Malique-Étude du Procédé et des Propriétés Physiques Conférées, du petit échantillon à l’échelle de la Planche. Ph.D. Thesis, Université de Lorraine, Lorraine, France, 2022. [Google Scholar]
- Beck, G. Leachability and decay resistance of wood polyesterified with sorbitol and citric acid. Forests 2020, 11, 650. [Google Scholar] [CrossRef]
- Wu, M.; Emmerich, L.; Kurkowiak, K.; Militz, H. Fire resistance of pine wood treated with phenol-formaldehyde resin and phosphate-based flame retardant. Wood Mater. Sci. Eng. 2023, 18, 1933–1939. [Google Scholar] [CrossRef]
- Rowell, R.M. Chemical modification of wood: A short review. Wood Mater. Sci. Eng. 2006, 1, 29–33. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Karlsson, O.; Jones, D. Wood Modification Technologies: Principles, Sustainability, and the Need for Innovation; CRC Press: Boca Raton, FL, USA, 2021; pp. 115–195. [Google Scholar]
- Östman, B.; Brandon, D.; Frantzich, H. Fire safety engineering in timber buildings. Fire Saf. J. 2017, 91, 11–20. [Google Scholar] [CrossRef]
- Lin, C.F.; Karlsson, O.; Das, O.; Mensah, R.A.; Mantanis, G.I.; Jones, D.; Sandberg, D. High leach-resistant fire-retardant modified pine wood (Pinus sylvestris L.) by in situ phosphorylation and carbamylation. ACS Omega 2023, 8, 11381–11396. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, J.; Militz, H.; Wang, F.; Wang, Q.; Mai, C.; Xiao, Z. Thermo-oxidative decomposition and combustion behavior of Scots pine (Pinus sylvestris L.) sapwood modified with phenol-and melamine-formaldehyde resins. Wood Sci. Technol. 2016, 50, 1125–1143. [Google Scholar] [CrossRef]
- McKenna, S.T.; Jones, N.; Peck, G.; Dickens, K.; Pawelec, W.; Oradei, S.; Harris, S.; Stec, A.A.; Hull, T.R. Fire behaviour of modern façade materials—Understanding the Grenfell Tower fire. J. Hazard. Mater. 2019, 368, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Praticò, Y.; Ochsendorf, J.; Holzer, S.; Flatt, R.J. Post-fire restoration of historic buildings and implications for Notre-Dame de Paris. Nat. Mater. 2020, 19, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, E.; Dréan, V.; Girardin, B.; Benameur, F.; Fateh, T. Reconstruction of Grenfell Tower fire. Part 1: Lessons from observations and determination of work hypotheses. Fire Mater. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Deldicque, D.; Rouzaud, J.N. Temperatures reached by the roof structure of Notre-Dame de Paris in the fire of April 15th 2019 determined by Raman paleothermometry. C. R. Géoscience 2020, 352, 7–18. [Google Scholar] [CrossRef]
- Rowell, R.M.; Dietenberger, M.A. Thermal properties, combustion, and fire retardancy of wood. In Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2012; pp. 121–151. [Google Scholar]
- Popescu, C.M.; Pfriem, A. Treatments and modification to improve the reaction to fire of wood and wood-based products—An overview. Fire Mater. 2020, 44, 100–111. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Regulatory Strategy for Flame Retardants; European Chemicals Agency: Helsinki, Finland, 2023.
- Marney, D.C.O.; Russell, L.J. Combined fire retardant and wood preservative treatments for outdoor wood applications–a review of the literature. Fire Technol. 2008, 44, 1–14. [Google Scholar] [CrossRef]
- LeVan, S.L.; Tran, H.C. The role of boron in flame-retardant treatments. In Proceedings of the First International Conference on Wood Protection with Diffusible Preservatives, Nashville, TN, USA, 28–30 November 1990; Forest Products Research Society: Madison, WI, USA, 1990; pp. 39–41. [Google Scholar]
- Obanda, D.N.; Shupe, T.F.; Barnes, H.M. Reducing leaching of boron-based wood preservatives—A review of research. Bioresour. Technol. 2008, 99, 7312–7322. [Google Scholar] [CrossRef]
- Lowden, L.A.; Hull, T.R. Flammability behaviour of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jiang, L.; Renner, J.S.; Xu, Q. Characterisation of the fire behaviour of wood: From pyrolysis to fire retardant mechanisms. J. Therm. Anal. Calorim. 2023, 148, 1407–1422. [Google Scholar] [CrossRef]
- Lin, C.F.; Karlsson, O.; Mantanis, G.I.; Sandberg, D. Fire performance and leach resistance of pine wood impregnated with guanyl-urea phosphate/boric acid and a melamine-formaldehyde resin. Eur. J. Wood Wood Prod. 2020, 78, 107–111. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of wood with silicon compounds. Inorganic silicon compounds and sol-gel systems: A review. Wood Sci. Technol. 2004, 37, 339–348. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Taghiyari, H.R. Innovative wood surface treatments based on nanotechnology. Coatings 2019, 9, 866. [Google Scholar] [CrossRef]
- Araby, S.; Philips, B.; Meng, Q.; Ma, J.; Laoui, T.; Wang, C.H. Recent advances in carbon-based nanomaterials for flame retardant polymers and composites. Compos. Part B Eng. 2021, 212, 108675. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Tapken, M.; Namyslo, J.C.; Kaufmann, D.E. Chemical improvement of surfaces. Part 6: Enhanced flame retardancy of Scots pine sapwood by covalent modification with phosphorus and boron functionalized benzoates. Holzforschung 2021, 75, 83–90. [Google Scholar] [CrossRef]
- Lake, K.D.; Brown, D.C.; McLeod, D.C. New drug therapy for kidney stones: A review of cellulose sodium phosphate, acetohydroxamic acid, and potassium citrate. Drug Intell. Clin. Pharm. 1985, 19, 530–539. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.A.; Pyzik, P.L.; Rubenstein, J.E.; Hamdy, R.F.; Kossoff, E.H. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics 2009, 124, e300–e304. [Google Scholar] [CrossRef]
- Krieger, N.S.; Asplin, J.R.; Frick, K.K.; Granja, I.; Culbertson, C.D.; Ng, A.; Bushinsky, D.A. Effect of potassium citrate on calcium phosphate stones in a model of hypercalciuria. J. Am. Soc. Nephrol. 2015, 26, 3001–3008. [Google Scholar] [CrossRef]
- Gao, W.; Chen, K.; Yang, R.; Yang, F. Process for coating of reconstituted tobacco sheet with citrates. J. Anal. Appl. Pyrolysis 2015, 114, 138–142. [Google Scholar] [CrossRef]
- Kraybill, H.R. Effect of some alkali salts upon fire-holding capacity of tobacco. Bot. Gaz. 1917, 64, 42–56. [Google Scholar] [CrossRef]
- Liu, C.; Parry, A. Potassium organic salts as burn additives in cigarettes. Contrib. Tob. Nicotine Res. 2003, 20, 341–347. [Google Scholar] [CrossRef]
- Shao, N.; Xue, F.; Hou, L.; Li, D.; Gao, Y.; Zhu, X. Effects of ternary potassium-containing intumescent flame retardant coating on the combustion and thermal degradation properties of reconstituted tobacco sheet. Thermochim. Acta 2019, 678, 178310. [Google Scholar] [CrossRef]
- Shao, N.; Qu, Y.; Hou, L.; Hu, Y.; Tian, Z.; Gao, Y.; Zhu, X. Effect of starch-based flame retardant on the thermal degradation and combustion properties of reconstituted tobacco sheet. Cellulose 2021, 28, 741–755. [Google Scholar] [CrossRef]
- Zhou, S.; He, Q.; Wang, X.; Ning, M.; Yang, Y.; Xu, Y.; She, S. An insight into the roles of exogenous potassium salts on the thermal degradation of flue-cured tobacco. J. Anal. Appl. Pyrolysis 2017, 123, 385–394. [Google Scholar] [CrossRef]
- Voelkert, J.C. Fire and Fire Extinguishment: A Brief Guide to Fire Chemistry and Extinguishment Theory for Fire Equipment Service Technicians; Amerex: Trussville, AL, USA, 2009; pp. 3–5. [Google Scholar]
- Fang, F.; Zhang, X.; Meng, Y.; Gu, Z.; Bao, C.; Ding, X.; Tian, X. Intumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf. Coat. Technol. 2015, 262, 9–14. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, G. The novel epoxy/PEPA phosphate flame retardants: Synthesis, characterization and application in transparent intumescent fire resistant coatings. Prog. Org. Coat. 2016, 97, 1–9. [Google Scholar] [CrossRef]
- Zhao, B.; Kolibaba, T.J.; Lazar, S.; Grunlan, J.C. Facile two-step phosphazine-based network coating for flame retardant cotton. Cellulose 2020, 27, 4123–4132. [Google Scholar] [CrossRef]
- EN CEN. Durability of Wood and Wood-Based Products—Accelerated Ageing of Treated Wood Prior to Biological Testing—Leaching Procedure; European Committee for Standardization: Brussels, Belgium, 2020. [Google Scholar]
- Deutsches Institut Für Normung e.V. DIN 52184: Prüfung von Holz; Bestimmung der Quellung und Schwindung; Beuth: Berlin, Germany, 1979.
- Pries, M.; Mai, C. Fire resistance of wood treated with a cationic silica sol. Eur. J. Wood Wood Prod. 2013, 71, 237–244. [Google Scholar] [CrossRef]
- ISO 11925-2:2020; Reaction to Fire Tests—Ignitability of Products Subjected to Direct Impingement of Flame—Part 2: Single-Flame Source Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 13927:2015; Simple Heat Release Test Using a Conical Radiant Heater and a Thermopile Detector. International Organization for Standardization: Geneva, Switzerland, 2015.
- Deutsches Institut Für Normung e.V. DIN 52186: Testing of Wood; Bending Test; Beuth: Berlin, Germany, 1978.
- Deutsches Institut für Normung e.V. DIN 52189: Testing of Wood; Determination of Impact Bending Strength; Beuth: Berlin, Germany, 1981.
- USDA Forest Products Laboratory. Wood Handbook: Wood as an Engineering Material; U.S. Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010.
- Mao, Z.; Yang, C.Q. IR spectroscopy study of cyclic anhydride as intermediate for ester crosslinking of cotton cellulose by polycarboxylic acids. V. Comparison of 1,2,4-butanetricarboxylic acid and 1,2,3-propanetricarboxylic acid. J. Appl. Polym. Sci. 2001, 81, 2142–2150. [Google Scholar] [CrossRef]
- Lin, C.F.; Karlsson, O.; Martinka, J.; Rantuch, P.; Garskaite, E.; Mantanis, G.I.; Sandberg, D. Approaching highly leaching-resistant fire-retardant wood by in situ polymerization with melamine formaldehyde resin. ACS Omega 2021, 6, 12733–12745. [Google Scholar] [CrossRef]
- Gindl, W.; Zargar-Yaghubi, F.; Wimmer, R. Impregnation of softwood cell walls with melamine-formaldehyde resin. Bioresour. Technol. 2003, 87, 325–330. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Hill, C.A.S. The sorption of water vapour by anhydride-modified softwood. Wood Sci. Technol. 2003, 37, 221–231. [Google Scholar] [CrossRef]
- Stamm, A.J.; Seborg, R.M. Resin-treated plywood. Ind. Eng. Chem. 1939, 31, 897–902. [Google Scholar] [CrossRef]
- Imamura, Y.; Kajita, H.; Higuchi, N. Modification of wood by treatment with low molecular phenol-formaldehyde resin (1). Influence of neutral and alkaline resins. In Proceedings of the 48th Annual Meeting of Japan Wood Research Society, Shizuoka, Japan, 3–5 April 1998; pp. 3–5. [Google Scholar]
- Frihart, C.R. Adhesive interactions with wood. In Fundamentals of Composite Processing: Proceedings of a Workshop; USDA Forest Service, Forest Products Laboratory: Madison, WI, USA, 2004; pp. 29–38. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: Chichester, UK; New York, NY, USA, 2004. [Google Scholar]
- Hasan, M.; Despot, R.; Katovic, D.; Vukusic, S.B.; Bogner, A.; Jambrekovic, V. Citric acid–promising agent for increased biological effectiveness of wood. In Proceedings of the 3rd European Conference on Wood Modification, Cardiff, UK, 15 October 2007; pp. 275–278. [Google Scholar]
- Vukusic, S.B.; Katovic, D.; Grgac, S.F.; Trajkovic, J.; Sefc, B.; Voncina, B. Study of the wood modification process with polycarboxylic acids and microwave treatment. Wood Res. 2010, 55, 121–130. [Google Scholar]
- Shao, H.; Sun, H.; Yang, B.; Zhang, H.; Hu, Y. Facile and green preparation of hemicellulose-based film with elevated hydrophobicity via cross-linking with citric acid. RSC Adv. 2019, 9, 2395–2401. [Google Scholar] [CrossRef]
- Meints, T.; Hansmann, C.; Müller, M.; Liebner, F.; Gindl-Altmutter, W. Highly effective impregnation and modification of spruce wood with epoxy-functional siloxane using supercritical carbon dioxide solvent. Wood Sci. Technol. 2018, 52, 1607–1620. [Google Scholar] [CrossRef]
- Östman, B.; Voss, A.; Hughes, A.; Jostein Hovde, P.; Grexa, O. Durability of fire retardant treated wood products at humid and exterior conditions—Review of literature. Fire Mater. 2001, 25, 95–104. [Google Scholar]
- Yi, X.; Cao, S.; Hao, X.; Wang, Z.; Huang, Y.; Li, L.; Guo, C. Dimensionally stable, flame-retardant, and leach-resistant furfurylated wood prepared by incorporating ammonium polyphosphate and nano-silica. Polym. Adv. Technol. 2023, 34, 2501–2514. [Google Scholar] [CrossRef]
- Grinins, J.; Biziks, V.; Rizikovs, J.; Irbe, I.; Militz, H. Evaluation of water related properties of birch wood products modified with different molecular weight phenol-formaldehyde oligomers. Holzforschung 2021, 75, 908–916. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Zhang, S.; Li, J.; Wang, J. Flammability and physical–mechanical properties assessment of wood treated with furfuryl alcohol and nano-SiO2. Eur. J. Wood Wood Prod. 2015, 73, 457–464. [Google Scholar] [CrossRef]
- Rowell, R.M.; Pettersen, R.; Han, J.S.; Rowell, J.S.; Tshabalala, M.A. Cell wall chemistry. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 33–72. [Google Scholar]
- Ahmed, S.A.; Morén, T.; Sehlstedt-Persson, M.; Blom, Å. Effect of oil impregnation on water repellency, dimensional stability and mold susceptibility of thermally modified European aspen and downy birch wood. J. Wood Sci. 2017, 63, 74–82. [Google Scholar] [CrossRef]
- Emmerich, L.; Altgen, M.; Rautkari, L.; Militz, H. Sorption behavior and hydroxyl accessibility of wood treated with different cyclic N-methylol compounds. J. Mater. Sci. 2020, 55, 16561–16575. [Google Scholar] [CrossRef]
- Altgen, M.; Altgen, D.; Klüppel, A.; Rautkari, L. Effect of curing conditions on the water vapor sorption behavior of melamine formaldehyde resin and resin-modified wood. J. Mater. Sci. 2020, 55, 11253–11266. [Google Scholar]
- Rowell, R.M. Physical and mechanical properties of chemically modified wood. In Chemical Modification of Lignocellulosic Materials; Routledge: London, UK, 1996; Volume 12, pp. 295–310. [Google Scholar]
- Ružinská, E.; Mitterová, I.; Zachar, M. The study of selected fire-technical characteristics of special wood products surface treatment by environmentally problematic coatings. Adv. Mater. Res. 2014, 1001, 373–378. [Google Scholar]
- Kačíková, D.; Kubovský, I.; Eštoková, A.; Kačík, F.; Kmeťová, E.; Kováč, J.; Ďurkovič, J. The influence of nanoparticles on fire retardancy of pedunculate oak wood. Nanomaterials 2021, 11, 3405. [Google Scholar] [CrossRef]
- Östman, B. Acceptance criteria for products according to the cone calorimeter. Fire Mater. 2023, 47, 848–850. [Google Scholar] [CrossRef]
- Terzi, E.; Kartal, S.N.; White, R.H.; Shinoda, K.; Imamura, Y. Fire performance and decay resistance of solid wood and plywood treated with quaternary ammonia compounds and common fire retardants. Eur. J. Wood Wood Prod. 2011, 69, 41–51. [Google Scholar] [CrossRef]
- Chung, Y.J. Comparison of combustion properties of native wood species used for fire pots in Korea. J. Ind. Eng. Chem. 2010, 16, 15–19. [Google Scholar]
- Benzarti, K.; Colin, X. Understanding the durability of advanced fibre-reinforced polymer (FRP) composites for structural applications. In Advanced Fibre-Reinforced Polymer (FRP) Composites for Structural Applications; Woodhead Publishing: Sawston, UK, 2013; Volume 12, pp. 361–439. [Google Scholar]
- Mikkola, E.; Wichman, I.S. On the thermal ignition of combustible materials. Fire Mater. 1989, 14, 87–96. [Google Scholar]
- Steen-Hansen, A.; Kristoffersen, B. Prediction of fire classification for wood-based products. A multivariate statistical approach based on the cone calorimeter. Fire Mater. 2007, 31, 207–223. [Google Scholar]
- Babrauskas, V. Heat release rates. In SFPE Handbook of Fire Protection Engineering; Springer: New York, NY, USA, 2016; pp. 799–904. [Google Scholar]
- Winandy, J.E.; Rowell, R.M. Chemistry of wood strength. In Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; p. 303. [Google Scholar]
- Bicke, S.; Mai, C.; Militz, H. Modification of beech veneers with low molecular weight phenol formaldehyde for the production of plywood: Durability and mechanical properties. In Proceedings of the European Conference on Wood Modification, Ljubljana, Slovenia, 17–18 September 2012; pp. 363–366. [Google Scholar]
- Hosseinpourpia, R.; Adamopoulos, S.; Mai, C. Tensile strength of handsheets from recovered fibers treated with N-Methylol melamine and 1,3-dimethylol-4,5-dihydroxyethyleneurea. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Xiao, Z.; Xie, Y.; Militz, H.; Mai, C. Effects of modification with glutaraldehyde on the mechanical properties of wood. Holzforschung 2010, 64, 475–482. [Google Scholar] [CrossRef]
- Bal, B.C.; Bektaş, İ. The effects of some factors on the impact bending strength of laminated veneer lumber. BioResources 2012, 7. [Google Scholar] [CrossRef]
- Bučar, D.G.; Merhar, M. Impact and dynamic bending strength determination of Norway spruce by impact pendulum deceleration. BioResources 2015, 10, 4740–4750. [Google Scholar]
- Mai, C.; Xie, Y.; Xiao, Z.; Bollmus, S.; Vetter, G.; Krause, A.; Militz, H. Influence of the modification with different aldehyde-based agents on the tensile strength of wood. In Proceedings of the European Conference on Wood Modification, Cardiff, UK, 15–16 October 2007; Bangor University: Bangor, UK, 2007; pp. 49–56. [Google Scholar]
- Lopes, D.B.; Mai, C.; Militz, H. Mechanical properties of chemically modified Portuguese pinewood. Maderas. Cienc. Y Tecnol. 2015, 17, 179–194. [Google Scholar]
- Hosseinpourpia, R.; Adamopoulos, S.; Mai, C. Effects of acid pre-treatments on the swelling and vapor sorption of thermally modified Scots pine (Pinus sylvestris L.) wood. BioResources 2018, 13, 331–345. [Google Scholar] [CrossRef]
- Pandey, K.K.; Pitman, A.J. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar]
- Traoré, M.; Kaal, J.; Martínez Cortizas, A. Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef]
- Cai, M.; Fu, Z.; Cai, Y.; Li, Z.; Xu, C.; Xu, C.; Li, S. Effect of impregnation with maltodextrin and 1,3-dimethylol-4,5-dihydroxyethyleneurea on Poplar wood. Forests 2018, 9, 676. [Google Scholar] [CrossRef]
- Cai, M.; Fu, Z.; Cai, Y.; Zhang, Y. Study on improving the fixation rate of impregnated poplar wood with maltodextrin and 1,3-dimethylol-4,5-dihydroxyethyleneurea. Appl. Sci. 2019, 9, 3237. [Google Scholar] [CrossRef]
- Emmerich, L.; Bollmus, S.; Militz, H. Wood modification with DMDHEU (1,3-dimethylol-4,5-dihydroxyethyleneurea)–State of the art, recent research activities and future perspectives. Wood Mater. Sci. Eng. 2019, 14, 3–18. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Schwanninger, M.J.C.R.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Gao, J.; Guo, H.; Chen, Y.; Cheng, Q.; Via, B.K. Wood veneer dyeing enhancement by ultrasonic-assisted treatment. BioResources 2015, 10, 1198–1212. [Google Scholar] [CrossRef][Green Version]
- Hu, J.; Wang, X. Modification Mechanisms and Properties of Poplar Wood via Grafting with 2-Hydroxyethyl Methacrylate/N,N′-methylenebis(acrylamide) onto Cell Walls. Polymers 2023, 15, 1861. [Google Scholar] [CrossRef] [PubMed]



| Chemicals | Additional | pH Value |
|---|---|---|
| SorCA (1:3; 30%) | TPC (10%) | 2.97 |
| SorCA (1:3; 30%) | TPC (20%) | 4.12 |
| SorCA (1:3; 30%) | TPC (30%) | 4.81 |
| Control | n/a | n/a |
| TPC (30%) | n/a | 8.62 |
| SorCA (1:3; 30%) | n/a | 1.49 |
| Citric acid (30%); TPC (30%) | n/a | 4.90 |
| Specimen Size (mm3) | Test type | Replicates per Collective |
|---|---|---|
| 20 × 20 × 10 | Dimensional stability | 10 |
| 4 × 13 × 125 | Flame retardant | 5 |
| 20 × 90 × 250 | Flame retardant | 2 |
| 18 × 100 × 100 | Flame retardant | 5 |
| 10 × 10 × 160 | Mechanical properties | 20 |
| Designation | Ignition (Yes/No) | Flame-Out Time (s) | Soot Cone Height (mm) | Pass/Fail * (Yes/No) |
|---|---|---|---|---|
| SorCA 30%; TPC 10% | No | n/a | 63 | Yes |
| SorCA 30%; TPC 20% | No | n/a | 78 | Yes |
| SorCA 30%; TPC 30% | No | n/a | 72.5 | Yes |
| Control | Yes | 18 | 101.5 | Yes |
| TPC 30% | No | n/a | 65.6 | Yes |
| SorCA 30% | No | n/a | 72 | Yes |
| CA 30%; TPC 30% | No | n/a | 74.5 | Yes |
| Designation | Ignition Time (s) | 1PHRR | Time to 1PHRR (s) | Peak HRR | Time to Peak HRR (s) | THR at 600 s | Mass Loss (%) |
|---|---|---|---|---|---|---|---|
| SorCA 30%; TPC 30% before EN 84 | 88 (±18) | 32.1 (±4.6) | 190 | 75.9 (±1.3) | 1260 | 16.4 (±1.5) | 67.1 (±2) |
| SorCA 30%; TPC 30% after EN 84 | 22 (±4) | 80.2 (±5.1) | 145 | 149.8 (±28.5) | 565 | 48.2 (±8.8) | 78.3 (±3.7) |
| TPC 30% before EN 84 | 161.3 (±7) | 16.4 (±18) | 180 | 72 (±21.4) | 1640 | 9.3 (±0.9) | 44.6 (±31.2) |
| TPC 30% after EN 84 | 15.6 (±3) | 78.1 (±2.8) | 120 | 140.5 (±18.9) | 530 | 50.2 (±4.5) | 76.6 (±4.3) |
| Designation | Ignition Time (s) | 1PHRR | Time to 1PHRR (s) | Peak HRR | Time to Peak HRR (s) | THR at 600 s | Mass Loss (%) |
|---|---|---|---|---|---|---|---|
| SorCA 30%; TPC 10% | 28.8 (±6) | 100.7 (±1.5) | 105 | 137.9 (±8.7) | 670 | 54.6 (±2.9) | 86.2 (±2.5) |
| SorCA 30%; TPC 20% | 18.2 (±5) | 109.1 (±2.7) | 60 | 159.1 (±16) | 540 | 63.8 (±1.9) | 86.9 (±1.6) |
| SorCA 30%; TPC 30% | 22 (±4) | 80.2 (±5.1) | 145 | 149.8 (±28.5) | 565 | 58.2 (±8.8) | 88.3 (±3.7) |
| Control | 32.8 (±4) | 97.1 (±3.9) | 65 | 144.4 (±7.1) | 750 | 49.1 (±3.6) | 91.4 (±9.3) |
| TPC 30% | 15.6 (±3) | 78.1 (±2.8) | 120 | 140.5 (±18.9) | 530 | 60.2 (±4.5) | 86.6 (±4.3) |
| SorCA 30% | 28.4 (±2) | 115 (±3.9) | 65 | 134.3 (±3.4) | 725 | 52.9 (±1.0) | 80.6 (±6.8) |
| CA 30%; TPC 30% | 20.8 (±12) | 97.2 (±2.8) | 105 | 171.4 (±16.1) | 525 | 61.3 (±2.8) | 89.7 (±4.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, S.; Chabert, A.J.; Militz, H. Fixation of Tripotassium Citrate Flame Retardant Using a Sorbitol and Citric Acid Wood-Modification Treatment. Materials 2024, 17, 5377. https://doi.org/10.3390/ma17215377
Yun S, Chabert AJ, Militz H. Fixation of Tripotassium Citrate Flame Retardant Using a Sorbitol and Citric Acid Wood-Modification Treatment. Materials. 2024; 17(21):5377. https://doi.org/10.3390/ma17215377
Chicago/Turabian StyleYun, Sanghun, Adèle Jane Chabert, and Holger Militz. 2024. "Fixation of Tripotassium Citrate Flame Retardant Using a Sorbitol and Citric Acid Wood-Modification Treatment" Materials 17, no. 21: 5377. https://doi.org/10.3390/ma17215377
APA StyleYun, S., Chabert, A. J., & Militz, H. (2024). Fixation of Tripotassium Citrate Flame Retardant Using a Sorbitol and Citric Acid Wood-Modification Treatment. Materials, 17(21), 5377. https://doi.org/10.3390/ma17215377








