Synthesis and Characterization of Cellulose Microfibril-Reinforced Polyvinyl Alcohol Biodegradable Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Whisker Synthesis
2.3. Cast Film Preparation
2.4. Characterizations
2.4.1. Morphological Characterization
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. Thermal Analysis
2.4.4. Mechanical Properties of Cast Films
3. Results and Discussion
3.1. CNC/CMF Characterization
3.1.1. Morphological Characterization
3.1.2. FTIR
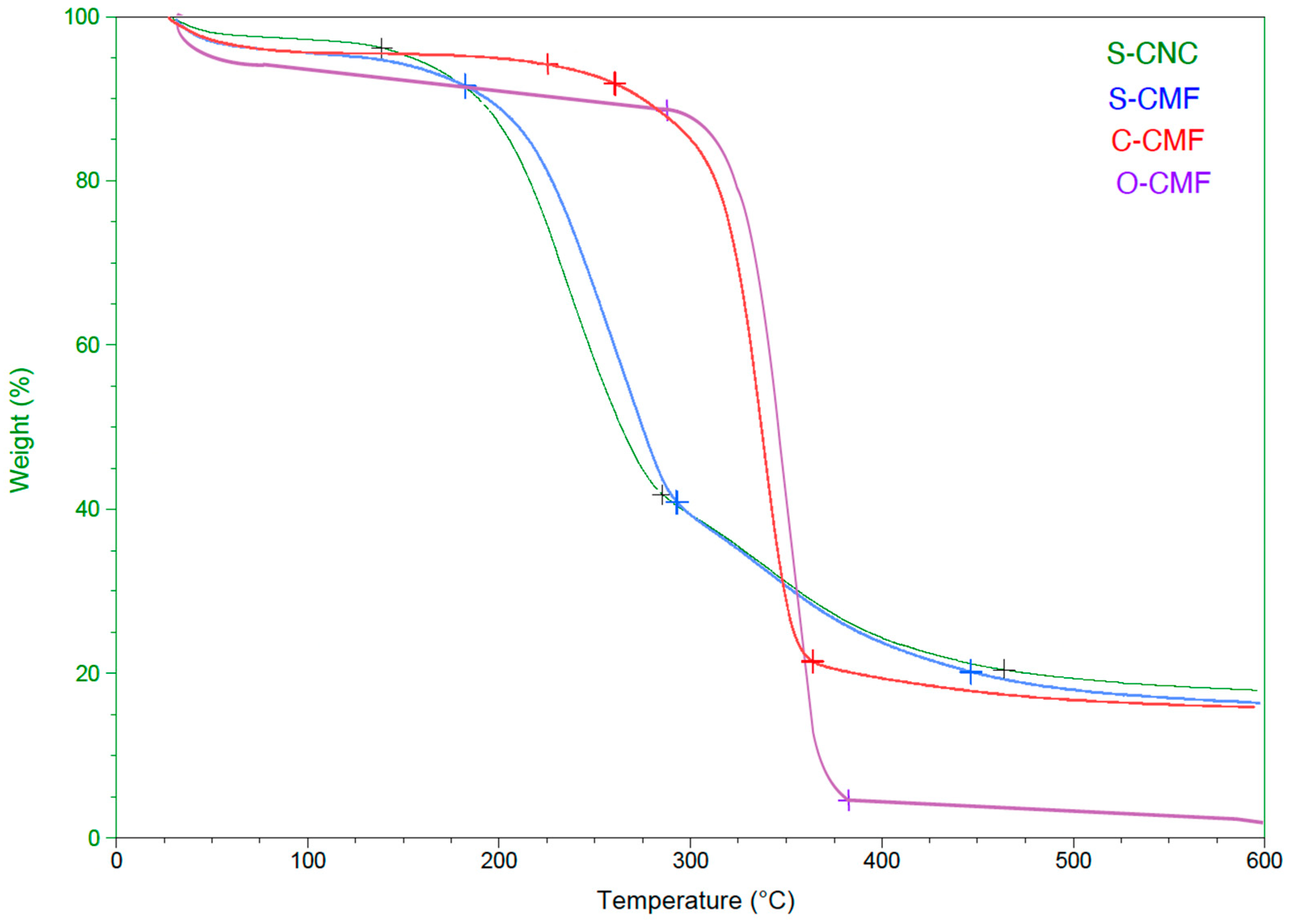
3.1.3. Thermal Analysis
3.2. Cast Film Characterization
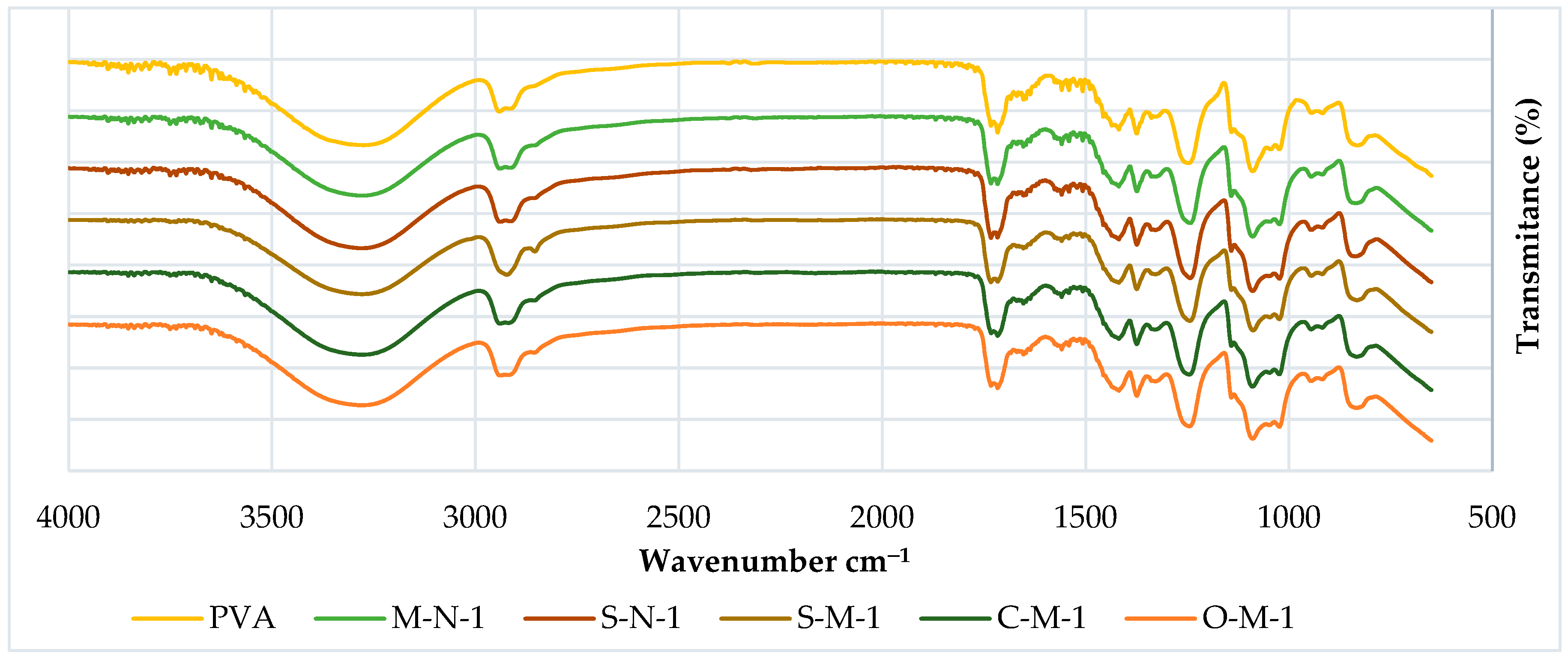
3.2.1. FTIR
3.2.2. Thermal Analysis
TGA
DSC
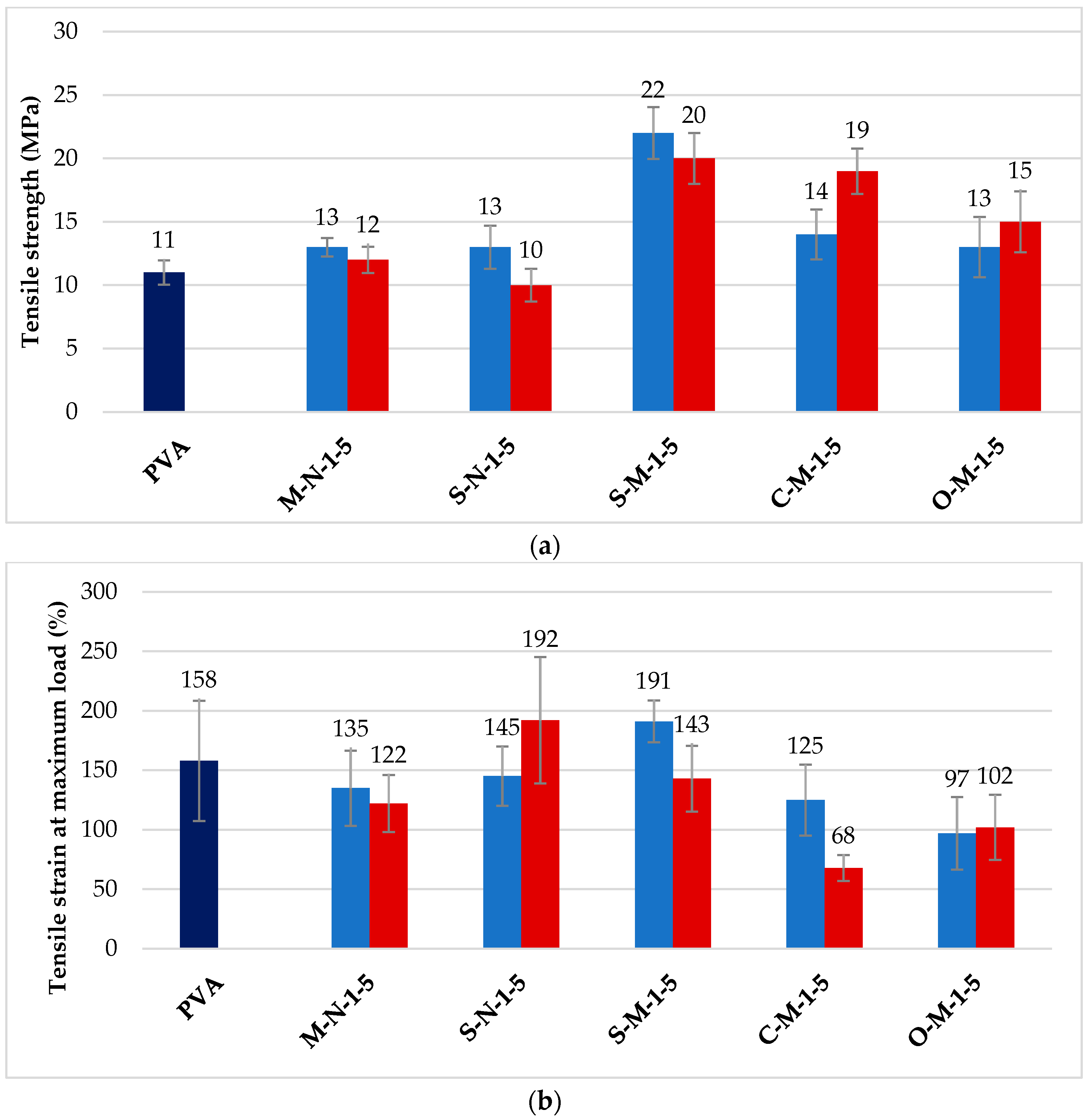
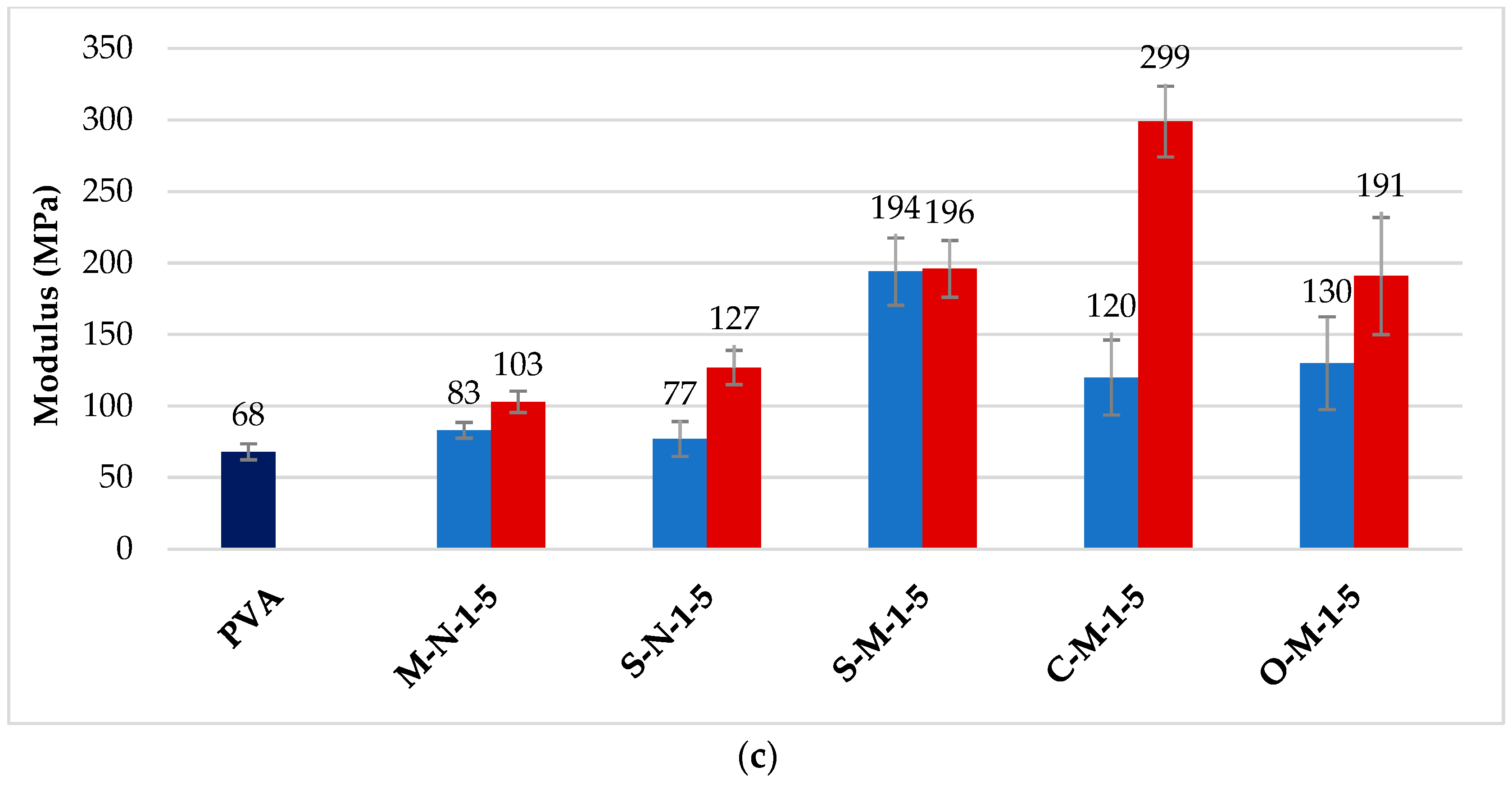
3.3. Mechanical Properties of Cast Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Wu, Q.; Zhao, X.; Huang, Z.; Cao, J.; Li, J.; Liu, S. Enhanced thermal and mechanical properties of PVA composites formed with filamentous nanocellulose fibrils. Carbohydr. Polym. 2014, 113, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. BioResources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sain, M. Processing of cellulose nanofiber-reinforced composites. J. Reinf. Plast. Compos. 2005, 24, 1259–1268. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline nanocellulose—Preparation, modification, and properties. In Cellulose-Fundamental Aspects and Current Trends; InTech: London, UK, 2015; Volume 7. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial hemp fibers: An overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Chanet, G.; Morin-Crini, N. Applications of hemp in textiles, paper industry, insulation and building materials, horticulture, animal nutrition, food and beverages, nutraceuticals, cosmetics and hygiene, medicine, agrochemistry, energy production and environment: A review. Environ. Chem. Lett. 2020, 18, 1451–1476. [Google Scholar] [CrossRef]
- Kaur, G.; Kander, R. The Sustainability of Industrial Hemp: A Literature Review of Its Economic, Environmental, and Social Sustainability. Sustainability 2023, 15, 6457. [Google Scholar] [CrossRef]
- Crowley, J.G. The Performance of Cannabis Sativa (HEMP) as a Fibre Source for Medium Density Fibre Board (MDF); European Agricultural Guidance and Guarantee Fund: Brussels, Belgium, 2001. [Google Scholar]
- González-García, S.; Hospido, A.; Feijoo, G.; Moreira, M.T. Life cycle assessment of raw materials for non-wood pulp mills: Hemp and flax. Resour. Conserv. Recycl. 2010, 54, 923–930. [Google Scholar] [CrossRef]
- Miller, J. Cellulose Nanomaterials Production Summary—November, 2022. In The Biobased Market Directory of Cellulose Nanomaterials (May 2023); TAPPI Nano Division: Peachtree Corners, GA, USA, 2023; pp. 14–15. [Google Scholar]
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, R.K.; Tadesse, M.G. Preparation and characterization of carboxylated cellulose nanocrystals from Oxytenanthera abyssinica (Ethiopian lowland bamboo) cellulose via citric acid anhydrous hydrolysis catalyzed by sulfuric acid. Biomass Convers. Biorefin. 2023, 1–17. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, Y.; Jiang, F. Sustainable isolation of nanocellulose from cellulose and lignocellulosic feedstocks: Recent progress and perspectives. Carbohydr. Polym. 2021, 267, 118188. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 1–25. [Google Scholar] [CrossRef]
- Ji, H.; Xiang, Z.; Qi, H.; Han, T.; Pranovich, A.; Song, T. Strategy towards one-step preparation of carboxylic cellulose nanocrystals and nanofibrils with high yield, carboxylation and highly stable dispersibility using innocuous citric acid. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Liu, K.; Liu, H.; Xu, T.; Zhang, S.; Chen, X.; Zhang, R.; Li, H.; Xie, H.; et al. Sustainable preparation of bifunctional cellulose nanocrystals via mixed H2SO4/formic acid hydrolysis. Carbohydr. Polym. 2021, 266, 118107. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Liu, Y. Two characteristic cellulose nanocrystals (CNCs) obtained from oxalic acid and sulfuric acid processing. Cellulose 2019, 26, 8351–8365. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, F.; Zanoaga, M.; Teaca, C.A.; Nechifor, M.; Shahzad, A. Modified hemp fibers intended for fiber-reinforced polymer composites used in structural applications—A review. I. Methods of modifications. Polym. Compos. 2019, 41, 5–31. [Google Scholar] [CrossRef]
- El Miri, N.; El Achaby, M.; Fihri, A.; Larzek, M.; Zahouily, M.; Abdelouahdi, K.; Barakat, A.; Solhy, A. Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites. Carbohydr. Polym. 2016, 137, 239–248. [Google Scholar] [CrossRef]
- Uddin, A.J.; Araki, J.; Gotoh, Y. Toward “Strong” Green Nanocomposites: Polyvinyl Alcohol Reinforced with Extremely Oriented Cellulose Whiskers. Biomacromolecules 2011, 12, 617–624. [Google Scholar] [CrossRef]
- Meree, C.E.; Schueneman, G.T.; Meredith, J.C.; Shofner, M.L. Rheological behavior of highly loaded cellulose nanocrystal/poly(vinyl alcohol) composite suspensions. Cellulose 2016, 23, 3001–3012. [Google Scholar] [CrossRef]
- Popescu, M.C.; Dogaru, B.I.; Goanta, M.; Timpu, D. Structural and morphological evaluation of CNC reinforced PVA/Starch biodegradable films. Int. J. Biol. Macromol. 2018, 116, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, A.; Teramoto, Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and how to incorporate them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Boroujeni, F.M.; Sharzehee, M. Synthetic oligomers with urea binding and their role as crosslinkers in improving the printing quality on cotton fabric. Fibers Polym. 2023, 24, 855–867. [Google Scholar] [CrossRef]
- Ulaganathan, R.K.; Senusi, N.A.M.; Amin, M.A.M.; Razab, M.K.A.A.; Ismardi, A.; Abdullah, N.H. Effect of cellulose nanocrystals (CNC) on PVA/CNC bio-nanocomposite film as potential food packaging application. Mater. Today Proc. 2020, 66, 3150–3153. [Google Scholar] [CrossRef]
- Lu, J.; Wang, T.; Drzal, L.T. Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part A Appl. Sci. Manuf. 2008, 39, 738–746. [Google Scholar] [CrossRef]
- Roohani, M.; Habibi, Y.; Belgacem, N.M.; Ebrahim, G.; Karimi, A.N.; Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J. 2008, 44, 2489–2498. [Google Scholar] [CrossRef]
- Revol, J.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Bondancia, T.J.; de Aguiar, J.; Batista, G.; Cruz, A.J.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of nanocellulose using citric acid in a biorefinery concept: Effect of the hydrolysis reaction time and techno-economic analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- ASTM E2550–11; Standard Test Method for Thermal Stability by Thermogravimetry. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- Zhang, P.P.; Tong, D.S.; Lin, C.X.; Yang, H.M.; Zhong, Z.K.; Yu, W.H.; Wang, H.; Zhou, C.H. Effects of acid treatments on bamboo cellulose nanocrystals. Asia-Pac. J. Chem. Eng. 2014, 9, 686–695. [Google Scholar] [CrossRef]
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Lu, F.; Yao, J. New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2007, 15, 149–159. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and properties of high quality natural cellulose fibers from cornstalks. Polymer 2005, 46, 5494–5500. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Rosa, M.; Medeiros, E.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Tan, X.Y.; Hamid, S.B.A.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenergy 2018, 81, 584–591. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly efficient and sustainable preparation of carboxylic and thermostable cellulose nanocrystals via FeCl3-catalyzed innocuous citric acid hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Yang, X.; Xie, H.; Du, H.; Zhang, X.; Zou, Z.; Zou, Y.; Liu, W.; Lan, H.; Zhang, X.; Si, C. Facile extraction of thermally stable and dispersible cellulose nanocrystals with high yield via a green and recyclable FeCl3-catalyzed deep eutectic solvent system. ACS Sustain. Chem. Eng. 2019, 7, 7200–7208. [Google Scholar] [CrossRef]
- Angles, M.N.; Dufresne, A. Plasticized starch/tunicin whiskers nanocomposite materials. 2. Mechanical behavior. Macromolecules 2001, 34, 2921–2931. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Balaji, A.N.; Rajan, S.T.K.; Ramanujam, N.R. Preparation of bio-eco based cellulose nanomaterials from used disposal paper cups through citric acid hydrolysis. Carbohydr. Polym. 2020, 235, 115997. [Google Scholar] [CrossRef]
- Thomas, P.S.; Guerbois, J.; Russell, G.F.; Briscoe, B.J. FTIR study of the thermal degradation of poly (vinyl alcohol). J. Ther. Anal. Calorim. 2001, 64, 501–508. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Kumar, H.N.; Prabhakar, M.N.; Prasad, C.V.; Rao, K.M.; Reddy, T.A.K.; Rao, K.C.; Subha, M. Compatibility studies of chitosan/PVA blend in 2% aqueous acetic acid solution at 30 °C. Carbohydr. Polym. 2010, 82, 251–255. [Google Scholar] [CrossRef]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N. Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Balavairavan, B.; Saravanakumar, S.S. Characterization of ecofriendly poly (vinyl alcohol) and green banana peel filler (GBPF) reinforced bio-films. J. Polym. Environ. 2021, 29, 2756–2771. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef]
- Li, W.; Yue, J.; Liu, S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly (vinyl alcohol) composites. Ultrason. Sonochem. 2012, 19, 479–485. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Studies on the mechanical, thermal, morphological and barrier properties of nanocomposites based on poly (vinyl alcohol) and nanocellulose from sugarcane bagasse. J. Ind. Eng. Chem. 2014, 20, 462–473. [Google Scholar] [CrossRef]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G.; McCall, R.D. Preparation and characterization of poly(vinyl alcohol) nanocomposites made from cellulose nanofibers. J. Appl. Polym. Sci. 2009, 113, 2238–2247. [Google Scholar] [CrossRef]
- Kaboorani, A.; Riedl, B.; Blanchet, P.; Fellin, M.; Hosseinaei, O.; Wang, S. Nanocrystalline cellulose (NCC): A renewable nano-material for polyvinyl acetate (PVA) adhesive. Eur. Polym. J. 2012, 48, 1829–1837. [Google Scholar] [CrossRef]
- Lee, S.; Mohan, D.J.; Kang, I.; Doh, G.; Lee, S.; Han, S.O. Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading. Fibers Polym. 2009, 10, 77–82. [Google Scholar] [CrossRef]
- Silvério, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crops Prod. 2013, 44, 427–436. [Google Scholar] [CrossRef]
- Yee, T.W.; Choy, L.J.; Rahman, W.A.W.A. Mechanical and water absorption properties of poly (vinyl alcohol)/sago pith waste biocomposites. J. Compos. Mater. 2011, 45, 1201–1207. [Google Scholar]
- Fortunati, E.; Puglia, D.; Luzi, F.; Santulli, C.; Kenny, J.M.; Torre, L. Binary PVA bio-nanocomposites containing cellulose nanocrystals extracted from different natural sources: Part I. Carbohydr. Polym. 2013, 97, 825–836. [Google Scholar] [CrossRef]
- Kord, B.; Malekian, B.; Yousefi, H.; Najafi, A. Preparation and characterization of nanofibrillated Cellulose/Poly (Vinyl Alcohol) composite films. Maderas Cienc. Technol. 2016, 18, 743–752. [Google Scholar] [CrossRef]



| Samples | T (°C) | t (min) | w/w (%) | CNC Yield (%) | CMF Yield (%) | Total Yield (%) |
|---|---|---|---|---|---|---|
| Sulfuric Acid Hydrolysis | 65–70 | 60 | 64 | 41.9 | 45.5 | 87.3 |
| Citric Acid Hydrolysis | 95–105 | 240 | 80 | 3.7 | 91.9 | 95.5 |
| Oxalic Acid Hydrolysis | 100–110 | 60–70 | 70 | 5.9 | 62.5 | 68.4 |
| Samples | Length | Width | (L/W) | ||
|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Mode | |
| S-CNC | 20–300 nm | 100 nm | 15–100 nm | 60 nm | 2.5 |
| S-CMF | 300 nm–120 μm | 20 μm | 150 nm–20 μm | 5 μm | 2.5 |
| C-CMF | 1–400 μm | 100 μm | 300 nm–22 μm | 7 μm | 5.5 |
| O-CMF | 900 nm–140 μm | 23 μm | 200 nm–20 μm | 5 μm | 3.5 |
| PEAK | HEMP | S-CMF | C-CMF | O-CMF | BOND SOURCE |
|---|---|---|---|---|---|
| 3335 | X | X | X | X | O-H (cellulose) |
| 2900 | X | X | X | X | C-H (cellulose) |
| 1730 | X | C=O (ester) | |||
| 1720 | X | X | X | C=O (carbonyl) | |
| 1640 | X | X | O-H (water) | ||
| 1500–1600 | X | X | X | aromatic ring (lignin) |
| Sample | Td (°C) | MC (%) | Mass Change (%) |
|---|---|---|---|
| S-CNC | 150 | 4 | 80 |
| S-CMF | 170 | 6 | 82 |
| C-CMF | 230 | 5 | 86 |
| O-CMF | 290 | 9 | 95 |
| Samples | Tg (°C) | Tm (°C) | ∆Hm (J/g) | Xc (%) | Tc (°C) | Td (°C) | MC (%) | TMC (%) |
|---|---|---|---|---|---|---|---|---|
| PVA | 66 | 175 | 18 | 11 | 127 | 264 | 6 | 92 |
| M-N-1 | 60 | 188 | 28 | 18 | 153 | 257 | 7 | 95 |
| S-N-1 | 65 | 185 | 22 | 14 | 147 | 261 | 4 | 59 |
| S-M-1 | 60 | 187 | 22 | 14 | 154 | 270 | 10 | 90 |
| C-M-1 | 60 | 188 | 27 | 17 | 155 | 269 | 5 | 89 |
| O-M-1 | 62 | 186 | 21 | 13 | 149 | 271 | 6 | 99 |
| M-N-5 | 48 | 185 | 26 | 17 | 153 | 264 | 13 | 94 |
| S-N-5 | 59 | 187 | 23 | 15 | 163 | 266 | 17 | 96 |
| S-M-5 | 55 | 186 | 27 | 18 | 158 | 269 | 12 | 96 |
| C-M-5 | 61 | 179 | 20 | 13 | 142 | 269 | 14 | 97 |
| O-M-5 | 68 | 188 | 19 | 12 | 150 | 258 | 11 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroujeni, F.M.; Fioravanti, G.; Kander, R. Synthesis and Characterization of Cellulose Microfibril-Reinforced Polyvinyl Alcohol Biodegradable Composites. Materials 2024, 17, 526. https://doi.org/10.3390/ma17020526
Boroujeni FM, Fioravanti G, Kander R. Synthesis and Characterization of Cellulose Microfibril-Reinforced Polyvinyl Alcohol Biodegradable Composites. Materials. 2024; 17(2):526. https://doi.org/10.3390/ma17020526
Chicago/Turabian StyleBoroujeni, Fatemeh Mahdiyeh, Gabriella Fioravanti, and Ronald Kander. 2024. "Synthesis and Characterization of Cellulose Microfibril-Reinforced Polyvinyl Alcohol Biodegradable Composites" Materials 17, no. 2: 526. https://doi.org/10.3390/ma17020526
APA StyleBoroujeni, F. M., Fioravanti, G., & Kander, R. (2024). Synthesis and Characterization of Cellulose Microfibril-Reinforced Polyvinyl Alcohol Biodegradable Composites. Materials, 17(2), 526. https://doi.org/10.3390/ma17020526






