Molecular Dynamics Simulation and Experimental Study of Mechanical Properties of Graphene–Cement Composites
Abstract
1. Introduction
2. Experimental and Simulation Methods
2.1. Experimental Methods
2.2. Simulation Methods
3. Result and Discussion
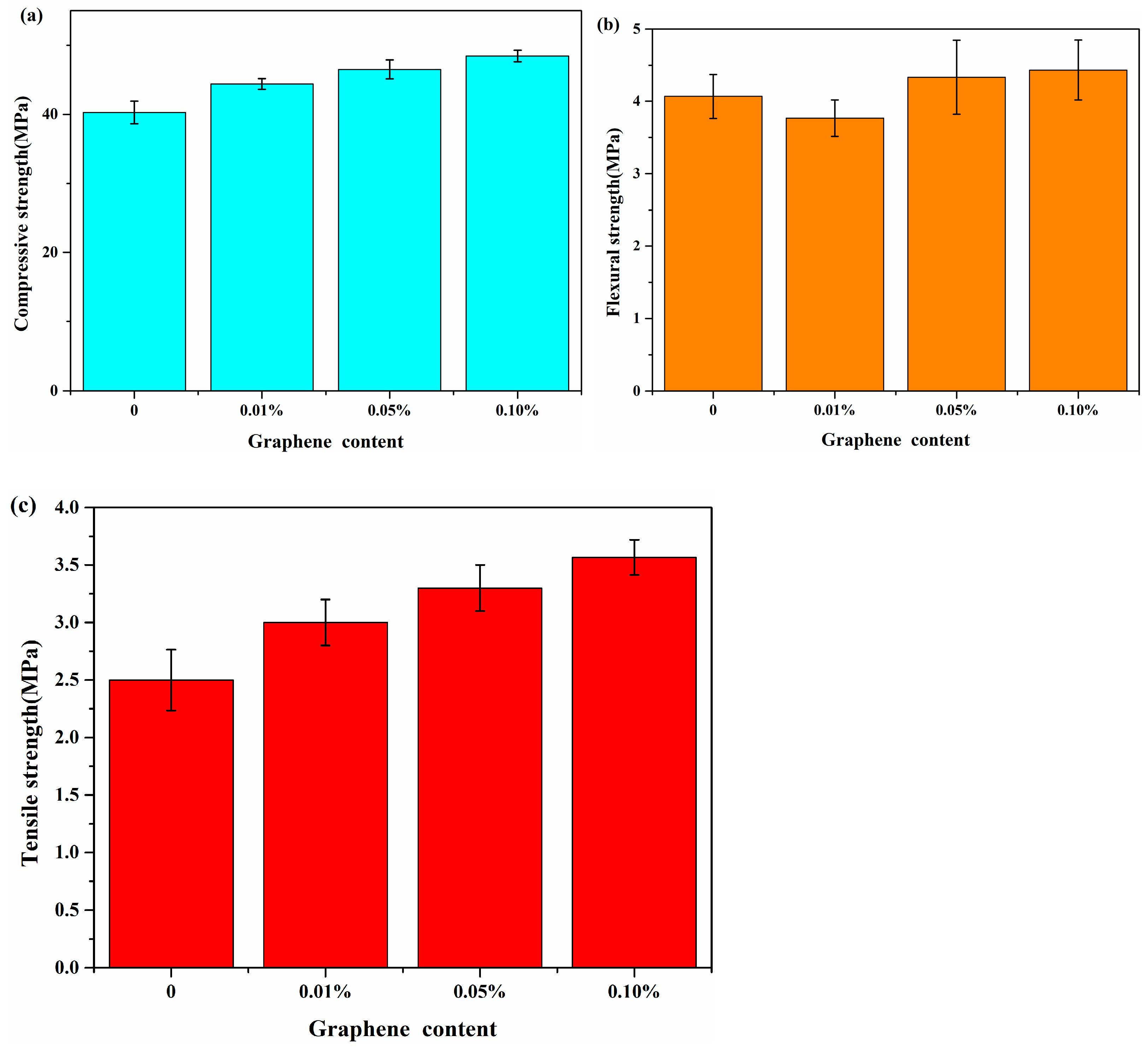
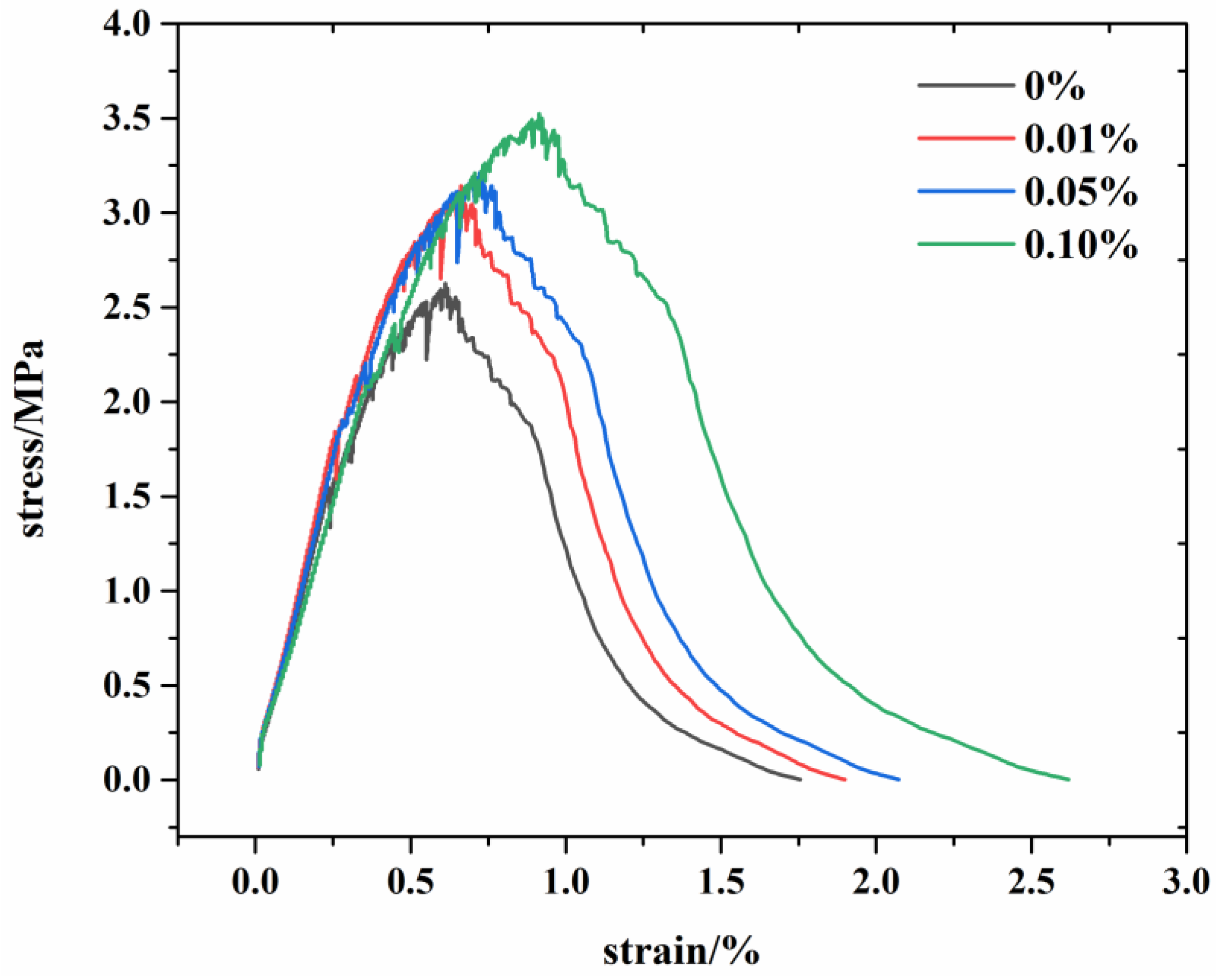
3.1. The Mechanical Behavior in Experiment
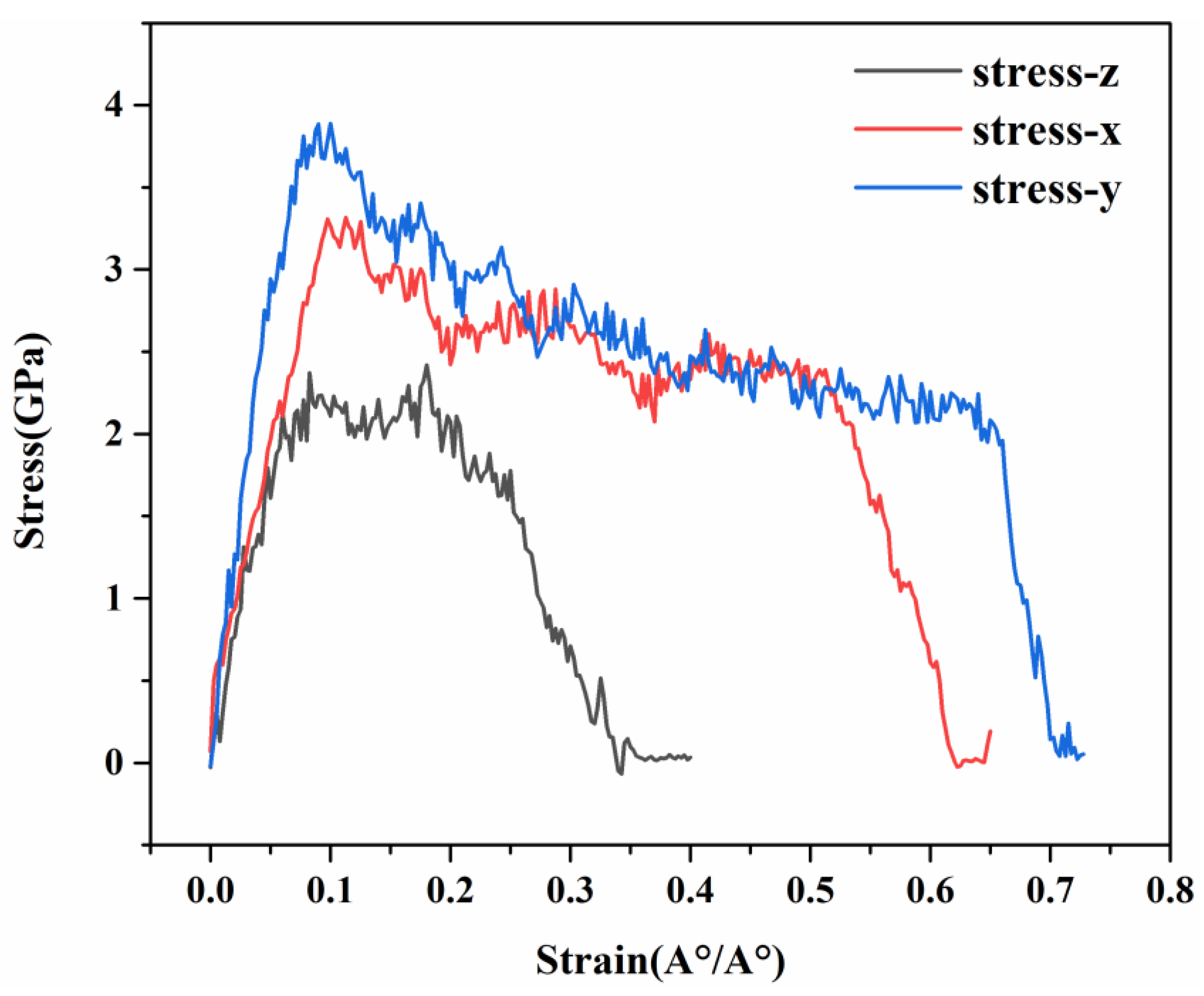
3.2. Mechanical Properties of C-S-H in Three Directions
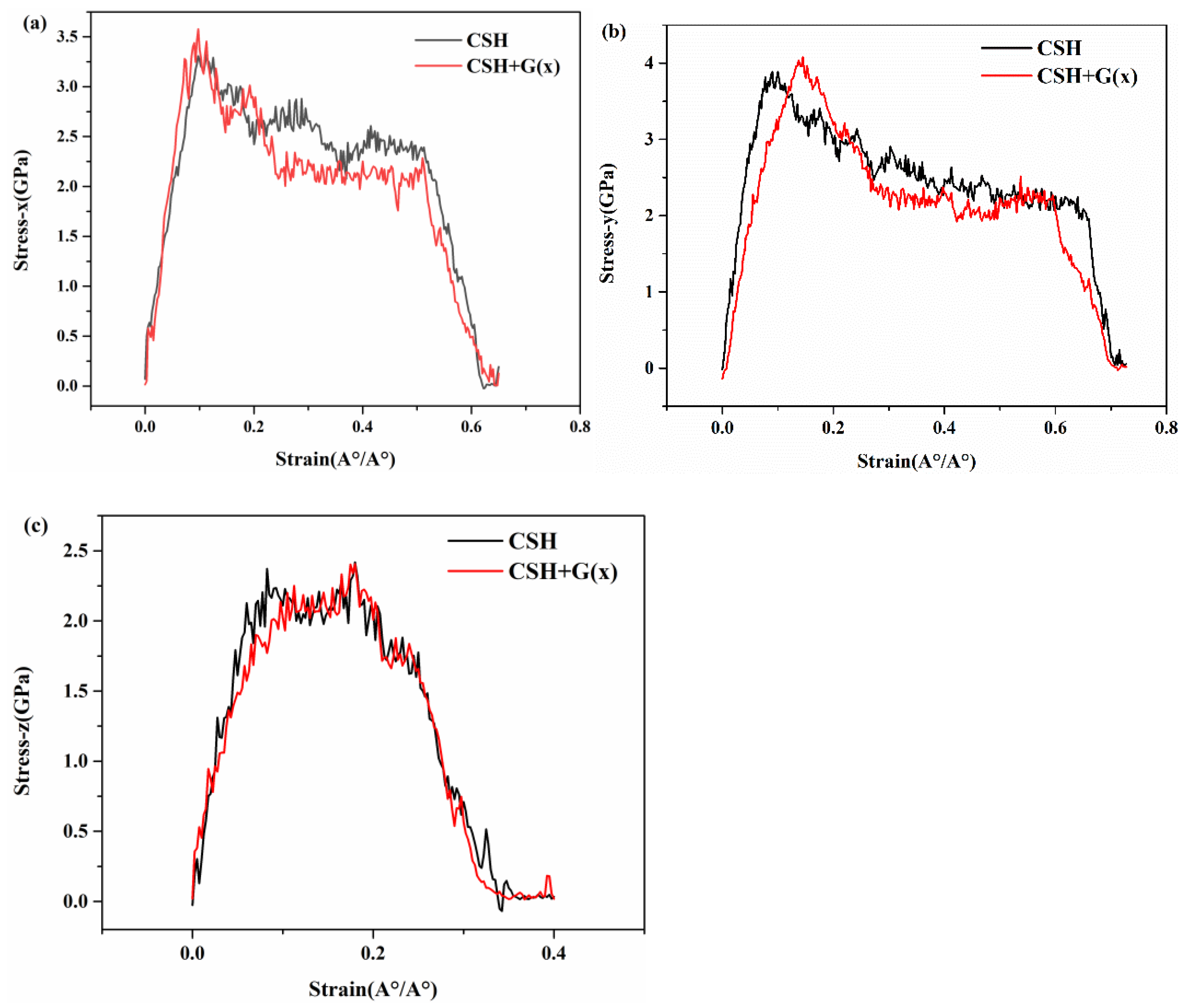
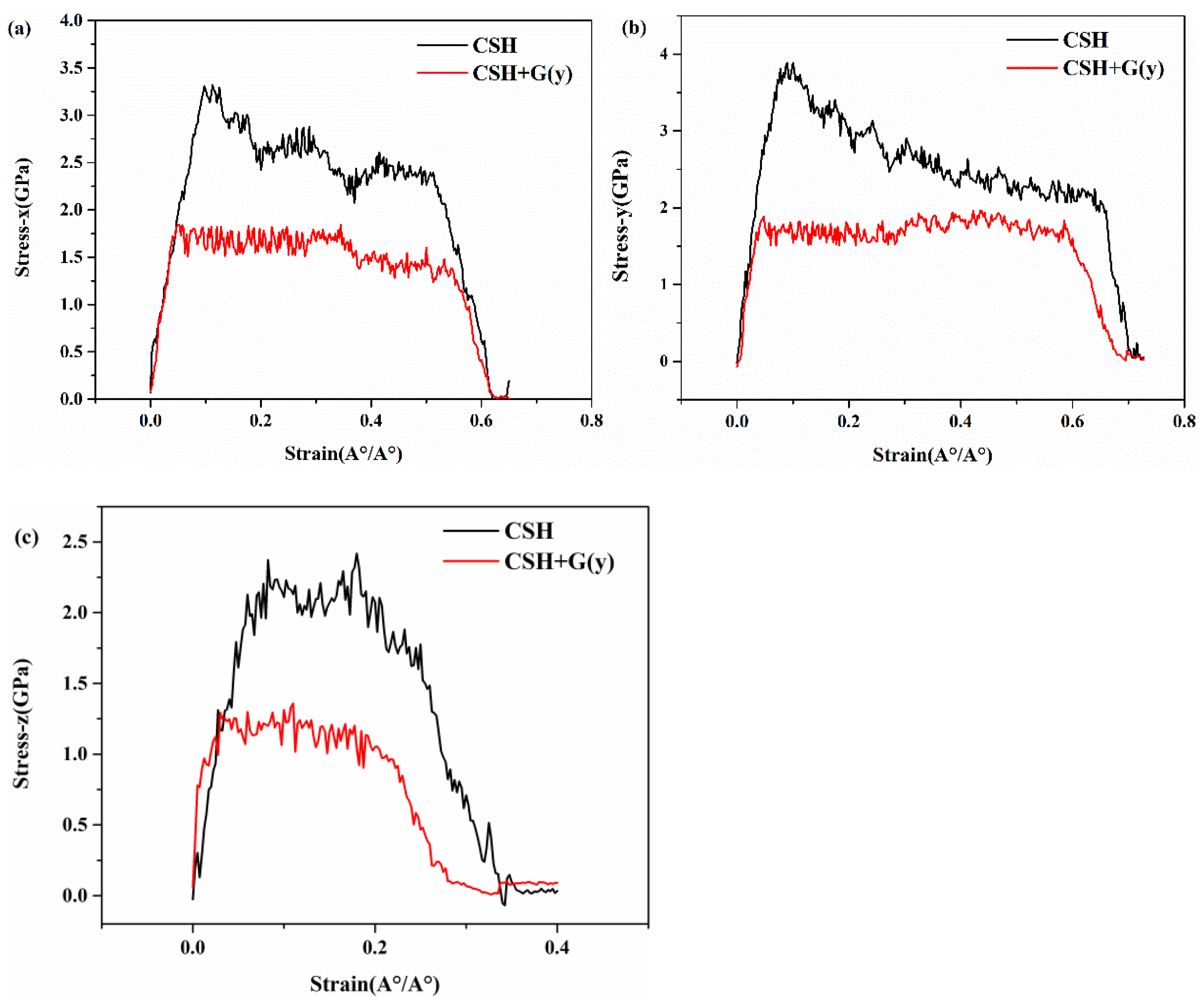
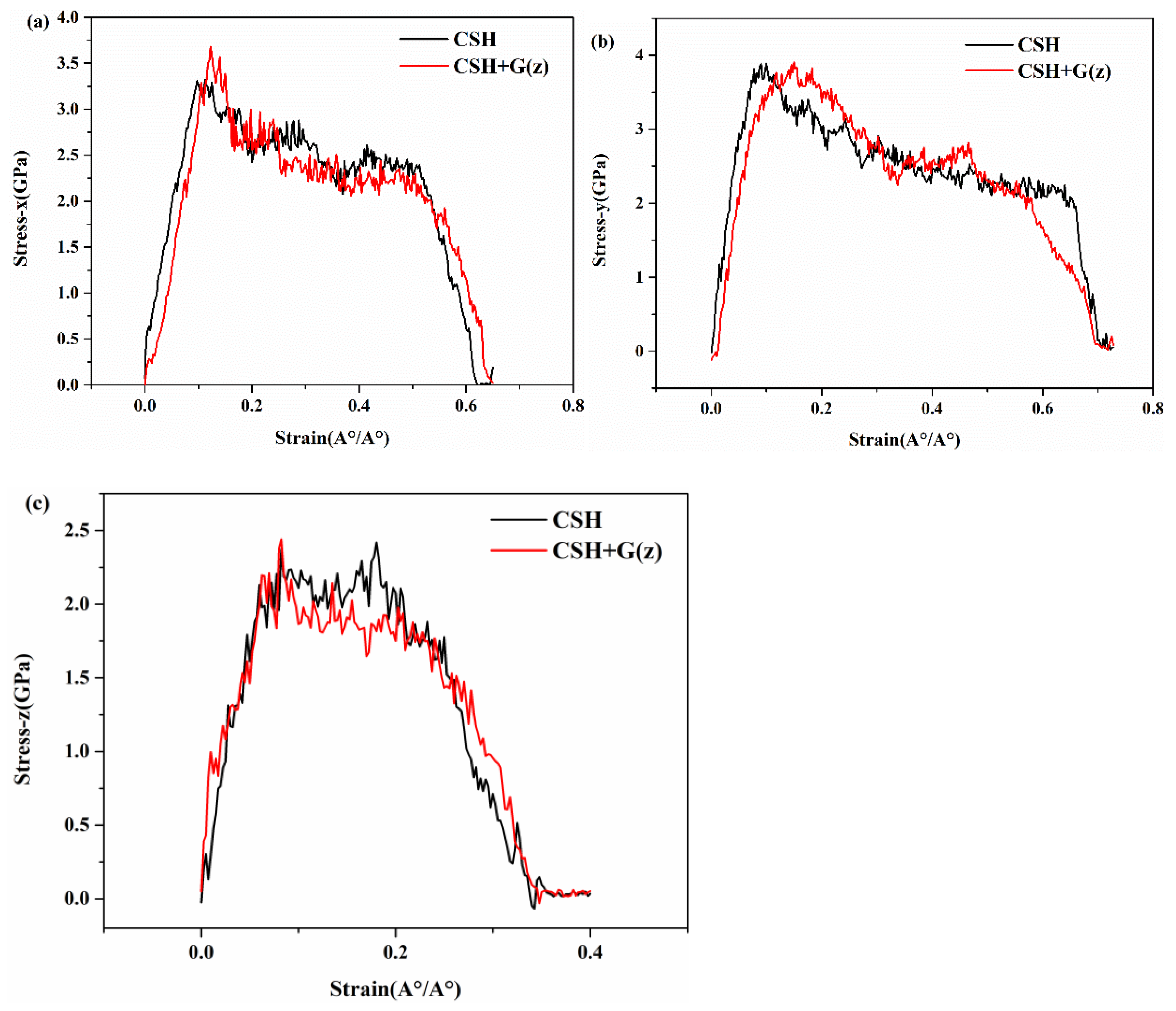
3.3. Mechanical Properties of G/C-S-H in Three Directions
3.4. Effect of Temperature and Strain Rate on Mechanical Properties
3.4.1. Effect of Temperature
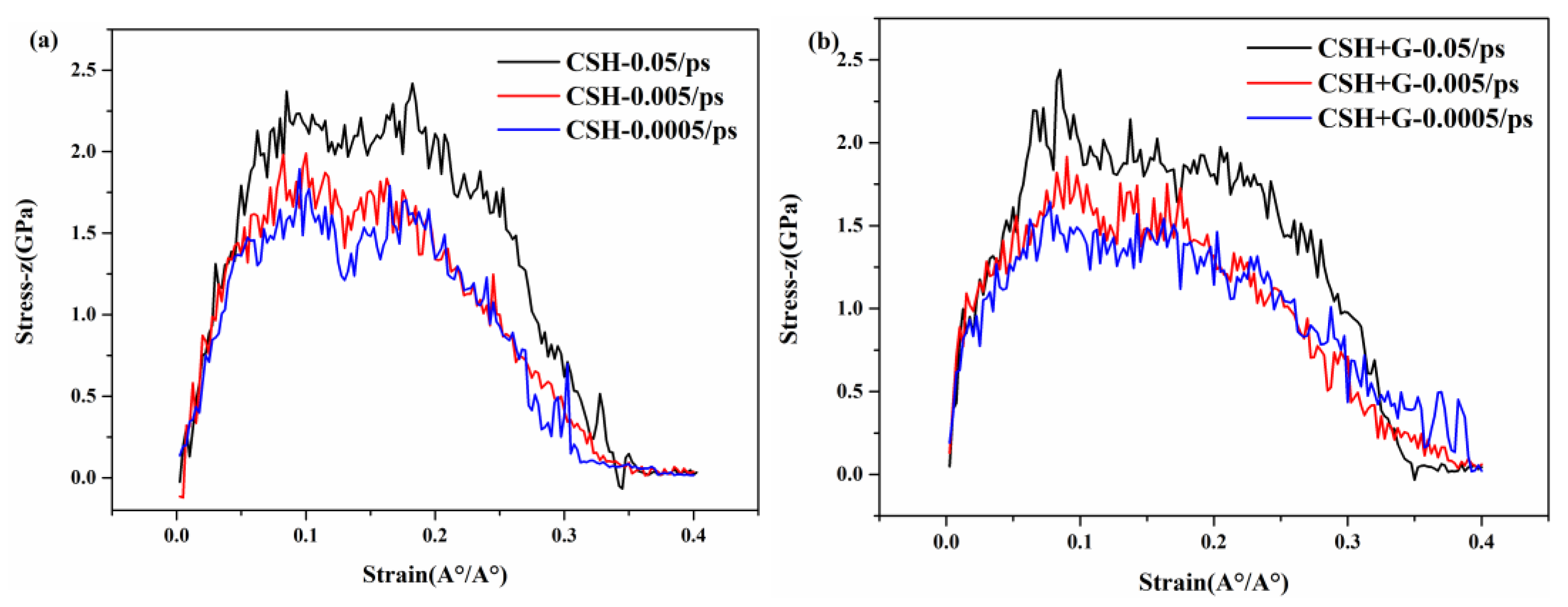
3.4.2. Effect of Loading Rate
3.5. Effect of Defect of Graphene on Mechanical Properties
4. Conclusions
- The experimental results show that in the G/C-S-H composites, the compressive, flexural, and tensile strengths were increased by up to 21.9%, 12.19%, and 34.6%, respectively. This was attributed to the bridging effect of graphene on the control of microcracks in the cement matrix.
- The uniaxial tensile mechanical properties of C-S-H and G/C-S-H in the three directions were simulated using molecular dynamics methods, and the results show that the C-S-H gel exhibited different mechanical properties in the three directions. The tensile strength of C-S-H in the x, y, and z directions is 3.31 Gpa, 3.74 Gpa, and 2.37 Gpa, respectively. The uniaxial tensile stress–strain curve of C-S-H in the x direction is basically consistent with the trend in the y direction, and the tensile stress in the z direction is lower, which is caused by the different layered structures of the C-S-H in the three directions.
- By inserting graphene in the x, y, and z directions of C-S-H, the tensile process of G/C-S-H was simulated. The results showed that the insertion of graphene in the x and z directions increased the tensile strength in the three directions, while the insertion of graphene in the y direction decreased the tensile strength of the G/C-S-H in the three directions.
- The results of uniaxial tensile simulations of C-S-H and G/C-S-H at different temperatures show that the tensile stress decreases slowly with increasing temperature. Meanwhile, the uniaxial tensile strength of C-S-H and G/C-S-H increases with the increase in loading rate.
- The effects of three kinds of defects and concentrations of graphene on the uniaxial tensile properties of G/C-S-H were studied. The tensile stress of monovacancy vacancy defects and diatomic vacancy defects at different concentrations is ordered as follows: . And the tensile strength of S-W defects at different concentrations is ordered as follows: .
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Shahsavari, R.; Buehler, M.J.; Pellenq, R.J.; Ulm, F. First-principles study of elastic constants and interlayer interactions of complex hydrated oxides: Case study of tobermorite and jennite. J. Am. Ceram. Soc. 2009, 92, 2323–2330. [Google Scholar] [CrossRef]
- Hamid, S.A. The crystal structure of the 11Å natural tobermorite Ca2.25 [Si3O7.5 (OH)1.5]·1H2O. Z. Für Krist.-Cryst. Mater. 1981, 154, 189–198. [Google Scholar] [CrossRef]
- Merlino, S.; Bonaccorsi, E.; Armbruster, T. The real structure of tobermorite 11A: Normal and anomalous forms, OD character and polytypic modifications. Eur. J. Mineral. 2001, 13, 577–590. [Google Scholar] [CrossRef]
- Qomi, M.A.; Krakowiak, K.; Bauchy, M.; Stewart, K.; Shahsavari, R.; Jagannathan, D.; Brommer, D.; Baronnet, A.; Buehler, M.; Yip, S.; et al. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef]
- Hou, D.; Zhu, Y.; Lu, Y.; Li, Z. Mechanical properties of calcium silicate hydrate (C–S–H) at nano-scale: A molecular dynamics study. Mater. Chem. Phys. 2014, 146, 503–511. [Google Scholar] [CrossRef]
- Dolado, J.S.; Griebel, M.; Hamaekers, J.; Heber, F. The nano-branched structure of cementitious calcium–silicate–hydrate gel. J. Mater. Chem. 2011, 21, 4445–4449. [Google Scholar] [CrossRef]
- Kovačević, G.; Persson, B.; Nicoleau, L.; Nonat, A.; Veryazov, V. Atomistic modeling of crystal structure of Ca1.67SiHx. Cem. Concr. Res. 2015, 67, 197–203. [Google Scholar] [CrossRef]
- Bauchy, M.; Qomi, M.J.; Ulm, F.J.; Pellenq, R.M. Order and disorder in calcium–silicate–hydrate. J. Chem. Phys. 2014, 140, 214503. [Google Scholar] [CrossRef]
- Wan, X.; Hou, D.; Zhao, T.; Wang, L. Insights on molecular structure and micro-properties of alkali-activated slag materials: A reactive molecular dynamics study. Constr. Build. Mater. 2017, 139, 430–437. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Konsta-Gdoutos, M.S.; Shah, S.P. Carbon nanofiber cementitious composites: Effect of debulking procedure on dispersion and reinforcing efficiency. Cem. Concr. Compos. 2013, 36, 25–32. [Google Scholar] [CrossRef]
- Mokhtar, M.; Abo-El-Enein, S.; Hassaan, M.; Morsy, M.; Khalil, M. Mechanical performance, pore structure and micro-structural characteristics of graphene oxide nano platelets reinforced cement. Constr. Build. Mater. 2017, 138, 333–339. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.A.; Sapidis, G.; Papastergiadis, E. Fundamental properties of epoxy resin-modified cement grouts. Constr. Build. Mater. 2016, 125, 184–195. [Google Scholar] [CrossRef]
- Murray, S.J.; Subramani, V.J.; Selvam, R.P.; Hall, K.D. Molecular dynamics to understand the mechanical behavior of cement paste. Transp. Res. Rec. 2010, 2142, 75–82. [Google Scholar] [CrossRef]
- Bauchy, M.; Laubie, H.; Qomi, M.A.; Hoover, C.G.; Ulm, F.J.; Pellenq, R.M. Fracture toughness of calcium–silicate–hydrate from molecular dynamics simulations. J. Non-Cryst. Solids 2015, 419, 58–64. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.M.; Li, W.G.; Li, C.Y.; Sanjayan, J.G.; Duan, W.H.; Li, Z. Effects of graphene oxide agglomerates on workability, hydration, microstructure and compressive strength of cement paste. Constr. Build. Mater. 2017, 145, 402–410. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Sabziparvar, A.; Hosseini, E.; Chiniforush, V.; Korayem, A. Barriers to achieving highly dispersed graphene oxide in cementitious composites: An experimental and computational study. Constr. Build. Mater. 2019, 199, 269–278. [Google Scholar] [CrossRef]
- Hou, D.; Lu, Z.; Li, X.; Ma, H.; Li, Z. Reactive molecular dynamics and experimental study of graphene-cement composites: Structure, dynamics and reinforcement mechanisms. Carbon 2017, 115, 188–208. [Google Scholar] [CrossRef]
- Bunch, J.S.; van der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Electromechanical resonators from graphene sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, J. Interfacial heat transfer behavior of graphene-based filler and calcium-silicate-hydrate in cement composites. Int. J. Heat Mass Transf. 2021, 176, 121165. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiong, C.; Peng, Z.; Huang, J.; Chang, H. Molecular dynamics simulation of the interfacial interaction mechanism between functional groups on graphene-based two-dimensional matrix and calcium silicate hydrate. Constr. Build. Mater. 2021, 284, 122804. [Google Scholar] [CrossRef]
- Alkhateb, H.; Al-Ostaz, A.; Cheng, A.H.-D.; Li, X. Materials genome for graphene-cement nanocomposites. J. Nanomechanics Micromechanics 2013, 3, 67–77. [Google Scholar] [CrossRef]
- Mao, S.; Yao, W. Enhancing the mechanical properties of calcium silicate hydrate by engineering graphene oxide structures via molecular dynamics simulations. Mol. Simul. 2023, 49, 351–364. [Google Scholar] [CrossRef]
- Pellenq, R.J.M.; Kushima, A.; Shahsavari, R.; Van Vliet, K.J.; Buehler, M.J.; Yip, S.; Ulm, F.J. A realistic molecular model of cement hydrates. Proc. Natl. Acad. Sci. USA 2009, 106, 16102–16107. [Google Scholar] [CrossRef]
- Puibasset, J.; Pellenq, R.J.M. Water adsorption on hydrophilic mesoporous and plane silica substrates: A grand canonical Monte Carlo simulation study. J. Chem. Phys. 2003, 118, 5613–5622. [Google Scholar] [CrossRef]
- Silva, W.R.L.; Němeček, J.; Štemberk, P. Application of multiscale elastic homogenization based on nanoindentation for high performance concrete. Adv. Eng. Softw. 2013, 62, 109–118. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- Wu, X.; Han, Q. Thermal conductivity of defective graphene: An efficient molecular dynamics study based on graphics processing units. Nanotechnology 2020, 31, 215708. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lan, F.; Wang, Y.; Lin, X.; Zhao, Y.; Zhen, Q.; Chen, D. Molecular Dynamics Simulation and Experimental Study of Mechanical Properties of Graphene–Cement Composites. Materials 2024, 17, 410. https://doi.org/10.3390/ma17020410
Li H, Lan F, Wang Y, Lin X, Zhao Y, Zhen Q, Chen D. Molecular Dynamics Simulation and Experimental Study of Mechanical Properties of Graphene–Cement Composites. Materials. 2024; 17(2):410. https://doi.org/10.3390/ma17020410
Chicago/Turabian StyleLi, Henggan, Fupeng Lan, Yulin Wang, Xiaotian Lin, Yan Zhao, Qi Zhen, and Dehong Chen. 2024. "Molecular Dynamics Simulation and Experimental Study of Mechanical Properties of Graphene–Cement Composites" Materials 17, no. 2: 410. https://doi.org/10.3390/ma17020410
APA StyleLi, H., Lan, F., Wang, Y., Lin, X., Zhao, Y., Zhen, Q., & Chen, D. (2024). Molecular Dynamics Simulation and Experimental Study of Mechanical Properties of Graphene–Cement Composites. Materials, 17(2), 410. https://doi.org/10.3390/ma17020410





