Abstract
With the advancement of industrial economies, incidents involving spills of petroleum products have become increasingly frequent. The resulting pollutants pose significant threats to air, water, soil, plant and animal survival, as well as human health. In this study, microcrystalline cellulose served as the matrix and benzoyl peroxide (BPO) as the initiator, while butyl acrylate (BA) and N,N′-methylene bisacrylamide (MBA) were employed as graft monomers. Through free radical graft polymerization, cellulose-graft-poly(butyl acrylate-N,N′-methylene bisacrylamide) [Cell-g-P(BA-MBA)], possessing oil-adsorbing properties, was synthesized. The chemical structure, elemental composition, surface morphology and wetting properties of the graft polymerization products have been characterized, using infrared spectroscopy, elemental analysis, scanning electron microscopy and contact angle testing. The adsorption properties of Cell-g-P(BA-MBA) for various organic solvents and oils were then assessed. The experimental results demonstrated that Cell-g-P(BA-MBA) exhibited a maximum adsorption capacity of 37.55 g/g for trichloromethane. Adsorption kinetics experiments indicated a spontaneous and exothermic process involving physical adsorption, conforming to the Freundlich isotherm model. Furthermore, adsorption kinetics experiments revealed that Cell-g-P(BA-MBA) displayed favorable reuse and regeneration performance, maintaining its adsorption capacity essentially unchanged over fifteen adsorption–desorption cycles.
1. Introduction
With rapid development of modern society and economic systems, demand for various petroleum products and organic solvents has surged significantly. However, in the exploration, transportation, storage, processing, refining and utilization of petroleum, leakage accidents frequently occur, resulting in substantial waste of fossil resources and simultaneously causing severe ecological and environmental problems [1,2]. Notable tragedies include the Gulf of Mexico oil spill in 2010 and the MV Wakashio (Mauritius) oil spill in 2020 [3,4,5]. Therefore, there is a pressing need to promptly and effectively manage oil pollution to minimize economic losses and mitigate harm to ecological environments and human health.
Currently, treatment methods for oil pollution can be broadly categorized into physical, in situ combustion, chemical and biological methods [6,7]. Among these, physical methods employing oil-absorbing materials prove to be one of the swiftest approaches for removing oil spills from water surfaces [8]. In comparison with chemical and in situ combustion methods, oil-adsorbing materials have minimal or no negative impact on the environment [9,10,11,12]. These materials can be classified into three main groups: natural inorganic materials, natural organic materials and synthetic organic materials [13]. Variations in lipophilicity, mechanical strength, oil-adsorption capacity, oil retention properties and recyclability exist among these materials. Polymers, such as polyurethane, polypropylene and polyacrylate, are widely utilized as oil-adsorbing materials [2,14,15].
Ideal oil-adsorbent materials should possess such properties as high oil adsorption, high oil retention, low water adsorption and reusability, in addition to being low-cost, abundant, biodegradable and mechanically strong [16]. Considering these criteria, biopolymer materials derived from agricultural and industrial wastes are gaining increased attention [17,18].
Cellulose, widely available and inexpensive, exhibits good biodegradability, biocompatibility and mechanical properties [19,20]. M. D. Teli et al. successfully prepared natural banana fiber oil-adsorbing materials using potassium persulfate as an initiator, achieving the highest adsorption capacity for 14.45 g/g of crude oil at a monomer ratio of 1.5:1 [21]. Yang et al. synthesized RSMs-g-P(MMA-co-BA), a modified oil-adsorbing material derived from rapeseed hectoliter, using free radical graft polymerization. This modification improved mechanical and oil-adsorption properties of the polymer, maintaining an 83.42% adsorption capacity after eight repeated oil adsorptions [22]. Another study by Rong et al. involved surface activation and vinyltriethoxysilane modification of carbonized pollen particles, resulting in a three-dimensional composite resin material with an adsorption capacity of 58.8 g/g for carbon tetrachloride [23]. S Vigu et al. enhanced the oil-adsorption capacity of nettle fibers through butyl acrylate graft polymerization, achieving maximum adsorption capacities of 36.60 g/g for crude oil and 25.56 g/g for vegetable oil, surpassing those of commercial polypropylene materials [24].
In this study, a novel cellulose-based oil-adsorbing material, Cell-g-P(BA-MBA), was prepared through free radical graft polymerization using microcrystalline cellulose as a substrate, demonstrating excellent oil–water separation performance. The successful preparation of Cell-g-P(BA-MBA) was confirmed through infrared spectral analysis, elemental analysis, scanning electron microscopy analysis and contact angle analysis. This study has also investigated the adsorption kinetics, adsorption isotherm and adsorption thermodynamics of Cell-g-P(BA-MBA) for oil adsorption, as well as oil–water separation and regeneration performance.
2. Materials and Methods
2.1. Materials and Reagents
Microcrystalline cellulose (MCC) with a particle size of ≤25 μm (Aladdin’s reagent) served as the matrix, while butyl acrylate (BA) of analytical reagent grade, provided by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. was utilized as the monomer. Initiator benzoyl peroxide (BPO) of chemically pure grade was obtained from Jiangsu Strong Functional Chemistry Co., Ltd. Changshu, China. while the cross-linking agent was N,N′-methylene bis-acrylamide (MBA) (analytical reagent grade Macklin reagent, Shanghai, China). All materials and reagents were used as received without further purification.
2.2. Adsorbent Preparation and Characterization
1 g of Pre-soaked cellulose was introduced into a 250 mL four-necked flask and subjected to nitrogen purging for 30 min at 70 °C in a constant-temperature water bath. A prepared initiator solution of 0.09 g of benzoyl peroxide (BPO) was weighed. Under nitrogen protection, stirring commenced and the pre-prepared BPO solution was slowly added over 20 min. Subsequent to a 30 min addition of the initiator solution, 7 g of butyl acrylate (BA) monomer was added dropwise within 10 min. Following the addition of BA monomer for 30 min, a reactive monomer solution containing MBA and the remaining BA was added dropwise. The MBA dosage was 0.35 wt% of the BA monomer dosage, and the dropwise addition of the monomer solution lasted for 30 min. Upon completion of the reaction, the crude product underwent filtration and multiple washes with acetone and deionized water to eliminate unreacted initiator and monomer. Soxhlet extraction with acetone as the solvent was then conducted for 24 h to remove homopolymer of butyl acrylate. The resulting product was subjected to a vacuum drying oven at 60 °C until a constant weight was achieved. The dried product was then weighed. The reaction principle is illustrated in Scheme 1.
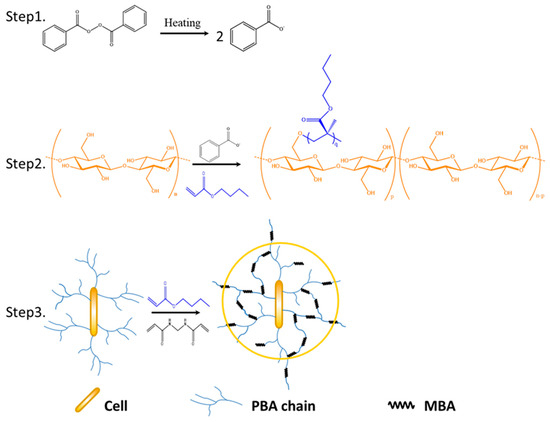
Scheme 1.
Synthetic route of Cell-g-P(BA-MBA).
The preparation of Cell-g-PBA resin followed the same procedure as described above, excluding the addition of MBA during the process. Similarly, the preparation of P(BA-MBA) resin reflected the above process, with the exception that cellulose was omitted from the preparation steps.
Structure and morphology of products were examined using an FEI Nova NanoSEM 450 field emission scanning electron microscope from FEI, Hillsboro, OR, USA, operating at an accelerating voltage of 5 kV. Sample powders were affixed and coated with gold using conductive adhesive. Infrared spectra of microcrystalline cellulose, Cell-g-PBA, P(BA-MBA) and Cell-g-(PBA-MBA) were obtained with a VERTEX70 infrared spectrometer from Bruker, Germany, within a scanning range of 4000–400 cm−1. Prior to testing, samples were subjected to a 12 h drying period in a vacuum drying oven. Elemental analysis experiments determined the carbon (C), hydrogen (H) and nitrogen (N) content of microcrystalline cellulose, Cell-g-PBA, Cell-g-(PBA-MBA) and P(BA-MBA). The Vario EL elemental analyzer from Elementar, Germany, employing helium as purge and carrier gas, served as the testing instrument. The water contact angle of samples was measured using a DSA25 contact angle meter from Kruss, Hamburg, Germany. This test, conducted at room temperature, involved the application of 5 μL water droplets on a dry, flat sample surface whenever possible.
2.3. Adsorption Capacity of Oils and Organic Solvents
Adsorption capacity of Cell-g-P(BA-MBA) for 10 different oils and organic solvents was determined using the weighing method. Initially, dry oil-adsorbing material with mass m1 (approximately 0.1 g) was weighed and enclosed in a non-woven bag. Subsequently, it was fully immersed in 150 mL of respective oils or organic solvents at room temperature. After 12 h of oil adsorption, the material was promptly removed and drained for 1 min until no more droplets fell. Residual oils on the sample surface were wiped off using filter paper, and the mass of the sample (m2) was immediately recorded. This approach minimized the impact of oil and solvent volatilization on the experimental results. The adsorption capacity (Q) of Cell-g-P(BA-MBA) for various oils and organic solvents was calculated using Equation (1):
where Q (g/g) denotes the adsorption capacity per unit mass of dry oil-adsorbing material and m1 (g) and m2 (g) denote the mass of oil-adsorbing material before and after adsorption, respectively.
A control experiment using microcrystalline cellulose as the oil-adsorbing material was conducted under identical conditions.
Adsorption experiments of Cell-g-P(BA-MBA) and microcrystalline cellulose on different oils and organic solvents were repeated three times under consistent experimental conditions, with the final average value considered as the experimental result.
2.4. Oil–Water Separation Performance
To demonstrate the practical application of Cell-g-P(BA-MBA) in oil contamination treatment, 5 mL of Sudan III-stained toluene was dropwise added to a beaker containing 100 mL of water. Subsequently, 0.3 g of Cell-g-P(BA-MBA) was weighed and immersed into the oil–water mixture. The observation, photography and documentation were conducted at two-minute intervals.
2.5. Regeneration Performance
The solvent elution method was employed for regenerating Cell-g-P(BA-MBA), using hexane as the extractant [25]. The specific experimental procedure involved weighing 0.1 g of the oil-adsorbing material, placing it in a nonwoven bag and adsorbing it in 150 mL of gasoline for 12 h at room temperature. After draining and weighing to record the mass, the adsorption capacity was calculated according to Equation (1). Subsequently, the saturated oil-adsorbing material was immersed in 30 mL of hexane solution for 5 h. Following removal, draining and weighing, the mass was recorded and the adsorption capacity was calculated again using Equation (1). This procedure was repeated 15 times in gasoline and hexane to determine the adsorption capacity of Cell-g-P(BA-MBA) after each adsorption and desorption. Each set of experiments was repeated three times under the same conditions, and the final average value was considered as the experimental result.
3. Results and Discussion
3.1. Characterization of Polymers
The surface morphology of Microcrystalline Cellulose (MCC), Cell-g-Poly(butyl acrylate) (Cell-g-PBA), Cell-g-Poly(butyl acrylate-co-N,N′-methylenebisacrylamide) (Cell-g-P(BA-MBA)) and Poly(butyl acrylate-co-N,N′-methylenebisacrylamide) (P(BA-MBA)) was observed and analyzed using scanning electron microscopy, as depicted in Figure 1. In Figure 1a, the pristine microcrystalline cellulose appears rod-like with uneven sizes, having a longitudinal length of ≤25 μm. Upon magnification, the microcrystalline cellulose surface is observed to be nearly smooth with slight folds. Figure 1b,c reveal that polymer chains are cross-linked and entangled, accumulating on the microcrystalline cellulose surface, resulting in rougher surfaces for Cell-g-PBA and Cell-g-P(BA-MBA). Figure 1d illustrates the smooth surface morphology of P(BA-MBA). The enlarged view in Figure 1c reveals abundant folds and a small pore structure on the surface of Cell-g-P(BA-MBA), enhancing oil molecule penetration and increasing the specific surface area, thereby improving the oil adsorbent performance [26].
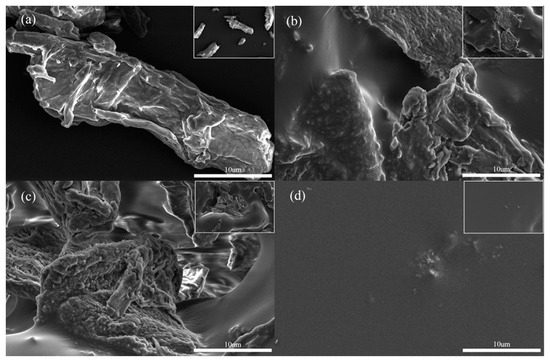
Figure 1.
Scanning electron micrographs of (a) MCC, (b) Cell-g-PBA, (c) Cell-g-P(BA-MBA) and (d) P(BA-MBA).
To affirm successful preparation of Cell-g-P(BA-MBA), chemical structures of microcrystalline cellulose, Cell-g-PBA, Cell-g-P(BA-MBA) and P(BA-MBA) were characterized and comparatively analyzed using Fourier Transform Infrared (FT-IR) spectroscopy, as presented in Figure 2. In infrared spectra of microcrystalline cellulose (curve (a)), characteristic peaks include a broad adsorption band with a maximum at near 3335 cm−1 corresponding to the stretching vibrational adsorption of the hydroxyl H-O bond [27], a weak band with a maximum at 2893 cm−1 indicating the stretching vibrational adsorption of the methylene C-H bond [28] and a weak symmetric stretching vibrational adsorption band with a maximum at 1159 cm−1 for the C-O-C bond [29]. Cell-g-PBA and Cell-g-P(BA-MBA) exhibit new peaks, such as asymmetric and symmetric stretching vibrational adsorption peaks of methyl and methylene C-H bonds at 2959 cm−1 and 2874 cm−1 [30], a strong band with a maximum at 1730 cm−1 due to the stretching vibration of the C=O bond of the ester carbonyl group and an asymmetric stretching vibrational adsorption band with a maximum at 1450 cm−1 of the methyl C-H bond [22]. These peaks align with those in the infrared spectra of P(BA-MBA), confirming successful grafting of the P(BA-MBA) copolymer onto microcrystalline cellulose. Furthermore, the intensity of the symmetric stretching vibration adsorption peak of the C-O-C bond at 1159 cm−1 in curves (b) and (c) is significantly higher than that of microcrystalline cellulose, which can be attributed to the increased C-O-C content in the ester group following butyl acrylate grafting.
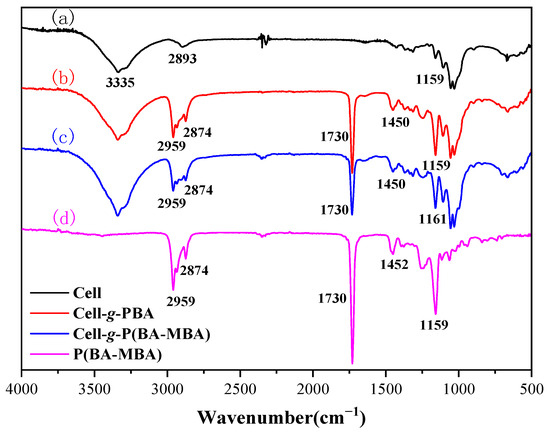
Figure 2.
FT-IR spectra of (a) MCC, (b) Cell-g-PBA, (c) Cell-g-P(BA-MBA) and (d) P(BA-MBA).
Elemental analysis was conducted to determine contents of C, H and N in microcrystalline cellulose, Cell-g-PBA, Cell-g-P(BA-MBA) and P(BA-MBA). The results, presented in Table 1, indicate significantly higher C and H contents in Cell-g-PBA compared to microcrystalline cellulose, affirming successful grafting of butyl acrylate onto microcrystalline cellulose. The presence of element N in Cell-g-P(BA-MBA) and P(BA-MBA) confirm successful grafting of the MBA monomer onto cellulose. Notably, the small amount of elemental N detected in the elemental analysis reflects the use of a minimal MBA monomer quantity in the preparation process to maintain optimal oil-adsorbing properties.

Table 1.
Content of C, H, N in microcrystalline cellulose, Cell-g-PBA, Cell-g-P(BA-MBA) and P(BA-MBA).
Wetting properties, crucial for oil-adsorbing material applications in oil–water separation, were assessed using water contact angle tests. Figure 3a reveals a water contact angle of 18.9° for MCC due to the hydrophilic nature of its surface hydroxyl groups. Figure 3b demonstrates that Cell-g-P(BA-MBA) attains a water contact angle of 101.3°, resulting from entangled polymer chains formed upon grafting hydrophobic BA monomers onto MCC. The presence of folds and a small pore structure on the surface of Cell-g-P(BA-MBA) enhances its hydrophobicity and lipophilicity, offering an excellent oil–water separation performance.
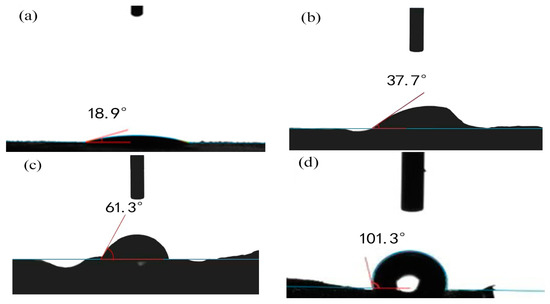
Figure 3.
Images of water contact angles of (a) MCC, (b) P(BA-MBA), (c) Cell-g-PBA and (d) Cell-g-P(BA-MBA).
3.2. Adsorption Property Testing
Oil adsorption experiments were conducted to evaluate the adsorption capacity of Cell-g-P(BA-MBA) for various oils and organic solvents under identical conditions. The results, illustrated in Figure 4 and Figure 5, reveal a substantial influence of oil and solvent types on the adsorption capacity of Cell-g-P(BA-MBA). The material exhibits maximum average adsorption capacities of 37.55 g/g for trichloromethane, 18.72 g/g for toluene, 9.46 g/g for acetone, 7.37 g/g for N,N-dimethylformamide and 10.41 g/g for gasoline. Figure 5 shows that the maximum average adsorption capacity of Cell-g-P(BA-MBA) for n-hexane, diesel oil, soybean oil, motor oil and vacuum pump oil was in the range of 0.54 g/g–0.90 g/g. The order of adsorption capacity for different organic solvents is trichloromethane > toluene > acetone > N,N-dimethylformamide > hexane. This order correlates with the density of the selected organic solvents, suggesting that a higher density positively impacts the adsorption capacity. Additionally, the adsorption capacity of Cell-g-P(BA-MBA) for N,N-dimethylformamide, despite its lower density than acetone, is attributed to differences in polarity, where acetone interacts more strongly with Cell-g-P(BA-MBA). From Figure 5, it can be seen that the adsorption capacity of microcrystalline cellulose on diesel oil, soybean oil, motor oil and vacuum pump oil is significantly higher than that of Cell-g-P(BA-MBA), which is due to the fact that the microcrystalline cellulose used in the oil-adsorption experiments is in the form of a powder and its specific surface area is much larger than that of the Cell-g-P(BA-MBA) oil-adsorbent material, and in addition, the four kinds of oils have a larger viscosity and are therefore more likely to be adsorbed on the surface of the microcrystalline cellulose [31].
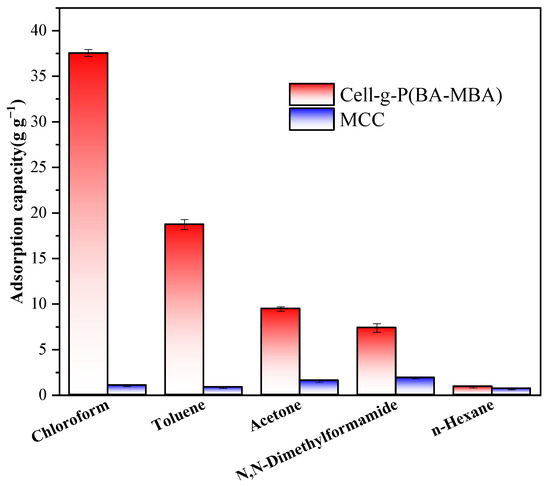
Figure 4.
Adsorption capacity of Cell-g-P(BA-MBA) for selected organic solvents.
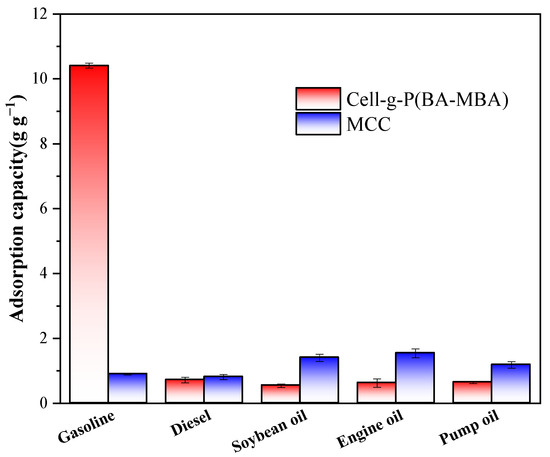
Figure 5.
Adsorption capacity of Cell-g-P(BA-MBA) for selected oils.
Cell-g-P(BA-MBA) demonstrates superior oil-adsorption performance compared to several other oil-adsorbing materials, as indicated in Table 2.

Table 2.
Comparison of oil-adsorption capacity of several reported adsorbents.
3.3. Adsorption Kinetics
The oil-adsorption mechanism of Cell-g-P(BA-MBA) was systematically examined through adsorption kinetic experiments. Figure 6 and Figure 7 illustrate adsorption capacities of trichloromethane, toluene and gasoline over varying contact times. These graphs reveal a consistent pattern across three solvents, wherein adsorption rates for trichloromethane, toluene and gasoline were notably rapid within the initial 180 min. Subsequently, the rate of increase in oil-adsorption capacity diminished and the adsorption rate gradually decelerated over the subsequent 7 h. By the 10th hour of contact time, the adsorption process approached near-equilibrium levels. This stabilization persisted beyond the 10 h mark, signifying that adsorption equilibrium was nearly attained.
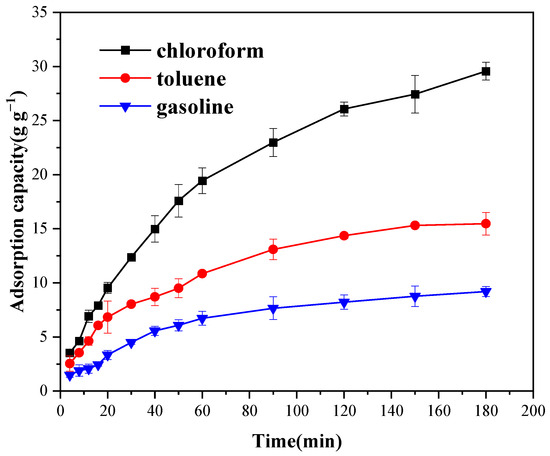
Figure 6.
Influence of contact time on oil-adsorption capacity of Cell-g-P(BA-MBA) for oils and organic solvents within 180 min.
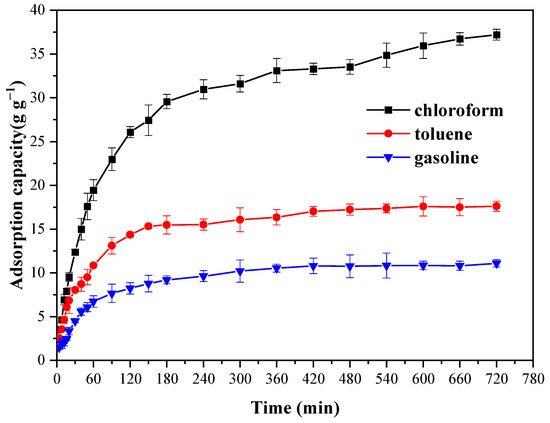
Figure 7.
Influence of contact time on oil-adsorption capacity of Cell-g-P(BA-MBA) for oils and organic solvents within 720 min.
To elucidate the adsorption mechanism of Cell-g-P(BA-MBA) on oil and organic solvents, both a pseudo-first-order kinetic model and pseudo-second-order kinetic model were employed for non-linear fitting of the experimental data [36]. The pseudo-first-order and pseudo-second-order equation can be expressed correctly in nonlinear forms:
where qe and qt are the amounts of adsorbate uptake per mass of adsorbent at equilibrium and at any time t (min), respectively; k1 (min−1) and k2 (g/g min−1) are the rate constant of the pseudo-first-order and pseudo-second-order equation, respectively. The outcomes of this fitting process are depicted in Figure 8 and Figure 9. The relevant kinetic modeling parameters, including k1 (min−1), k2 (g/g min−1), qe, calc (g/g) and R2, are detailed in Table 3.
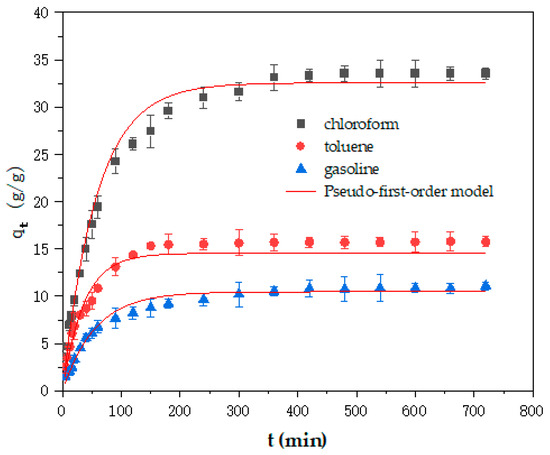
Figure 8.
The pseudo-first-order kinetics of the Cell-g-P(BA-MBA) for the adsorption of different organic solvents and crude oil.
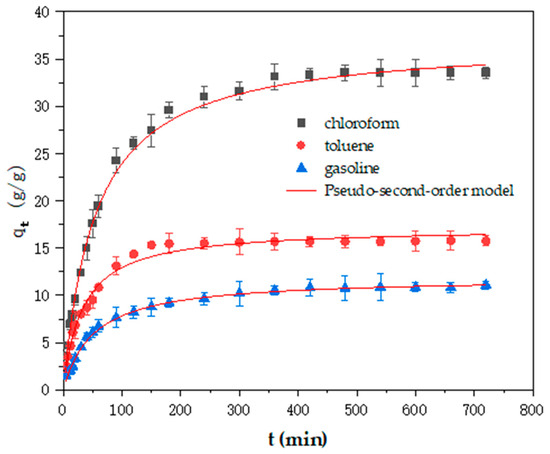
Figure 9.
The pseudo-second-order kinetics of the Cell-g-P(BA-MBA) for the adsorption of different organic solvents and crude oil.

Table 3.
Related parameters of pseudo-first-order kinetic model and pseudo-second-order kinetic model.
The analysis of Figure 8 and Figure 9 shows that the fitting degree of the pseudo-second-order kinetic model is significantly improved compared with the pseudo-first-order kinetic model. Upon scrutinizing the data, it becomes apparent that the correlation coefficients (R2 values) for the pseudo-second-order kinetic model in the adsorption process of Cell-g-P (BA-MBA) with three solvents consistently exceeded 0.98. Furthermore, the equilibrium adsorption capacities for trichloromethane, toluene and gasoline, as fitted by the pseudo-second-order kinetic model, closely reflected the actual values obtained from oil-adsorption experiments, registering at 36.98 g/g, 17.16 g/g and 11.88 g/g, respectively.
Upon comparison of the rate constants (k2) derived from the pseudo-second-order kinetic model, it was discerned that the adsorption rates of Cell-g-P(BA-MBA) for the three solvents followed the sequence of trichloromethane > toluene > gasoline. This order is consistent with the order of the adsorption capacity of Cell-g-P(BA-MBA) for the three solvents. The fitting results by the nonlinear method of the pseudo-second-order kinetic model are basically the same as the experimental data.
3.4. Adsorption Isotherm
The examination of adsorption equilibrium data through theoretical or empirical equations is crucial for understanding the interaction between adsorbent materials and adsorbates. The experimental data for gasoline adsorption were subjected to non-linear fitting using Langmuir and Freundlich isothermal adsorption models [37]. The Langmuir and Freundlich isothermal adsorption equation can be expressed correctly in nonlinear forms:
where qe is the amount of adsorbate uptake per mass of adsorbent at equilibrium, qm denotes the maximum amount of sorbate, KL is the thermodynamic equilibrium constant (a dimensionless quantity), Ce is the molar (or molal) concentration in the solution at the equilibrium and KF and n are constant.
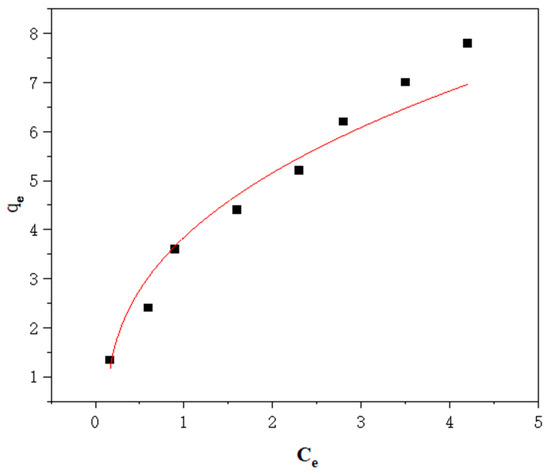
Figure 10.
Sorption of gasoline onto Cell-g-P(BA-MBA); data fitted according to the Langmuir model.

Figure 11.
Sorption of gasoline onto Cell-g-P(BA-MBA); data fitted according to the Freundlich model.

Table 4.
Related parameters of Langmuir adsorption isotherm and Freundlich adsorption isotherm.
Comparing the correlation parameters computed by the Langmuir and Freundlich adsorption isothermal models, it was evident that the linear correlation coefficient of the Freundlich adsorption isothermal model (R2 = 0.99094) was significantly higher than that of the Langmuir adsorption isothermal model (R2 = 0.94653). This observation suggests that the Freundlich adsorption isothermal model is more suitable for describing the adsorption process of Cell-g-P(BA-MBA) oil-adsorbing materials [38]. Unlike the Langmuir model, which assumes ideal conditions of uniform adsorption in a monomolecular layer, the Cell-g-P(BA-MBA) adsorbent material’s surface is non-homogeneous, and multilayer adsorption occurs during the adsorption process. Furthermore, the n value, representing adsorption intensity, falls between 1 and 2, indicating a moderate adsorption process [39].
3.5. Adsorption Thermodynamics
The results of fitting the experimental data on adsorption thermodynamics are depicted in Figure 12, and specific thermodynamic parameters are outlined in Table 5. As observed in Figure 12, the linear correlation coefficient R2 = 0.997, indicating a robust fit. From the table, it is evident that ∆G0 values are consistently negative across all temperatures, signifying the spontaneous nature of the adsorption process of Cell-g-P(BA-MBA) on gasoline. Absolute values of ∆G0 increase with decreasing temperature, suggesting that lower temperatures favor the adsorption process. According to the literature, the reported ∆G0 value for physical adsorption falls between −20 and 0 kJ mol−1, and the ∆G0 values obtained at different temperatures in this study are within this range, affirming the occurrence of physical adsorption during the adsorption process. Negative ∆H0 values indicate an exothermic adsorption process, with lower temperatures being more favorable, aligning with the previous observation. The magnitude of the ∆H0 value, less than 40 kJ mol−1, supports the notion that the adsorption process is classified as physisorption, further confirming the existence of physisorption in the adsorption process. Additionally, a negative ∆S0 value signifies a reduction in interfacial disorder during the adsorption process, indicative of a disorder-to-order transformation [40].
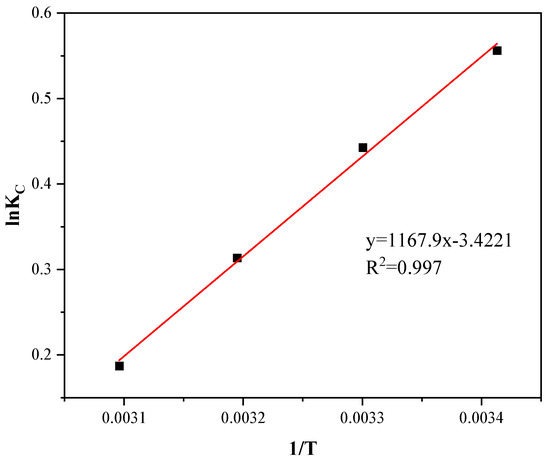
Figure 12.
Logarithm of the equilibrium constant for the sorption of gasoline onto Cell-g-P(BA-MBA) against 1/T.

Table 5.
Thermodynamic parameters for oil adsorption of Cell-g-P(BA-MBA) in gasoline/water system.
3.6. Oil-Water Separation Performance
The results of the oil–water separation experiments are depicted in Figure 13. Adsorption of toluene by Cell-g-P(BA-MBA) is remarkably swift; as illustrated in the figure, toluene on water surface is completely removed after approximately 12 min. Furthermore, throughout the adsorption process, the oil-adsorbing material consistently floated on the water surface, showcasing commendable buoyancy and enabling straightforward recovery. This experiment underscores that Cell-g-P(BA-MBA) exhibits notable advantages, including excellent buoyancy, a rapid oil-adsorption rate, outstanding oil–water separation performance and facile recyclability. These attributes position it as a promising candidate for deployment in emergency oil pollution treatment.
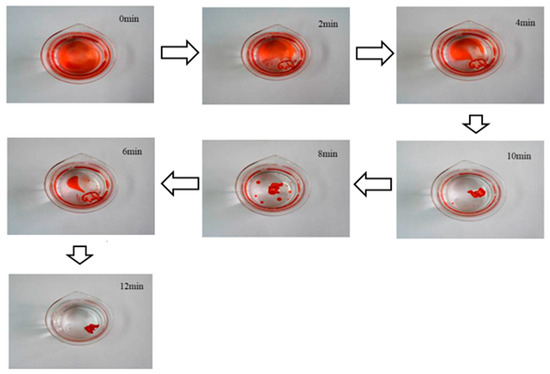
Figure 13.
Dynamic process of toluene adsorption on water surface by Cell-g-P(BA-MBA).
3.7. Regeneration Performance
Regeneration performance is a crucial property of oil-adsorbing materials in practical applications. In experiments, oil-adsorbing materials were reclaimed through solvent elution, and gasoline could be easily desorbed using n-hexane. The trend of the adsorption capacity of Cell-g-P(BA-MBA) on gasoline during 15 adsorption–desorption cycles is presented in Figure 14. After 15 cycles, the adsorption capacity of Cell-g-P(BA-MBA) on gasoline remained essentially unchanged compared to the initial adsorption, with no significant loss of sample mass. Cellulose in the Cell-g-P(BA-MBA) oil-adsorbing material contributed to a certain degree of mechanical strength, preserving the three-dimensional network structure. Furthermore, this experiment utilized a gentler solvent elution method for reuse, preventing damage to the structure of the Cell-g-P(BA-MBA) oil-adsorbing material in contrast to methods like drying and extrusion. These findings underscore the excellent regeneration properties of the Cell-g-P(BA-MBA) oil-adsorbing material.
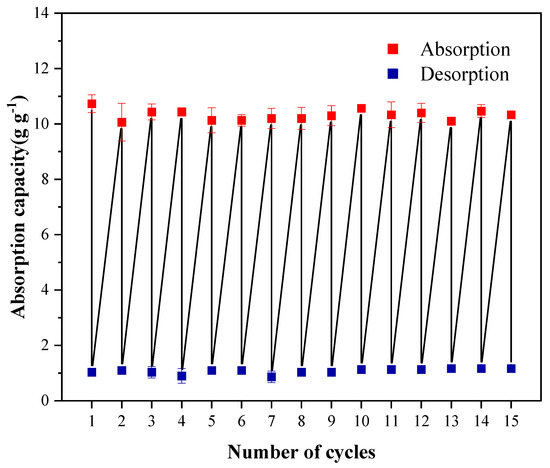
Figure 14.
Recyclability of Cell-g-P(BA-MBA) adsorbents in gasoline.
4. Conclusions
In this study, butyl acrylate was grafted onto cellulose through free radical graft polymerization to fabricate a Cell-g-P(BA-MBA) oil-adsorbing material. The results from oil-adsorption experiments revealed that the maximum average adsorption capacities of Cell-g-P(BA-MBA) for trichloromethane, toluene, acetone, N,N-dimethylformamide and gasoline were 37.55 g/g, 18.72 g/g, 9.46 g/g, 7.37 g/g and 10.41 g/g, respectively. In addition, the maximum average adsorption capacity for n-hexane, diesel, soybean oil, engine oil and vacuum pump oil fell in the range of 0.54 g/g to 0.90 g/g. Adsorption kinetics experiments indicated that the adsorption process adhered to the quasi-secondary kinetic model, with the adsorption rate inversely correlated with the polarity of oil and organic solvent. Adsorption isotherm experiments demonstrated a non-uniform multilayer adsorption process, aligning with the Freundlich isotherm adsorption model. Thermodynamic investigations revealed the spontaneous and exothermic nature of Cell-g-P(BA-MBA) adsorption on gasoline, with lower temperatures favoring the adsorption process. The adsorption of toluene on the water surface confirmed the rapid oil treatment capability of Cell-g-P(BA-MBA). Regeneration performance experiments demonstrated that the oil-adsorption capacity of Cell-g-P(BA-MBA) remained essentially unchanged after 15 adsorption–desorption cycles. The prepared Cell-g-P(BA-MBA) exhibited notable adsorption capacities for various oils and organic solvents, coupled with economic efficiency, a straightforward preparation process and reusability, suggesting broad prospects for application due to these exceptional properties.
Author Contributions
Conceptualization, F.-J.M.; Methodology, X.-G.W.; Investigation, P.-X.G., X.-G.W. and J.-X.W.; Writing—original draft, P.-X.G.; Writing—review and editing, P.-X.G. and M.-Q.Y.; Supervision, M.-Q.Y.; Project administration, X.-G.W.; Funding acquisition, F.-J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Mao-Qian Yang was employed by the company Shandong Jianke Construction Materials Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hubbe, M.A.; Rojas, O.J.; Fingas, M.; Gupta, B.S. Cellulosic Substrates for Removal of Pollutants from Aqueous Systems: A Review. 3. Spilled Oil and Emulsified Organic Liquids. BioResources 2013, 8, 3038–3097. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Z.; Lu, Y.; Liu, Y.; Yang, D.; Li, Q.; Qiu, F. Recent progress and future prospects of oil-absorbing materials. Chin. J. Chem. Eng. 2019, 27, 1282–1295. [Google Scholar] [CrossRef]
- Atlas, R.M.; Hazen, T.C. Oil Biodegradation and Bioremediation: A Tale of the Two Worst Spills in US History. Environ. Sci. Technol. 2011, 45, 6709–6715. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.G. Ecological Impacts of the Deepwater Horizon Oil Spill: Implications for Immunotoxicity. Toxicol. Pathol. 2012, 40, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, A.G.; Nelson, R.K.; Gagnon, M.M.; Holman, A.I.; Reddy, C.M.; Sutton, P.A.; Grice, K. MV Wakashio grounding incident in Mauritius 2020: The world’s first major spillage of Very Low Sulfur Fuel Oil. Mar. Pollut. Bull. 2021, 171, 112917. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.M.T.M.; Saleem, J.; Bazargan, A.; Duarte, J.L.D.S.; McKay, G.; Meili, L. Sorption as a rapidly response for oil spill accidents: A material and mechanistic approach. J. Hazard. Mater. 2021, 407, 124842. [Google Scholar] [CrossRef] [PubMed]
- Patalano, A.; Villalobos, F.; Pena, P.; Jauregui, E.; Ozkan, C.; Ozkan, M. Scaling sorbent materials for real oil-sorbing applications and environmental disasters. MRS Energy Sustain. 2019, 6, e3. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Remediation technologies for marine oil spills: A critical review and comparative analysis. Am. J. Environ. Sci. 2011, 7, 423–440. [Google Scholar] [CrossRef]
- Ifelebuegu, A.O.; Johnson, A. Nonconventional low-cost cellulose- and keratin-based biopolymeric sorbents for oil/water separation and spill cleanup: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 964–1001. [Google Scholar] [CrossRef]
- Karan, C.P.; Rengasamy, R.S.; Das, D. Oil spill cleanup by structured fibre assembly. Indian J. Fibre Text. Res. 2011, 36, 190–200. [Google Scholar]
- Nyankson, E.; Rodene, D.; Gupta, R.B. Advancements in Crude Oil Spill Remediation Research after the Deepwater Horizon Oil Spill. Water Air Soil Pollut. 2016, 227, 29. [Google Scholar] [CrossRef]
- Zhang, N.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A Review on Oil/Water Mixture Separation Material. Ind. Eng. Chem. Res. 2020, 59, 14546–14568. [Google Scholar] [CrossRef]
- Dhaka, A.; Chattopadhyay, P. A review on physical remediation techniques for treatment of marine oil spills. J. Environ. Manag. 2021, 288, 112428. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, L.; Zhang, D.; Chen, X.; Xue, Y.; Guan, Y.; Li, Y. Mesoporous carbon spheres modified polyurethane sponge/phase change material composites: Photothermal conversion, thermal storage and efficient recovery of high-viscosity crude oil. Carbon 2023, 214, 118372. [Google Scholar] [CrossRef]
- Lee, H.; Trinh, B.M.; Crawford, E.A.; Mekonnen, T.H. Thermoplastic elastomer melt-blown fiber mats for oil spill remediation: Fabrication, oil uptake, and gel formation studies. Sep. Purif. Technol. 2023, 326, 124761. [Google Scholar] [CrossRef]
- El Allaoui, B.; Chakhtouna, H.; Zari, N.; Benzeid, H.; Qaiss, A.E.; Bouhfid, R. Superhydrophobic alkylsilane functionalized cellulose beads for efficient oil/water separation. J. Water Process. Eng. 2023, 54, 104015. [Google Scholar] [CrossRef]
- Mahmodi, G.; Bafti, R.R.; Boroujeni, N.I.; Pradhan, S.; Dangwal, S.; Sengupta, B.; Vatanpour, V.; Sorci, M.; Fathizadeh, M.; Bikkina, P.; et al. Improving cellulose acetate mixed matrix membranes by incorporating hydrophilic MIL-101(Cr)-NH2 nanoparticles for treating dye/salt solution. Chem. Eng. J. 2023, 477, 146736. [Google Scholar] [CrossRef]
- Kadili, J.A.; Abdullah, A.H.; Johari, I.S.; Zainuddin, N.; Jamil, S. Highly efficient and reusable superhydrophobic 3D polyurethane nanocomposite foam for remediation of oil polluted water. J. Porous Mater. 2023. [Google Scholar] [CrossRef]
- Quoc Ba, T.; Son Truong, N.; Duong Khanh, H.; Tuan Du, T.; Dat Minh, H.; Do, N.H.N.; Thao Phuong, L.; Le, P.K.; Le, D.K.; Nhan, P.-T.; et al. Cellulose-based aerogels from sugarcane bagasse for oil spill-cleaning and heat insulation applications. Carbohydr. Polym. 2020, 228, 115365. [Google Scholar]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petru, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef]
- Teli, M.D.; Valia, S.P. Grafting of Butyl Acrylate on to Banana Fibers for Improved Oil Absorption. J. Nat. Fibers 2016, 13, 470–476. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Almohsin, A.; Alsharaeh, E.H. Syntheses and fabrication of mesoporous styrene-co-methyl methacrylate-graphene composites for oil removal. Diam. Relat. Mater. 2022, 130, 109494. [Google Scholar] [CrossRef]
- Iordanskii, A.; Karpova, S.; Olkhov, A.; Borovikov, P.; Kildeeva, N.; Liu, Y. Structure-morphology impact upon segmental dynamics and diffusion in the biodegradable ultrafine fibers of polyhydroxybutyrate-polylactide blends. Eur. Polym. J. 2019, 117, 208–216. [Google Scholar] [CrossRef]
- Viju, S.; Brindha, R.; Thilagavathi, G. Surface modification of nettle fibers by grafting to improve oil sorption capacity. J. Ind. Text. 2019, 50, 1314–1329. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, T.; Yang, D.; Qiu, F.; Rong, J.; Xu, J.; Fang, J. The synthesis of hierarchical porous Al2O3/acrylic resin composites as durable, efficient and recyclable absorbents for oil/water separation. Chem. Eng. J. 2017, 309, 522–531. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Kang, Y.; Wang, A. Investigation of oil sorption capability of PBMA/SiO2 coated kapok fiber. Chem. Eng. J. 2013, 223, 632–637. [Google Scholar] [CrossRef]
- Littunen, K.; Hippi, U.; Johansson, L.-S.; Osterberg, M.; Tammelin, T.; Laine, J.; Seppala, J. Free radical graft copolymerization of nanofibrillated cellulose with acrylic monomers. Carbohydr. Polym. 2011, 84, 1039–1047. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Preparation and properties of kapok fiber enhanced oil sorption resins by suspended emulsion polymerization. J. Appl. Polym. Sci. 2013, 127, 2184–2191. [Google Scholar] [CrossRef]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana fiber Cellulose Nano Crystals grafted with butyl acrylate for heavy metal lead (II) removal. Int. J. Biol. Macromol. 2019, 131, 461–472. [Google Scholar]
- Siddiqui, M.N.; Redhwi, H.H.; Charitopoulou, D.; Achilias, D.S. Effect of organomodified clay on the reaction kinetics, properties and thermal degradation of nanocomposites based on poly(styrene-co-ethyl methacrylate). Polym. Int. 2014, 63, 766–777. [Google Scholar] [CrossRef]
- Devi, P.; Singh, J.; Rehmaan, H.; Kaushik, R.D. Novel Aloe-Vera and allyl methacrylate-based antimicrobial copolymer nanoemulsion for coating: An in-situ approach. J. Polym. Res. 2022, 29, 511. [Google Scholar] [CrossRef]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Pan, Q. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]
- Rizvi, A.; Chu, R.K.M.; Lee, J.H.; Park, C.B. Superhydrophobic and Oleophilic Open-Cell Foams from Fibrillar Blends of Polypropylene and Polytetrafluoroethylene. ACS Appl. Mater. Interfaces 2014, 6, 21131–21140. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, M.Y.; Yurtov, E.V. Pickering emulsions: Structure, properties and the use as colloidosomes and stimuli-responsive emulsions. Russ. Chem. Rev. 2022, 91, RCR5024. [Google Scholar] [CrossRef]
- Yan, L.; Li, Q.; Wang, X.; Song, H.; Chi, H.; Qiao, Y.; Zhai, Y.; Liu, D. Synthesis and Absorption Performance of Acrylic Ester and Hollow Fiber MgO Nanoparticle Resin Composite. Polym.-Plast. Technol. Eng. 2017, 56, 1857–1865. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Aldawsari, Y.F.; Alsharaeh, E.H. Three-dimensionallycross-linkedstyrene-methyl methacrylate-divinylbenzene terpolymer networks for organic solvents and crude oil absorption. J. Appl. Polym. Sci. 2021, 138, 9. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Mir Mohamad Sadeghi, G. Effect of Liquefied Lignin Content on Synthesis of Bio-based Polyurethane Foam for Oil Adsorption Application. J. Polym. Environ. 2020, 28, 892–905. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Safari, G.H.; Sharafi, K.; Kamani, H.; Jaafari, J. Adsorption of 4-Nitrophenol on calcium alginate-multiwall carbon nanotube beads: Modeling, kinetics, equilibriums and reusability studies. Int. J. Biol. Macromol. 2021, 185, 66–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).